Abstract
The corrections of photon attenuation, scatter, and depth-dependent blurring improve image quality in myocardial perfusion single-photon emission computed tomography (SPECT) imaging; however, the combined corrections induce artifacts. Here, we present the single correction method of depth-dependent blurring and its impact for myocardial perfusion distribution in phantom and clinical studies. The phantom and clinical patient images were acquired with two conditions: circular and noncircular orbits of gamma cameras yielded constant and variable depth-dependent blurring, respectively. An iterative reconstruction with the correction method of depth-dependent was used to reconstruct the phantom and clinical patient images. We found that the single correction method improved the robustness of phantom images whether the images contained constant or variable depth-dependent blurring. The myocardial perfusion databases generated from 72 normal patients exhibited uniform perfusion distribution of whole myocardium. In summary, the single correction method of depth-dependent blurring with iterative reconstruction is helpful for myocardial perfusion SPECT.
Subject terms: Ischaemia, Radionuclide imaging
Introduction
Iterative reconstruction1, such as ordered-subset expectation maximization (OSEM)2, is an indispensable technology in single-photon emission computed tomography (SPECT) imaging to correct depth-dependent blurring3–9, photon attenuation10, and scatter11 in nuclear medicine12–16. The iterative reconstruction technology with resolution recovery (RR) algorithm improves the signal-to-noise ratio of counts in myocardial perfusion SPECT imaging (MPI), and is feasible for performing half-time or half-dose SPECT acquisition3,4.
With respect to attenuation correction (AC), scatter correction (SC), and RR algorithm, our previous study exhibited the optimal OSEM reconstruction condition incorporating these processing16. Although the AC improves photon attenuation in the body, artifacts induced by AC, such as apical thinning may occur in SPECT images17–20. Furthermore, since attenuation-corrected myocardial perfusion images differ greatly from uncorrected images, nuclear medicine physicians usually have to compare corrected and non-corrected images to diagnose myocardial ischemia and infarction in clinical practice21,22. Although normal databases of myocardial perfusion SPECT have been created in Japanese subjects by the Japanese Society of Nuclear Medicine working group23–25, they were mainly based on non-AC images, and effects of OSEM were not evaluated. Therefore, we hypothesized that OSEM with RR (OSEMRR) could aid the interpretation of MPI without artifacts, and OSEMRR is also helpful in the reconstruction of projection images. A large number of studies have been published using OSEMRR regarding the improvement of FHWM and signal-to-noise ratio in basic phantom studies and wall thickness of the myocardium in clinical studies26,27. However, the improvement of distribution of myocardial perfusion is crucial to determine ischemia or infarction of the myocardium in MPI. To best of our knowledge in OSEMRR processing, the count distribution of the whole myocardium using a polar map has only been reported by Zoccarato et al. research group14,15,28. However, the group focused on the comparison of gamma camera differences and reconstruction algorithm differences using count distribution. Moreover, uniform myocardial perfusion distribution was not determined when the number of iterations was change. Our motivation for this research was to provide the uniform count distribution with less depth-dependent blurring in myocardial perfusion SPECT images.
The aim of this study was to confirm that the myocardial perfusion SPECT distribution derived from OSEMRR in both phantom and clinical studies reduces the differences between circular and non-circular orbits compared to reconstruction with FBP. Consequently, we generated a normal database (NDB) for OSEMRR derived from patients with a low likelihood of coronary artery disease (CAD) and evaluated myocardial perfusion distribution.
Materials and Methods
Cone-shape phantom
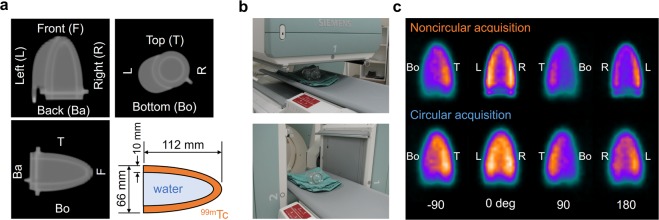
An acrylic cone-shape phantom has a height of 112 mm and a basal diameter of 66 mm (Fig. 1a). The compartment for the radioisotope was filled with 64 MBq of 99mTc-pertechnetate, and an inner cavity was filled with non-radioactive water. The volumes of compartments for radioisotope and water were 118 mL and 93 mL, respectively. The noncircular orbit with the 360-degree rotation of gamma cameras was applied to the phantom acquisition in close proximity to the surface of the phantom and bed (Fig. 1b). A circular orbit was also applied in this phantom study.
Figure 1.
A cone-shape phantom. (a) A coronal image (upper left), a transaxial image (upper right), a sagittal image (bottom left) and the structure of the phantom (bottom right). (b) The positions of gamma cameras in the minimum (upper) and maximum (bottom) gaps between the phantom and gamma cameras in NCA. (c) The projection images derived from CA and NCA with the positions of gamma cameras at 0, ±90, and 180 degrees. NCA, noncircular acquisition; and CA, circular acquisition.
