Abstract
Chronic traumatic encephalopathy (CTE) is a progressive neurodegenerative disorder linked to repetitive head impacts and has been associated with amyotrophic lateral sclerosis (ALS), a fatal, degenerative neuromuscular disorder. The Department of Veterans Affairs Biorepository Brain Bank (VABBB) is a tissue repository that collects antemortem disease progression data and postmortem central nervous system tissue from veterans with ALS. We set out to determine the frequency of co-morbid ALS and CTE in the VABBB cohort and to characterize the clinical, genetic, and pathological distinctions between participants with ALS only and those with both ALS and CTE (ALS+CTE). Of 155 participants, 9 (5.8%) had neuropathologically confirmed ALS+CTE. Participants with ALS+CTE were more likely to have a history of traumatic brain injury (p < 0.001), served during the first Persian Gulf War (p < 0.05), and to have more severe tau pathology within the frontal cortex and spinal cord (p < 0.05). The most common exposures to head impacts included contact sports (n = 5) and military service (n = 2). Clinically, participants with ALS+CTE were more likely to have bulbar onset ALS (p = 0.006), behavioral changes (p = 0.002), and/or mood changes (p < 0.001). Overall, compared with ALS in isolation, comorbid ALS+CTE is associated with a history of TBI and has a distinct clinical and pathological presentation.
Keywords: Amyotrophic lateral sclerosis, Chronic traumatic encephalopathy, Military veterans, Motor neuron degeneration, Motor neuron disease, Traumatic brain injury
INTRODUCTION
Chronic traumatic encephalopathy (CTE) is a progressive neurodegenerative disease associated with repetitive head impacts (RHI) and exposure to blast injury. Repetitive head impacts, including concussion and subconcussion, may result from a number of sources including participation in contact sports, military-related activities, and motor vehicle accidents (1–4). The clinical presentation of CTE is typically insidious and often occurs years after the traumatic exposure (1, 2, 4–6). The clinical syndrome of CTE continues to be refined but includes behavioral, mood, and cognitive dysfunction as well as occasional motor symptoms that are typically extrapyramidal (1, 2). Symptoms may include headache, depression, and difficulty with concentration, attention, and memory.
Pathologically, CTE is characterized by accumulation of hyperphosphorylated tau (ptau) that is aggregated in neurons, astrocytes, and cell processes around small vessels and in the depths of the cerebral sulci (7). The stage of CTE (I to IV) is based on the extent and severity of ptau pathology (8). Briefly, stage I CTE is characterized by isolated perivascular foci of ptau present at the sulcal depths. In stage II, multiple perivascular lesions are present, the ptau pathology extends to involve the superficial cortical layers of the gyral crest. In stage III, there is increased cortical ptau pathology as well as medial temporal lobe ptau pathology, e.g. hippocampus, amygdala, entorhinal cortex. By stage IV, there is widespread cortical involvement as well as abnormal ptau accumulation within the diencephalon, brainstem, and cerebellum. Transactive response DNA-binding protein of 43 kDa (TDP-43) pathology is also frequent in CTE, including TDP-43-immunoreactive neuronal cytoplasmic inclusions and dot-like structures (6, 7). Thus far, CTE can only be definitely diagnosed postmortem, and the prevalence and incidence of the disorder remain unknown (2, 5–9).
Amyotrophic lateral sclerosis (ALS) is a progressive, fatal neurodegenerative disorder involving motor neuron dysfunction. Onset may include upper or lower motor neuron symptoms, which are typically progressive with eventual involvement of upper and lower motor neurons. Cognition is also affected in a majority of cases at some point in the disease course (10). Incidence estimates for ALS range from about 2 to 3 per 100 000 people per year; ALS is more common in males and military veterans (11, 12). ALS onset is most often sporadic, with approximately 10% of cases considered familial. There is a known genetic component for approximately 5%–10% of sporadic cases and 60% of familial cases (12, 13). As a result, environmental factors are believed to play an important role in the development of ALS (12).
One such environmental risk factor may be traumatic brain injury (TBI). Chen et al found that individuals were 11 times more likely to develop ALS if they reported multiple head injuries within the last 10 years prior to diagnosis (14), although other studies have not found a modifying effect of head injury on ALS (15). US military veterans have an increased frequency of ALS, and the veteran population has higher head injury prevalence than the comparable civilian population (16–18). Moreover, in a veteran cohort, those with head injury, especially head injuries within 15 years prior to diagnosis, were found to be significantly more likely to have developed ALS (16).
We set out to determine the frequency of occurrence of co-morbid CTE within an ALS brain bank and to examine the demographic, functional, and pathological distinctions between participants with ALS only and those with ALS+CTE. We hypothesized that veterans with both ALS+CTE would be more likely to have had a TBI or to have served for longer periods in the military than veterans with ALS only. We further tested the hypotheses that participants with ALS+CTE would be more likely to present with behavioral, mood, and cognitive disorders and to have more ptau pathology in their frontal cortex and spinal cord.
MATERIALS AND METHODS
Participants
The Department of Veterans Affairs Biorepository Brain Bank (VABBB) is a longitudinal research project and tissue repository that collects extensive functional, disease progression, and neuropathological data from enrolled veterans across the United States (18). The VABBB follows veterans with ALS or related motor neuron disorders during life and then collects, processes, and stores CNS tissue after death (19). Since its inception in 2006, the VABBB has enrolled a total of 287 veterans with ALS. Some veterans (16 with ALS and 1 with ALS+CTE) were enrolled postmortem by family members. Veterans who elect to enroll in the study consent to the collection of health and functional data as well as to the donation of their whole brain, spinal cord, and cerebrospinal fluid at the time of their death. Procedures were in accordance with ethical standards required by the VA Boston Healthcare System Institutional Review Board and VA Office of Research and Development. Of the 287 total participants enrolled, 156 were deceased with a complete neuropathological evaluation with cortical and spinal cord tau immunostaining for the current analyses. One participant did not meet criteria for neuropathological diagnosis of ALS and was excluded, resulting in a total of 155 deceased participants selected for the study.
Clinical Assessment
Longitudinal clinical data was collected via telephone interview of the participant (or proxy in cases where the participant was unable to speak), questionnaires completed via the mail, and medical record review by study staff using the VA’s Compensation and Pension Record Interchange medical record system (18). Demographic information collected included gender, race and ethnicity, occupation, and military service information including branch, number of years served, deployment history, and combat history. Information collected that was relevant to ALS included the clinical diagnosis as determined by a neurologist, site and age of onset, family history of ALS, and other neurological diagnoses.
