Abstract
Background
Maternal macronutrient intake is likely to play a pivotal role in fetoplacental growth. Male fetuses grow faster and their growth is more responsive to maternal size.
Objective
We assessed the role of fetal sex in modifying the effect of maternal macronutrient intake on the risk of small-for-gestational-age (SGA) birth.
Design
This was a prospective, observational cohort study of 2035 births from an urban South Asian Indian population. Maternal intakes of total energy and macronutrients were recorded by validated food-frequency questionnaires. The interaction of trimester 1 macronutrient intake with fetal sex was tested on the outcome of SGA births.
Results
The prevalence of SGA was 28%. Trimester 1 macronutrient composition was high in carbohydrate and low in fat (means ± SDs—carbohydrate: 64.6% ± 5.1%; protein: 11.5% ± 1.1%; and fat: 23.9% ± 4.4% of energy). Higher carbohydrate and lower fat consumption were each associated with an increased risk of SGA [adjusted OR (AOR) per 5% of energy (95% CI): carbohydrate: 1.15 (1.01, 1.32); fat: 0.83 (0.71, 0.97)] specifically among male births (males: n = 1047; females: n = 988). Dietary intake of >70% of energy from carbohydrate was also associated with increased risk (AOR: 1.67; 95% CI: 1.00, 2.78), whereas >25% of energy from fat intake was associated with decreased risk (AOR: 0.61; 95% CI: 0.41, 0.90) of SGA in male births.
Conclusions
Higher carbohydrate and lower fat intakes early in pregnancy were associated with increased risk of male SGA births. Therefore, we speculate that fetal sex acts as a modifier of the role of maternal periconceptional nutrition in optimal fetoplacental growth.
Keywords: fetal sex, maternal diet, fetal growth restriction, SGA, placenta
INTRODUCTION
In a country like India, where a large number of small-for-gestational-age (SGA) infants are born, the maternal diet plays an important role in fetal growth, unlike in developed countries where prematurity is the most common reason for low birth weight (LBW) (1). Many studies have analyzed associations between maternal diet during pregnancy and periconceptionally with birth outcomes such as birth weight or the risk of being born SGA. Higher maternal intakes of micronutrients such as vitamin B-12, vitamin C, folate, and vitamin D have been reported to be associated with improved birth weight (2–4). Meta-analyses of interventions with micronutrients have consistently reported a reduction in the risk of LBW and of SGA births with multiple micronutrient supplementation (5, 6). Reports of associations between intakes of macronutrients and neonate size from observational studies have been inconsistent. Although Godfrey et al. (7) reported lower placental and birth weights among mothers with high carbohydrate intake early in pregnancy in a prospective observational study, Mathews et al. (8) found no such associations for macronutrient intake. A low maternal intake of SFAs or of seafood, a source of the long-chain PUFA DHA, has been reported to be associated with LBW (9, 10). On the other hand, Chong et al. (11) recently reported that maternal protein intake was not associated with offspring birth weight in a multiethnic Asian cohort of 835 pregnant women from the Growing Up in Singapore Towards Healthy (GUSTO) Study. Nevertheless, from the same GUSTO study, 2 recent reports highlighted the associations between maternal macronutrient composition and neonatal and infant body composition (12, 13).
Starting early in trimester 2, male fetuses grow faster than their female counterparts, leading not just to larger size but also to sex-specific intrauterine growth patterns (14). Male fetal growth, both in terms of birth weight and neonatal body composition, has been reported to be more sensitive to maternal weight and height, which are likely indicators of maternal periconceptional nutrition status (15–18). On the other hand, female neonatal body composition is likely modulated by the maternal inflammatory environment as indicated by maternal plasma IL-6 and C-reactive protein (16). Therefore, fetal sex could be a modifier of the associations between maternal macronutrient intakes and fetal growth. However, none of the studies examining such associations have investigated the role of fetal sex as a modifier.
The primary objective of this study was to evaluate the role of fetal sex as a modifier of associations between maternal macronutrient intake and birth outcome. This analysis provides increased understanding of the role of fetal sex in modulating the effects of in utero environmental exposures.
METHODS
Study design
We used a prospective observational cohort study design. The study population is part of a prospective observational cohort of pregnant women established at St. John's Research Institute and the Departments of Obstetrics and Gynecology and Pathology of St. John's Medical College Hospital, Bangalore, India, to study the effect of maternal determinants on birth outcomes (2). The experimental protocols were approved by the institutional ethical review board. All of the study participants gave written informed consent at enrollment.