Anthropomorphic torso phantom
We utilized the anthropomorphic torso phantom configured with the cardiac, pulmonary and hepatic components (Kyoto Kagaku, Kyoto, Japan). The left ventricular (LV) myocardium and liver were filled with 185 and 19 MBq of Tc-99m pertechnetate, respectively. The left and right ventricular cavities were filled with non-radioactive water. The circular-orbit acquisition (CA) and noncircular-orbit acquisition (NCA) with the 360-degree rotation of gamma cameras were used for the anthropomorphic torso phantom study. Four plastic circular defects with a 20 mm diameter were placed in the mid-anterior, lateral, inferior, and septal walls.
Study population
We retrospectively included 37 female and 35 male patients with a low likelihood of CAD from Kanazawa University Hospital. 99mTc-sestamibi or 99mTc-tetrofosmin MPI was performed with NCA of gamma cameras. The mean age of male and female patients was 68 ± 16 and 67 ± 15 years, respectively (p = n. s.). The mean height and weight were 151 ± 5 cm and 50 ± 6 kg for females, and 165 ± 8 cm and 66 ± 15 kg for males, respectively (p < 0.0001 for both). Approval for this study was obtained from the ethics committee of the Kanazawa University. The current study was performed in accordance with the ethical guidelines of the Declaration of Helsinki. Due to our retrospective study, informed consent was obtained from all subjects in the form of opt-out. Patients who rejected participation in our study were excluded.
Image acquisition and data processing
In both phantoms and clinical studies, SPECT acquisition was performed with a dual-head gamma camera (Symbia T6 hybrid SPECT/CT scanner, Siemens Healthcare K.K., Tokyo, Japan) equipped with a low-energy high-resolution collimator. The pixel size was 6.6 mm for a 64 × 64 matrix. A photopeak energy window of 99mTc was set at 15% centered at 140 keV. In the phantom study, acquisition time was set as 5 seconds per projection. 60 projection images with CA and NCA of gamma cameras were obtained. In the clinical study, we performed stress and rest gated MPI with 16 frames per cardiac cycle on the hybrid SPECT/CT scanner. MPI was performed with a 360-degree NCA with 60 projections at 40 minutes after injection of 99mTc tracers of 300–370 MBq. The acquisition time was set as 35 seconds per projection.
Data analysis
We used two-reconstruction processing methods: OSEMRR and filtered back projection (FBP) in the phantom and clinical studies. The resolution recovery processing is based on a Flash 3D algorithm (Siemens Healthcare K.K., Tokyo, Japan)29. The Flash 3D algorithm was not incorporated into the FBP processing. In the phantom study, when the subset was constantly set as 15, the number of iterations was set at 1, 5, 10, 15, and 20. In the clinical study, subsets and iterations were set as 15 and 8, respectively. We utilized a Gaussian post-filter for OSEMRR and a Butterworth filter for FBP in both studies. The full width at half maximum of the Gaussian filter was 13.2 mm. The cutoff frequency and order for the Butterworth filter were 0.68 Nyquist and 8. All OSEMRR and FBP processing was performed using e.soft version 8.1 (Siemens Healthcare K.K., Tokyo, Japan).
In the cone-shape phantom and anthropomorphic torso phantom studies, circumferential profile analysis was used to assess SPECT count profiles30,31. The SPECT count profile was presented on a line chart as a function of angle, the origin of which corresponds to the 12 o’clock position on the phantom images. The angles were measured in a clockwise direction relative to an origin at 12 o’clock. The anterior, lateral, inferior, and septal regions of myocardium were defined as 315–45°, 45–135°, 135–225°, and 225–315° in the anthropomorphic torso phantom, respectively. The defect regions of myocardium were also defined as 345–15° for the anterior wall, 75–105° for the lateral wall, 165–195° for the inferior wall, and 255–285° for the septal wall in the anthropomorphic torso phantom with defect, respectively. The SPECT count profiles were evaluated in the central transaxial slice of the cone phantom and the central short axial slice of the anthropomorphic torso phantom. A count ratio of anterolateral to the anteroseptal region was calculated as segments 8 and 14 divided by segments 12 and 16 in the quantitative analysis of the NDB using a standard 17-segment model32. The count ratio of the anterolateral to the inferior region and that of the anterolateral to the anterior region were also calculated as segments 10 and 15 divided by segments 12 and 16, and segments 7 and 13 divided by segments 12 and 16, respectively. Polar maps were generated with quantitative perfusion SPECT (QPS) version 2008.1 (Cedars-Sinai Medical Center, Los Angeles, CA, USA).
The compatibility of Gaussian and Butterworth filters was evaluated in the anthropomorphic phantom study. When the cutoff frequency of Butterworth filter was set as 0.68 Nyquist in our clinical condition, the FWHM of Gaussian filter was changed from 3.3 to 16.5 mm. SPECT count profiles for the central short axial slice of the cardiac component were compared in both Gaussian and Butterworth filters. The comparative evaluation of OSEMRR and FBP reconstructions with or without Gaussian filtering was also performed. SPECT count distributions were calculated for OSEMRR and FBP images without Gaussian filtering, and a fast Fourier transform was used to convert the SPECT count distributions into the frequency domain using ImageJ version 1.52p (NIH, Bethesda, Maryland, USA).
