Abstract
Background
‘Last-line’ antimicrobial usage has promoted the emergence of MDR bacteria. Production of Klebsiella pneumoniae carbapenemases (KPCs) is increasingly common and leads to resistance to most antimicrobials. However, ceftazidime/avibactam demonstrates activity against KPC-producing strains. Ceftazidime/avibactam in the empirical setting remains unknown.
Methods
Strains underwent genetic analysis evaluating blaKPC presence/production and MICs were determined. Four strains were assessed in an in vitro, one-compartment pharmacokinetic (PK)/pharmacodynamic (PD) model for 96 h. The following bolus dosing exposures were tested: 2.5 g of ceftazidime/avibactam every 8 h, 2 g of meropenem every 8 h, 1.25 mg/kg polymyxin B every 12 h, amikacin ‘once-daily dosing’ (peak of 70–80 mg/L), tigecycline at 200 mg ×1 dose followed by 100 mg every 12 h, and a drug-free growth control.
Results
Thirty blaKPC-producing strains were evaluated; 97% of strains were ceftazidime/avibactam susceptible with MIC50/MIC90 values of 0.38/1.5 mg/L (range 0.032–16 mg/L). Two K. pneumoniae strains, one Klebsiella oxytoca strain and one Citrobacter freundii strain underwent further analysis in PK/PD models. Ceftazidime/avibactam displayed potent activity with a reduction of 4.23 ± 0.42 cfu/mL from the initial inoculum at 96 h. Against susceptible isolates, amikacin displayed similar activity compared with ceftazidime/avibactam at 96 h, although this was not demonstrated against all strains. Polymyxin B produced comparable activity to ceftazidime/avibactam against two strains. Neither meropenem nor tigecycline produced effective killing and were comparable to the drug-free growth control at 96 h.
Conclusions
bla KPC-producing organisms demonstrated susceptibility to ceftazidime/avibactam and bactericidal activity was observed in the PK/PD model. Based on these data, ceftazidime/avibactam is a valuable agent for treating KPC-producing organisms and should be considered for treatment of infections caused by these pathogens.
Introduction
Gram-negative bacteria demonstrating resistance to antibacterial agents represent a significant public health burden worldwide. The emergence of isolates of Enterobacteriaceae producing carbapenemases, most frequently harbouring the Klebsiella pneumoniae carbapenemase (KPC) enzyme in the USA, as well as other countries, is of significant concern due to their ability to render all β-lactams ineffective. Fortunately, many of these carbapenem-resistant Enterobacteriaceae (CRE) are colonizers or are pathogenic in infections with inoculums that are relatively easy to treat, such as urinary tract infections.1,2 Mortality rates for more severe higher inoculum infections, however, including bacteraemia, are high and range ∼20%–70%.3–6
The poor chance of survival observed among patients with invasive Gram-negative infections producing KPCs is likely attributed to both significant delays in time to appropriate therapy and the lack of effective antimicrobial options, given that the majority of strains display resistance to the fluoroquinolones and carbapenems. While, polymyxins, aminoglycosides and tigecycline remain the most active in vitro options to treat these pathogens, these drugs are hindered by their inefficiency as monotherapeutic options and/or toxicity.7,8
The FDA approved ceftazidime/avibactam for the treatment of complicated intra-abdominal infections in combination with metronidazole, complicated urinary tract infections and hospital-acquired bacterial pneumonia and ventilator-associated pneumonia. While ceftazidime is a well-described ‘third-generation’ β-lactam, avibactam represents a unique class of non-β-lactam β-lactamase inhibitors, which demonstrates inhibitory activity against Ambler class A, C and some D β-lactamases.9In vitro studies demonstrated potent activity of ceftazidime/avibactam against KPC-producing strains with reported MIC50/90 values of 1/2 mg/L in one study and 0.5/2 mg/L in another, both well below the approved breakpoint of 8/4 mg/L.10–13
Early observational studies reporting experiences with ceftazidime/avibactam have been encouraging, but data are limited to small sample sizes. Additionally, ceftazidime/avibactam was frequently utilized as salvage therapy or in combination with other antimicrobials.14–16 The objective of this study was to evaluate the activity of ceftazidime/avibactam alone compared with standard-of-care agents in an in vitro pharmacokinetic (PK)/pharmacodynamic (PD) model simulating bacteraemia with KPC-producing pathogens.
