Abstract
Communication contributed to 4 important aspects of the Sierra Leone Trial to Introduce a Vaccine Against Ebola (STRIVE): recruiting participants, supporting Human Subjects Protection, building trust in the community to support the trial, and mitigating the impact of rumors and misinformation. Communication was particularly important because STRIVE was Sierra Leone’s first vaccine clinical trial and was implemented during a public health emergency. Communication efforts began months prior to trial launch, building awareness and support through sensitization sessions with stakeholders and community leaders. Community engagement activities continued throughout the trial to maintain relationships with leaders and stakeholders and disseminate accurate information, fostering trust in the trial. The communication team led recruitment with hundreds of information sessions for potential participants, facilitating the informed consent process. Communication efforts continued post-enrollment, supporting ongoing voluntary participation in the trial. Informal formative activities during the trial yielded insights on participants’ perceptions and information needs. While Centers for Disease Control and Prevention Institutional Review Board–approved activities and materials did not change, this flexible strategy allowed for responsive interactions with participants. The trial success and its community acceptance illustrated STRIVE’s successful communications efforts, owing in large part to this flexibility and commitment to community engagement.
Clinical Trials Registration
ClinicalTrials.gov [NCT02378753] and Pan African Clinical Trials Registry [PACTR201502001037220].
Keywords: Communications, Ebola, Ebola vaccine, clinical trial, Sierra Leone, community engagement, informed decision-making, informed consent
In late 2014, the Centers for Disease Control and Prevention (CDC), Sierra Leone’s Ministry of Health and Sanitation, and the College of Medicine and Allied Health Sciences, University of Sierra Leone, began planning STRIVE (the Sierra Leone Trial to Introduce a Vaccine Against Ebola). Planning took place at the height of the Ebola outbreak, with more than 9400 cases and 2700 deaths reported in Sierra Leone by the end of 2014 [1]. The CDC reported in December 2014 that healthcare workers (HCWs) were at 100-fold increased risk of Ebola, compared with the general adult population [2]. Because of this risk, STRIVE defined the trial population as healthcare workers and frontline workers (eg, ambulance drivers and burial teams) who provided care to people with confirmed or suspected Ebola. STRIVE staff were not eligible to be part of the study.
The outbreak was ongoing when the trial launched in April 2015, creating a constantly changing environment as the country struggled to end transmission. It was against this backdrop that STRIVE developed and implemented a flexible communication strategy to recruit participants, support Human Subjects’ Protection, build trust in the community, and mitigate potential rumors and misinformation.
BACKGROUND
Overview of STRIVE
STRIVE was an unblinded, individually randomized clinical trial to study the efficacy and safety of rVSV∆G-ZEBOV-GP vaccine. STRIVE randomized participants into 2 groups: those who were immediately vaccinated (ie, <7 days after enrollment) and those for whom vaccination was deferred until 18–24 weeks after enrollment. All participants were eligible to receive the vaccine by the end of the trial; there was no placebo. Participants were nonpregnant healthcare and frontline Ebola response workers aged ≥18 years. The trial was conducted in 5 districts of Sierra Leone and had 2 substudies, one to investigate safety and the other to investigate immunogenicity. Participants in the safety substudy kept a daily diary to record symptoms, and those in the immunogenicity substudy had blood specimens collected before vaccination and at set times after vaccination to assess the magnitude and durability of the immune response [3].
STRIVE surveillance staff followed participants from the time of enrollment to 6 months post-vaccination. Trial staff called participants monthly to monitor health and conducted home visits if they were unable to reach participants via telephone. Participants had access to a 24-hour hotline to call with any questions or health concerns. The hotline staff routed health issues to nurses and, if medically warranted, nurses referred cases to physicians participating in the trial. Medical care was free to STRIVE participants throughout their enrollment.