Statistical analyses
All continuous values were expressed as a mean ± standard deviation (SD). The differences in continuous variables were analyzed using Student’s t-test and Tukey-Kramer test. A paired t-test was used to analyze the differences in paired continuous data. All statistical tests were two-tailed, and a p-value of less than 0.05 was considered significant. These analyses were performed using the JMP version 11.2.1 (SAS Institute Inc., Cary, NC, USA) software.
Results
Cone-shape phantom
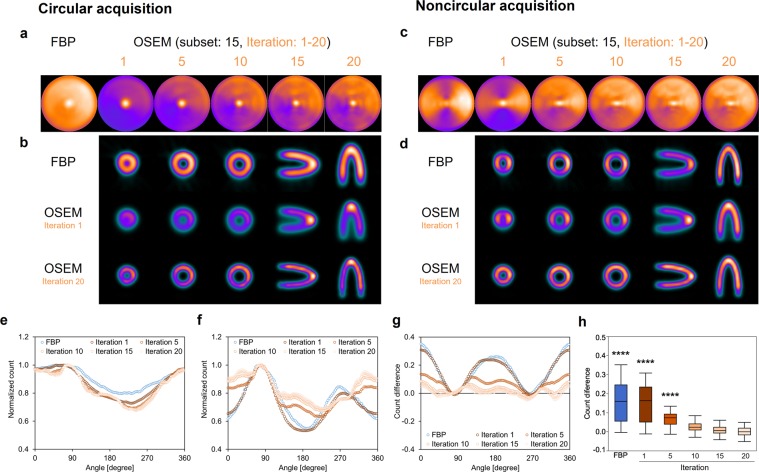
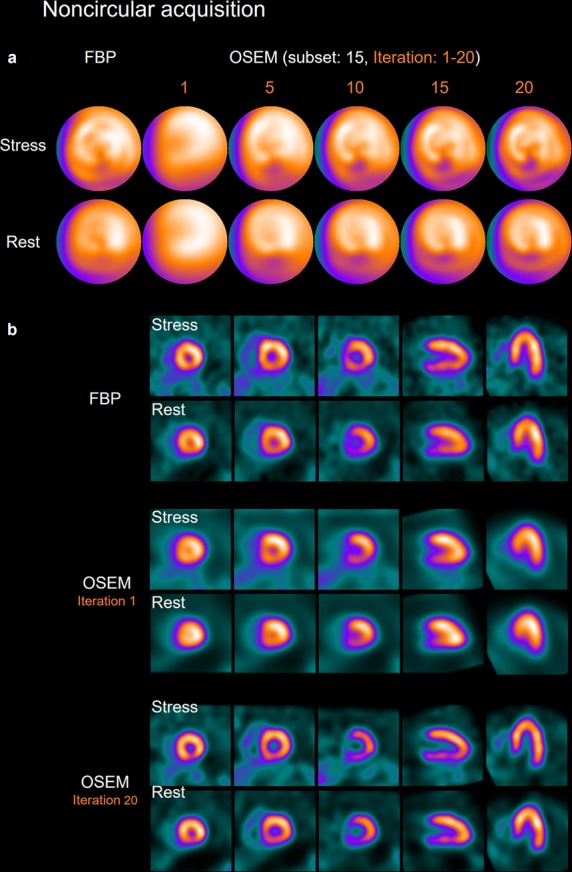
Figure 1 shows a cone-shape phantom and experimental condition. The projection images were obtained from NCA (Fig. 1b) and CA of gamma cameras. The rotation radius was constantly 230 mm in CA, and minimum and maximum radii were 91 mm and 230 mm in NCA, respectively. The superior resolution of projection images was visually shown at 0 and 180 degrees of gamma camera in NCA compared with CA (Fig. 1c). Acquired counts of the projection data ranged from 111 kilocounts/view at 91 mm to 62 kilocounts/view at 230 mm in NCA. The projection data acquired with circular and noncircular orbits of gamma cameras were reconstructed with FBP and OSEMRR (Fig. 2). Polar maps derived from CA and NCA were different greatly in FBP (Fig. 2a,c). Polar maps derived from CA and NCA were visually similar in OSEMRR with 10, 15, and 20 iterations except for the apex. Normalized SPECT count at the front region was higher in NCA than in CA in Supplementary Fig S1. An inner space of the cone phantom derived FBP and OSEMRR with one iteration was smaller in CA than in NCA (Fig. 2b,d). That derived from OSEMRR with 20 iterations was equivalent in CA and NCA. OSEMRR with 5, 10, 15, and 20 iterations yielded similar normalized count profiles in CA, and that with 10, 15, and 20 iterations also yielded similar normalized count profiles in NCA (Fig. 2e,f). The largest and smallest normalized count differences were exhibited in FBP (0.16 ± 0.11) and OSEMRR with 20 iterations (0.0026 ± 0.0059), respectively (p < 0.0001, Fig. 2g,h).