Materials and methods
Bacterial strains
A total of 30 clinical strains from the Detroit Medical Center underwent genetic analysis to confirm blaKPC production. blaKPC was amplified using established primers and each amplicon was sequenced.17 Four representative KPC strains (two K. pneumoniae, one Klebsiella oxytoca and one Citrobacter freundii) were utilized for in vitro PK/PD modelling experimentations.
Antimicrobials
Avibactam was provided by its manufacturer (Allergan, Parsippany, NJ, USA). Ceftazidime, meropenem, amikacin, polymyxin B and tigecycline were commercially purchased (Sigma Chemical Co., St Louis, MO, USA).
Antimicrobial susceptibility testing
In vitro antimicrobial susceptibility testing was performed on all 30 strains. Vitek®2 or Microscan was utilized when possible to determine MIC. For ceftazidime/avibactam, polymyxin B and tigecycline, Etests were utilized following methodology according to the manufacturer. Antibiotic containing plates for resistance screening used brain heart infusion agar (Difco, Detroit, MI, USA).
In vitro PK/PD model
An in vitro, one-compartment PK/PD model with a 250 mL capacity and input/outflow ports was used. The apparatus was prefilled with medium (Mueller–Hinton broth) and antimicrobials were administered as boluses over a 96 h time period. A starting inoculum of ∼1 × 106 cfu/mL was targeted for each experiment. Fresh medium was continuously supplied and removed from the compartment along with the drug via a peristaltic pump (Masterflex; Cole-Parmer Instrument Company, Chicago, IL, USA) at an appropriate rate to simulate the average human half-lives of the antimicrobials or at the longest half-life for the drug-free growth control. The apparatus was maintained at 37°C throughout the duration of experimentation. All experiments were performed in duplicate.
The antimicrobial regimen simulations evaluated as bolus doses were as follows: 2.5 g of ceftazidime/avibactam every 8 h (fCmax 93.2/13.6 mg/L, average t½ 2.7 h),18–20 2 g of meropenem every 8 h (fCmax 110 mg/L, average t½ 1 h),21,22 1.25 mg/kg polymyxin B every 12 h (fCmax 6.13 mg/L, average t½ 6 h),23,24 amikacin once-daily dosing to achieve a peak of 70–80 mg/L (average t½ 2 h),24–27 tigecycline at 200 mg × 1 dose followed by 100 mg every 12 h (fCmax 0.3 mg/L, average t½ 42 h)28 and a drug-free growth control.
PD analysis
Samples were removed at 0, 4, 8, 24, 32, 48, 72 and 96 h and serially diluted in cold 0.9% sodium chloride. Bacterial counts were determined by spiral plating appropriate dilutions using a Whitley automatic spiral plater (DW Scientific, Shipley, West Yorkshire, UK). Tryptic soy agar plates were incubated at 37°C for 24 h before colonies were counted. Antimicrobial killing was demonstrated via plotting mean ± SD colony counts (log10 cfu/mL) versus time with the lower limit of detection being 1 log10 cfu/mL. Bactericidal activity was defined as ≥3 log10 cfu/mL reduction from baseline.
PK analysis
PK samples were obtained, through the injection port of each model at appropriate timepoints throughout the model for verification of target antibiotic concentrations. All samples were stored at −80°C until ready for analysis. Ceftazidime/avibactam concentrations were sent out to Keystone Bioanalytical, Inc. for LC-MS analysis.29 All other drug concentrations were determined by bioassay, as previously described.30–32 In brief, blank 0.635 cm discs were spotted with 10 μL of the standards or samples. Each standard was tested in duplicate by placing the disc on antibiotic medium agar plate no. 11, which was inoculated with a 0.5 McFarland suspension of the test organism. Plates were incubated for 24 h at 37°C at which time the zone sizes were measured using a protocol reader (Protocol; Microbiology International, Frederick, MD, USA). The half-life, AUC0–24, peak concentrations and time above MIC were determined utilizing PK Analyst software (version 1.10; MicroMath Scientific Software, Salt Lake City, UT, USA) using the linear trapezoidal method.