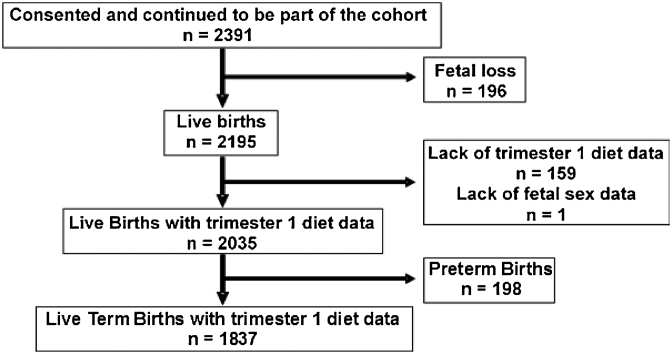
Pregnant women (aged 17–40 y) attending routine antenatal care in trimester 1 of gestation were invited to participate in the study. The recruitments for the current analysis were made between 2002 and 2014. Women were excluded if they had multiple gestations; a clinical diagnosis of chronic illness (e.g., type 2 diabetes, hypertension, heart disease, thyroid disease, and epilepsy); or a positive test for hepatitis B surface antigen, HIV, or syphilis; or if they planned to deliver at a location other than St. John's Medical College Hospital. A total of 2391 women consented to participate in the study and continued to be part of the cohort. There were 196 fetal losses recorded and 2195 live births. Because the objective of the current study was to evaluate the role of fetal sex in modifying the associations between maternal macronutrient intakes in trimester 1 and SGA births, 159 were excluded from analysis due to lack of maternal trimester 1 dietary data and another birth was excluded due to missing fetal sex data. Data from the remaining 2035 births were analyzed. Of these, 198 were preterm births. As such, data from the 1837 full-term births were considered for similar analysis of the full-term births.
The subjects’ age and obstetric history were recorded at recruitment. Routine antenatal tests were carried out at recruitment and, as part of routine antenatal care, folic acid, iron, and calcium supplements, and tetanus toxoid were provided. Assessment of gestational age was made from the first day of the last menstrual period and was confirmed by ultrasound within 2 wk of the initial visit. Height was measured to the nearest 0.1 cm on a calibrated stadiometer. Body weight was recorded (Soehnle, Darmstadt, Germany) to the nearest 0.1 kg during each monthly visit. Calculation of gestational weight gain (kilograms per week) was made between measurements during each trimester. During the course of pregnancy, the morbidity and clinical outcomes were recorded. Neonatal anthropometric measurements and birth outcomes were recorded at delivery. Maternal complications at delivery, such as premature rupture of membrane, were also recorded by the research assistants. Infant birth weight was measured to the nearest 0.01 kg on a digital weighing scale (BWS 101; Phoenix) and birth length measured on a standard infantometer to the nearest 0.1 cm. Information on infant sex was recorded. Birth outcomes were categorized as preterm births (born at <37 wk of gestation), LBW (birth weight <2.5 kg), and SGA infant (birth weight <10th percentile for gestational age) (19).
Selection bias could influence the modification of the association between maternal macronutrient intake and the risk of SGA birth by fetal sex if either female or male births were preferentially retained in the cohort. The likelihood of such a bias was low due to a legislative ban on prenatal sex determination (except for clinical reasons) in India (20). Nevertheless, we chose to address this bias, first by comparing the number of female and male births, and second by comparing the maternal characteristics of female and male births.
Statistical analysis
Categorical data are presented as n (%) and continuous data are presented as means ± SDs or as medians (quartile 1, quartile 3). Continuous variables were compared between the groups by independent-samples t test, whereas categorical variables (parity and maternal education) were compared by chi-square test. Loss to follow-up was addressed by comparing baseline data of pregnancies that were lost to follow-up with those that continued to be part of the cohort. The effect of interaction of maternal macronutrient intake with fetal sex on the incidence of SGA was tested as an independent variable in multiple variable logistic regression. Maternal age, education, parity, height, and weight at recruitment; fetal sex; and total energy intake were covariates. The adjusted ORs (AORs), 95% CIs, and P values for SGA were reported. Fetal sex-specific ORs of SGA with maternal macronutrient intakes as independent variables were examined in a multiple variable logistic regression with fetal sex–specific maternal macronutrient intakes as independent variables with the above-listed covariates. Two-sided Pvalues <0.05 were considered significant. Macronutrient intakes were adjusted for total energy intake by the nutrient density method (21). As part of sensitivity analyses, the above analysis was repeated after adjusting macronutrient intakes for total energy intake by the regression residual method (21) and separately, specifically in the term births. All of the analyses were conducted by using the SPSS program (version 18.0; SPSS).
RESULTS
From the St. John's birth cohort, 2035 births were analyzed for the current study (Figure 1). Baseline data available among the 159 pregnancies (7.2%) that were excluded from the final analysis due to lack of trimester-1 diet data were similar to the 2036 pregnancies for which trimester-1 diet data were available [age: 24.3 ± 3.8 compared with 24.4 ± 3.8 y; P = 0.722; height: 1.55 ± 0.07 compared with 1.56 ± 0.06 m; P = 0.073; BMI (kg/m2): 21.9 ± 4.4 compared with 21.7 ± 3.6; P = 0.535 (unpaired t test); and primiparous: 57% compared with 59%; P = 0.665; male births: 55% compared with 51%; P = 0.702 (chi-square test)]. However, the educational status of the subjects excluded was significantly lower (up to high school: 43% compared with 33%; P = 0.013, chi-square test). The birth variables of the 2 groups were similar (gestational age at birth: 38.7 ± 1.4 compared with 38.6 ± 1.5 wk; P = 0.372; birth weight: 2809 ± 488 compared with 2876 ± 450 g; P = 0.075, unpaired t test). Furthermore, another birth was excluded from the final analysis of the current study due to lack of availability of data on sex of the infant.
FIGURE 1.
Flowchart of participants included for analysis from the St. John's birth cohort.