Figure 2.
The cone phantom images and its count distributions. (a,c) Polar maps were generated with FBP and OSEMRR reconstructions in CA and NCA. The iterations changed from 1 to 20, and a subset was constantly 15 in OSEMRR. (b,d) Transaxial images in the front, central, and base, sagittal images, and coronal images of the cone phantom in CA and NCA. (e) Normalized count distributions of the central transaxial slice in CA. (f) Normalized count distributions of the central transaxial slice in NCA. (g) Count differences between CA and NCA. (h) The box-and-whisker plot of the count differences. The count differences obtained from FBP and OSEMRR with 1, 5, 10, and 15 iterations were compared with OSEMRR with 20 iterations. (****P < 0.0001, Tukey-Kramer test). Error bars are SD of the mean. Abbreviations as in Fig. 1.
Anthropomorphic torso phantom: normal myocardium model
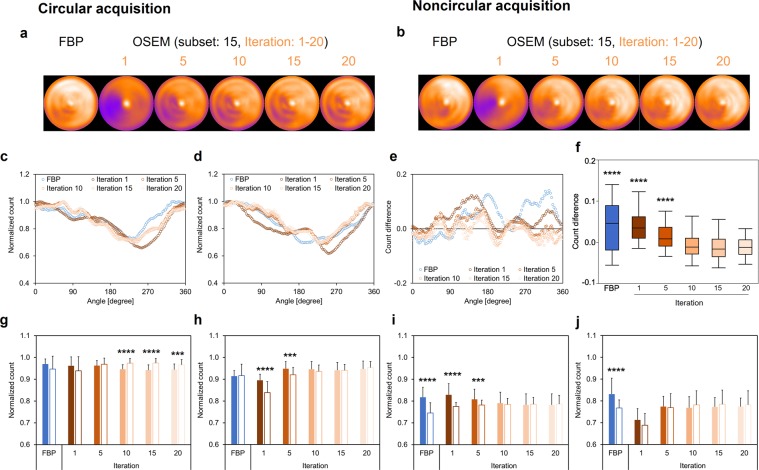
The polar maps of the anthropomorphic torso phantom were generated to confirm the difference between FBP and OSEMRR in both CA and NCA. Polar maps derived from CA and NCA were visually similar in FBP and OSEMRR (Fig. 3a,b). OSEMRR with 10, 15, and 20 iterations yielded similar normalized count profiles in CA and NCA (Fig. 3c,d). A lower normalized count difference was exhibited in OSEMRR with 10 iterations (−0.0061 ± 0.029), whereas higher normalized count differences were exhibited in FBP (0.039 ± 0.058) and OSEMRR with 1 iteration (0.040 ± 0.037), (p < 0.0001, Fig. 3e,f). No significant differences between CA and NCA were exhibited in FBP and OSEMRR with 1 and 5 iterations in the anterior wall (Fig. 3g), in FBP and OSEMRR with 10, 15, and 20 iterations in the lateral wall (Fig. 3h), in OSEMRR with 10, 15, and 20 iterations in the inferior wall (Fig. 3i), and in OSEMRR with 1, 5,10, 15, and 20 iterations in the septal wall (Fig. 3j).
Figure 3.
The anthropomorphic torso phantom images and its count distributions. Polar maps were generated with FBP and OSEMRR reconstructions in CA (a) and NCA (b). The iterations changed from 1 to 20, and a subset was constantly 15 in OSEMRR. (c) Normalized count distributions of the short axis image in CA. (d) Normalized count distributions of the short axis image in NCA. (e) Count differences between CA and NCA. (f) The box-and-whisker plot of the count differences. The count differences obtained from FBP and OSEMRR with 1, 5, 10, and 15 iterations were compared with OSEMRR with 20 iterations. (g) Normalized counts in the anterior wall of myocardium. (h) Normalized counts in the lateral wall of myocardium. (i) Normalized counts in the inferior wall of myocardium. (j) Normalized counts in the septal wall of myocardium. Solid and white bars denote CA and NCA, respectively. Error bars are SD of the mean. (***P < 0.001 and, ****P < 0.0001, Tukey-Kramer test in f and student’s t test in g–j). Abbreviations as in Fig. 1.