Resistance
Emergence of resistance (treatment emergent resistance) was evaluated daily by plating 100 μL samples from the model on plates supplemented at a concentration 3× the MIC of the tested antimicrobial. Plates were examined for growth after 48 h of incubation at 37°C. Resistant colonies growing on screening plates were evaluated by Etest or broth microdilution methods to determine the MIC.
Statistical analysis
Changes in cfu/mL at 96 h were compared by one-way analysis of variance with Tukey’s post hoc test. All statistical analyses were performed using SPSS Statistical Software (Release 22.0; IBM Corp., Armonk, NY, USA).
Results
Susceptibility testing
The MIC results for the isolates evaluated are summarized in Table 1. A total of 29 of 30 (97%) of isolates were ceftazidime/avibactam susceptible with MIC50 and MIC90 values of 0.38 and 2 mg/L (range 0.032–16 mg/L) despite only five of the isolates being ceftazidime susceptible. Strains 6R, 11R, 4299 and 4329 were evaluated in the PK/PD models. These strains were MDR and were not susceptible to ceftazidime, meropenem or ciprofloxacin. Two strains (4299 and 4329) were susceptible to amikacin with MICs of ≤2 and 4 mg/L, respectively. Only one strain (4329) was susceptible to trimethoprim/sulfamethoxazole. All four strains were susceptible to ceftazidime/avibactam, polymyxin B and tigecycline.
Table 1.
Susceptibilities (mg/L) of study organisms
| Isolate | Organism | CZA | CAZ | MEM | AMK | TOB | CIP | LVX | SXT | TGC | PMB |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | K. pneumoniae | 0.25 | 4 | >8 | ≤2 | ≤0.5 | ≤0.5 | ≤1 | ≤1 | 0.19 | 0.125 |
| 3 | Escherichia coli | 0.5 | ≤0.5 | >8 | ≤8 | ≤2 | ≤0.5 | ≤1 | ≤1 | 2 | 0.19 |
| 4 | K. pneumoniae | 0.38 | >16 | >8 | >32 | >8 | >2 | >4 | >2 | 1.5 | 0.38 |
| 6 | K. oxytoca | 0.94 | >16 | >8 | >32 | >8 | >2 | >4 | >2 | 3 | 0.38 |
| 10 | K. pneumoniae | 0.38 | >16 | >8 | 16 | >8 | >2 | >4 | >2 | 3 | 0.25 |
| 12 | K. pneumoniae | 0.75 | >16 | >8 | 32 | >8 | >2 | >4 | >2 | 1.5 | 0.38 |
| 14 | K. pneumoniae | 0.064 | >16 | >8 | 16 | >8 | >2 | >4 | >2 | 4 | 0.38 |
| 18 | K. pneumoniae | 0.25 | >16 | >8 | 32 | >8 | >2 | >4 | >2 | 1 | 0.38 |
| 20 | K. pneumoniae | 0.75 | >16 | >8 | 32 | >8 | >2 | >4 | >2 | 3 | 0.75 |
| 21 | K. pneumoniae | 0.064 | >16 | >8 | ≤2 | >8 | >2 | >4 | ≤1 | 3 | 0.38 |