Overview of Communication
There were 4 major considerations for the STRIVE communication team: working in a clinical-trial-naive population, implementing Sierra Leone’s first vaccine clinical trial, conducting research in the midst of a national emergency, and adapting to the rapidly changing nature of emergency response. Because Sierra Leone did not have a long history of clinical trials, it was important that potential participants understood clinical trial processes, principals of Human Subjects Protection, and their rights as part of the trial. It was also important that STRIVE staff communicating with participants understood how to effectively communicate to facilitate voluntary, informed decision-making. As the first vaccine clinical trial, STRIVE needed to help potential participants understand what an experimental vaccine meant and provide information about the risk/benefits and unknowns about the vaccine, as well as manage the expectations of the vaccine licensure process. Conducting research during an epidemic presented unique challenges, the most important of which was to ensure that communication efforts were integrated with overall response communication activities and that STRIVE activities did not impact the Ebola response efforts. Finally, STRIVE communications needed to have a strategy that acknowledged the rapidly changing environment of an outbreak. Information about the virus was evolving, locations of case clusters changed over time, and individuals’ perception of their risk of contracting Ebola changed as the context changed. STRIVE needed to be able to adapt to the changes in the environment in which it was working.
Communication contributed to 4 important aspects of STRIVE: recruiting participants, supporting Human Subjects Protection, building trust in the community to support the trial, and mitigating the impact of rumors and misinformation. Strategies and activities were based on STRIVE’s communication framework, which used a tailored social ecology model to identify and reach specific spheres that influence a potential participant’s decision-making (Table 1). The framework used 3 approaches: ongoing formative activities, to identify and address emerging issues; anthropological understandings, so communication were culturally appropriate and understandable; and participatory communication, so potential and enrolled participants, as well as stakeholder and trusted leaders, could provide insights and participants would feel empowered to make informed decisions. All communication efforts used risk communications principles, including transparency, conveying risk and benefits, and acknowledging unknowns. A brief overview of formative activities and development of the approach are included in this article.
Table 1.
General Communication Concepts
| Communication Concept | Activities Associated With Concept |
|---|---|
| Behavior change communication | Tailored messaging and activities designed to promote specific positive behaviors |
| Formative activities | Research and other activities, such as stakeholder engagement and key informant insights, that inform the development of strategies that are socioculturally relevant and effective |
| Formative research | Research to inform strategy development, including communication channels, messages and materials; often qualitative (eg, involving interviews and focus group discussions) but can be quantitative (eg, surveys) |
| Participatory communication | Engages target audiences as stakeholders to ensure communications are culturally relevant, clear, and accessible |
| Risk communication | Provides information that helps people understand personal risk and make informed decisions, and that is timely, accurate, and understandable in the midst of an emergency; common principals include transparency, conveying risk/benefit, and what is known and unknown |
| Social math | Presents statistics, numbers, and other data in a real-life, relatable context to make them easier to understand, meaningful, and interesting to the audience |
This article focuses on the 5 major activities conducted by the communications team—materials development, sensitization activities, informational/educational activities, staff communication capacity development, and community engagement—and how those activities were structured and implemented to support Human-Subjects-Protection principals. The article also outlines lessons learned from STRIVE and recommendations for similar clinical trial communication efforts during outbreaks.
FORMATIVE ACTIVITIES
The STRIVE communication strategy was informed in part by institutional review board–approved mixed-methods research, including both quantitative (surveys) and qualitative (in-depth interviews and focus group discussions) methods.
Such formative research is a valuable component of communication planning but was not sufficient to fully inform the communication strategy for STRIVE. STRIVE relied on other, nonresearch formative activities to provide real-time, robust insight as participant and stakeholder understanding of clinical trials—and the outbreak—evolved. These activities fell into 3 categories. First, stakeholder and partner engagement activities, which included workshops with community stakeholder and social mobilizers, national and district government briefings, and national response integration. Stakeholders, identified through formative research and local partner input, included paramount and district chiefs, and religious and cultural leaders. Second, key informant insight activities included informal trial site and hospital site visits and input from the trial’s cultural subject matter experts (ie, Sierra Leonean pharmacists and Peace Corps language and cultural facilitators). Third, participant observation and feedback activities including information gleaned from questions asked at information sessions and informal feedback from participants.