Anthropomorphic torso phantom: myocardial defects model
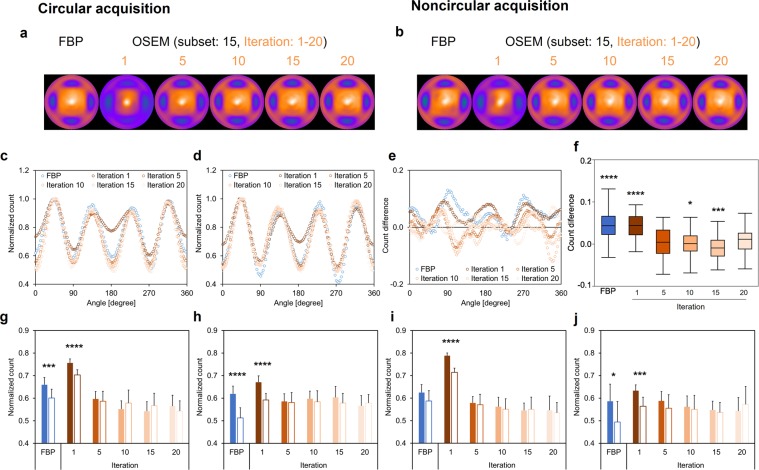
The polar maps of the anthropomorphic torso phantom with myocardial defects were generated to confirm the difference between FBP and OSEMRR in both CA and NCA (Fig. 4a,b). FBP and OSEMRR with 5, 10, 15, and 20 iterations yielded similar normalized count profiles in CA and NCA (Fig. 4c,d). Significant higher normalized count differences were exhibited in FBP (0.044 ± 0.038) and OSEMRR with 1 iteration (0.043 ± 0.029) than in OSEMRR with 20 iterations (0.009 ± 0.030) (p < 0.0001 for all, Fig. 4e,f). No significant differences between CA and NCA were exhibited in OSEMRR with 5, 10, 15, and 20 iterations in the anterior (Fig. 4g), lateral (Fig. 4h), inferior (Fig. 4i), and septal walls (Fig. 4j). To confirm the compatibility of Gaussian and Butterworth filters, the phantom delineation created from Gaussian filter with 3.3-, 6.6-, 8.3-, 9.9-, 11.6-, 13.2-, 14.9-, and 16.5-mm FWHMs was compared with that from 0.64 Nyquist frequency of Butterworth filter in Supplementary Fig. S2. The smallest count difference was exhibited in the combination of Butterworth with 0.64 Nyquist frequency and Gaussian with 13.2-mm FHWM. OSEMRR and FBP reconstructions with or without Gaussian filtering was also compared in Supplementary Fig. S3. The amount of statistical noise component without Gaussian filter was higher in FPB than in OSEMRR. Although the effect of Gaussian filter for the noise reduction was similar in FBP and OSEMRR images, the SPECT count distribution for FBP significantly differed from that for OSEMRR in the mid and basal regions.
Figure 4.
The anthropomorphic torso phantom images and its count distributions. Four plastic circular defects were placed in the mid-anterior, lateral, inferior, and septal walls. Polar maps were generated with FBP and OSEMRR reconstructions in CA (a) and NCA (b). The iterations changed from 1 to 20, and a subset was constantly 15 in OSEMRR. (c) Normalized count distributions of the short axis image in CA. (d) Normalized count distributions of the short axis image in NCA. (e) Count differences between CA and NCA. (f) The box-and-whisker plot of the count differences. The count differences obtained from FBP and OSEMRR with 1, 5, 10, and 15 iterations were compared with OSEMRR with 20 iterations. (g) Normalized counts in the anterior defect of myocardium. (h) Normalized counts in the lateral defect of myocardium. (i) Normalized counts in the inferior defect of myocardium. (j) Normalized counts in the septal defect of myocardium. Solid and white bars denote CA and NCA, respectively. Error bars are SD of the mean. (*P < 0.05, ***P < 0.001, and ****P < 0.0001, Tukey-Kramer test in f and student’s t test in g–j). Abbreviations as in Fig. 1.
Clinical subjects
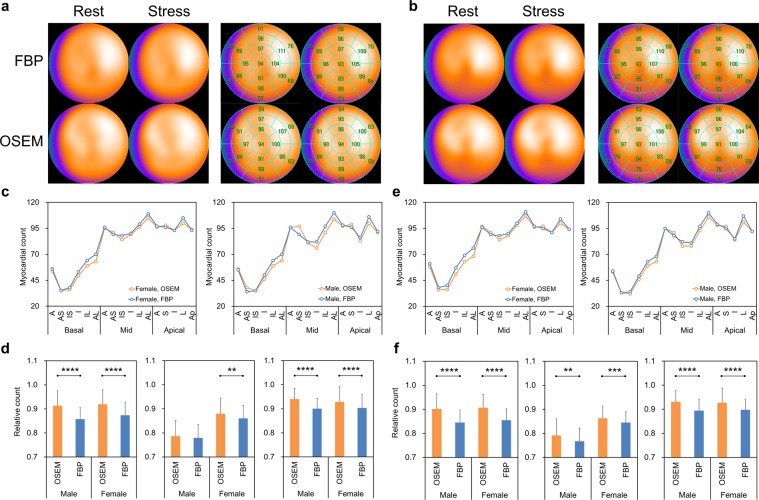
The NDBs were generated to confirm the difference of clinical myocardial perfusion distribution between FBP and OSEMRR in NCA (Fig. 5a,b). Myocardial perfusion counts were always high in the anterolateral region in both male and female NDBs. Moreover, those showed higher values in the anteroseptal region in OSEMRR. The segmental myocardial perfusion distributions derived from OSEMRR paralleled those from FBP in both male and female NDBs (Fig. 5c,e). Based on count ratios of anterolateral segments to anteroseptal segments, inferior segments, and anterior segments (Fig. 5d,f), significantly higher myocardial perfusion counts were exhibited in OSEMRR than in FBP. The clinical study demonstrated that slight ischemia was delineated in the inferior regions in FBP and OSEMRR with 10, 15, and 20 iterations (Fig. 6a). The delineation of the LV cavity by OSEMRR with 20 iterations was superior to that by FBP (Fig. 6b).