| 22 | K. pneumoniae | 0.032 | ≤1 | >8 | ≤2 | ≤0.5 | ≤0.5 | ≤1 | ≤1 | 2 | 0.38 |
| 24 | K. pneumoniae | 0.75 | >16 | 8 | 16 | >8 | >2 | >4 | >2 | 3 | 0.38 |
| 30 | K. pneumoniae | 2 | >16 | >8 | 32 | >8 | >2 | >4 | >2 | 3 | 0.5 |
| 34 | K. pneumoniae | 0.75 | >16 | >8 | 32 | >8 | >2 | >4 | >2 | 2 | 0.5 |
| 35 | K. pneumoniae | 0.38 | >16 | >8 | ≤4 | >8 | >2 | >4 | >2 | 3 | 0.5 |
| 36 | K. pneumoniae | 3 | >16 | >8 | 32 | >8 | >2 | >4 | >2 | 0.38 | 1 |
| 38 | K. pneumoniae | 2 | >16 | >8 | 32 | >8 | >2 | >4 | >2 | 2 | 0.5 |
| 43 | K. pneumoniae | 0.5 | >16 | >8 | 32 | >8 | >2 | >4 | >2 | 3 | 0.38 |
| 45 | K. pneumoniae | 0.125 | >16 | >8 | 32 | >8 | >2 | >4 | >2 | 4 | 0.38 |
| 46 | E. coli | 1 | >16 | >8 | 16 | >8 | >2 | ≤1 | >2 | 3 | 2 |
| 4091 | K. pneumoniae | 0.047 | >16 | ≥16 | ≤2 | >16 | ≥4 | >4 | >320 | 0.75 | 0.19 |
| 4164 | K. oxytoca | 16 | >16 | >8 | ≤2 | 4 | >2 | 2 | >40 | 1.5 | 128 |
| 4234 | K. pneumoniae | 0.125 | 4 | ≥16 | ≤2 | ≤1 | 1 | ≤1 | 40 | 2 | 0.38 |
| 4299 | C. freundii | 0.19 | >16 | ≥16 | ≤2 | 8 | ≥4 | >4 | >320 | 6 | 0.38 |
| 4329 | K. pneumoniae | 0.5 | >16 | ≥16 | 4 | ≤1 | ≥4 | >4 | ≤20 | 1.5 | 0.75 |
| 1R | K. pneumoniae | 1.5 | >16 | >8 | >32 | ≤1 | >2 | >4 | >2 | 1.5 | 1 |
| 3R | K. pneumoniae | 0.047 | >16 | >8 | 32 | >8 | >2 | >4 | >2 | 3 | 0.5 |
| 5R | K. pneumoniae | 0.38 | 4 | >8 | >32 | 2 | ≤0.5 | ≤1 | ≤1 | 1.5 | 0.75 |
| 6R | K. oxytoca | 0.75 | >16 | >8 | 32 | >8 | >2 | >4 | >2 | 1 | 0.5 |
| 11R | K. pneumoniae | 0.19 | >16 | >8 | >32 | >8 | >2 | >4 | >2 | 3 | 0.38 |
CZA, ceftazidime/avibactam; CAZ, ceftazidime; MEM, meropenem; AMK, amikacin; TOB, tobramycin; CIP, ciprofloxacin; LVX, levofloxacin; SXT, trimethoprim/sulfamethoxazole; TGC, tigecycline; PMB, polymyxin B.
In vitro PK/PD model
The observed fCmax and t½ values for amikacin were 74.6 ± 5.9 mg/L (target 70 mg/L) and 2.12 ± 0.2 h (target 2 h). The observed fCmax and t½ values for meropenem were 104 ± 7.4 mg/L (target 110 mg/L) and 0.96 ± 0.2 h (target 1 h). The observed fCmax and t½ values for polymyxin B were 6.2 ± 0.9 mg/L (target 6.13 mg/L) and 5.92 ± 0.5 h (target 6 h). The observed fCmax and t½ values for tigecycline were 0.4 ± 0.1 mg/L (target 0.3 mg/L) and 38.4 ± 4.1 h (target 42 h). The observed fCmax and t½ values for ceftazidime/avibactam were 93.1 ± 5.14/12.9 ± 2.07 mg/L (target 93.2/13.6 mg/L) and 2.5 ± 0.25 h (target 2.7 h), respectively.