The anthropometric characteristics of the 2035 mothers at enrollment and of the neonates are summarized in Table 1. The follow-up of the pregnancies was done until birth. The mean birth weight of the neonates was 2875 g. Approximately 28% of the neonates were born SGA and 51% of the births were male births. As expected, the male neonates were heavier at birth (male: 2914 g; female: 2834 g). Because the primary objective of this study was to evaluate the role of fetal sex as a modifier of associations between maternal macronutrient intake and birth outcome, we first ascertained whether maternal trimester-1 intake of total energy or macronutrients (adjusted for total energy intake according to the nutrient density method and expressed as percentage of energy) was overtly different between the male and female births or the SGA and appropriate-for-gestational-age births. We did not find any such differences in all 2035 births (Table 1) or separately in the 1837 term births (Supplemental Table 1).
TABLE 1.
Maternal sociodemographic and neonatal characteristics and maternal trimester-1 dietary intakes by birth outcome and fetal sex1
| Characteristics | All births | AGA | SGA | P | Male births | Female births | P |
|---|---|---|---|---|---|---|---|
| n | 2035 | 1458 | 577 | 1047 | 988 | ||
| Maternal | |||||||
| Age, y | 24.4 ± 3.8 | 24.5 ± 3.9 | 24.0 ± 3.7 | 0.005 | 24.4 ± 3.8 | 24.3 ± 3.9 | 0.521 |
| Weight at recruitment, kg | 52.5 ± 9.5 | 53.4 ± 9.5 | 50.2 ± 9.2 | <0.001 | 52.5 ± 9.3 | 52.4 ± 9.7 | 0.731 |
| Height, m | 1.6 ± 0.1 | 1.6 ± 0.1 | 1.5 ± 0.1 | <0.001 | 1.6 ± 0.1 | 1.6 ± 0.1 | 0.220 |
| Primiparous,2n (%) | 1200 (59) | 835 (57) | 365 (63) | 0.013 | 611 (58) | 589 (60) | 0.564 |
| Education,2n (%) | <0.001 | 0.247 | |||||
| Up to high school | 675 (33) | 445 (31) | 230 (40) | 335 (32) | 340 (34) | ||
| PUC/diploma and above | 1360 (67) | 1013 (70) | 347 (60) | 712 (68) | 648 (66) | ||
| Maternal dietary intake (trimester 1) | |||||||
| Energy, kcal/d | 1910 ± 517 | 1918 ± 521 | 1890 ± 504 | 0.260 | 1900 ± 487 | 1921 ± 546 | 0.346 |
| Carbohydrate, % of energy | 64.6 ± 5.1 | 64.5 ± 5.1 | 64.8 ± 4.9 | 0.175 | 64.5 ± 5.0 | 64.7 ± 5.1 | 0.351 |
| Protein, % of energy | 11.5 ± 1.1 | 11.5 ± 1.1 | 11.5 ± 1.0 | 0.816 | 11.5 ± 1.0 | 11.5 ± 1.1 | 0.847 |
| Fat, % of energy | 23.9 ± 4.4 | 24.0 ± 4.5 | 23.6 ± 4.3 | 0.109 | 24.0 ± 4.4 | 23.8 ± 4.5 | 0.267 |
| Neonate | |||||||
| Birth weight, g | 2875 ± 450 | 3015 ± 439 | 2523 ± 234 | <0.001 | 2914 ± 461 | 2834 ± 435 | <0.001 |
| SGA, n (%) | 577 (28) | — | — | — | 311 (30) | 266 (27) | 0.164 |
| Gestational age at birth, wk | 38.6 ± 1.5 | 38.5 ± 1.7 | 38.9 ± 1.0 | <0.001 | 38.6 ± 1.6 | 38.7 ± 1.5 | 0.250 |
All values are means ± SDs unless otherwise indicated; n = 2035. P values were derived by using independent-samples t test unless otherwise indicated. AGA, appropriate-for-gestational age; PUC, pre-University course; SGA, small-for-gestational age.
P values were derived by using chi-square test.
Differential effects by fetal sex of maternal trimester 1 macronutrient intake on SGA birth were evaluated in logistic regression models. We included maternal age, education, parity, height, and weight at recruitment and total energy intake per day in trimester 1 as covariates (Table 2). The interaction tended to be significant for carbohydrate intake and was significant for fat intake (per 5% of energy for both; P = 0.09 and P = 0.05, respectively). We further assessed the fetal sex-specific effect of maternal macronutrient intake on SGA (Table 3). The risk of an SGA birth was significantly increased with higher carbohydrate intake (AOR per 5% of energy: 1.15; 95% CI: 1.01, 1.32; P = 0.04) or lower fat intake (AOR per 5% of energy: 0.83; 95% CI: 0.71, 0.97; P = 0.02) specifically in male births (n = 1047). No such association was seen in female births (n = 988).
TABLE 2.