Figure 5.
Myocardial perfusion distributions in clinical patients. (a) Polar maps created from 37 female patients with a low likelihood of CAD. The 17-segment polar maps were shown in the right panel. (b) Polar maps created from 35 male patients with a low likelihood of CAD. The 17-segment polar maps were also shown in the right panel. (c) Stress myocardial count distributions derived from FBP and OSEMRR in male (right) and female (left) patients. (d) Relative count ratios of anterolateral segments to anteroseptal segments (left), inferior segments (middle), and anterior segments (right) in stress condition. (e) Rest myocardial count distributions derived from FBP and OSEMRR in male (right) and female (left) patients. (f) Relative count ratios of anterolateral segments to anteroseptal segments (left), inferior segments (middle), and anterior segments (right) in rest condition. Error bars are SD of the mean. (**P < 0.01, ***P < 0.001, and ****P < 0.0001. Paired student’s t test). A, anterior; AS, anteroseptal; S, septal; IS, interoseptal; I, inferior; IL, inferolateral; L, lateral; and AL, anterolateral.
Figure 6.
Clinical stress-rest polar maps (a) and SPECT images (b) in a 60-year-old male patient with slight ischemia in the inferior wall. Height and weight were 167 cm and 66 kg, respectively. All images were acquired with the noncircular orbit of gamma cameras. The Iterations changed from 1 to 20, and a subset was constantly 15.
Discussion
This present study showed that OSEMRR could yield similar count distributions in CA and NCA in the phantom study. In the anthropomorphic torso phantom study with defects, defect delineation was equivalent between CA and NCA in OSEMRR processing. Clinical myocardial perfusion showed a more uniform distribution in OSEMRR than FBP in the NDB. Since the rotation radius varies greatly in SPECT acquisition, we would suggest the use of OSEMRR in nuclear cardiology study. However, the OSEMRR processing with 10, 15, and 20 iterations should need to yield superior myocardial perfusion.
Although our study revealed that OSEMRR compensated for myocardial perfusion counts of NDBs in the anterior, anteroseptal, and inferior walls, uniform myocardial count distribution in the whole myocardium has not yet been shown in OSEMRR processing. Even if attenuation and scatter corrections are used in MPI, uniform myocardial perfusion would not be observed17,33 because the anterior and inferior myocardial counts are influenced by respiratory motion owing to diaphragmatic movement in digital phantom studies34–36. Regarding the myocardial count distribution, maximum myocardium movement is observed in the apex37, and then myocardial 99mTc distribution will be blurred. Consequently, further corrections for cardiac and respiratory movements are needed to find the true myocardial perfusion distribution.
The OSEM technology can easily be integrated with the RR algorithm. Furthermore, our results showed no disadvantages and artifacts owing to using OSEMRR in the phantom scans and clinical NDBs. Although the FBP has commonly been used to reconstruct slices from projection images in nuclear cardiology, we highly recommend the use of OSEMRR in daily clinical practice. Moreover, manufacturers have developed their own version of RR algorithms for OSEM iterative reconstruction, such as Astonish by Philips Medical Systems, Milpitas, CA, USA38, Evolution for Cardiac by GE Healthcare, Waukesha, WI, USA39, and wide beam reconstruction by UltraSPECT, Haifa, Israel3,39. Consequently, the optimal number of iterations and filtering parameters should need to be investigated by OSEMRR fitted for each SPECT imaging system.
The effects of OSEMRR in CA and NCA were evaluated in the cone-shape phantom, anthropomorphic torso phantom, and clinical patient studies. Although the orbit of the camera for the cone phantom experiment did not represent a clinical condition, our aim of this experiment was to confirm the SPECT count distribution of RR in SPECT acquisition using a simplified model. In the anthropomorphic torso phantom without defects, count distributions between FBP and OSEM seemed to be similar. However, defects delineation was different between CA and NCA in FBP. Myocardial perfusion counts of clinical NDB were corrected with OSEMRR in the deep regions of the heart: anterior, anteroseptal, and inferior walls. The OSEMRR processing could contribute to producing uniform myocardial perfusion counts even without the attenuation correction.
