Our trial of a single-tablet regimen containing elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate in antiretroviral therapy–naive HIV-2–infected individuals for 48 weeks, in a resource-limited setting, demonstrated favorable immunovirologic outcomes and was well tolerated.
Keywords: HIV-1
Abstract
Background
There is an urgent need for safe and effective antiretroviral therapy (ART) for human immunodeficiency virus type 2 (HIV-2) infection. We undertook the first clinical trial of a single-tablet regimen containing elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate (E/C/F/TDF) to assess its effectiveness in HIV-2–infected individuals in Senegal, West Africa.
Methods
HIV-2–infected, ART-naive adults with World Health Organization stage 3–4 disease or CD4 count <750 cells/μL were eligible for this 48-week, open-label trial. We analyzed HIV-2 viral loads (VL), CD4 counts, clinical and adverse events, mortality, and loss to follow-up.
Results
We enrolled 30 subjects who initiated E/C/F/TDF. Twenty-nine subjects completed 48 weeks of follow-up. The majority were female (80%). There were no deaths, no new AIDS-associated clinical events, and 1 loss to follow-up. The median baseline CD4 count was 408 (range, 34–747) cells/μL, which increased by a median 161 (range, 27–547) cells/μL at week 48. Twenty-five subjects had baseline HIV-2 VL of <50 copies/mL of plasma. In those with detectable HIV-2 VL, the median was 41 (range, 10–6135) copies/mL. Using a modified intent-to-treat analysis (US Food and Drug Administration Snapshot method), 28 of 30 (93.3%; 95% confidence interval, 77.9%–99.2%) had viral suppression at 48 weeks. The 1 subject with virologic failure had multidrug-resistant HIV-2 (reverse transcriptase mutation: K65R; integrase mutations: G140S and Q148R) detected at week 48. There were 8 grade 3–4 adverse events; none were deemed study related. Adherence and acceptability were good.
Conclusions
Our data suggest that E/C/F/TDF, a once-daily, single-tablet-regimen, is safe, effective, and well tolerated. Our findings support the use of integrase inhibitor–based regimens for HIV-2 treatment.
Clinical Trials Registration
Human immunodeficiency virus type 2 (HIV-2) is endemic in West Africa, with limited global spread primarily to countries with socioeconomic ties to the region [1–3]. There are an estimated 1–2 million HIV-2–infected individuals worldwide, including those dually infected with both human immunodeficiency virus type 1 (HIV-1) and HIV-2 [4–6]. Compared to HIV-1, the natural history of HIV-2 infection is characterized by a much longer asymptomatic stage, significantly lower plasma viral loads (pVLs), slower decline in CD4 cell count, lower mortality rate due to AIDS, lower rates of mother-to-child transmission, and lower rates of genital shedding and sexual transmission [7–16]. The reasons for these differences between HIV-1 and HIV-2 have yet to be fully explored.
Despite its lower virulence, a significant proportion of HIV-2–infected individuals progress to AIDS and may benefit from antiretroviral therapy (ART). In contrast to the significant amount of science and clinical experience guiding treatment of HIV-1 in developed and low- and middle-income countries [17, 18], little is known about ART directed against HIV-2. To date there has not been a single randomized clinical trial of ART for HIV-2 infection [2, 19], even though it has been >3 decades since the discovery of HIV-2 [20] and >2 decades since the landmark studies showed the benefit of highly active ART for people infected with HIV-1 [21–23]. Currently, 6 different classes of antiretrovirals (ARVs) are approved for treatment of HIV-1 (nucleoside/nucleotide reverse transcriptase inhibitors [NRTIs], nonnucleoside reverse transcriptase inhibitors [NNRTI], protease inhibitors [PIs], fusion inhibitors, CCR5-coreceptor entry inhibitors, and integrase inhibitors [INIs]), of which 4 show at least some in vitro activity against HIV-2 [24–33]. HIV-2 is intrinsically resistant to NNRTIs and the fusion inhibitor enfuvirtide (T-20) [24, 25, 34]. HIV-2 also exhibits intrinsic resistance to several HIV-1 PIs, with most studies indicating that saquinavir, lopinavir, and darunavir are the only potent PI against HIV-2 replication; however, PI cross-resistance is frequent [33, 35]. The INIs (raltegravir, elvitegravir, and dolutegravir) are highly potent against HIV-2 isolates [30, 31, 36, 37].
National and international guidelines [17, 18, 38, 39] for ART for HIV-2 infection are primarily based on cohort studies, small case series, individual case reports, in vitro data, and extrapolation from HIV-1. As is true for HIV-1, triple-NRTI regimens appear to be inferior to boosted PI-based regimens (ie, 2 NRTIs plus a PI) [40–42]. The most experience in treating HIV-2–infected individuals is with a fixed-dose combination of lopinavir/ritonavir plus 2 NRTIs (typically azidothymidine and lamivudine [3TC]), with HIV-2 viral suppression rates and CD4 count increases often substantially less than those seen in HIV-1 [42–44].
Anecdotal evidence from published case reports and clinical series of combination ARV regimens containing INIs suggest potential utility for treating HIV-2 infection [32, 45–50]. Overall, current ARV regimens for HIV-2 infection are suboptimal and substantially less effective than those for HIV-1, and new ART options for HIV-2 infection are urgently needed. We report the first trial of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate (E/C/F/TDF; Stribild, Gilead Sciences), an INI-based, single-tablet regimen, in HIV-2–infected, ARV-naive adults living in Senegal, West Africa.