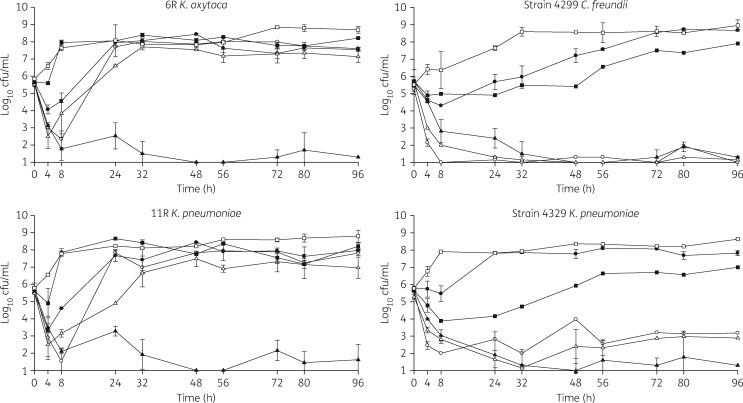
The changes in log10 cfu/mL for the tested regimens against the four strains are displayed in Figure 1. Against all strains, ceftazidime/avibactam demonstrated bactericidal activity with an average reduction of 4.23 ± 0.42 cfu/mL from the starting inoculum within 8–24 h. By 96 h, ceftazidime/avibactam was statistically the most effective antimicrobial against three of the four strains (P < 0.01). However, against strain 4299, both amikacin and polymyxin B were comparable to ceftazidime/avibactam. Amikacin displayed potent killing (3.85 ± 0.72 reduction in starting inoculum) within the first 8 h in all strains. However, regrowth was noted at 24 h in amikacin non-susceptible strains 6 R and 11 R (MICs 32 and ≥32 mg/L, respectively). Similar to amikacin, polymyxin B demonstrated bactericidal activity within 8 h (3.08 ± 0.84 reduction in starting inoculum) and continued throughout the duration of the experiment (3.78 ± 1.45 reduction in starting inoculum) against two of the strains (4299 and 4329). However, in the other two strains (6 R and 11R), sustained killing was not observed. Tigecycline reduced the initial inoculum within the first 4–8 h but regrowth was noted in all strains by 24 h. As expected due to meropenem resistance in all strains (MICs >8 mg/L for four strains evaluated in PK/PD models), meropenem activity at 96 h was comparable to the drug-free growth control in all strains evaluated, with regrowth occurring as early as 8 h in two of the four strains and by 24 h in the remaining two.
Figure 1.
PK/PD graphs for four study pathogens in in vitro models. Open circles, amikacin; filled triangles, ceftazidime/avibactam; filled circles, meropenem; open triangles, polymyxin B; filled squares, tigecycline; open squares, drug-free growth control.
Resistance emergence
Isolates with MICs that were higher than the baseline MICs were not detected on resistance screening plates.
Discussion
Studies have demonstrated that inappropriate empirical coverage, particularly for bloodstream infections, yields higher mortality compared with appropriate therapy.33 In light of the increasing presence and degree of Gram-negative antimicrobial resistance, clinicians face an increasing challenge when attempting to cover empirically the most likely pathogens with an agent that has retained activity against those organisms. In centres with a high prevalence of CRE, this is particularly challenging, as effective and safe treatment options are limited.
In this evaluation, in an in vitro model, we demonstrated that initial monotherapy with ceftazidime/avibactam displays potent bactericidal activity against CRE regardless of resistance to other classes of antimicrobials. Bactericidal activity was noted in all strains evaluated and susceptibility was restored despite high prevalence to ceftazidime resistance. While tigecycline displayed an initial kill in the first 4–8 h of experimentation, it was not anticipated that this agent would produce a sustained killing effect due to low concentrations utilized for the simulation of a bloodstream infection. However, this agent may still be beneficial for other infection types where the drug achieves high concentrations. In two strains (6R and 11R) treated with polymyxin B, regrowth was noted despite no development of resistance. However, this could be due to limitations of Etest methodology for the polymyxins with up to 20% of isolates being falsely reported as susceptible.34,35 Additionally, there is potential that resistant mutants could have been pumped out of the model system due to the type of in vitro modelling utilized with high flow rates.