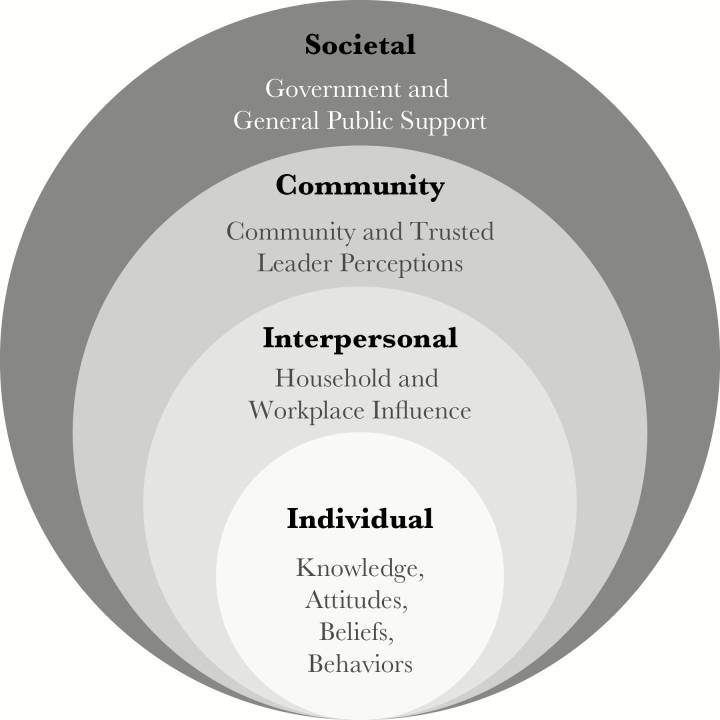
Formative activities were continuous, so communication efforts could take advantage of emerging insights, within the scope of approved activities, as needed throughout the trial. STRIVE used a modified social ecology model to reach people in different spheres of the social ecology and foster understanding of factors particular to each sphere that could affect the trial’s communication goals (Figure 1) [4]. The social ecology model shows how an issue is influenced at multiple societal levels. STRIVE communication team adapted the model to address cultural norms around participatory decision-making, acknowledging interpersonal influences, community perceptions, and societal support.
Figure 1.
STRIVE modified ecological model.
Key insights that informed STRIVE’s communications activities included concerns regarding safety (including unfounded concerns the vaccine could cause Ebola) and the unknown level of protection from an experimental vaccine, the limited understanding of differences between approved and experimental vaccines, the need for a high level of trust that the trial was providing complete and accurate information [5], the need for clear and transparent information about the trial protocol and vaccine licensure process, the importance of culturally relevant communications and materials, and the need for staff training on communications designed to inform about STRIVE without promoting or influencing participation and, more broadly, to facilitate Human Subjects Protection.
KEY AUDIENCES
STRIVE identified 3 main audiences: potential participants, enrolled participants, and key stakeholders. STRIVE did not include the public as a primary audience because it did not want to actively engage an audience that was not eligible for a potentially protective intervention during a deadly outbreak.
STRIVE’s target population was diverse in socioeconomic status, cultural background, education, and literacy level. The eligible population included physicians with advanced degrees and significant understanding of immunization, young student nurses just beginning their education, and burial workers with more-limited general literacy and health literacy. This meant that, in addition to different literacy levels, there were also varied cultural understandings of concepts such as voluntary and informed decision-making. This presented challenges in how to provide information that was understandable to varied demographics .
Information was developed to meet the needs of the 3 audiences:
Potential participant information focused on ensuring informed consent
Enrolled participant information focused on facilitating ongoing voluntary participation
Stakeholder information focused on providing high level clinical trial and experimental vaccine information as well as updates on trial logistics and milestones to allow them to be informed and trusted spokespersons for the trial in their communities.
While the STRIVE communications team did not proactively engage the general public, it did grant all requests for public-facing communication such as radio interviews.
CORE COMMUNICATION ACTIVITIES
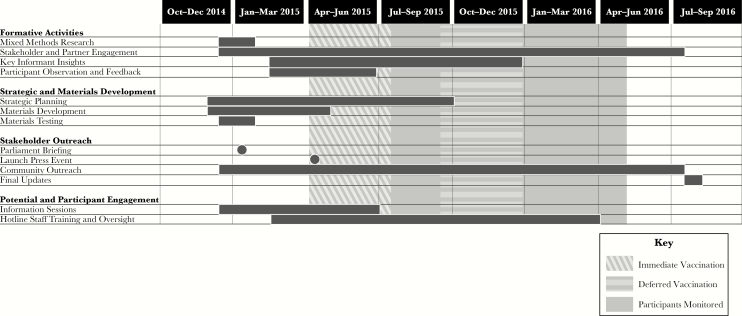
STRIVE’s communication goals were achieved by implementing a communication strategy based on the principles described previously and by addressing the needs identified during formative activities. Efforts began months prior to the launch of the trial and continued for 18 months (Figure 2).