TBI was defined as any single event leading to neurological symptoms, ranging from mild to severe events, and including situations that resulted in loss of consciousness or hospitalization. RHI were defined as an extended period of engagement in an activity associated with head impacts, including contact sports play and bull riding. Cognitive impairment was determined based on a clinical history of dementia, short- or long-term memory dysfunction, and recurrent episodes of confusion or a lack of situational awareness, as reported by families or in the medical record. A behavioral disorder was considered present when there was documented history of anger, mania, irritability, aggression, violent actions, inappropriate behavior, emotional lability, or decreased capacity for self-direction that was noted by the family or the medical record to be a departure from previous normal behavior. Mood disorders were determined based on a documented clinical diagnosis of depression or anxiety. Pseudobulbar affect was considered a separate neurological diagnosis and was not included as a behavioral or mood disorder.
Motor Function Assessment
Motor function status was assessed throughout the time of participation in the study. Beginning at enrollment, and continuing semi-annually, veterans participated in a telephone interview with a VABBB researcher during which they discussed changes in functionality, reviewed their medication list, and completed the ALS Functional Rating Scale-revised (ALSFRS-R). The ALSFRS-R is a widely used and validated measure to evaluate functional status in ALS (20). The administration of the ALSFRS-R by trained non-clinicians has been demonstrated to have exemplary inter-rater, intra-rater, and telephone-administered reliability (21). This scale consists of 12 questions divided into 4 sub-categories, which consist of 3 questions each. These sub-scales evaluate gross motor function, fine motor function, respiratory function, and bulbar function. The maximum total score of the ALSFRS-R is a 48, and the maximum score on each sub-scale is 12 (22, 23).
Longitudinal data from the ALSFRS-R were used to evaluate functional status, both at individual points in time, such as at enrollment or at death, as well as to evaluate progression of functional status over time. For this cohort, ALSFRS-R scores at death data were available for participants with ALS only (n = 128), and those with ALS+CTE (n = 8). ALSFRS-R scores were not available for participants who were enrolled postmortem. Thus, 18 participants with ALS only and one participant with ALS+CTE did not have ALSFRS-R data available. To determine ALSFRS-R change scores (ΔFS) over time, a score of 48 (maximum score, indicative of healthy functioning) was assigned at the time of symptom onset, and a ΔFS score was calculated as follows: ΔFS = (48 – ALSFRS-R at baseline)/(disease duration), with baseline referring to the baseline assessment completed shortly after enrollment (24, 25). This score, the ΔFS score, has been used to indicate functional progression and disease duration in other ALS cohorts (25).
Neuropathological Examination
Neuropathological processing followed previously established procedures (18) and included a comprehensive analysis designed to screen for all known neurodegenerative pathologies. Neuropathologists were blinded to clinical features of the participants. Neuropathological diagnoses were based on well-defined criteria. Briefly, ALS was defined as the degeneration of upper and lower motor neurons, degeneration of lateral and ventral corticospinal tracts of the spinal cord, and loss of anterior horn cells from cervical, thoracic and lumbar spinal cord with gliosis (19, 26). For a subset of cases, the TDP-43 stage was determined using the criteria of Brettschneider et al (27). Tau pathology in the dorsolateral frontal cortex and spinal cord was scored on a semiquantitative scale from 0 to 3. CTE was diagnosed using the recent consensus criteria that include ptau accumulation within neurons, astrocytes, and cell processes in an irregular and patchy distribution that is perivascular and concentrated within the depths of sulci (7). The criteria for Alzheimer disease were based on the presence of amyloid-ß neuritic plaques and ptau neurofibrillary tangles according to the most recent NIA Alzheimer Association’s guidelines (28, 29), including Thal phase for amyloid (30), Braak and Braak staging of neurofibrillary tangles (31, 32), and the overall density of neuritic plaques based on CERAD criteria (33). The diagnosis of Lewy body disease was based on the presence and distribution of α-synuclein-positive Lewy bodies and was considered brainstem-predominant, limbic or transitional Lewy body disease, and neocortical or diffuse Lewy body disease as defined by McKeith criteria (34) and Braak staging (35, 36). Neuropathological diagnosis of frontotemporal lobar degeneration (FTLD) was based on predominant involvement of the frontal and temporal lobes and characteristic immunohistochemistry for ptau, TDP-43, and p-TDP-43 using established criteria (37–39).
Genetic Analysis
Genotyping was done using an updated version of the NeuroX Illumina genotyping array, NeuroChip. NeuroChip tests for 179 467 genetic variants associated with various neurological diseases, as well as testing a separate set of 306 670 variants (40). In addition, samples were also run using Illumina’s OmniExpress chip, which conducts an assay on 730 525 single nucleotide polymorphisms. Analyses were performed at the National Institute on Aging in Bethesda, Maryland.
Statistical Analysis
All statistical analyses were performed using IBM SPSS Statistics for Windows, version 20 (IBM Corp., Armonk, NY). Non-parametric independent samples Mann-Whitney U tests were performed for all categorical data in which ranking distributions or means were compared between groups. Fisher exact tests of variance were performed for group comparisons on categorical data counts. Pearson chi-squared tests of variance with Bonferroni corrections were conducted for multiple group comparisons on categorical data counts. An alpha level of 0.05 was used for all statistical tests.
RESULTS
Cohort Summary
One hundred fifty-five participants with a clinical diagnosis of motor neuron disease and a comprehensive neuropathological workup were included in this cohort. The mean age at death was 70.3 years, and the median was 71.8 years. ALS disease duration ranged from 6 to 504 months with a mean duration of 126 months and median of 96 months. The cohort was predominantly male (99%). One hundred thirty-eight participants were White (89%), 4 were Black or African American (3%), 1 was Asian (<1%), and 10 did not provide information (8%). With regards to ethnicity, 139 (89%) of participants were not Hispanic or Latino, 2 (1%) were Hispanic or Latino, and 14 (10%) did not provide information. Veterans previously served in the following branches of the US Armed Forces: Army 71 (46%), Navy 38 (24%), Air Force 31 (19%), Marines 8 (6%), Coast Guard 2 (1%), Army and Air Force 1 (<1%), and information was unavailable for 4 (3%). Mean time in active military service, based on available information for 149 participants, was 6.1 years. Twenty-six (17%) had a family history of neurological disease, and of this group 13 (8%) had a family history specific for ALS.