Given the challenges with treating CRE, ceftazidime/avibactam is a viable therapeutic option. However, in clinical settings where ceftazidime/avibactam has been employed, it is frequently prescribed in combination or as salvage therapy. In a multicentre study evaluating clinical outcomes in 60 CRE-infected patients treated with ceftazidime/avibactam, microbiological cure and clinical success were observed in 53% and 65% of patients, respectively.14 The majority of these patients demonstrated a high degree of acute illness with invasive infections. What makes the impact of ceftazidime/avibactam particularly difficult to interpret is that roughly half of the patients received concomitant therapy most commonly with an aminoglycoside, polymyxin and/or tigecycline.
Several studies evaluated ceftazidime/avibactam for salvage therapy with high cure rates (74%) and low mortality (8%).15,36 However, it is important to note that combination therapy was common and occurred in 66%–85% of patients. One study reported outcomes in 37 CRE-infected patients treated with ceftazidime/avibactam demonstrated similar clinical success in 59% of patients.14 Unlike the previously mentioned evaluations, the majority of patients in this evaluation received monotherapy (70%) with a clinical success rate of 58%.
There are several limitations of the current study. First, experiments were only conducted for 96 h with only four strains. While no ceftazidime/avibactam resistance development was noted during this time, organisms can develop resistance after 96 h of therapy. However, development of resistance after 96 h has only been described in three ceftazidime/avibactam-treated patients to date.37 Many patients receive Gram-negative antimicrobial therapy prior to the identification of a carbapenem-resistant organism. In our experiment, isolates were not subjected to antimicrobial exposure prior to the receipt of ceftazidime/avibactam. Combination therapy is often utilized to treat these organisms and this was not assessed. Lastly, all analyses were conducted based upon simulation of normal renal clearance; therefore, altered killing may be observed in patients with decreased or increased renal function.
In conclusion, in this in vitro model, ceftazidime/avibactam was efficacious against carbapenem-resistant organisms, specifically those producing KPC enzymes. In patients at high risk for CRE infection caused by KPC production, ceftazidime/avibactam monotherapy appears to be an effective empirical therapeutic agent although future studies with combination therapy are still warranted.
Acknowledgements
We thank Allergan for providing avibactam powder.
Funding
This work was supported by Forest Laboratories now owned by Allergan.
R. A. B. receives grant funding from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award numbers R01AI100560, R01AI063517, R21AI114508 and R01AI072219. This study was supported in part by funds and/or facilities provided by the Cleveland Department of Veterans Affairs, award number 1I01BX001974 to R. A. B. from the Biomedical Laboratory Research & Development Service of the VA Office of Research and Development and the Geriatric Research Education and Clinical Center VISN 10.
Transparency declarations
K. E. B. has received grant funding from Allergan. J. M. P. is a consultant, speaker and/or receives grant funding from Allergan, Merck, Medicines Company, Achaogen, Shionogi and Zavante. K. S. K. has received grant funding from Merck and Allergan and consults for Merck, Allergan, Melinda, Achaogen, Shionogi and Zavante. H. D. W. and R. A. B.: none to declare.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Veterans Affairs.