METHODS
The trial was conducted at the Service des Maladies Infectieuses et Tropicales, Ibrahima Diop Mar, Centre Hospitalier National Universitaire de Fann, Universite Cheikh Anta Diop de Dakar, Senegal. The trial was approved by the University of Washington Institutional Review Board (IRB00005647; FWA00006878) and the Senegal Ethics Committee (IRB00002659; FWA00002691); all participants provided written informed consent. The trial was registered at ClinicalTrials.gov (NCT02180438). HIV-2–infected, ART-naive adults with World Health Organization (WHO) stage 3 or 4 disease [17] or CD4 counts <750 cells/μL were eligible for this open-label trial, with planned enrollment of 30 subjects and follow-up for 48 weeks. Exclusion criteria included HIV-1 or HIV-1/HIV-2 dual infection, pregnancy or breastfeeding, CD4 count >750 cells/μL, known allergy or contraindication to E/C/F/TDF, and/or active tuberculosis (secondary to contraindication of rifampin with E/C/F/TDF). A once-daily fixed-dose combination of elvitegravir (150 mg), cobicistat (150 mg), emtricitabine (200 mg), and tenofovir disoproxil fumarate (300 mg) (ie, E/C/F/TDF) was provided by the manufacturer (Gilead Sciences) and dispensed monthly to study subjects. Individuals with CD4 counts <350 cells/μL were given cotrimoxazole per WHO guidelines [17]. Enrolled subjects were monitored every 4 weeks for clinical and immunovirologic outcomes as well as adverse events (AEs).
For this 48-week analysis, changes in HIV-2 pVL, CD4 cell counts, AEs, all-cause mortality, and loss to follow-up were analyzed. CD4 counts were measured by the point-of-care Alere Pima CD4 test. HIV-2 pVL was measured on the Abbott m2000sp/rt platform (Abbott Molecular Diagnostics) [51]. The limit of quantification of the HIV-2 pVL assay was defined at <10 copies/mL; HIV-2 RNA detection was possible but not quantifiable below this limit [51]. There is considerable debate in the HIV-2 field regarding appropriate endpoints for clinical trials, given that a significant proportion of ART-naive, HIV-2–infected individuals maintain viral control and have undetectable or very low pVLs, and would meet HIV-1 criteria for “viral suppression” or “elite control” (<50 copies/mL) without ART [2, 19]. Given the potential problems involved with using only HIV-2 viral control/suppression at the primary biomarker endpoint, we used multiple prespecified endpoints, and these are reported independently. We used a modified intent-to-treat (mITT) analysis with noncompleter or missing data equal to failure, as well as the 48-week US Food and Drug Administration (FDA) Snapshot method, to define virologic failure. Prespecified primary outcomes were: death or new WHO stage 3 or 4 event or virologic failure (FDA Snapshot [HIV-2 pVL >50 and >400 copies/mL]) at 48 weeks. Prespecified secondary outcomes were grade 3 or 4 AEs (using the National Institute of Allergy and Infectious Diseases, Division of AIDS criteria), CD4 T-cell count at 48 weeks less than baseline, <50 cells/μL CD4 T-cell increase at 48 weeks from baseline, switching off E/C/F/TDF prior to 48 weeks, and/or development of drug resistance mutations to tenofovir disoproxil fumarate, emtricitabine, or elvitegravir. HIV-2 drug resistance mutations were evaluated in dried blood spot samples in 1 individual with virologic failure as previously described [29, 31]. HIV-2 drug resistance mutations were determined using the Stanford HIV resistance database (https://hivdb.stanford.edu) and the HIV-2EU resistance database (http://www.hiv-grade.de/HIV2EU/deployed/grade.pl?program=hivalg). Data were recorded on paper case report forms and stored in REDCap (https://redcap.iths.org). Statistical analyses were performed using Stata SE 14 software (StataCorp LLC, College Station, Texas). A complete trial protocol is available in the Supplementary Data.
RESULTS
The baseline characteristics of the 30 adult ARV-naive, HIV-2–infected subjects enrolled in the trial are shown in Table 1. The median age was 49 years, and the majority of participants were female. Seven individuals had WHO stage 3 or 4 HIV disease at entry. The median CD4 count was 408 cells/μL, with 4 individuals with CD4 counts <200 cells/μL, 7 individuals with CD4 counts between 200 and 350 cells/μL, 11 individuals with CD4 counts between 351 and 500 cells/μL, and 8 individuals with CD4 counts between 501 and 750 cells/μL. At study entry, HIV-2 pVL was detectable in 73%. Ten percent of participants were coinfected with hepatitis B virus.
Table 1.
Characteristics of Antiretroviral-naive Human Immunodeficiency Virus Type 2–infected Subjects Initiating Elvitegravir, Cobicistat, Emtricitabine, and Tenofovir Disoproxil Fumarate
| Characteristic | No. (%) or Median (IQR) |
| Screened | 35 |
| Enrolled | 30 |
| Female sex | 24 (80) |
| Age, median (range) | 49 (24–65) |
| Year of HIV diagnosis, median (IQR) | 2008 (2005–2015) |
| WHO stagea | |
| 1 | 13 (43.3) |
| 2 | 9 (30.0) |
| 3 | 6 (20.0) |
| 4 | 1 (3.3) |
| BMI, kg/m2, median (IQR) | 21.3 (18.0–29.0) |
| CD4 count, cells/μL | |
| Median (range) | 408 (34–747) |
| <200 | 4 (13.3) |
| 200–350 | 7 (23.3) |
| 351–500 | 11 (36.7) |
| 501–750 | 8 (26.7) |
| HIV-2 plasma viral load | |
| Not detected | 8 (26.7) |
| Detected, <10 copies/mL (quantifiable limit) | 7 (23.3) |
| Detected, copies/mL, median (IQR; range)b | 41 (28–53; 10–6135) |
| HBsAg positive | 3 (10) |
| Creatinine, mg/dL, median (IQR) | 0.8 (0.7–1.0) |
| Hemoglobin, g/dL median (IQR) | 12.3 (10.8–13.4) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: BMI, body mass index; HBsAg, hepatitis B surface antigen; HIV, human immunodeficiency virus; IQR, interquartile range; WHO, World Health Organization.
aMissing WHO stage: n = 1.
bAmong those with quantifiable viral loads.