Of the 155 participants, 152 had available clinical medical records. All 3 of the cases missing clinical medical records were participants with ALS only. Sixteen (11%) had a record of at least 1 TBI, 12 (8%) had a record of exposure to a form of RHI (e.g. participation in contact sports), and 7 (5%) had a history of both TBI and RHI. Overall, there were a total of 21 participants with a history of either TBI or RHI: 9 (43%) had a pathological diagnosis of CTE (CTE-1-9) and 12 (57%) did not (ALS-1-12, Table 1). Of the 16 participants with a history of TBI, 8 (50%) received a co-morbid CTE diagnosis at autopsy. For the 152 participants with clinical records, 40 (26%) had noted mood changes or a mood disorder diagnosis, 12 (8%) had behavioral changes, and 12 (8%) had cognitive impairment. In the entire cohort, 153 (99%) had neuropathologically confirmed ALS and 2 (1%) had motor neuron disease with prominent lower motor degeneration consistent with primary muscular atrophy. Onset site of motor neuron dysfunction varied. Of the 153 participants with a neuropathologically confirmed ALS diagnosis, 65 (42%) of participants had lower extremity onset, 41 (27%) participants had upper extremity onset, 21 (14%) of participants had bulbar onset, and one (<1%) participant had respiratory onset. Onset site was unknown for the remaining 25 (16%) of participants. Age at onset of ALS symptoms was known for 140 participants, and the mean age at onset was 59.6 years.
Table 1.
Demographic and Pathological Features of Participants With a History of Traumatic Brain Injury or Repetitive Head Impacts With ALS and CTE (CTE-1-9) and ALS Only (ALS-1-12)
| Case | Age at Death | Onset | Disease Duration (Months) | Weight Change | TBI History | RHI Exposure | Clinical Diagnosis | CTE Stage | UMN Degeneration Ranked | Cervical LMN Degeneration Ranked | Thoracic LMN Degeneration Ranked | Lumbar LMN Degeneration Ranked | TDP-43 Inclusions | Braak NFT Stage | CERAD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTE-1 | 42 | UE | 203 | −62.1 | + | + | ALS | III | +++ | + + + | + + + | + + + | + | III | 0 |
| CTE-2 | 64 | LE | 81 | 9.9 | + | + | ALS | I | + | + + + + | + + + + | + + + + | + | 0 | 0 |
| CTE-3 | 53 | UE/Bulbar | 110 | 19.2 | − | + | ALS | II | +++ | + + + | + + + | + + + | + | I | 0 |
| CTE-4 | 80 | LE | 288 | −8.2 | + | − | ALS | II | + + + | + + | + + | + + + | − | III | 1 |
| CTE-5 | 79 | Bulbar | 87 | −25 | + | + | ALS | IV | +++ | + + + | + + + | + + + | + | II | 1 |
| CTE-6 | 87 | LE | 164 | −33 | + | + | ALS | II | + | + | + | + + | − | III | 0 |
| CTE-7 | 56 | Bulbar | 51 | −15.6 | + | ALS | III | + + + + | + + + + | + + + + | + + + + | + | II | 1 | |
| CTE-8 | 55 | LE/Bulbar | 35 | −68.3 | + | + | ALS | III | + + + | + + + | + + + | + + + | + | III | 0 |
| CTE-9 | 54 | Bulbar | 54 | −26.5 | + | − | ALS | II | + + + | + + + + | + + + | + + + | + | II | 0 |
| ALS-1 | 44 | UE | 90 | −40 | + | ALS | 0 | ++++ | ++++ | ++++ | ++ | − | 0 | 0 | |
| ALS-2 | 75 | LE | 228 | + | + | ALS | 0 | ++ | ++ | ++ | + | + | 0 | 0 | |
| ALS-3 | 84 | LE | 180 | −11.6 | + | + | ALS | 0 | ++ | ++ | +++ | +++ | − | III | 1 |
| ALS-4 | 73 | LE | 192 | −21 | − | + | ALS | 0 | ++++ | + | + | + | + | III | 0 |
| ALS-5 | 80 | LE | 164 | −53 | + | ALS | 0 | + | ++ | + | + | − | II | 1 | |
| ALS-6 | 57 | UE | 105 | −20.7 | − | + | ALS | 0 | ++ | ++++ | ++++ | ++++ | + | I | 0 |
| ALS-7 | 70 | LE | 120 | 20 | − | + | ALS | 0 | ++ | +++ | +++ | + | II | 0 | |
| ALS-8 | 73 | LE | 60 | −28.8 | + | ALS | 0 | ++ | +++ | +++ | +++ | + | II | 0 | |
| ALS-9 | 73 | LE | 192 | −16 | + | ALS | 0 | + | +++ | +++ | +++ | − | I | 1 | |
| ALS-10 | 88 | LE | 84 | −3 | − | + | ALS | 0 | + | +++ | +++ | +++ | − | III | 1 |
| ALS-11 | 69 | UE | 96 | −78 | + | ALS | 0 | +++ | +++ | +++ | +++ | + | III | 1 | |
| ALS-12 | 57 | Bulbar | 96 | −90 | + | ALS | 0 | +++ | ++++ | ++++ | ++++ | + | II | 0 |
Data are displayed as exact values, as values in established neuropathological ranking systems, or as a (+/−) system as appropriate. (+) indicates the presence of a given variable and (−) indicates the absence of history or pathological marker; for ranked columns of degeneration severity is scored from 0 to ++++.
ALS, amyotrophic lateral sclerosis; CTE, chronic traumatic encephalopathy; TBI, traumatic brain injury; RHI, repetitive head impacts; UMN, upper motor neuron; LMN, lower motor neuron; TDP-43, transactive response DNA binding protein 43; NFT, neurofibrillary tangle; CERAD, Consortium to Establish a Registry for Alzheimer’s Disease (0–3).
Neuropathological Assessment of CTE
Of the 155 participants with pathologically confirmed ALS or related MND, 9 participants (5.8%) were neuropathologically diagnosed with CTE. The pathologic features of participants with a history of TBI or repetitive head impacts with co-morbid ALS and CTE or with ALS alone are summarized in Table 1. The brains of all participants were examined macroscopically for changes of CTE, including cerebral atrophy, ventricular dilation, and atrophy of the thalamus, hypothalamus, and mammillary bodies. Septal abnormalities could not be optimally assessed due to the midline cut necessary to process half the cerebrum for freezing and half for fixation. In one participant with CTE, CTE-1, there was a dusky discoloration of the leptomeninges over the left dorsolateral frontal cortex including the motor strip suggestive of a remote subarachnoid hemorrhage. CTE-4 had a cavitary lesion (4 cm) within the right gyrus rectus consistent with his history of severe TBI. Five participants with CTE showed mild to moderate atrophy of the frontal lobes (CTE-2, CTE-3, CTE-4, CTE-5, and CTE-8). Temporal atrophy was found in 2 (CTE-5 and CTE-8), mammillary body atrophy in one (CTE-3); thinning of the posterior aspect of the corpus callosum in 5 (CTE-3, CTE-4, CTE-5, CTE-6, CTE-8, and CTE-9), and mild hippocampal atrophy in 2 (CTE-5 and CTE-8).