Interactions between the effects of maternal macronutrient intake in trimester 1 (adjusted for total energy intake by the nutrient density method) and fetal sex on adjusted ORs for SGA birth1
| P | |||
|---|---|---|---|
| Dietary intakes | P-interaction(dietary intake × fetal sex) | Dietary intake | Fetal sex |
| Energy, per 100 kcal/d | 0.62 | 0.91 | 0.35 |
| Carbohydrate, per 5% of energy | 0.09 | 0.72 | 0.12 |
| Protein, % of energy | 0.92 | 0.88 | 0.79 |
| Fat, per 5% of energy | 0.05 | 0.71 | 0.03 |
n = 2035. Multiple variable logistic regression was used with maternal age, education, parity, height, and weight at recruitment; fetal sex; and total energy intake as covariates. SGA, small-for-gestational age.
TABLE 3.
Fetal sex-specific effect of maternal macronutrient intakes in trimester 1 on AORs for SGA birth1
| Male births (n = 1047) | Female births (n = 988) | |||
|---|---|---|---|---|
| Dietary intakes | SGA, AOR (95% CI) | P | SGA, AOR (95% CI) | P |
| Energy, per 100 kcal/d | 0.99 (0.96, 1.02) | 0.56 | 1.00 (0.98, 1.03) | 0.91 |
| Carbohydrate, per 5% of energy | 1.15 (1.01, 1.32) | 0.04 | 0.97 (0.84, 1.12) | 0.72 |
| Protein, % of energy | 1.00 (0.88, 1.14) | 0.99 | 1.01 (0.89, 1.16) | 0.88 |
| Fat, per 5% of energy | 0.83 (0.71, 0.97) | 0.02 | 1.03 (0.88, 1.21) | 0.71 |
n = 2035. Multiple variable logistic regression was used with fetal sex-specific maternal macronutrient intakes and maternal age, education, parity, height, and weight at recruitment; fetal sex; and total energy intake as covariates. AOR, adjusted OR; SGA, small-for-gestational age.
We next grouped the births according to low, adequate, and high maternal intake of macronutrients in trimester 1. The dietary recommendations for pregnant mothers in India are as follows—protein: 10–15% of energy and fat: 20–25% of energy, with the rest derived from carbohydrates (22). Accordingly, we grouped the births as follows—carbohydrate intake: low, <60%; adequate, 60–70%; and high, >70% of energy; protein intake: low, <10%; adequate, 10–20%; and high, >20% of energy; and fat intake: low, <20%; adequate, 20–25%; and high, >25% of energy. The maternal demographic variables according to these groups are summarized in Supplemental Table 2. Mothers with low carbohydrate intake or high fat intake were older and better educated (P < 0.001).
On analyzing the fetal sex-specific effect of adequate or high maternal macronutrient intake on SGA birth with the low-intake group as the referent group, we observed a significant increase in the risk of an SGA birth for high carbohydrate intake (Table 4). A significant decrease in the risk of an SGA birth was observed for high fat intake. These associations were again specific to male births.
TABLE 4.
Fetal sex-specific effect of different amounts of maternal macronutrient intake in trimester 1 on AORs for SGA birth1
| Male births (n = 1047) | Female births (n = 988) | ||||
|---|---|---|---|---|---|
| Dietary intakes and groups | SGA, % | SGA, AOR (95% CI) | P | SGA, AOR (95% CI) | P |
| Carbohydrate | |||||
| <60% of energy | 25 | 1.00 | 1.00 | ||
| 60–70% of energy | 28 | 1.41 (0.96, 2.07) | 0.08 | 1.06 (0.72, 1.56) | 0.79 |
| >70% of energy | 29 | 1.67 (1.002, 2.780) | 0.049 | 0.81 (0.48, 1.38) | 0.44 |
| Protein | |||||
| <10% of energy | 26 | 1.00 | 1.00 | ||
| 10–20% of energy | 28 | 1.17 (0.68, 2.02) | 0.57 | 1.34 (0.73, 2.46) | 0.35 |
| >20% of energy | 29 | 1.02 (0.58, 1.82) | 0.94 | 1.54 (0.82, 2.91) | 0.18 |
| Fat | |||||
| <20% of energy | 30 | 1.00 | 1.00 | ||
| 20–25% of energy | 26 | 0.91 (0.62, 1.32) | 0.60 | 1.13 (0.76, 1.68) | 0.55 |
| >25% of energy | 26 | 0.61 (0.41, 0.90) | 0.01 | 1.04 (0.69, 1.56) | 0.87 |
n = 2035. Multiple variable logistic regression was used with fetal sex-specific maternal macronutrient intakes and maternal age, education, parity, height, and weight at recruitment; fetal sex; and total energy intake as covariates. AOR, adjusted OR; SGA, small-for-gestational age.
We performed 2 separate sensitivity analyses for the above findings. First, we repeated the multiple logistic regressions using maternal macronutrient intakes adjusted for total energy intake by the nutrient density method specifically for the full-term births. Similar to the findings in the total 2035 births, the association of SGA birth with maternal carbohydrate intake tended to be modified by fetal sex and that with maternal fat intake was modified significantly by fetal sex (P = 0.06 and 0.03 respectively; Supplemental Table 3). The risk of an SGA birth was significantly increased with higher carbohydrate intake or lower fat intake for the full-term male births [AOR (95% CI) per 5% of energy: 1.19 (1.03, 1.34); P = 0.02; and 0.80 (0.67, 0.94); P = 0.01, respectively; n = 946; Supplemental Table 4], whereas no such effect was seen for the female births (n = 891). We could not separately analyze these findings in the preterm births due to the small sample size (n = 198). However, gestational age did not influence these relations in the full-term births because, on repeating the multiple logistic regressions with final gestational age as an added covariate, the association of maternal carbohydrate and fat intake continued to be modified similarly by fetal sex (P = 0.06 and 0.03, respectively).