Our study has several limitations. We did not compare CA and NCA in the clinical study. No optimal reconstruction condition for the OSEMRR was used in the clinical studies. However, a small number of OSEM iterations yielded insufficient compensation for the RR in the phantom and clinical studies (Figs. 2–4 and 6). Moreover, optimal smoothing filtering conditions for Gaussian and Butterworth filter was not evaluated. This is because OSEMRR incorporating Butterworth filter is not supported by the Siemens data processing system. However, the effect of smoothing filtering condition, which was used in this study, has already been examined in our previous study16. Since quantitative scoring of myocardial perfusion using NDBs was not performed in the present clinical study, summed stress/rest/difference scores could be calculated to confirm the difference between OSEMRR and FBP.
Conclusion
We clarified the myocardial perfusion distribution corrected with RR in CA and NCA of SPECT imaging in the cone-shape and anthropomorphic torso phantom studies. The phantom study characterized the relationship between iterations and count distribution in OSEMRR processing. Moreover, the count distribution derived from NCA paralleled those from CA. When NDBs were created to evaluate the myocardial perfusion distributions in clinical studies, OSEMRR is recommended to compensate for the depth-dependent blurring in myocardial perfusion counts on the 17-segment polar map.
Supplementary information
Acknowledgements
The authors thank nuclear medicine technologists Minoru Tobisaka, Shigeto Matsuyama, Hironori Kojima, Takahiro Konishi, Keita Sakuta, and Haruka Koshida (Kanazawa University Hospital, Kanazawa, Japan) for their technical and clinical assistance. We would also like to thank the Cambridge English Correction service (https://cambridge-correction.com) for English language editing. This study was partly funded by JSPS KAKENHI Grants (Numbers 18K15649 and 15K09947).
Author contributions
K.O. drafted the manuscript and K.N. edited it. K.O., K.N. and S.M. performed data analysis and interpretation. K.O. performed the statistical analysis of the data and K.N. confirmed it. H.Y., T.S. and M.O. provided technical support for SPECT data acquisition. K.N., M.H. and S.K. participated in the conception and study design. All authors reviewed the manuscript and approved the final manuscript.
Competing interests
K. Nakajima has collaborative research work with Siemens Healthcare K.K., Tokyo, Japan.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-019-56097-4.
References
- 1.Shepp LA, Vardi Y. Maximum likelihood reconstruction for emission tomography. IEEE Trans Med Imaging. 1982;1:113–122. doi: 10.1109/TMI.1982.4307558. [DOI] [PubMed] [Google Scholar]
- 2.Hudson HM, Larkin RS. Accelerated image reconstruction using ordered subsets of projection data. IEEE Trans Med Imaging. 1994;13:601–609. doi: 10.1109/42.363108. [DOI] [PubMed] [Google Scholar]
- 3.DePuey EG, et al. Ordered subset expectation maximization and wide beam reconstruction “half-time” gated myocardial perfusion SPECT functional imaging: a comparison to “full-time” filtered backprojection. J Nucl Cardiol. 2008;15:547–563. doi: 10.1016/j.nuclcard.2008.02.035. [DOI] [PubMed] [Google Scholar]
- 4.Zafrir N, et al. Feasibility of myocardial perfusion imaging with half the radiation dose using ordered-subset expectation maximization with resolution recovery software. J Nucl Cardiol. 2012;19:704–712. doi: 10.1007/s12350-012-9552-7. [DOI] [PubMed] [Google Scholar]
- 5.Kohli V, King MA, Pan TS, Glick SJ. Compensation for distance-dependent resolution in cardiac-perfusion SPECT: Impact on uniformity of wall counts and wall thickness. Ieee Transactions on Nuclear Science. 1998;45:1104–1110. doi: 10.1109/23.681986. [DOI] [Google Scholar]
- 6.James O, Pagnanelli R, Neto SB. Resolution recovery and noise regularization in nuclear cardiology. J Nucl Cardiol. 2017;24:138–141. doi: 10.1007/s12350-016-0532-1. [DOI] [PubMed] [Google Scholar]
- 7.Pan TS, Luo DS, Kohli V, King MA. Influence of OSEM, elliptical orbits and background activity on SPECT 3D resolution recovery. Phys Med Biol. 1997;42:2517–2529. doi: 10.1088/0031-9155/42/12/015. [DOI] [PubMed] [Google Scholar]
- 8.Tashiro K, et al. Clinical usefulness of a collimator distance dependent resolution recovery in myocardial perfusion SPECT: a clinical report from a single institute. Ann Nucl Med. 2011;25:133–137. doi: 10.1007/s12149-010-0436-0. [DOI] [PubMed] [Google Scholar]