The regions most frequently involved by CTE ptau pathology were the middle frontal gyrus (8/9), superior temporal gyrus (9/9), inferior parietal lobule (7/9), and hippocampus (7/9). Each participant was assigned a stage corresponding to the CTE stages proposed by McKee et al (8). One participant was stage I, 4 participants were stage II, 3 participants were stage III, and one participant was stage IV. Figure 1 shows the pathology of participants CTE-5 and CTE-8. CTE-5 had both TDP-43 and tau pathology within the spinal cord and middle frontal cortex. Abundant neurofibrillary tangles and ptau-positive processes were present around blood vessels and at the sulcal depths most severely in the frontal cortex (Fig. 1F). Additional examples of ptau pathology present within neurons and processes in a CTE pattern at the sulcal depths and around blood vessels are shown for participant CTE-8 (Fig. 1G–I). All participants with ALS+CTE had tau-positive NFTs and 4/9 had tau-positive glial tangles. Beta-amyloid plaques were present in 5 participants (CTE-4, CTE-5, CTE-6, CTE-7, and CTE-8). TDP-43 inclusions were present in 7 participants and involved the frontal cortex in 3 participants (CTE-1, CTE-7, and CTE-5). CTE-5 had TDP-43 intra-neuronal cytoplasmic inclusions within the dentate gyrus of the hippocampus as well as the frontal and temporal cortex and met criteria for FTLD-TDP.
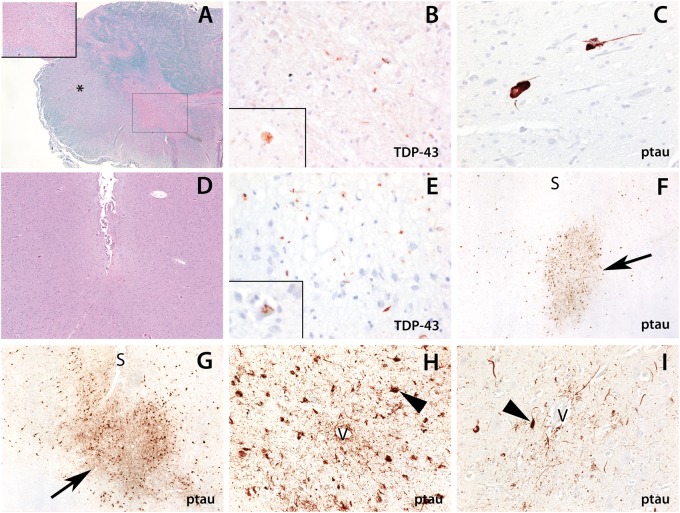
Figure 1.
Neuropathological features of ALS with chronic traumatic encephalopathy in CTE-5 (A–F) and CTE-8 (G–I). (A–C) There is marked degeneration of the spinal cord. (A) Luxol H&E shows degeneration of the lateral corticospinal tracts (*, ×20 magnification) and loss of anterior horn cells (box and inset, ×200 magnification). (B) An immunostain for phosphorylated TDP-43 (pTDP-43) shows rare cytoplasmic inclusions within the remaining anterior horn cells (×200 and inset, ×400 magnification). (C) Immunostaining (AT8) shows scattered ptau-positive neurofibrillary tangles and threads within the anterior horn (×400 magnification). (D–F) The cortex shows the changes of chronic traumatic encephalopathy. (E) There are pTDP-43-positive neurites and cytoplasmic inclusions that are predominant within the sulcal depths (×200 magnification) and occasional skein-like inclusions within neurons (inset, ×400 magnification). (F, G) Ptau pathology is present focally at the sulcal depths of the frontal cortex with perivascular neurofibrillary tangles and threads in CTE-5 (F) and CTE-8 (G) ([S], sulcus; arrows highlight the foci of ptau pathology; ×40 magnification). (H, I) Perivascular ptau neurofibrillary tangles and processes in CTE-8 ([V], blood vessel; arrowheads highlight neurofibrillary tangles; ×200 magnification).
History of Head Impacts
All 9 participants with ALS+CTE were male, and all had a history of some form of head impact: 8 had a history of at least 1 moderate to severe TBI, and 6 had confirmed history of RHI from contact or alternative sports or military-related activities. In 2 participants, head impact exposure was associated with military service. Eight donors were White, and one was Asian. CTE-1 was an Army veteran of the Persian Gulf War era who played football, basketball, and baseball for 4 years each, intermural sports during college, and who suffered one TBI that resulted in a loss of consciousness; CTE-2 was an Army veteran of the Vietnam era who participated in bull riding and suffered 3 TBI’s as a result of falling out of a helicopter, a serious motor vehicle accident, and a grenade blast; CTE-3 was a Navy veteran of the Persian Gulf War era that reportedly suffered mild head impacts due to repetitive landings on air craft carriers, which he performed throughout his 25 years of service. CTE-4 was an Air Force veteran of the Korean era who denied history of repetitive head injury, but suffered a TBI from a fall that resulted in a fractured skull; CTE-5 was an Army veteran of the Korean era who played football, basketball, participated in boxing, and suffered one TBI that resulted in a loss of consciousness; CTE-6 was a Merchant Marine veteran of World War II who played football for 8 years, and suffered 2 sports-related concussions; CTE-7 was a Navy veteran of the post-Vietnam era for whom a history of RHI was unknown, but who suffered a TBI due to a fall, resulting in hospitalization and 8 stiches; CTE-8 was an Air Force veteran of the post-Vietnam era who played football, baseball, and basketball for 4 years, was a mountain biker, and suffered a TBI due to a motorcycle accident that led to subsequent behavioral changes; finally, CTE-9 was a Marine veteran of the Persian Gulf War era for whom a history of contact sports play was unknown, but who had a history of falls secondary to his ALS, one of which resulted in hospitalization for a concussion.
Demographic, Clinical, and Genetic Characteristics
Demographic, clinical, and genetic data are compared for participants with ALS+CTE and ALS participants without CTE in Table 2. Participants with ALS+CTE had a slightly younger, but non-significant, age at death (63.3 years) than ALS alone (70.7 years; p = 0.128). As expected, a history of TBI was significantly more prevalent among participants with ALS+CTE than among those with ALS alone (8/9 ALS+CTE [89%] vs 8/146 ALS [5%], p < 0.001). Clinically, participants with ALS+CTE were more likely to present with bulbar-onset ALS symptoms compared to ALS alone (56% vs 14% p = 0.006). In addition, participants with ALS+CTE were significantly more likely to have mood disorders (78% vs 23%, p = 0.001) as well as behavioral disorders (44% vs 5%, p = 0.002). There was no significant difference between the frequencies of cognitive impairment. Mean age at onset was younger in participants with co-morbid CTE by about 7 years, although this did not reach significance (52.9 years vs 59.9 years, p = 0.096). Mean disease duration was not significantly different between groups. There were also no apparent differences between groups in the progression of ALS symptoms: the ALSFRS-R score at death (12.9 vs 12.8, p = 0.989) and the ΔFS (−0.5 vs −0.6, p = 0.689) were not significantly different between the groups.