Figure 2.
STRIVE communications strategy.
The STRIVE communication team focused on 5 activities. These activities were informed by the formative activities and developed on the basis of the framework. Communication efforts supported Good Clinical Practice and Human Subjects Protection standards [6, 7]. The Sierra Leone National Ebola Response Centre Social Mobilisation Pillar (one of 9 sector-specific pillars managing the response), the CDC Institutional Review Board, and the Sierra Leone Ethics and Scientific Review Committee approved communication activities and materials.
Materials Development
STRIVE developed a suite of more than a dozen materials (Figure 3), ranging from flip books and presentations to posters and fact sheets. The materials were written in English, Sierra Leone’s official language. Krio is most commonly spoken but has a shorter history as a written language [8]. STRIVE and local experts decided materials should be in English and use illustrations and other visual elements that could be easily understood by those with lower literacy levels. The materials used the formative research to guide message development and supported human subjects protection with detailed information on the rights of potential and enrolled participants. The National Ebola Response Centre Social Mobilization Pillar reviewed, provided edits to, and approved all materials, to provide cultural insights and ensure the materials were consistent with overarching Ebola response communications
Figure 3.
STRIVE materials examples. STRIVE materials included fact sheets, posters, flip books, and safety and instructional cards as part of the enrollment packet. Messages used the Krio word marklate for “vaccine.” Materials relied heavily on visual communications to address varied literacy among potential participants. Illustrations were gender and job inclusive. Key messages about voluntary participation, confidentiality, and safety were reiterated across materials.
The materials were designed to address the needs of the target audience: plain language and illustrated concepts were used to provide understandable information about complex issues involved in vaccine clinical trials to the diverse demographics within the target audiences. A synopsis of prior research, as well as known possible side effects, were outlined in the materials, to address safety concerns and facilitate informed decision-making. The materials were created using risk communications principles, conveying transparency and unknowns regarding the experimental vaccine. The content in materials given to potential and enrolled participants overlapped, but information for potential participants focused on building knowledge about clinical trials and an experimental vaccine, whereas enrolled participants received materials that reinforced ongoing voluntary participation, including information on enrollment, postvaccination instructions and side effects information, and Ebola surveillance activities. Materials were branded with the partners’ logos to show leadership of the Government of Sierra Leone and reinforce legitimacy and trust.
As printed materials were approved as part of the protocol, edits to them would require new approval and protocol amendments. During the fast pace of research in emergencies, edits and reprinting were unrealistic, and, more importantly, consistency of messaging would suffer, so the materials focused on facts regarding the clinical trial and vaccine that were not likely to change. Thus, the materials were not part of the flexible strategy. Rather, they provided the foundation for other activities where messaging could be tailored as needed to address emerging issues. STRIVE staff disseminated materials in a variety of ways, from presentations to take-home packets for participants, making sure that messages, channels, and timing were coordinated.
Additional types of materials were more adaptive and included messaging guidance on specific issues, such as accessing health benefits as part of STRIVE. Also, because routine blood specimen draws are uncommon in Sierra Leone, some participants had concerns with the amount of blood specimens drawn during the immunogenicity subtrial. To address this concern, the communications team used visual communications and social math concepts to create a diorama, with a jug containing 5 L (the approximate amount of blood in a person’s body) of red-dyed water and a small bottle with about 15 mL (the amount of blood collected when a blood specimen is drawn) of water. These props provided a concrete way of understanding the blood specimen amount that would be drawn, to help individuals make an informed decision regarding participation in the immunogenicity subtrial.