There were no deaths or new, on treatment, WHO stage 3 or 4 clinical events. There was 1 loss to follow-up/self-withdrawal at week 4.
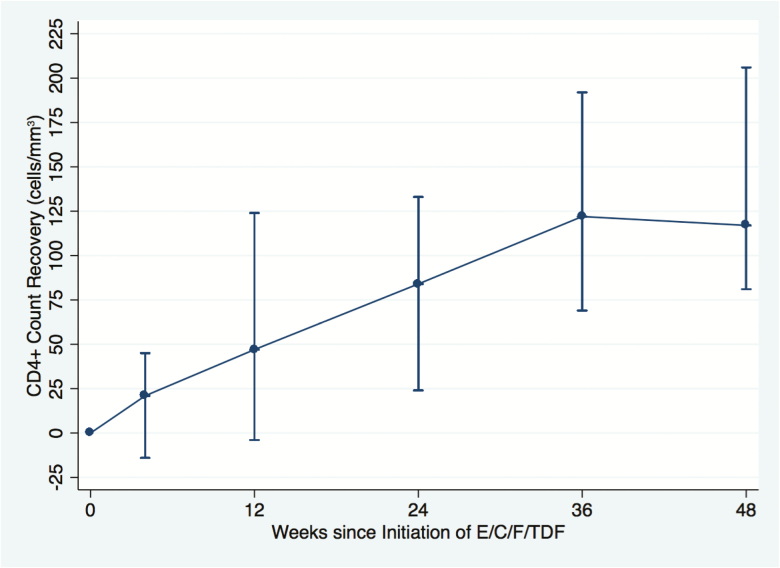
Using mITT analysis from week 0, CD4 count trajectory over 48 weeks is shown in Figure 1, and CD4 count increases from study entry (overall and stratified by CD4 count at entry) for those participants who completed 48 weeks of E/C/F/TDF are shown in Table 2. Of note, there was a median increase of >100 CD4 cells/μL over 48 weeks at all strata (<200, 200–350, 351–500, and 501–750 cells/μL) of the CD4 count at entry (Table 2). In addition, CD4 increases were similar irrespective of whether HIV-2 viral load was detectable or undetectable (cutoff <10 copies/mL) prior to initiation of ART (median CD4 count change over 48 weeks: 129 vs 182 cells/μL, respectively, P > .05; Mann-Whitney U test).
Figure 1.
Modified intent-to-treat analysis of CD4 cell count trajectory of human immunodeficiency virus type 2 (HIV-2)–infected patients on elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate (E/C/F/TDF) from week 0 to week 48 (n = 29/30). Missing CD4 data at week 0 (n = 1). Bars indicate median CD4 count change from baseline (with interquartile range).
Table 2.
Forty-eight-week CD4 Count Trajectory by Baseline CD4 Count at Antiretroviral Therapy Initiation With Elvitegravir, Cobicistat, Emtricitabine, and Tenofovir Disoproxil Fumarate
| Baselinea CD4 Count, Cells/μL | CD4 Increase at 48 wkb, Median (Range) |
|---|---|
| Overallc (387; 34–747) | 161 (27–547) |
| <200 | 121 (64–271) |
| 200–350 | 161 (58–241) |
| 351–500 | 111 (36–375) |
| 501–750 | 195 (27–547) |
aBaseline = screening visit.
bIn those individuals completing 48 weeks of antiretroviral therapy (n = 29).
cFor all CD4 count strata.
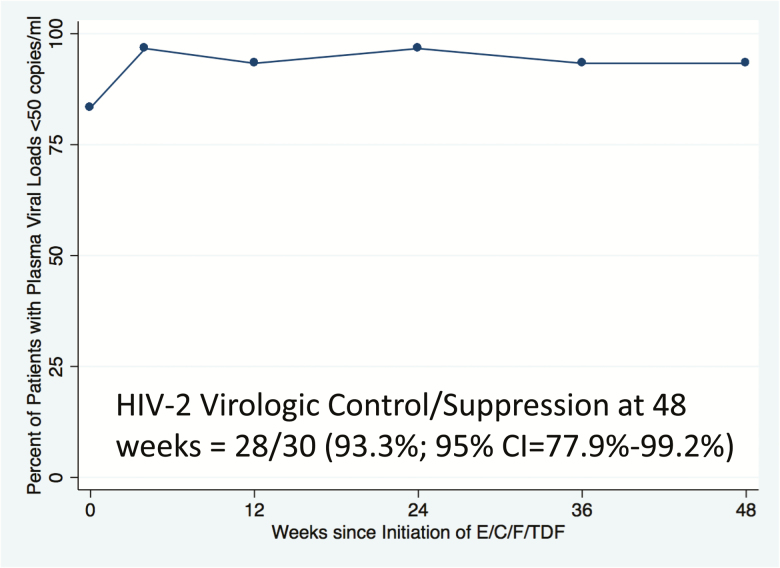
HIV-2 virologic outcomes are shown in Figure 2. We used an mITT analysis of virologic suppression (HIV-2 pVL <50 and <400 copies/mL) of HIV-2–infected patients on E/C/F/TDF over 48 weeks (n = 30), with viral load >50 copies/mL (n = 1 at weeks 36 and 48), noncompleter (n = 1), or missing (week 12, n = 1) equaling failure. Prespecified primary and secondary outcomes are shown in Table 3. The 1 participant with initial virologic failure (HIV-2 pVL = 236 copies/mL at week 36) did not show evidence of INI resistance at week 36 (genotyping for NRTI resistance was attempted but nonobtainable). However, this individual developed multidrug-resistant (MDR) HIV-2 by week 48, with mutations in both reverse transcriptase (K65R) and integrase (G140S + Q148R). These mutations predict high-level NRTI resistance to 3TC/emtricitabine and potential tenofovir disoproxil fumarate and abacavir resistance, as well as pan-INI resistance [52].