Table 2.
Demographic, Clinical, and Genetic Findings
| ALS (n = 146) | ALS + CTE (n = 9) | p Value | ||
|---|---|---|---|---|
| Demographics | ||||
| Age at death (yrs) | 70.7 ± 9.9 | 63.3 ± 15.5 | 0.128‡ | |
| Gender, n male (%) | 143 (98%) | 9 (100%) | 0.835§ | |
| History of TBI, n (%) | 8 (5%) | 8 (89%) | <0.001§ | |
| RHI Exposure, n (%)* | – | 6 (67%) | – | |
| Family history of ALS, +/− (%) | 12/133 (8.3%) | 1/9 (11%) | 0.558§ | |
| Clinical characteristics | ||||
| Age at onset, years | 59.9 ± 12.1 | 52.9 ± 15.0 | 0.096‡ | |
| Disease duration, months | 126.9 ± 99.2 | 119.2 ± 83.7 | 0.912‡ | |
| Bulbar onset, +/− (%) | 17/108 (14%) | 5/4 (56%) | 0.006§ | |
| ALSFRS-R at death | 12.8 ± 7.8 | 12.9 ± 12 | 0.989‡ | |
| Progression rate (ΔFS)† | −0.6 ± 0.8 | −0.5 ± 0.5 | 0.689‡ |
|
| Cognitive impairment, +/− (%) | 11/135 (8%) | 1/8 (11%) | 0.525§ | |
| Behavioral disorder, +/− (%) | 8/138 (5%) | 4/5 (44%) | 0.002§ | |
| Mood disorder, +/− (%) | 33/113 (23%) | 7/2 (78%) | 0.001§ | |
| Genetic mutations | ||||
| Number tested | 93 (63%) | 5 (56%) | ||
| Known mutations** | 9 (10%) | 0 (0%) | 1.00§ | |
| C9orf72 | 3 (3%) | 0 (0%) | 0.306§ | |
Data are displayed as mean ± SD, percentage of cohort (%), or median (25th, 75th range) as appropriate.
ALS, amyotrophic lateral sclerosis; CTE, chronic traumatic encephalopathy; TBI, traumatic brain injury; RHI, repetitive head impacts; ALSFRS-R, ALS Functional Rating Scale-revised; C9orf72, chromosome 9 open reading frame 72; age at onset and disease duration available in 131 ALS participants; bulbar onset information available in 125 ALS participants.
RHI data was not available for the majority of ALS-only cases.
Progression rate (ΔFS) calculated as ΔFS = (ALSFRS at baseline – 48)/(disease duration).
Mann-Whitney U test.
Fisher exact test.
Known mutations included C9orF, FUS, NEK1, SOD1, TARDPB, PFN1, SETX, DCTN1, and OPTN.
There were no significant differences between groups in the presence of a family history of ALS. One of the 9 participants with ALS+CTE (CTE-4) had a family history of ALS, but genetic testing on this subject did not reveal any known ALS mutations. Ninety-eight participants were tested postmortem for genetic mutations known to be associated with neurological disease. No mutations were found in any of the 5 tested participants with ALS+CTE. Nine participants in the ALS only group tested positive for a known genetic mutation, and 3 of these participants had a C9orf72 mutation. Between the 2 groups, differences in the number of mutations were not significant for C9orf72 mutations (0% vs 3%, p = 0.36), or for all tested mutations (0% vs 10%, p = 1.00), although this comparison is likely underpowered.
Military history was different between groups. There were no significant differences in total number of years of service or in military branch between participants with ALS and ALS+CTE. However, the service era distribution was significantly different between groups (χ2 = 17.7, p = 0.013) such that participants with ALS+CTE were less likely to serve in Vietnam (p < 0.05) and more likely to have served in the first Persian Gulf War (p < 0.05, Table 3).
Table 3.
Military Service History
| (n = 141) | ALS + CTE (n = 9) | p Value | |
|---|---|---|---|
| Service duration, years ± SD | 6.15 ± 6.84 | 7.28 ± 8.86 | 0.639* |
| Branch, n (%) | 0.732† | ||
| Army | 28 (20%) | 3 (33%) | |
| Air Force | 68 (48%) | 3 (33%) | |
| Navy | 35 (25%) | 3 (33%) | |
| Marines | 8 (6%) | 0 (0%) | |
| Coast Guard | 2 (1%) | 0 (0%) | |
| Service era, n (%) | 0.013† | ||
| WWII | 10 (7%) | 0 (0%) | |
| Post-WWII | 2 (1%) | 0 (0%) | |
| Korean | 18 (13%) | 2 (22%) | |
| Post-Korean | 17 (13%) | 0 (0%) | |
| Vietnam | 63 (46%) | 1 (11%) | <0.05‡ |
| Post-Vietnam | 11 (8%) | 2 (22%) | |
| Persian Gulf War 1 | 11 (8%) | 4 (44%) | <0.05‡ |
| Multiple eras | 4 (3%) | 0 (0%) |
WWII, World War II.
Mann-Whitney U test.
χ2 test for proportions.
Post-hoc z-test with Bonferroni correction for multiple comparisons; service era data available for 136 participants with ALS.
Pathological Findings in Participants With CTE
Pathological findings were compared for participants with ALS+CTE and participants with ALS without CTE in Table 4. There were no significant differences in brain weight or severity of upper motor neuron or lower motor neuron degeneration. Both the frequency of tau pathology and its severity were increased within the middle frontal cortex (p = 0.002 and p < 0.001 respectively) in participants with ALS+CTE compared to participants with ALS alone. Furthermore, within ALS+CTE participants there was a trend toward increased frequency of tau pathology at all levels of the spinal cord, and the severity of tau pathology was significantly increased with higher median semiquantitative tau pathology scores within cervical (p = 0.024), thoracic (p = 0.014), and lumbar (p = 0.007) regions. There was no significant difference in the frequency of TDP-43 inclusions between groups (78% vs 71%, p = 1.000). However, there was a significantly increased TDP-43 stage using the criteria of Brettschneider et al in participants with ALS+CTE (27). There was a non-significant increased frequency of co-morbid frontotemporal lobar degeneration in participants with ALS+CTE vs participants with only ALS (11% vs 3%, p = 0.306). There were no significant differences between groups in the frequency of co-morbid Lewy body disease, median Braak NFT stage, median CERAD score, or median Thal phase.