Sensitization Activities
Sensitization activities, largely led by in-country partners, the Ministry of Health and Sanitation and the College of Medicine and Allied Health Sciences, were activities that took place prior to the launch of STRIVE. From December 2014 through April 2015, about 50 meetings with stakeholders, including community and government leaders (at the national, district, and local levels), took place. These meetings were held at hospital and healthcare facilities, local government offices, and community centers across Sierra Leone because it was unknown at that time where STRIVE would be conducted. Activities were designed to introduce the idea and increase awareness of vaccine clinical trials; to solicit insight in key cultural, communication, and Human-Subjects-Protection considerations; and to answer questions regarding vaccine clinical trial science and processes. As these activities took place prior to the finalization of the STRIVE protocol, they did not provide specific information regarding the STRIVE trial or a candidate vaccine. However, they were instrumental first steps in building important relationships that would be the focus of community outreach, and they provided an informational foundation for stakeholders, allowing them to be trusted sources of information about potential Ebola vaccine clinical trials in their communities.
Informational and Educational Outreach
STRIVE conducted >100 information sessions (during April–June 2016), reaching thousands of potential participants. Sessions involved a range of audience sizes, from small groups to groups comprising >100 individuals, and were conducted at hospitals, clinics and Ebola holding centers and treatment units, and STRIVE enrollment centers. The sessions used STRIVE English-language materials, such as flip books or presentations, to relay information in a clear and consistent manner, but outreach was conducted by local STRIVE staff in the audience’s language, most often Krio but infrequently Mende or Temne. To address cultural norms about engaging influencers prior to decision-making, STRIVE conducted information sessions in each district at least a week before the vaccination sites opened and continued them while the sites remained open for enrollment. Attendees received materials to help facilitate conversations with family members or others involved in the decision-making process. Outreach was designed to be participatory to foster empowerment; as part of the information sessions, attendees had the opportunity to ask questions after the presentation. STRIVE communications staff used information gained during the question-and-answer exchanges to identify recurring themes and tailor informal communications to address them. These tailored messages were provided at subsequent information sessions, through a hotline for participants, and regularly updated key messages distributed to STRIVE staff to assist them in delivering clear, consistent, and understandable information.
Staff Communications Capacity Development
STRIVE staff included >350 Sierra Leoneans, as well as several hundred people deployed from the CDC (mainly on a 6-week rotational basis, with a small core staff residing in Sierra Leone). Most members of the STRIVE staff were not professional communicators and had little knowledge of best practices for public health communication. Therefore, the STRIVE communication team provided formal and informal training and mentorship on communication issues to the STRIVE staff, primarily focusing on interpersonal communication and interaction that supported Human Subjects Protections. Training continued throughout the trial, to reinforce skills and address emerging issues. The communication training that STRIVE staff members received helped them create a safe and respectful environment that facilitated dialogue. Examples of training included understanding body language, to ascertain a participant’s understanding, and how to frame answers, to not imply judgment or inadvertently influence decision-making.
For staff conducting information sessions, the training resulted in information session attendees being very engaged, sometimes extending the question-and-answer period following the presentation for more than an hour. For staff conducting informed consent, the trainings helped to create an environment where participants felt empowered to ask questions, communicating in ways that helped participants understand complex issues and reminding participants of their rights.
Community Engagement
STRIVE reached out to hundreds of community stakeholders and leaders to build respectful and trusting partnerships. The communication team considered community engagement a relationship-building process and, as such, met with key district stakeholders, including district-level Ministry of Health and Sanitation leaders, local government officials, hospital and healthcare leaders, and paramount chiefs, prior to launch and about every 6 weeks throughout the trial, to provide updates and solicit feedback. Updates included status reports on the trials progress, based on key messages, and addressed any questions and concerns from stakeholders. Human Subjects Protection concepts and other facts about STRIVE and the vaccine were reinforced so stakeholders could be trusted sources of information in their communities. STRIVE continued community engagement throughout the study, including national- and district-level final updates to convey the interim results of the trial and explain the licensure process after STRIVE's completion. Community engagement efforts increased trust in the trial, provided key insights, and developed trusted spokespersons.
DISCUSSION
The STRIVE communication experience helps fill a gap in the communication literature on a systematic approach to communication to support Human Subjects Protection in a clinical trial in a resource-limited setting during a public health emergency. There are a number of important lessons learned and recommendations resulting from the STRIVE experience. Six overarching lessons learned are detailed below, and a chart of STRIVE-specific issues and solutions can be found in Table 2.
Table 2.