For the next sensitivity analysis, we adjusted maternal macronutrient intake for total energy intake by the regression residual method instead of the nutrient density method. A trend for interaction between carbohydrate intake (per 25 g/d) and fetal sex was observed (P = 0.07), whereas the interaction between fat intake (per 10 g/d) and fetal sex was significant (P = 0.04; Supplemental Table 5). The AORs for SGA births were significantly higher for carbohydrate intake and lower for fat intake specifically in the male births [AOR (95% CI) per 5% of energy: 1.16 (1.00, 1.33); P = 0.046; and 0.84 (0.73, 0.98); P = 0.02, respectively; n = 1047; Supplemental Table 6].
DISCUSSION
Based on the underlying assumption that birth outcome and offspring health are associated with maternal nutrition in a fetal sex-specific manner, we tested the hypothesis that fetal sex will modify associations between maternal diet during pregnancy and fetal growth and birth outcome. We observed a significantly increased risk of SGA births, specifically in male infants, with increasing maternal trimester 1 carbohydrate intake or decreasing fat intake.
Fetal sex is known to modulate fetal growth as well as neonatal body composition (14, 15). It has also been recently reported to be associated with the risk of maternal complications during and after pregnancy, such as gestational diabetes mellitus and preeclampsia, which could potentially be related to the effect of fetal sex on maternal vascular and hormonal adaptation to pregnancy (23–26). At its extreme, Trivers and Willard (27) hypothesized better reproductive success for the parents if more male offspring were produced (higher sex ratio at birth) when maternal conditions during and before pregnancy were optimal. Although evidence for their hypothesis exists in the animal kingdom, evidence in humans exists only for situations leading to extreme imbalances in food availability, such as famines or maternal stress during pregnancy (28–32). Nevertheless, it is well recognized that male fetuses are more sensitive to maternal exposures to suboptimal conditions (33, 34). For instance, the influence of maternal conditions such as gestational diabetes mellitus on neonatal adiposity is evident primarily in the male fetuses (35). This could even have consequences on other aspects of fetal development. It was recently reported that in a mouse model of maternal diet–driven obesity, the male fetal brain transcriptome was more sensitive to maternal diet than the female brain, which is in line with evidence of a higher risk of neurodevelopmental impairment in male fetuses exposed to obstetric risk factors such as preeclampsia or SGA birth (36, 37). Multiple studies that used animal models have observed fetal sex-specific effects of manipulating maternal diet on fetal growth as well as on cardiovascular development and hemodynamics (32, 38). However, the effect of fetal sex as a modifier of associations between maternal diet and fetal growth has not been explored in humans to the best of our knowledge.
The fetal sex-specific modulation of the association between maternal macronutrient intake in trimester 1 and the risk of SGA birth held even when macronutrient intake was adjusted for total energy intake (21). For the main analysis, we used the nutrient density model of adjusting for total energy intake. This model has been used in national dietary guidelines and recommendations. Therefore, it is traditionally used for epidemiologic studies for ease of comparison with dietary recommendations for the target population. We performed a sensitivity analysis using the widely regarded regression residual method to adjust macronutrient intake for total energy intake. Our findings were consistent in this sensitivity analysis. We further conducted a sensitivity analysis for our findings restricting our analysis to the full-term births and observed similar findings, which points to the robustness of our observations.
There can be potentially multiple, interlinked biological explanations for our observations. The increased risk of SGA births with modulations of maternal diet that we observed is likely to be due to the constitutionally weaker nature of male offspring, making them more prone to growth restriction and, in extreme cases, mortality, under suboptimal conditions in utero (39). Furthermore, the fetal programming hypothesis that suboptimal in utero exposures such as maternal malnutrition sets up the stage for a higher risk of adult-onset noncommunicable diseases such as coronary artery disease, obesity, hypertension, and type 2 diabetes was initially proposed on the basis of data on men (40). Current evidence for the fetal programming hypothesis has also been reported for male offspring (41).
The placenta could be a mediator of fetal sex-specific effects of in utero exposures on fetal growth and later life outcomes (42). The placenta has the same sex as the fetus due to the fetal origin of the placental cells. This can explain the sex-specific responses, functional as well as regulatory (epigenetic and transcriptional), of the placenta to in utero exposures (43). In a recent meta-analysis of gene expression data from nonpathological term placentas, >140 genes were reported to be differentially expressed between placentas from male and female births (44). A higher expression of genes associated with maintenance of pregnancy, placental development, and maternal tolerance of the developing fetus was observed in placentas from the female births, providing mechanistic explanation for better robustness of the female placenta to withstand adverse in utero exposures.