Table 4.
Neuropathological Findings
| ALS (n = 146) | ALS + CTE (n = 9) | p Value | |
|---|---|---|---|
| Brain weight (g), n tested | 1307 ± 135, 141 | 1355 ± 198, 9 | 0.386* |
| Corticospinal tract degeneration, median (25th, 75th range) | 2, (1, 3) | 3 (1, 3) | 0.362* |
| LMN degeneration, median (25th, 75th range) | |||
| Cervical | 3 (2, 3) | 3 (2, 4) | 0.419* |
| Thoracic | 3 (2, 3) | 3 (2, 3) | 0.745* |
| Lumbar | 3 (2, 3) | 3 (3, 3) | 0.276* |
| TDP-43 inclusions, +/− (%) | 101/42 (71%) | 7/2 (78%) | 1.000* |
| TDP-43 stage, median (25th, 75th range) | 0 (0, 1) | 1 (0, 3) | 0.006* |
| Lewy body disease, +/− (%) | 21/125 (14%) | 1/8 (11%) | 1.000* |
| Frontotemporal lobar degeneration, +/− (%) | 5/141 (3%) | 1/8 (11%) | 0.306* |
| Braak NFT stage, median (25th, 75th range) | 2 (1, 3) | 2.5 (2, 3) | 0.171* |
| CERAD score, median (25th, 75th range) | 0.5 (0, 1) | 0.5 (0, 1) | 0.788* |
| Thal phase, median (25th, 75th range) | 2 (1, 2) | 1 (1, 1) | 0.310* |
| Tau pathology | |||
| Middle frontal | |||
| Frequency, +/− (%) | 12/113 (10%) | 8/1 (89%) | <0.001† |
| Severity, median (25th, 75th range) | 0 (0, 0) | 1 (0, 2) | <0.001* |
| Cervical | |||
| Frequency, +/− (%) | 51/49 (51%) | 6/1 (86%) | 0.080† |
| Severity, median (25th, 75th range) | 0 (0, 1) | 1 (1, 2) | 0.024* |
| Thoracic | |||
| Frequency, +/− (%) | 34/56 (38%) | 5/2 (71%) | 0.090† |
| Severity, median (25th, 75th range) | 0 (0, 1) | 1 (0, 2) | 0.014* |
| Lumbar | |||
| Frequency, +/− (%) | 25/69 (27%) | 4/3 (57%) | 0.102† |
| Severity, median (25th, 75th range) | 0 (0, 0) | 1 (0, 2) | 0.007* |
Data are displayed as mean ± SD, percentage of cohort tested, or median (25th, 75th range) as appropriate. Corticospinal tract degeneration, LMN degeneration, and tau pathology severity are based on semiquantitative scores that range from 0 to 3.
ALS, amyotrophic lateral sclerosis; CTE, chronic traumatic encephalopathy; TDP-43, transactive response DNA binding protein 43; LMN, lower motor neuron; NFT, neurofibrillary tangle; CERAD, Consortium to Establish a Registry for Alzheimer’s Disease.
Mann-Whitney U test.
Fisher exact test.
DISCUSSION
In this cohort of 155 veterans with pathologically confirmed ALS or related MND, 9 participants (5.8%) had a co-morbid diagnosis of CTE. Participants co-morbid for CTE were significantly more likely to have a history of TBI, to have bulbar onset ALS, and to have had behavioral and mood changes, including depression and anxiety disorders. Participants with ALS+CTE also had significantly more ptau pathology in the frontal cortex and at all levels of the spinal cord.
TBI from a variety of sources were associated with the development of CTE in this cohort. Of participants with a positive history of TBI, 50% were found to have co-morbid CTE. A history of head impact was present in all participants with ALS+CTE and consisted of a single or multiple mild to severe TBI, RHI, or both. The frequency of TBI was significantly higher among participants with ALS+CTE than among participants with ALS only. Within participants with ALS+CTE, RHI exposure was due to contact sports exposure (e.g. tackle football) in 4 (44%), military service (frequent aircraft carrier landings) in one (11%), and both (bull riding and grenade blast exposure) in one (11%). Most of these exposures have been previously related to the development of CTE, including bull riding, which was recently reported in association with CTE (41). In addition, individuals with ALS are at higher risks for falls compared to the general population and therefore may have an increased risk for developing CTE (42).
A total of 98 participants were assessed for major genetic variations associated with ALS, such as SOD1, FUS, and C9orf72. Of the 9 participants who tested positive for an ALS-linked mutation, none were co-morbid for CTE, and only CTE-4 had a family history of ALS. Although the sample size is limited, the lack of known ALS-associated genetic variation in ALS+CTE supports the idea that RHI may be an environmental cause of ALS as well as CTE. The study of additional ALS subjects with CTE or a history of exposure to RHI will help determine whether there is an interaction between trauma and known ALS genetic risk factors.
Participants with co-morbid ALS+CTE were significantly more likely to have bulbar onset ALS. Age of onset was lower in participants with CTE, though this difference did not reach statistical significance. This is similar to a previous report of increased frequency of bulbar onset ALS and a younger age of onset in a group of soccer players (43). Although bulbar onset ALS has been associated with faster disease progression (44), ΔFS rates were not significantly different between participants with ALS+CTE and ALS only although this analysis is likely underpowered due to the small number of participants with CTE.
CTE is often associated with mood and behavioral dysfunction (6). In this study, participants with ALS+CTE had significantly more reported behavioral changes and mood disorders compared to participants with ALS alone. Participants with ALS have been shown to have an elevated frequency of minor to major depressive symptoms of approximately 27% (42), which is similar to the frequency of mood disorders amongst the VABBB ALS participants (26%). In contrast, in participants with ALS+CTE, the frequency of mood disorders was much higher (78%). There were no significant differences in the frequency of cognitive impairment between groups. However, the prevalence of cognitive impairment, as well as mood and behavioral dysfunction, may be underreported as detection of these changes can be masked by communication difficulties caused by progressive ALS symptomology.