Communication Issues and Solutions During the STRIVE Clinical Trial
| Issues | Solutions |
|---|---|
| Varied audience socioeconomic status, education level, medical understanding | Develop illustrated, plain language materials: STRIVE had suite of more than a dozen materials, ranging from frequently asked questions to flip charts. Incorporate anthropological understandings of health and medical concepts into materials: STRIVE incorporated culturally appropriate communication on complex issues, such as immunity. Create multiple ways to convey information (eg, written, key messages for conversations and dioramas): STRIVE created multiple formats to illustrate amount of blood drawn during blood specimen collection (an uncommon practice in Sierra Leone) |
| Strong need for community trust and support | Engage community stakeholders and social mobilizers to guide strategic development: STRIVE held community workshop months prior to launch to identify community priorities and needs. Build and maintain community stakeholder relationships: STRIVE held recurring (around every 6 weeks) meetings with local stakeholders in all trial districts. Employ local subject matter experts in communication to ensure cultural understanding and relevancy: STRIVE employed Sierra Leonean pharmacists and Peace Corp language and cultural facilitators as part of communication team |
| Unfamiliarity with informed decision-making communications | Incorporate Human Subjects Protection rights into all potential and enrolled participant materials and messages: STRIVE emphasized the voluntary nature of participation, confidentiality, and the rights of participants (and nonparticipants). Ensure trial staff are trained on informed decision-making communication: STRIVE conducted trainings and mentorship on communications issues, such as reading body language, interpersonal communication, and noncoercive communication |
| Accustomed to behavior change designed to solicit a particular outcome | Focus on transparency in communications: STRIVE communications materials were clear on potential benefits and risks, as well as what was unknown about the vaccine |
| Emerging administrative issues as trial evolved | Have way for participants to make trial leadership aware of concerns: STRIVE had a 24-hour hotline that participants could call with any questions concerns, and operators were debriefed every other week to identify emerging issues such as questions regarding follow-up, appointments, or reimbursements |
1. Define phases of effort. In the fast-moving environment of an emergency, clinical trial research teams without a strong strategy run the risk of being reactive rather than proactive. The STRIVE communication team built a detailed model that identified major phases (ie, development, initial implementation, and close down of the study) and key milestones (ie, closing of enrollment and hiatuses of vaccination sites) to be ahead of such activities, so that community, participants, and staff were aware of milestones and changes and the risk of rumors was negated. The model also helped identify areas where communications could support other activities integral to the management of the trial.
2. Design a flexible strategy. Complex humanitarian emergencies are dynamic environments where information is constantly evolving and changing. Conducting clinical research in this environment necessitates the ability to tailor information quickly. It was important, as part of the strategy, to use risk communications principles particularly foreshadowing change and to communicating transparently so STRIVE’s reputation remained strong even when its messages shifted. Although a flexible communication strategy offers great value in communicating with participants and stakeholders in a clinical trial, there are important limitations. STRIVE could not be flexible in the types of communication activities conducted, as those activities were required to conform with the trial’s approved protocol. Similarly, STRIVE did not adapt materials once they were approved by the institutional review board. The flexibility in the strategy did, however, allow for nuanced informal conversations within approved activities. By identifying recurring questions at information sessions, staff were able to address these questions up front in a manner that showed respect and transparency.
3. Integrate communications efforts with response communication efforts. Engage with partners’ communication teams across the response. Building these relationships helped STRIVE ensure its messages were in line with response efforts, provided opportunities for collaboration, and provided insights to emerging issues that may impact STRIVE.
4. Invest in communication training for all staff. Training, particularly in interpersonal communication skills, helps create an environment that facilitates informed decision-making and the informed consent process. This is especially important because clinical trial communication is different in important ways from other types of communication that the staff are more familiar with, such as those of behavior-change campaigns.
5. Build and maintain strong community partners. Community engagement must be an ongoing relationship, requiring consistency and time, not a so-called one-and-done activity. STRIVE invested extensive resources and staffing, including a long-term CDC communication field team lead with previous experience working in Sierra Leone, to develop and maintain valuable community partnerships. These partnerships helped create and maintain trust in the trial and its staff, provided valuable insights to help navigate the changing environment, and built trusted intermediaries in the community at the district and chiefdom levels. STRIVE, in general, had good community support because of these efforts.