Another question that needs explanation is why there was a differential association of maternal fat intake with the risk of SGA births, specifically in the male infants. Positive associations between maternal intakes of SFAs, α-linolenic acid , and long-chain n–3 PUFAs and birth weight have been reported from the current cohort (9, 10). However, fetal sex-specific associations have not been reported previously. Mechanistically, one possible explanation emanates from the observation that activity of carnitine palmitoyltransferase 1a (CPT-1a) was reduced by half in CPT-1a+/− heterozygote male mice, but no such effect was seen in the female mice (45). Because CPT-1a is the rate-limiting enzyme for transferring long-chain fatty acids into the mitochondria for fatty acid oxidation, a differential effect of reduction in gene dosage of CPT-1a on its activity in the male sex could possibly explain higher sensitivity of the male fetal growth on the maternal supply of fatty acids, although this needs to be tested.
Carbohydrates in the diet are the major determinants of postprandial glucose concentrations, and low-carbohydrate diets have been reported to result in significant reductions in postprandial glucose and insulin concentrations (46). Although hyperglycemia during embryogenesis is associated with increased congenital anomalies and birth defects in diabetic mothers, frequent, postprandial episodic hyperglycemia arising from high-carbohydrate maternal diets could still lead to dysregulated fetal growth (47). Gill-Randall et al. (48) reported that transferring embryos from euglycemic female Wistar rats to pseudo-pregnant hyperglycemic Goto Kakizaki rats resulted in offspring that were significantly lighter at 6 wk of age and developed hyperglycemia later. A possible mechanistic explanation could be the modulation of the insulin receptor signaling pathway. Recently, we reported a positive association of placental growth factor receptor–bound protein 10 (GRB10) expression with placental weight and birth weight specifically in male births (49). Because GRB10 is considered to be a negative regulator of insulin signaling, a positive association between GRB10 expression and birth weight can be explained in terms of its ability to tune down the extent of insulin signaling that is "detrimental" to fetal growth, specifically in the male births.
The primary strengths of this study are analyses of maternal dietary data in light of a rich collection of maternal and neonatal data, from births spanning 13 y in a South Asian Indian urban setting that has seen substantial economic and demographic transition. A limitation of this study lies in its observational nature, with the dietary data collected in the form of food-frequency questionnaires from a single tertiary-care hospital in urban South India. Similar studies conducted in rural settings or in settings where the population has different habitual dietary patterns will further clarify the role that fetal sex plays in modulating the effect of maternal diet on fetal growth. This will build the platform for understanding the underlying maternal and fetoplacental signaling mechanisms on one hand and for providing guidance for personalized advice in maternal and neonatal outcomes on the other.
Supplementary Material
ACKNOWLEDGEMENTS
We acknowledge the contribution of the research assistants, Nancy Nandidha, Roopashree C, Aruna BS, and Arogya Mary, who collected the samples and data.
The authors’ responsibilities were as follows—AM, RJB, CPD and AVK: designed the research; PD and AT: conducted the research; AM, TT, and RJB: analyzed the data, performed the statistical analyses, and interpreted the results; AM: wrote the manuscript and had primary responsibility for the final content of the manuscript; and all authors: critically reviewed the manuscript and read and approved the final manuscript. The authors declared no conflicts of interest.
Notes
Supported by the Department of Biotechnology, Government of India (grant sanction order 102/IFD/SAN/749/2014-2015 and BT/PR10632/PFN/20/838/2013 to AM and AVK; 102/IFD/SAN/3325/2013-2014 to AM, PD, and AVK) and institutional intramural funding. CPD was supported in part by the NIH (K24DK104676) and the Feed the Future Food Innovation Lab for Nutrition, which is funded by the US Agency for International Development (USAID).
Supplemental Tables 1–6 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used:
- AOR
adjusted odds ratio
- CPT-1a
carnitine palmitoyltransferase 1a
- GRB10
growth factor receptor-bound protein 10
- LBW
low birth weight
- SGA
small-for-gestational age
REFERENCES
- 1. UNICEF, Progress for children: a world fit for children statistical review. No. 6, 2007. Available from: http://www.unicef.org/progressforchildren/2007n6/files/Progress_for_Children_-_No._6.pdf (accessed March 3, 2017). [Google Scholar]
- 2. Muthayya S, Kurpad A, Duggan C, Bosch R, Dwarkanath P, Mhaskar A, Mhaskar R, Thomas A, Vaz M, Bhat S et al.. Low maternal vitamin B12 status is associated with intrauterine growth retardation in urban South Indians. Eur J Clin Nutr. 2006;60:791–801. [DOI] [PubMed] [Google Scholar]