Pathologically, there was significantly increased ptau pathology in the frontal cortex and all levels of the spinal cord in participants within ALS+CTE compared to ALS alone. The frontal cortex ptau pathology of CTE may underlie the increased frequency of behavioral and mood disorders in participants with ALS+CTE. A recent study demonstrated phosphorylation of tau at Thr175 and Thr231 in individuals with CTE and ALS+CTE, but not in participants with sporadic ALS without CTE (45). It remains to be determined whether the increased ptau pathology in the spinal cord reflects traumatic injury primarily to the brain or spinal region, or both. Although there were no significant differences between the degree of degeneration in the upper or lower motor neuron pathways in ALS+CTE compared to ALS alone, assessment at death when the disease is advanced may preclude detection of changes earlier in the disease course. There were also no significant differences in the frequencies of comorbid neurodegenerations, including FTLD, LBD, and AD pathological changes.
The presence of comorbid ALS+CTE in an ALS cohort parallel similar findings of CTE in neurodegenerative cohorts, e.g. 6% in an multiple system atrophy (MSA) cohort as reported by Koga et al (46), 6% in an American football CTE group (47) and 11.9%–32% in a group of various diseases reported by Ling et al (48). The prevalence of CTE found in an MSA cohort is similar to the prevalence found in this study (5.8%), and both populations are at a high risk of falls after the onset of neurological disease. In addition, veterans are at risk for CTE from exposure to multiple sources of trauma, including combat-related activities, blast exposures, training exercises, recreational activities, as well as motor vehicle accidents, falls, and contact sports participation. Moreover, individuals with a history of repetitive head impacts and CTE may be at risk for multiple neurodegenerations including β-amyloid deposition (49) and Lewy body disease (50).
LIMITATIONS
There are several limitations inherent to our study population and methodology. Veterans were initially enrolled via outreach to the Durham VA ALS Registry and subsequently through passive methods such as online articles, use of a website, and unsolicited referrals from clinics aware of the project, all of which may introduce bias. Methods for determination of TBI and RHI exposure were based on retrospective review, which may also introduce bias. Future studies utilizing prospective, longitudinal clinical data are necessary to confirm and expand associations among RHI, CTE, and ALS. Veterans are at increased risk for ALS development (51) and their exposure to combat, combat training, and contact sports may predispose them to head injuries to a greater degree than the general population, limiting the generalizability of the study. Although this is the largest screen of participants with ALS for CTE to date, the relatively small number of participants with ALS+CTE limits the power of many of the analyses.
CONCLUSIONS
We examined a cohort of 155 veteran ALS brain donors for co-morbid CTE and found it in 5.8% of participants. There were also distinct clinical and pathological differences between participants with ALS+CTE and ALS alone. Veterans with ALS+CTE had significantly higher frequencies of TBI exposure, bulbar onset ALS, mood and behavioral disorders, and increased ptau pathology in the frontal cortex and spinal cord. Future studies of non-veterans will help resolve whether these differences in clinical presentation and pathology are also found in contact sports participants with CTE+ALS.
ACKNOWLEDGMENTS
We are grateful to Dr Bryan Traynor (NIH—National Institute on Aging) for providing genetic testing on VABBB tissue samples, and to the Cooperative Studies Program (CSP) and CSP #500A, National Registry of Veterans with Amyotrophic Lateral Sclerosis for providing data on those participants who were previously enrolled in CSP #500A that later enrolled in the Veterans Affairs Biorepository Brain Bank. All content, statements, opinions, or views are solely of the author(s) and do not reflect official views of the Department of Veterans Affairs or the National Institutes of Health. We gratefully acknowledge all the veterans and their families whose participation and contributions made this work possible.
This work was supported by the Department of Veterans Affairs, Veterans Health Administration, Veterans Affairs Biorepository (BX002466); Clinical Sciences Research and Development Merit Award (I01-CX001038); National Center for PTSD; National Institute of Aging (RF1AG054156, R56AG057768); National Institute of Aging Boston University AD Center (P30AG13846; supplement 0572063345-5).
The authors have no duality or conflicts of interest to declare.
REFERENCES
- 1. McKee AC, Cantu RC, Nowinski CJ, et al. Chronic traumatic encephalopathy in athletes: Progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol 2009;68:709–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gavett BE, Stern RA, McKee AC.. Chronic traumatic encephalopathy: A potential late effect of sport-related concussive and subconcussive head trauma. Clin Sports Med 2011;30:179–88, xi [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bailes JE, Petraglia AL, Omalu BI, et al. Role of subconcussion in repetitive mild traumatic brain injury. J Neurosurg 2013;119:1235–45 [DOI] [PubMed] [Google Scholar]
- 4. Perry DC, Sturm VE, Peterson MJ, et al. Association of traumatic brain injury with subsequent neurological and psychiatric disease: A meta-analysis. J Neurosurg 2016;124:511–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gardner RC, Yaffe K.. Epidemiology of mild traumatic brain injury and neurodegenerative disease. Mol Cell Neurosci 2015;66:75–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Daneshvar DH, Goldstein LE, Kiernan PT, et al. Post-traumatic neurodegeneration and chronic traumatic encephalopathy. Mol Cell Neurosci 2015;66:81–90 [DOI] [PubMed] [Google Scholar]
- 7. McKee AC, Cairns NJ, Dickson DW, et al. The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol 2016;131:75–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McKee AC, Stern RA, Nowinski CJ, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain 2013;136:43–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Omalu B, Bailes J, Hamilton RL, et al. Emerging histomorphologic phenotypes of chronic traumatic encephalopathy in American athletes. Neurosurgery 2011;69:173–83 [DOI] [PubMed] [Google Scholar]
- 10. Strong MJ, Abrahams S, Goldstein LH, et al. Amyotrophic lateral sclerosis—Frontotemporal spectrum disorder (ALS-FTSD): Revised diagnostic criteria. Amyotroph Lateral Scler Frontotemporal Degener 2017;18:153–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bieniek KF, Ross OA, Cormier KA, et al. Chronic traumatic encephalopathy pathology in a neurodegenerative disorders brain bank. Acta Neuropathol 2015;130:877–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Logroscino G, Traynor BJ, Hardiman O, et al. Incidence of amyotrophic lateral sclerosis in Europe. J Neurol Neurosurg Psychiatry 2010;81:385–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tiryaki E, Horak HA.. ALS and other motor neuron diseases. Continuum (Minneap Minn) 2014;20:1185–207 [DOI] [PubMed] [Google Scholar]