Figure 2.
Modified intent-to-treat analysis (with 95% confidence interval [CI]) of virologic suppression (human immunodeficiency virus type 2 [HIV-2] plasma viral load <50 copies/mL) of HIV-2–infected patients on elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate (E/C/F/TDF) over 48 weeks (n = 30). Events: HIV-2 >50 copies/mL (weeks 36 and 48, n = 1), noncompleter (n = 1), missing (week 12, n = 1).
Table 3.
Prespecified Primary and Secondary Outcome Measures at 48 Weeks After Antiretroviral Therapy Initiation (n = 29a)
| Outcome | No. of Participants |
| Primary outcome | |
| Death | 0 |
| New WHO stage 3 or 4 event | 0 |
| Virologic failure: FDA Snapshot | |
| HIV-2 plasma viral load | |
| >50 copies/mL | 1b |
| >400 copies/mL | 0 |
| Secondary outcome | |
| Grade 3 or 4 adverse events | |
| Clinical | 1 |
| Laboratory | 7 |
| CD4 T-cell count less than baseline | 0 |
| CD4 T-cell increase <50/μL from baseline | 2 |
| Switching off E/C/F/TDF | 0 |
| HIV-2 drug resistance mutations | 1b RT = K65R IN = G140S, Q148R |
Abbreviations: E/C/F/TDF, elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate; FDA, US Food and Drug Administration; HIV-2, human immunodeficiency virus type 2; IN, integrase; RT, reverse transcriptase; WHO, World Health Organization.
aOne participant of the 30 enrolled was lost to follow-up/self-withdrew at week 4.
bAt virologic failure (week 36), the HIV-2 viral load was 236 copies/mL; data available for integrase only (no drug resistance mutation [DRM] detected); at 48 weeks the same study participant had a HIV-2 viral load = 214 copies/mL and DRM: K65R + G140S/Q148R.
E/C/F/TDF was well tolerated, and no clinical AEs were deemed medication-related (Table 4). Grade 3 or 4 laboratory abnormalities were not common (n = 7; Supplementary Table 1). The change in creatinine in the 29 subjects who completed 48 weeks was a median of +0.1 mg/dL (interquartile range [IQR], 0–0.3 mg/dL), and total cholesterol increased by a median of 18 (IQR, 1–42) mg/dL. Medication adherence as measured by self-report of pills missed in the last 7 days was generally good (8/29 subjects reported missing between 1 and 22 pills over the course of the study; total reported pills missed = 0.57%). Overall, acceptability of the regimen was high, as evidenced by the 96.7% study completion rate.
Table 4.
Clinical Adverse Events
| Adverse Event | Time | Grade | Outcome |
|---|---|---|---|
| Lower limb edema | Week 4 | Grade 1 | Resolved |
| Oral candidiasis | Week 4 | Grade 1 | Resolved (Rx fluconazole) |
| Vomiting/diarrhea | Week 4 | Grade 1 | Resolved |
| Abdominal pain/dyspepsia | Week 28 | Grade 1 | Resolved |
| Stroke (CVA) | Week 36–40 | Grade 4 | Partial improvement |
Abbreviations: CVA, cerebrovascular accident; Rx, prescription.
DISCUSSION
Effective first- and second-line ART regimens for HIV-2 infection have not been defined with well-controlled clinical trials, and no ARV drugs are currently approved for treatment of HIV-2 by the FDA or the European Medicines Agency. In our clinical trial of a once-daily single-tablet regimen of E/C/F/TDF in ART-naive, HIV-2–infected adults, there were no on-treatment AIDS-associated clinical events or deaths. HIV-2 virologic control/suppression was maintained or achieved (<50 copies/mL) in 93.3% (95% confidence interval, 77.9%–99.2%) of participants (28/30) at 48 weeks (mITT analysis). A primary endpoint occurred in 1 subject who had virologic failure at 48 weeks. The median CD4 count increase was 161 cells/μL in the 29 participants who completed 48 weeks in the study. Two trial participants meet the secondary endpoint for lack of CD4 count increase of at least 50 cells/μL at 48 weeks. Notably, there was no significant difference in CD4 count increase between individuals with vs without detectable HIV-2 pVL (cutoff <10 copies/mL) prior to initiation of E/C/F/TDF (129 vs 182 cells/μL, respectively), suggesting that there may be some immunological benefit in treating HIV-2–infected individuals with undetectable viral loads at baseline. Similarly, we observed robust CD4 cell count increases in all strata (<200, 200–350, 351–500, and 501–750 cells/μL) of pretreatment CD4 counts, suggesting that early treatment of HIV-2 may also confer some immunologic benefit.
Unfortunately, it is impossible to accurately compare the results of our trial with existing published data on ART outcomes in HIV-2–infected individuals. A recent systematic review of the subject, including 17 reports involving 976 HIV-2–infected patients (none of which were clinical trials) [40], concluded that the available data were insufficient to determine a preferred first-line regimen for HIV-2 infection. In addition, HIV-2 virologic suppression and/or failure rates at 12 months, as well as CD4 cell count trajectories, were often reported in an aggregate manner and were not broken down by individual ARV regimens.