6. Use cultural norms to support the scientific integrity of research. Careful consideration of cultural norms and needs can inform the development of methods that can support the scientific integrity of the research. For example, STRIVE used a balloting process for trial arm assignment in which participants selected a sealed envelope that contained a paper with “immediate” or “deferred” written on it. This process addressed an expressed need for transparency while supporting the need for randomization. Conversely, although formative research identified a desire among community members to see trial leadership be vaccinated before other participants, the protocol was not designed such that trial leaders met eligibility criteria. Subsequently, this was the single most common issue raised in information sessions, posing challenges to building community trust early on.
The diversity of communication activities and materials ensured that participants had access to accurate, timely, and consistent information. The flexibility in the strategy, tailoring messaging to specific audiences or issues and ensuring they were culturally relevant, helped ensure that participants understood the information.
Notes
Acknowledgments. This article is dedicated to Jerry Maxwell Bangura, Peace Corp language and cultural facilitator and valued member of the STRIVE communications team, who passed away shortly after STRIVE vaccination was complete. Jerry’s talent, expertise, and commitment to STRIVE helped ensure our communications efforts were a success.
We thank Ann Aikin, Joe Alcober, Rosalind Carter, Katy Clark, Emily Cramer, Dave Daigle, Stefanie Erskine, Paula Frew, Megan Frey, Yvonne Garcia, Rebecca Gold, Valerie Johnson, Karen Mason, Allison Maiuri, Ben Monroe, Kristen Nordlund, Kara Polen, Leslie Rodriguez, Liz Ryan, Jane Seward, and Karen Swails, for their efforts and contributions to STRIVE communications; and Barbara Mahon. for her contributions to the manuscript.
Disclaimer. The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial support. This work was supported by the Centers for Disease Control and Prevention, the Biomedical Advanced Research and Development Authority, and the National Institutes of Health, with additional support from the CDC Foundation.
Supplement sponsorship. This work is part of a supplement sponsored by the Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICJME Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: American Society for Tropical Medicine and Hygiene 65th Annual Meeting, Atlanta, Georgia, 13–17 November 2016. Abstract 1466.
References
- 1. World Health Organization. Health worker Ebola infections in Guinea, Liberia and Sierra Leone: a preliminary report Geneva: World Health Organization, 2015. http://apps.who.int/iris/bitstream/10665/171823/1/WHO_EVD_SDS_REPORT_ 2015.1_eng.pdf?ua=1&ua=1. Accessed 21 October 2016 [Google Scholar]
- 2. Kilmarx PH, Clarke KR, Dietz PM, et al. Ebola virus disease in health care workers—Sierra Leone. MMWR 2014; 63:1167–71. [PMC free article] [PubMed] [Google Scholar]
- 3. Samai M, Seward JF, Goldstein ST, et al. The Sierra Leone Trial to Introduce a Vaccine against Ebola: an evaluation of rVSVΔG-ZEBOV vaccine tolerability and safety during the West Africa Ebola outbreak. J Infect Dis 2018; 217:s6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McLaren L, Hawe P. Ecological perspectives in health research. J Epidemiol Community Health 2005; 59:6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. The AAA/Wenner-Gren Ebola Emergency Response Workshop, November 6–7, 2014: preliminary guidances and recommendations http://www.ebola-anthropology.net/wp-content/uploads/2014/11/Workshop-Preliminary-Guidances-and-Recommendations.pdf. Accessed 21 October 2016
- 6. Guidance for industry: E6 good clinical practice: consolidated guidance Silver Spring, MD: Department of Health and Human Services, Food and Drug Administration, 1996. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm073122.pdf. Accessed 21 October 2016 [Google Scholar]
- 7. Vanderpool HY. The ethics of research involving human subjects: facing the 21st century. Hagerstown, MD: University Publishing Group; 1996. [Google Scholar]
- 8. Jones ED, Sandred KI, Shrimpton N. Reading and writing Krio: proceedings of a workshop on health at the Institute of Public Administration and Management, University of Sierra Leone 1990. http://www.scsu.edu/files/english%20and%20modern%20languages/papers/varieties%20of%20krio%20and%20standard%20krio.pdf. Accessed 7 October 2017.