- 14. Chen H, Richard M, Sandler DP, et al. Head injury and amyotrophic lateral sclerosis. Am J Epidemiol 2007;166:810–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fournier CN, Gearing M, Upadhyayula SR, et al. Head injury does not alter disease progression or neuropathologic outcomes in ALS. Neurology 2015;84:1788–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schmidt S, Kwee LC, Allen KD, et al. Association of ALS with head injury, cigarette smoking and APOE genotypes. J Neurol Sci 2010;291:22–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weisskopf MG, Cudkowicz ME, Johnson N.. Military service and amyotrophic lateral sclerosis in a population-based cohort. Epidemiology 2015;26:831–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brady CB, Trevor KT, Stein TD, et al. The Department of Veterans Affairs Biorepository Brain Bank: A national resource for amyotrophic lateral sclerosis research. Amyotroph Lateral Scler Frontotemporal Degener 2013;14:591–7 [DOI] [PubMed] [Google Scholar]
- 19. Greenfield JG, Love S, Louis DN, et al. Greenfield's Neuropathology .8th Ed London: Hodder Arnold; 2008 [Google Scholar]
- 20. Kollewe K, Mauss U, Krampfl K, et al. ALSFRS-R score and its ratio: A useful predictor for ALS-progression. J Neurol Sci 2008;275:69–73 [DOI] [PubMed] [Google Scholar]
- 21. Kaufmann P, Levy G, Montes J, et al. Excellent inter‐rater, intra‐rater, and telephone‐administered reliability of the ALSFRS‐R in a multicenter clinical trial. Amyotroph Lateral Scler 2007;8:42–6 [DOI] [PubMed] [Google Scholar]
- 22. Cedarbaum JM, Stambler N, Performance of the Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS) in multicenter clinical trials J Neurol Sci 1997;152(Suppl 1):S1–S9 [DOI] [PubMed] [Google Scholar]
- 23. Cedarbaum JM, Stambler N, Malta E, et al. The ALSFRS-R: A revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J Neurol Sci 1999;169:13–21 [DOI] [PubMed] [Google Scholar]
- 24. Kimura F, Fujimura C, Ishida S, et al. Progression rate of ALSFRS-R at time of diagnosis predicts survival time in ALS. Neurology 2006;66:265–7 [DOI] [PubMed] [Google Scholar]
- 25. Labra J, Menon P, Byth K, et al. Rate of disease progression: A prognostic biomarker in ALS. J Neurol Neurosurg Psychiatry 2016;87:628–32 [DOI] [PubMed] [Google Scholar]
- 26. Brownell B, Oppenheimer DR, Hughes JT.. The central nervous system in motor neurone disease. J Neurol Neurosurg Psychiatry 1970;33:338–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brettschneider J, Del Tredici K, Toledo JB, et al. Stages of pTDP‐43 pathology in amyotrophic lateral sclerosis. Ann Neurol 2013;74:20–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Newell KL, Hyman BT, Growdon JH, et al. Application of the National Institute on Aging (NIA)-Reagan Institute criteria for the neuropathological diagnosis of Alzheimer disease. J Neuropathol Exp Neurol 1999;58:1147–55 [DOI] [PubMed] [Google Scholar]
- 29. Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: A practical approach. Acta Neuropathol 2012;123:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Thal DR, Rub U, Orantes M, et al. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology 2002;58:1791–800 [DOI] [PubMed] [Google Scholar]
- 31. Braak H, Braak E.. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991;82:239–59 [DOI] [PubMed] [Google Scholar]
- 32. Braak H, Braak E, Bohl J.. Staging of Alzheimer-related cortical destruction. Eur Neurol 1993;33:403–8 [DOI] [PubMed] [Google Scholar]
- 33. Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology 1991;41:479–86 [DOI] [PubMed] [Google Scholar]
- 34. McKeith IG. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): Report of the Consortium on DLB International Workshop. J Alzheimers Dis 2006;9:417–23 [DOI] [PubMed] [Google Scholar]
- 35. Del Tredici K, Rub U, De Vos RA, et al. Where does Parkinson disease pathology begin in the brain? J Neuropathol Exp Neurol 2002;61:413–26 [DOI] [PubMed] [Google Scholar]
- 36. Braak H, Del Tredici K.. Invited Article: Nervous system pathology in sporadic Parkinson disease. Neurology 2008;70:1916–25 [DOI] [PubMed] [Google Scholar]
- 37. Cairns NJ, Bigio EH, Mackenzie IR, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: Consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol 2007;114:5–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bigio EH. Update on recent molecular and genetic advances in frontotemporal lobar degeneration. J Neuropathol Exp Neurol 2008;67:635–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mackenzie IR, Neumann M, Bigio EH, et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: An update. Acta Neuropathol 2010;119:1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Blauwendraat C, Faghri F, Pihlstrom L, et al. NeuroChip, an updated version of the NeuroX genotyping platform to rapidly screen for variants associated with neurological diseases. Neurobiol Aging 2017;57:e9–e13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Keene CD, Latimer CS, Steele LM, et al. First confirmed case of chronic traumatic encephalopathy in a professional bull rider. Acta Neuropathol 2018;135:303–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kehyayan V, Korngut L, Jette N, et al. Profile of patients with amyotrophic lateral sclerosis across continuum of care. Can J Neurol Sci 2014;41:246–52 [DOI] [PubMed] [Google Scholar]
- 43. Chio A, Benzi G, Dossena M, et al. Severely increased risk of amyotrophic lateral sclerosis among Italian professional football players. Brain 2005;128:472–6 [DOI] [PubMed] [Google Scholar]
- 44. Chio A, Logroscino G, Hardiman O, et al. Prognostic factors in ALS: A critical review. Amyotroph Lateral Scler 2009;10:310–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Moszczynski AJ, Strong W, Xu K, et al. Pathologic Thr(175) tau phosphorylation in CTE and CTE with ALS. Neurology 2018;90:e380–e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Koga S, Dickson DW, Bieniek KF.. Chronic traumatic encephalopathy pathology in multiple system atrophy. J Neuropathol Exp Neurol 2016;75:963–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mez J, Daneshvar DH, Kiernan PT, et al. Clinicopathological evaluation of chronic traumatic encephalopathy in players of American football. JAMA 2017;318:360–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ling H, Holton JL, Shaw K, et al. Histological evidence of chronic traumatic encephalopathy in a large series of neurodegenerative diseases. Acta Neuropathol 2015;130:891–3 [DOI] [PubMed] [Google Scholar]
- 49. Stein TD, Montenigro PH, Alvarez VE, et al. Beta-amyloid deposition in chronic traumatic encephalopathy. Acta Neuropathol 2015;130:21–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Adams JW, Alvarez VE, Mez J, et al. Lewy body pathology and chronic traumatic encephalopathy associated with contact sports. J Neuropathol Exp Neurol 2018;77:757–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Saberi S, Stauffer JE, Schulte DJ, et al. Neuropathology of amyotrophic lateral sclerosis and its variants. Neurol Clin 2015;33:855–76 [DOI] [PMC free article] [PubMed] [Google Scholar]