Similarly, it is challenging to directly compare results between HIV-1 and HIV-2. Controlled trials of a single-tablet regimen of E/C/F/TDF in ART-naive, HIV-1–infected patients have demonstrated rates of viral load suppression that are similar to the rate found in our study (~88%–90%), with potentially higher CD4 count gains at 48 weeks of treatment (204–239 cells/μL) [53–56]. However, as is typical for HIV-2 infection, a substantial number of individuals in our study had low or undetectable HIV-2 VL prior to initiating ART, confounding direct comparisons of “virologic suppression.” Moreover, several retrospective cohort studies with non-INI-based regimens have also reported less robust CD4 cell count reconstitution in HIV-2–infected patients on ART compared with HIV-1, although these comparisons may have been confounded by differences in patient characteristics and ART regimens [41].
The 1 study participant (3.3%) with virologic failure had evidence of MDR HIV-2, with both NRTI and INI resistance mutations at week 48. Our data suggest that MDR was acquired on treatment with E/C/F/TDF, as the participant’s VL decreased from a pretreatment baseline of 487 copies/mL to undetectable by week 12, with viral rebound at week 36 (236 copies/mL). In addition, the dried blood spot sample from week 36 had no detectable INI-associated mutations, suggesting that MDR HIV-2 became the predominant genotype between weeks 36 and 48 of treatment (we were unable to amplify RT-encoding sequences from this time point to assess NRTI resistance). MDR HIV-2 has also been observed in a substantial number of patients failing initial PI-based regimens [33, 57, 58]. Given the intrinsic resistance of HIV-2 to NNRTI, the low genetic barrier to broad-spectrum NRTI resistance [29, 57], and the potential for cross-resistance among HIV-2–active PI [33, 35], second-line and salvage regimens for patients harboring MDR HIV-2 are severely limited. Although maraviroc appears to have activity against CCR5–tropic HIV-2 strains, testing for tropism is not routinely available, and CXCR4-tropic HIV-2 is commonly observed in individuals failing first-line regimens [32, 50].
Our study has several limitations. Study patients were followed intensively and likely received more care and monitoring than is typically available in Senegal and other resource-limited settings. Our study had a planned enrollment of 30 patients; this relatively small sample size is reflected in the large 95% confidence interval surrounding the estimate of viral suppression. Furthermore, rare AEs were unlikely to have been captured. Last, it should be noted that elvitegravir and cobicistat have the potential for drug–drug interactions with cytochrome P450 CYP3A- and CYP2D6-metabolized drugs. This interaction limits the concurrent use of elvitegravir and cobicistat with rifampin, thereby complicating treatment of HIV/tuberculosis coinfection, which is endemic in resource-limited settings.
Our findings, together with the forthcoming results from 3 other trials of first-line, INI-based ART for HIV-2 (NCT01605890, NCT02150993, and NCT03224338), will hopefully help define evidence-based guidelines for management of this oft-neglected infection. In addition, our data suggest that there is a potential benefit in treating HIV-2–infected individuals with either low or undetectable viral loads, as well as those with CD4 counts >500 cells/μL. Moreover, extrapolating from the wealth of data from HIV-1 infection, it would not be unreasonable to consider treatment for HIV-2 as soon as individuals are diagnosed and ready to start ART (ie, a “test and treat” approach) as well as for its potential public health benefits, (ie, “treatment as prevention”), with the goal of eventually eradicating HIV-2 from the human population [6].
For too long, outcomes of HIV-2–infected patients on ART in West Africa and other locales have been suboptimal, and new therapeutic options are needed. Our data suggest that E/C/F/TDF, a once-daily single-tablet regimen, is safe, effective, and well tolerated in this population. Our findings support the use of INI-based regimens for HIV-2 treatment. Efforts to make them available in HIV-2–endemic areas should be an urgent priority.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. Study design: R. A. S., S. E. H., P. S. S., M. S., G. S. G. Data collection: S. B., D. N. R., F. S., K. F. Data analysis: S. B., D. N. R., R. A. S., S. E. H., G. S. G. Data interpretation: D. N. R., R. A. S., S. E. H., P. S. S., M. S., G. S. G. Manuscript writing: S. B., D. N. R., R. A. S., F. S., S. E. H., P. S. S., M. S., G. S. G. All authors approved the final manuscript.
Acknowledgments. We thank the study participants. The University of Washington (UW)–Dakar HIV-2 Study Group also includes Fatou Traore, Marie Pierre Sy, Bintou Diaw, Mbaye Ndoye, Amadou Bale Diop, Marianne Fadam Diome (Clinique des Maladies Infectieuses Ibrahima Diop Mar, Centre Hospitalier National Universitaire Fann, Universite Cheikh Anta Diop de Dakar, Dakar, Senegal); Alassane Niang, Jean Jacques Malomar, ElHadji Ibrahima Sall, Ousseynou Cisse, Ibrahima Tito Tamba, Jean Philippe Diatta, Jacques Sambou, Raphael Bakhoum, Juliette Gomis (Centre de Sante de Ziguinchor, Ziguinchor, Casamance, Senegal); Noelle Benzekri, John Lin, Nancy Kiviat, Sally Leong, Sara Masoum, and Vincent Wu (UW, Seattle). Thanks to Carol Gallardo, Eleanor Espinosa, Ming Chang, and Bob Coombs at the UW Clinical Retrovirology laboratory.
Disclaimer. The study sponsors had no role in the design and conduct of the study; the collection, analysis, and interpretation of the data; or the preparation or decision to submit the manuscript
Financial support. This work was supported by Gilead Sciences, Inc (grant to G. S. G.) and support from the UW Center for AIDS Research (grant number AI-827757) and the AIDS Clinical Trials Group Laboratory Center (grant number AI-068636).