- 3. Rao S, Yajnik CS, Kanade A, Fall CHD, Margetts BM, Jackson AA, Shier R, Joshi S, Rege S, Lubree H et al.. Intake of micronutrient-rich foods in rural Indian mothers is associated with the size of their babies at birth: Pune maternal nutrition study. J Nutr. 2001;131:1217–24. [DOI] [PubMed] [Google Scholar]
- 4. Mannion C, Gray-Donald K, Koski K. Association of low intake of milk and vitamin D during pregnancy with decreased birth weight. CMAJ. 2006;174:1273–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kawai K, Spiegelman D, Shankar AH, Fawzi WW. Maternal multiple micronutrient supplementation and pregnancy outcomes in developing countries: meta-analysis and meta-regression. Bull World Health Organ. 2011;89:402–11B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bhutta ZA, Ahmed T, Black RE, Cousens S, Dewey K, Giugliani E, Haider BA, Kirkwood B, Morris SS, Sachdev H et al.. What works? Interventions for maternal and child undernutrition and survival. Lancet. 2008;371:417–40. [DOI] [PubMed] [Google Scholar]
- 7. Godfrey K, Robinson S, Barker D, Osmond C, Cox V. Maternal nutrition in early and late pregnancy in relation to placental and fetal growth. BMJ. 1996;312:410–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mathews F, Yudkin P, Neil A. Influence of maternal nutrition on outcome of pregnancy: prospective cohort study. BMJ. 1999;319:339–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Muthayya S, Dwarkanath P, Thomas T, Ramprakash S, Mehra R, Mhaskar A, Mhaskar R, Thomas A, Bhat S, Vaz M et al.. The effect of fish and omega-3 LCPUFA intake on low birth weight in Indian pregnant women. Eur J Clin Nutr. 2009;63:340–6. [DOI] [PubMed] [Google Scholar]
- 10. Mani I, Dwarkanath P, Thomas T, Thomas A, Kurpad AV. Maternal fat and fatty acid intake and birth outcomes in a South Indian population. Int J Epidemiol. 2016;45:523–31. [DOI] [PubMed] [Google Scholar]
- 11. Chong MF-F, Chia A-R, Colega M, Tint M-T, Aris IM, Chong Y-S, Gluckman P, Godfrey KM, Kwek K, Saw S-M, et al.. Maternal protein intake during pregnancy is not associated with offspring birth weight in a multiethnic Asian population. J Nutr. 2015;145:1303–10. [DOI] [PubMed] [Google Scholar]
- 12. Chen L-W, Aris IM, Bernard JY, Tint M-T, Colega M, Gluckman PD, Tan KH, Shek LP-C, Chong Y-S, Yap F et al.. Associations of maternal macronutrient intake during pregnancy with infant BMI peak characteristics and childhood BMI. Am J Clin Nutr. 2017;105(3):705–13. [DOI] [PubMed] [Google Scholar]
- 13. Chen L, Tint M, Fortier M V, Aris IM, Bernard JY, Colega M, Gluckman PD, Saw S, Chong Y, Yap F, et al.. Maternal macronutrient intake during pregnancy is associated with neonatal abdominal adiposity : the Growing Up in Singapore Towards Healthy Outcomes (GUSTO). J Nutr. 2016;146:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Melamed N, Meizner I, Mashiach R, Wiznitzer A, Glezerman M, Yogev Y. Fetal sex and intrauterine growth patterns. J Ultrasound Med. 2013;32:35–43. [DOI] [PubMed] [Google Scholar]
- 15. lampl M, Gotsch F, Kusanovic JP, Gomez R, Nien JK, Frongillo EA, Romero R. Sex differences in fetal growth responses to maternal height and weight. Am J Hum Biol. 2009;22:431–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. O'Tierney-Ginn P, Presley L, Minium J, Hauguel deMouzon S, Catalano PM. Sex-specific effects of maternal anthropometrics on body composition at birth. Am J Obs Gynecol. 2014;211:292.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mohanty C, Prasad R, Srikanth Reddy A, Ghosh JK, Singh TB, Das BK. Maternal anthropometry as predictors of low birth weight. J Trop Pediatr. 2006;52:24–9. [DOI] [PubMed] [Google Scholar]
- 18. Perkins JM, Subramanian SV, Smith GD, Özaltin E. Adult height, nutrition, and population health. Nutr Rev. 2016;74:149–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995;854:1–452. [PubMed] [Google Scholar]
- 20. Indian Government. The Pre-Natal Diagnostic Techniques (PNDT) act and rules. 2003. Available from: http://chdslsa.gov.in/right_menu/act/pdf/(accessed Februrary 27, 2017). [Google Scholar]
- 21. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65(Suppl):1220S–8S.; discussion: 1229S–31S. [DOI] [PubMed] [Google Scholar]
- 22. National Institute of Nutrition Indian Council of Medical Research. Dietary guidelines for Indians—a manual. 2nd ed Hyderabad (India): National Institute of Nutrition, Indian Council of Medical Research; 2010. [Google Scholar]
- 23. Retnakaran R, Shah BR. Fetal sex and the natural history of maternal risk of diabetes during and after pregnancy. J Clin Endocrinol Metab. 2015;100:2574–80. [DOI] [PubMed] [Google Scholar]
- 24. Jaskolka D, Retnakaran R, Zinman B, Kramer CK. Fetal sex and maternal risk of pre-eclampsia/eclampsia: a systematic review and meta-analysis. BJOG. 2017;124(4):553–60. [DOI] [PubMed] [Google Scholar]
- 25. Broere-Brown Z, Schalekamp-Timmermans S, Hofman A, Jaddoe V, Steegers E. Fetal sex dependency of maternal vascular adaptation to pregnancy: a prospective population-based cohort study. BJOG. 2016;123:1087–95. [DOI] [PubMed] [Google Scholar]