References
- 1. Tischendorf J, de Avila RA, Safdar N.. Risk of infection following colonization with carbapenem-resistant Enterobactericeae: a systematic review. Am J Infect Control 2016; 44: 539–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chiotos K, Tamma PD, Flett KB. et al. Multicenter study of the risk factors for colonization or infection with carbapenem-resistant Enterobacteriaceae in children. Antimicrob Agents Chemother 2017; 61: e01440-17.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tumbarello M, Viale P, Viscoli C. et al. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis 2012; 55: 943–50. [DOI] [PubMed] [Google Scholar]
- 4. Qureshi ZA, Paterson DL, Potoski BA. et al. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother 2012; 56: 2108–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Daikos GL, Tsaousi S, Tzouvelekis LS. et al. Carbapenemase-producing Klebsiella pneumoniae bloodstream infections: lowering mortality by antibiotic combination schemes and the role of carbapenems. Antimicrob Agents Chemother 2014; 58: 2322–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tumbarello M, Trecarichi EM, De Rosa FG. et al. Infections caused by KPC-producing Klebsiella pneumoniae: differences in therapy and mortality in a multicentre study. J Antimicrob Chemother 2015; 70: 2133–43. [DOI] [PubMed] [Google Scholar]
- 7. Livermore DM, Warner M, Mushtaq S. et al. What remains against carbapenem-resistant Enterobacteriaceae? Evaluation of chloramphenicol, ciprofloxacin, colistin, fosfomycin, minocycline, nitrofurantoin, temocillin and tigecycline. Int J Antimicrob Agents 2011; 37: 415–9. [DOI] [PubMed] [Google Scholar]
- 8. Chiu SK, Wu TL, Chuang YC. et al. National surveillance study on carbapenem non-susceptible Klebsiella pneumoniae in Taiwan: the emergence and rapid dissemination of KPC-2 carbapenemase. PLoS One 2013; 8: e69428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ehmann DE, Jahic H, Ross PL. et al. Kinetics of avibactam inhibition against Class A, C, and D β-lactamases. J Biol Chem 2013; 288: 27960–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shields RK, Clancy CJ, Hao B. et al. Effects of Klebsiella pneumoniae carbapenemase subtypes, extended-spectrum β-lactamases, and porin mutations on the in vitro activity of ceftazidime-avibactam against carbapenem-resistant K. pneumoniae. Antimicrob Agents Chemother 2015; 59: 5793–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Castanheira M, Farrell SE, Krause. et al. Contemporary diversity of β-lactamases among Enterobacteriaceae in the nine U.S. census regions and ceftazidime-avibactam activity tested against isolates producing the most prevalent β-lactamase groups. Antimicrob Agents Chemother 2014; 58: 833–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-Second Informational Supplement M100-ED28. CLSI, Wayne, PA, USA, 2018. [Google Scholar]
- 13. EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 8.0, 2018 http://www.eucast.org.
- 14. King M, Heil E, Kuriakose S. et al. Multicenter study of outcomes with ceftazidime-avibactam in patients with carbapenem-resistant Enterobacteriaceae infections. Antimicrob Agents Chemother 2017; 61: e00449-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Temkin E, Torre-Cisneros J, Beovic B. et al. Ceftazidime-avibactam as salvage therapy for infections caused by carbapenem-resistant organisms. Antimicrob Agents Chemother 2017; 61: e01964-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shields RA, Potoski BA, Haidar G. et al. Clinical outcomes, drug toxicity, and emergence of ceftazidime-avibactam resistance among patients treated for carbapenem-resistant Enterobacteriaceae infections. Clin Infect Dis 2016; 63: 1615–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van Duin D, Perez F, Rudin SD. et al. Surveillance of carbapenem-resistant Klebsiella pneumoniae: tracking molecular epidemiology and outcomes through a regional network. Antimicrob Agents Chemother 2014; 58: 4035–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Merdjan H, Rangaraju M, Tarral A.. Safety and pharmacokinetics of single and multiple ascending doses of avibactam alone and in combination with ceftazidime in healthy male volunteers: results of two randomized, placebo-controlled studies. Clin Drug Investig 2015; 35: 307–17. [DOI] [PubMed] [Google Scholar]