Potential conflicts of interest. G. S. G. has received research grants and support from the US National Institutes of Health, UW, the Bill & Melinda Gates Foundation, Gilead Sciences, Alere Technologies, Merck & Co, Janssen Pharmaceutica, Cerus Corporation, ViiV Healthcare, Roche Molecular Systems, and Abbott Molecular Diagnostics. P. S. S. is currently and employee of Gilead Sciences, Inc. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part at the 9th IAS Conference on HIV Science (IAS 2017), Paris, France, 23–26 July 2017.
Contributor Information
University of Washington–Dakar HIV-2 Study Group:
Fatou Traore, Marie Pierre Sy, Bintou Diaw, Mbaye Ndoye, Amadou Bale Diop, Marianne Fadam Diome, Alassane Niang, Jean Jacques Malomar, ElHadji Ibrahima Sall, Ousseynou Cisse, Ibrahima Tito Tamba, Jean Philippe Diatta, Jacques Sambou, Raphael Bakhoum, Juliette Gomis, Noelle Benzekri, John Lin, Nancy Kiviat, Sally Leong, Sara Masoum, Vincent Wu, Carol Gallardo, Eleanor Espinosa, Ming Chang, and Bob Coombs
References
- 1. De Cock KM, Adjorlolo G, Ekpini E et al. Epidemiology and transmission of HIV-2. Why there is no HIV-2 pandemic [published erratum appears in JAMA 1994 Jan 19;271(3):196] [see comments]. JAMA 1993; 270:2083–6. [DOI] [PubMed] [Google Scholar]
- 2. Gottlieb GS, Eholie SP, Nkengasong JN et al. A call for randomized controlled trials of antiretroviral therapy for HIV-2 infection in West Africa. AIDS 2008; 22:2069–72; discussion 2073–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Campbell-Yesufu OT, Gandhi RT. Update on human immunodeficiency virus (HIV)-2 infection. Clin Infect Dis 2011; 52:780–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ariën KK, Abraha A, Quiñones-Mateu ME, Kestens L, Vanham G, Arts EJ. The replicative fitness of primary human immunodeficiency virus type 1 (HIV-1) group M, HIV-1 group O, and HIV-2 isolates. J Virol 2005; 79:8979–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Joint United Nations Programme on HIV/AIDS. UNAIDS 2017. Available at: http://aidsinfo.unaids.org. Accessed 26 September 2017. [Google Scholar]
- 6. Gottlieb GS. Changing HIV epidemics: what HIV-2 can teach us about ending HIV-1. AIDS 2013; 27:135–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kanki PJ, Travers KU, MBoup S et al. Slower heterosexual spread of HIV-2 than HIV-1. Lancet 1994; 343:943–6. [DOI] [PubMed] [Google Scholar]
- 8. Simon F, Matheron S, Tamalet C et al. Cellular and plasma viral load in patients infected with HIV-2. AIDS 1993; 7:1411–7. [DOI] [PubMed] [Google Scholar]
- 9. Marlink R, Kanki P, Thior I et al. Reduced rate of disease development after HIV-2 infection as compared to HIV-1. Science 1994; 265:1587–90. [DOI] [PubMed] [Google Scholar]
- 10. The HIV infection in Newborns French Collaborative Study Group. Comparison of vertical human immunodeficiency virus type 2 and human immunodeficiency virus type 1 transmission in the French prospective cohort. Pediatr Infect Dis J 1994; 13:502–6. [PubMed] [Google Scholar]
- 11. Adjorlolo-Johnson G, De Cock KM, Ekpini E et al. Prospective comparison of mother-to-child transmission of HIV-1 and HIV-2 in Abidjan, Ivory Coast. JAMA 1994; 272:462–6. [PubMed] [Google Scholar]
- 12. Prazuck T, Yameogo JM, Heylinck B et al. Mother-to-child transmission of human immunodeficiency virus type 1 and type 2 and dual infection: a cohort study in Banfora, Burkina Faso. Pediatr Infect Dis J 1995; 14:940–7. [DOI] [PubMed] [Google Scholar]
- 13. Gottlieb GS, Sow PS, Hawes SE et al. Equal plasma viral loads predict a similar rate of CD4+ T cell decline in human immunodeficiency virus (HIV) type 1- and HIV-2-infected individuals from Senegal, West Africa. J Infect Dis 2002; 185:905–14. [DOI] [PubMed] [Google Scholar]
- 14. Gilbert PB, McKeague IW, Eisen G et al. Comparison of HIV-1 and HIV-2 infectivity from a prospective cohort study in Senegal. Stat Med 2003; 22:573–93. [DOI] [PubMed] [Google Scholar]
- 15. Gottlieb GS, Hawes SE, Agne HD et al. Lower levels of HIV RNA in semen in HIV-2 compared with HIV-1 infection: implications for differences in transmission. AIDS 2006; 20:895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hawes SE, Sow PS, Stern JE, Critchlow CW, Gottlieb GS, Kiviat NB. Lower levels of HIV-2 than HIV-1 in the female genital tract: correlates and longitudinal assessment of viral shedding. AIDS 2008; 22:2517–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents. Recommendations for a public health approach. 2010. Available at: http://apps.who.int/iris/bitstream/10665/44379/1/9789241599764_eng.pdf. Accessed 26 September 2017. [PubMed] [Google Scholar]
- 18. US Department of Health and Human Services. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2015. Available at: https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arv-guidelines/24/hiv-2-infection. Accessed 26 September 2017. [Google Scholar]