- 26. Enninga EAL, Nevala WK, Creedon DJ, Markovic SN, Holtan SG. Fetal sex-based differences in maternal hormones, angiogenic factors, and immune mediators during pregnancy and the postpartum period. Am J Reprod Immunol. 2015;73:251–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Trivers RL, Willard DE. Natural selection of parental ability to vary the sex ratio of offspring. Science. 1973;179:90–2. [DOI] [PubMed] [Google Scholar]
- 28. Clutton-Brock TH, Albon SD, Guinness FE. Maternal dominance, breeding success and birth sex ratios in red deer. Nature. 1984;308:358–60. [Google Scholar]
- 29. Sheldon BC, West SA. Maternal dominance, maternal condition, and offspring sex ratio in ungulate mammals. Am Nat. 2004;163:40–54. [DOI] [PubMed] [Google Scholar]
- 30. Ruckstuhl KE, Colijn GP, Amiot V, Vinish E. Mother's occupation and sex ratio at birth. BMC Public Health. 2010;10:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Song S. Does famine influence sex ratio at birth? Evidence from the 1959–1961 Great Leap Forward Famine in China. Proc Biol Sci. 2012;279:2883–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hernández-Julián R, Mansour H, Peters C. The effects of intrauterine malnutrition on birth and fertility outcomes: evidence from the 1974 Bangladesh famine. Demography. 2014;51:1775–96. [DOI] [PubMed] [Google Scholar]
- 33. McMillen MM. Differential mortality by sex in fetal and neonatal deaths. Science. 1979;204:89–91. [DOI] [PubMed] [Google Scholar]
- 34. Stevenson DK, Verter J, Fanaroff AA, Oh W, Ehrenkranz RA, Shankaran S, Donovan EF, Wright LL, Lemons JA, Tyson JE et al.. Sex differences in outcomes of very low birthweight infants: the newborn male disadvantage. Arch Dis Child Fetal Neonatal Ed. 2000;83: F182–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lingwood BE, Henry AM, D'Emden MC, Fullerton A-M, Mortimer RH, Colditz PB, Le Cao K-A, Callaway LK. Determinants of body fat in infants of women with gestational diabetes mellitus differ with fetal sex. Diabetes Care. 2011;34:2581–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Edlow AG, Guedj F, Pennings JLA, Sverdlov D, Neri C, Bianchi DW. Males are from Mars, and females are from Venus: sex-specific fetal brain gene expression signatures in a mouse model of maternal diet-induced obesity. Am J Obstet Gynecol. 2016;214:623e1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Spinillo A, Montanari L, Gardella B, Roccio M, Stronati M, Fazzi E. Infant sex, obstetric risk factors, and 2-year neurodevelopmental outcome among preterm infants. Dev Med Child Neurol. 2009;51:518–25. [DOI] [PubMed] [Google Scholar]
- 38. Zambrano E, Bautista CJ, Deás M, Martínez-Samayoa PM, González-Zamorano M, Ledesma H, Morales J, Larrea F, Nathanielsz PW. A low maternal protein diet during pregnancy and lactation has sex- and window of exposure-specific effects on offspring growth and food intake, glucose metabolism and serum leptin in the rat. J Physiol. 2006;571:221–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Eriksson J, Kajantie E, Osmond C, Thornburg K, Barker D. Boys live dangerously in the womb. Am J Hum Biol. 2010;22:330–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1:1077–81. [DOI] [PubMed] [Google Scholar]
- 41. Van Abeelen AFM, De Rooij SR, Osmond C, Painter RC, Veenendaal MVE, Bossuyt PMM, Elias SG, Grobbee DE, Van Der Schouw YT, Barker DJP et al.. The sex-specific effects of famine on the association between placental size and later hypertension. Placenta. 2011;32:694–8. [DOI] [PubMed] [Google Scholar]
- 42. Sferruzzi-Perri AN, Camm EJ. The programming power of the placenta. Front Physiol. 2016;7:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rosenfeld CS. Sex-specific placental responses in fetal development. Endocrinology. 2015;156:3422–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Buckberry S, Bianco-Miotto T, Bent SJ, Dekker GA, Roberts CT. Integrative transcriptome meta-analysis reveals widespread sex-biased gene expression at the human fetal-maternal interface. Mol Hum Reprod. 2014;20:810–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ji S, You Y, Kerner J, Hoppel CL, Schoeb TR, Chick WSH, Hamm DA, Daniel Sharer J, Wood PA. Homozygous carnitine palmitoyltransferase 1b (muscle isoform) deficiency is lethal in the mouse. Mol Genet Metab. 2008;93:314–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Arora SK, McFarlane SI. The case for low carbohydrate diets in diabetes management. Nutr Metab (Lond). 2005;2:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Simpson JL, Elias S, Martin AO, Palmer MS, Ogata ES, Radvany RA. Diabetes in pregnancy, Northwestern University series (1977–1981). Am J Obs Gynecol. 1983;146:263–70. [PubMed] [Google Scholar]
- 48. Gill-Randall R, Adams D, Ollerton RL, Lewis M, Alcolado JC. Type 2 diabetes mellitus—genes or intrauterine environment? An embryo transfer paradigm in rats. Diabetologia. 2004;47:1354–9. [DOI] [PubMed] [Google Scholar]
- 49. Mukhopadhyay A, Ravikumar G, Dwarkanath P, Meraaj H, Thomas A, Crasta J, Thomas T, Kurpad AV, Sridhar TS. Placental expression of the insulin receptor binding protein GRB10: relation to human fetoplacental growth and fetal gender. Placenta. 2015;36:1225–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.