- 19. Riccobene TA, Su SF, Rank D.. Single- and multiple-dose study to determine the safety, tolerability, and pharmacokinetics of ceftaroline fosamil in combination with avibactam in healthy subjects. Antimicrob Agents Chemother 2013; 57: 1496–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Product Information: AVYCAZ Intravenous Injection Powder, Ceftazidime Avibactam Intravenous Injection Powder. Cincinnati, OH, USA: Forest Pharmaceuticals, Inc; (per manufacturer), 2015. [Google Scholar]
- 21. Nilsson-Ehle I, Hutchison M, Haworth SJ. et al. Pharmacokinetics of meropenem compared to imipenem-cilastatin in young, healthy males. Eur J Clin Microbiol Infect Dis 1991; 10: 85–8. [DOI] [PubMed] [Google Scholar]
- 22. Cheatham SC, Kays MB, Smith DW. et al. Steady-state pharmacokinetics and pharmacodynamics of meropenem in hospitalized patients. Pharmacother 2008; 28: 691–8. [DOI] [PubMed] [Google Scholar]
- 23. Product Information: POLYMYXIN B Injection Powder, Polymyxin B Sulfate Injection Powder. Schaumburg, IL, USA: Sagent Pharmaceuticals, Inc; (per Daily Med), 2013. [Google Scholar]
- 24. Zavascki AP, Goldani LZ, Cao G. et al. Pharmacokinetics of intravenous polymyxin B in critically ill patients. Clin Infect Dis 2008; 47: 1298–304. [DOI] [PubMed] [Google Scholar]
- 25. Edwards CC. Concentration of gentamicin and amikacin in human kidneys. Antimicrob Agents Chemother 1976; 9: 925.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hansbrough JF, Clark JE, Reimer LG.. Concentrations of kanamycin and amikacin in human gallbladder bile and wall. Antimicrob Agents Chemother 1981; 20: 515–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Product Information: Amikin®, Amikacin. Princeton, NJ, USA: Apothecon, 1997. [Google Scholar]
- 28. Product Information: TYGACIL® Intravenous Injection, Tigecycline Intravenous Injection. Philadelphia, PA, USA: Wyeth Pharmaceuticals, Inc; (per FDA), 2013. [Google Scholar]
- 29. Xu A, Validation Report on an Analytical Procedure for the Quantification of Ceftazidime and Avibactam in Human NaF/KOX Plasma by LC/MS/MS. North Wales, PA, USA: Keystone Bioanalytical, 2015. [Google Scholar]
- 30. Mendez AS, Weisheimer V, Oppe TP. et al. Microbiological assay for the determination of meropenem in pharmaceutical dosage form. J Pharm Biomed Anal 2005; 37: 649–53. [DOI] [PubMed] [Google Scholar]
- 31. Barber KE, Werth BJ, Rybak MJ.. The combination of ceftaroline plus daptomycin allows for therapeutic de-escalation and daptomycin sparing against MRSA. J Antimicrob Chemother 2015; 70: 505–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hammerberg S, Sinai R, Marks MI.. Serum standards for the bioassay of aminoglycosides in cerebrospinal fluid. J Clin Pathol 1978; 31: 172–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Morata L, Cobos TN, Martínez JA. et al. Influence of multidrug resistance and appropriate empirical therapy on the 30-day mortality rate of Pseudomonas aeruginosa bacteremia. Antimicrob Agents Chemother 2012; 56: 4833–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kulengowski B, Ribes JA, Burgess DS, Polymyxin B.. Etest® compared to gold-standard broth microdilution in carbapenem-resistant Enterobacteriaceae exhibiting a wide range of polymyxin B MICs. Clin Microbiol Infect 2018; doi:10.1016/j.cmi.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 35. Singhal L, Sharma M, Verma S. et al. Comparative evaluation of broth microdilution with polystyrene and glass-coated plates, agar dilution, E-Test, Vitek, and disk diffusion for susceptibility testing of colistin and polymyxin B on carbapenem-resistant clinical isolates of Acinetobacter baumannii. Microb Drug Resist 2018; doi:10.1089/mdr.2017.0251. [DOI] [PubMed] [Google Scholar]
- 36. van Duin D, Lok JJ, Earley M. et al. Colistin versus ceftazidime/avibactam in the treatment of infections due to carbapenem-resistant Enterobacteriaceae. Clin Infect Dis 2017; 66: 163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shields RA, Chen L, Cheng S. et al. Emergence of ceftazidime-avibactam resistance due to plasmid-borne blaKPC-3 mutations during treatment of carbapenems-resistant Klebsiella pneumoniae infections. Antimicrob Agents Chemother 2017; 61: e02097–16. [DOI] [PMC free article] [PubMed] [Google Scholar]