- 19. Matheron S. HIV-2 infection: a call for controlled trials. AIDS 2008; 22:2073–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clavel F, Guétard D, Brun-Vézinet F et al. Isolation of a new human retrovirus from West African patients with AIDS. Science 1986; 233:343–6. [DOI] [PubMed] [Google Scholar]
- 21. Markowitz M, Saag M, Powderly WG et al. A preliminary study of ritonavir, an inhibitor of HIV-1 protease, to treat HIV-1 infection. N Engl J Med 1995; 333:1534–9. [DOI] [PubMed] [Google Scholar]
- 22. Collier AC, Coombs RW, Schoenfeld DA et al. Treatment of human immunodeficiency virus infection with saquinavir, zidovudine, and zalcitabine. AIDS Clinical Trials Group. N Engl J Med 1996; 334:1011–7. [DOI] [PubMed] [Google Scholar]
- 23. Carpenter CC, Fischl MA, Hammer SM et al. Antiretroviral therapy for HIV infection in 1997. Updated recommendations of the International AIDS Society-USA panel. JAMA 1997; 277:1962–9. [PubMed] [Google Scholar]
- 24. Witvrouw M, Pannecouque C, Van Laethem K, Desmyter J, De Clercq E, Vandamme AM. Activity of non-nucleoside reverse transcriptase inhibitors against HIV-2 and SIV. AIDS 1999; 13:1477–83. [DOI] [PubMed] [Google Scholar]
- 25. Witvrouw M, Pannecouque C, Switzer WM, Folks TM, De Clercq E, Heneine W. Susceptibility of HIV-2, SIV and SHIV to various anti-HIV-1 compounds: implications for treatment and postexposure prophylaxis. Antivir Ther 2004; 9:57–65. [PubMed] [Google Scholar]
- 26. Willey S, Peters PJ, Sullivan WM, Dorr P, Perros M, Clapham PR. Inhibition of CCR5-mediated infection by diverse R5 and R5X4 HIV and SIV isolates using novel small molecule inhibitors of CCR5: effects of viral diversity, target cell and receptor density. Antiviral Res 2005; 68:96–108. [DOI] [PubMed] [Google Scholar]
- 27. Roquebert B, Damond F, Collin G et al. Polymorphism of HIV-2 intregrase gene and in vitro phenotypic susceptibiltity of HIV-2 clinical isolates to intergrase inhibitors: raltegravir and evitegravir. Antivir Ther 2007; 12:S92. [Google Scholar]
- 28. Damond F, Lariven S, Roquebert B et al. Virological and immunological response to HAART regimen containing integrase inhibitors in HIV-2-infected patients. AIDS 2008; 22:665–6. [DOI] [PubMed] [Google Scholar]
- 29. Smith RA, Anderson DJ, Pyrak CL, Preston BD, Gottlieb GS. Antiretroviral drug resistance in HIV-2: three amino acid changes are sufficient for classwide nucleoside analogue resistance. J Infect Dis 2009; 199:1323–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Smith RA, Raugi DN, Kiviat NB et al. ; University of Washington-Dakar HIV-2 Study Group Phenotypic susceptibility of HIV-2 to raltegravir: integrase mutations Q148R and N155H confer raltegravir resistance. AIDS 2011; 25:2235–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Smith RA, Raugi DN, Pan C et al. ; University of Washington-Dakar HIV-2 Study Group Three main mutational pathways in HIV-2 lead to high-level raltegravir and elvitegravir resistance: implications for emerging HIV-2 treatment regimens. PLoS One 2012; 7:e45372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Visseaux B, Charpentier C, Hurtado-Nedelec M et al. ; French ANRS HIV-2 Cohort (ANRS CO 05 VIH-2) In vitro phenotypic susceptibility of HIV-2 clinical isolates to CCR5 inhibitors. Antimicrob Agents Chemother 2012; 56:137–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Raugi DN, Smith RA, Ba S et al. ; University of Washington-Dakar HIV-2 Study Group Complex patterns of protease inhibitor resistance among antiretroviral treatment-experienced HIV-2 patients from Senegal: implications for second-line therapy. Antimicrob Agents Chemother 2013; 57:2751–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Poveda E, Rodes B, Toro C, Soriano V. Are fusion inhibitors active against all HIV variants?AIDS Res Hum Retroviruses 2004; 20:347–8. [DOI] [PubMed] [Google Scholar]
- 35. Desbois D, Roquebert B, Peytavin G et al. ; French ANRS HIV-2 Cohort (ANRS CO 05 VIH-2) In vitro phenotypic susceptibility of human immunodeficiency virus type 2 clinical isolates to protease inhibitors. Antimicrob Agents Chemother 2008; 52:1545–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Roquebert B, Damond F, Collin G et al. ; French ANRS HIV-2 Cohort (ANRS CO 05 VIH-2) HIV-2 integrase gene polymorphism and phenotypic susceptibility of HIV-2 clinical isolates to the integrase inhibitors raltegravir and elvitegravir in vitro. J Antimicrob Chemother 2008; 62:914–20. [DOI] [PubMed] [Google Scholar]
- 37. Charpentier C, Larrouy L, Collin G et al. ; French ANRS HIV-2 Cohort (ANRS CO 05 VIH-2) In-vitro phenotypic susceptibility of HIV-2 clinical isolates to the integrase inhibitor S/GSK1349572. AIDS 2010; 24:2753–5. [DOI] [PubMed] [Google Scholar]
- 38. Gilleece Y, Chadwick DR, Breuer J et al. ; BHIVA Guidelines Subcommittee British HIV Association guidelines for antiretroviral treatment of HIV-2-positive individuals 2010. HIV Med 2010; 11:611–9. [DOI] [PubMed] [Google Scholar]
- 39. Yeni P. Prise en charge médicale des personnes infectéespar le VIH. 2010. Available at: http://apps.who.int/medicinedocs/documents/s19815fr/s19815fr.pdf. Accessed 26 September 2017. [Google Scholar]
- 40. Ekouevi DK, Tchounga BK, Coffie PA et al. Antiretroviral therapy response among HIV-2 infected patients: a systematic review. BMC Infect Dis 2014; 14:461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Drylewicz J, Eholie S, Maiga M et al. ; International epidemiologic Databases to Evaluate AIDS (IeDEA) West Africa Collaboration First-year lymphocyte T CD4+ response to antiretroviral therapy according to the HIV type in the IeDEA West Africa collaboration. AIDS 2010; 24:1043–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Benard A, van Sighem A, Taieb A et al. ; ACHIEV2E Collaboration Study Group Immunovirological response to triple nucleotide reverse-transcriptase inhibitors and ritonavir-boosted protease inhibitors in treatment-naive HIV-2-infected patients: the ACHIEV2E Collaboration Study Group. Clin Infect Dis 2011; 52:1257–66. [DOI] [PubMed] [Google Scholar]
- 43. Bénard A, Damond F, Campa P et al. ; ANRS CO5 HIV-2 Cohort Study Group Good response to lopinavir/ritonavir-containing antiretroviral regimens in antiretroviral-naive HIV-2-infected patients. AIDS 2009; 23:1171–3. [DOI] [PubMed] [Google Scholar]
- 44. Peterson I, Togun O, de Silva T et al. Mortality and immunovirological outcomes on antiretroviral therapy in HIV-1 and HIV-2-infected individuals in The Gambia. AIDS 2011; 25:2167–75. [DOI] [PubMed] [Google Scholar]
- 45. Zheng Y, Lambert C, Arendt V, Seguin-Devaux C. Virological and immunological outcomes of elvitegravir-based regimen in a treatment-naïve HIV-2-infected patient. AIDS 2014; 28:2329–31. [DOI] [PubMed] [Google Scholar]
- 46. Garrett N, Xu L, Smit E, Ferns B, El-Gadi S, Anderson J. Raltegravir treatment response in an HIV-2 infected patient: a case report. AIDS 2008; 22:1091–2. [DOI] [PubMed] [Google Scholar]
- 47. Descamps D, Peytavin G, Visseaux B et al. Dolutegravir in HIV-2-infected patients with resistant virus to first-line integrase inhibitors from the French Named Patient Program. Clin Infect Dis 2015; 60:1521–7. [DOI] [PubMed] [Google Scholar]
- 48. Requena S, Treviño A, Cabezas T et al. ; Spanish HIV-2 Study Group Drug resistance mutations in HIV-2 patients failing raltegravir and influence on dolutegravir response. J Antimicrob Chemother 2017; 72:2083–8. [DOI] [PubMed] [Google Scholar]
- 49. Treviño A, Cabezas T, Lozano AB et al. Dolutegravir for the treatment of HIV-2 infection. J Clin Virol 2015; 64:12–5. [DOI] [PubMed] [Google Scholar]
- 50. Visseaux B, Hurtado-Nedelec M, Charpentier C et al. ; ANRS CO 05 HIV-2 Cohort Molecular determinants of HIV-2 R5-X4 tropism in the V3 loop: development of a new genotypic tool. J Infect Dis 2012; 205:111–20. [DOI] [PubMed] [Google Scholar]
- 51. Chang M, Gottlieb GS, Dragavon JA et al. Validation for clinical use of a novel HIV-2 plasma RNA viral load assay using the Abbott m2000 platform. J Clin Virol 2012; 55:128–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Charpentier C, Camacho R, Ruelle J et al. HIV-2EU-supporting standardized HIV-2 drug-resistance interpretation in Europe: an update. Clin Infect Dis 2015; 61:1346–7. [DOI] [PubMed] [Google Scholar]
- 53. Cohen C, Elion R, Ruane P et al. Randomized, phase 2 evaluation of two single-tablet regimens elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate versus efavirenz/emtricitabine/tenofovir disoproxil fumarate for the initial treatment of HIV infection. AIDS 2011; 25:F7–12. [DOI] [PubMed] [Google Scholar]
- 54. DeJesus E, Rockstroh JK, Henry K et al. ; GS-236-0103 Study Team Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate versus ritonavir-boosted atazanavir plus co-formulated emtricitabine and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet 2012; 379:2429–38. [DOI] [PubMed] [Google Scholar]
- 55. Sax PE, DeJesus E, Mills A et al. ; GS-US-236-0102 Study Team Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus co-formulated efavirenz, emtricitabine, and tenofovir for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3 trial, analysis of results after 48 weeks. Lancet 2012; 379:2439–48. [DOI] [PubMed] [Google Scholar]
- 56. Zolopa A, Sax PE, DeJesus E et al. ; GS-US-236-0102 Study Team A randomized double-blind comparison of coformulated elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate versus efavirenz/emtricitabine/tenofovir disoproxil fumarate for initial treatment of HIV-1 infection: analysis of week 96 results. J Acquir Immune Defic Syndr 2013; 63:96–100. [DOI] [PubMed] [Google Scholar]
- 57. Gottlieb GS, Badiane NM, Hawes SE et al. ; University of Washington-Dakar HIV-2 Study Group Emergence of multiclass drug-resistance in HIV-2 in antiretroviral-treated individuals in Senegal: implications for HIV-2 treatment in resource-limited West Africa. Clin Infect Dis 2009; 48:476–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Charpentier C, Eholié S, Anglaret X et al. ; IeDEA West Africa Collaboration Genotypic resistance profiles of HIV-2-treated patients in West Africa. AIDS 2014; 28:1161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.