- 9.Zhang B, Zeng GL. High-resolution versus high-sensitivity SPECT imaging with geometric blurring compensation for various parallel-hole collimation geometries. IEEE Trans Inf Technol Biomed. 2010;14:1121–1127. doi: 10.1109/TITB.2010.2050145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ficaro EP, et al. Simultaneous transmission/emission myocardial perfusion tomography. Diagnostic accuracy of attenuation-corrected 99mTc-sestamibi single-photon emission computed tomography. Circulation. 1996;93:463–473. doi: 10.1161/01.cir.93.3.463. [DOI] [PubMed] [Google Scholar]
- 11.Ichihara T, Ogawa K, Motomura N, Kubo A, Hashimoto S. Compton scatter compensation using the triple-energy window method for single- and dual-isotope SPECT. J Nucl Med. 1993;34:2216–2221. [PubMed] [Google Scholar]
- 12.Links JM, et al. Combined corrections for attenuation, depth-dependent blur, and motion in cardiac SPECT: a multicenter trial. J Nucl Cardiol. 2000;7:414–425. doi: 10.1067/mnc.2000.108350. [DOI] [PubMed] [Google Scholar]
- 13.Narayanan MV, et al. Human-observer receiver-operating-characteristic evaluation of attenuation, scatter, and resolution compensation strategies for (99m)Tc myocardial perfusion imaging. J Nucl Med. 2003;44:1725–1734. [PubMed] [Google Scholar]
- 14.Zoccarato O, et al. Differences in polar-map patterns using the novel technologies for myocardial perfusion imaging. J Nucl Cardiol. 2017;24:1626–1636. doi: 10.1007/s12350-016-0500-9. [DOI] [PubMed] [Google Scholar]
- 15.Zoccarato O, et al. Comparative analysis of iterative reconstruction algorithms with resolution recovery for cardiac SPECT studies. A multi-center phantom study. J Nucl Cardiol. 2014;21:135–148. doi: 10.1007/s12350-013-9821-0. [DOI] [PubMed] [Google Scholar]
- 16.Okuda K, et al. Optimization of iterative reconstruction parameters with attenuation correction, scatter correction and resolution recovery in myocardial perfusion SPECT/CT. Ann Nucl Med. 2014;28:60–68. doi: 10.1007/s12149-013-0785-6. [DOI] [PubMed] [Google Scholar]
- 17.Okuda K, et al. Cause of apical thinning on attenuation-corrected myocardial perfusion SPECT. Nucl Med Commun. 2011;32:1033–1039. doi: 10.1097/MNM.0b013e32834b69e1. [DOI] [PubMed] [Google Scholar]
- 18.Johnson KM, Johnson HE, Dowe DA. Left ventricular apical thinning as normal anatomy. J Comput Assist Tomogr. 2009;33:334–337. doi: 10.1097/RCT.0b013e3181870356. [DOI] [PubMed] [Google Scholar]
- 19.Purser NJ, Armstrong IS, Williams HA, Tonge CM, Lawson RS. Apical thinning: real or artefact? Nucl Med Commun. 2008;29:382–389. doi: 10.1097/MNM.0b013e3282f4a22e. [DOI] [PubMed] [Google Scholar]
- 20.Links JM, Becker LC, Anstett F. Clinical significance of apical thinning after attenuation correction. J Nucl Cardiol. 2004;11:26–31. doi: 10.1016/j.nuclcard.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Xin WC, et al. Is there an incremental value to use myocardial perfusion imaging with or without CT attenuation for the diagnosis of coronary artery disease? A study in Chinese patients. Hell J Nucl Med. 2018;21:48–54. doi: 10.1967/s002449910706. [DOI] [PubMed] [Google Scholar]
- 22.Sharma P, Patel CD, Karunanithi S, Maharjan S, Malhotra A. Comparative accuracy of CT attenuation-corrected and non-attenuation-corrected SPECT myocardial perfusion imaging. Clin Nucl Med. 2012;37:332–338. doi: 10.1097/RLU.0b013e31823ea16b. [DOI] [PubMed] [Google Scholar]
- 23.Nakajima K, et al. Creation and characterization of Japanese standards for myocardial perfusion SPECT: database from the Japanese Society of Nuclear Medicine Working Group. Ann Nucl Med. 2007;21:505–511. doi: 10.1007/s12149-007-0059-2. [DOI] [PubMed] [Google Scholar]
- 24.Nakajima K. Normal values for nuclear cardiology: Japanese databases for myocardial perfusion, fatty acid and sympathetic imaging and left ventricular function. Ann Nucl Med. 2010;24:125–135. doi: 10.1007/s12149-009-0337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakajima K, et al. Normal values and standardization of parameters in nuclear cardiology: Japanese Society of Nuclear Medicine working group database. Ann Nucl Med. 2016;30:188–199. doi: 10.1007/s12149-016-1065-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Onishi H, Motomura N, Fujino K, Natsume T, Haramoto Y. Quantitative performance of advanced resolution recovery strategies on SPECT images: evaluation with use of digital phantom models. Radiol Phys Technol. 2013;6:42–53. doi: 10.1007/s12194-012-0168-z. [DOI] [PubMed] [Google Scholar]
- 27.DePuey E. Gordon. Advances in SPECT camera software and hardware: Currently available and new on the horizon. Journal of Nuclear Cardiology. 2012;19(3):551–581. doi: 10.1007/s12350-012-9544-7. [DOI] [PubMed] [Google Scholar]
- 28.Scabbio C, et al. Impact of non-specific normal databases on perfusion quantification of low-dose myocardial SPECT studies. J Nucl Cardiol. 2019;26:775–785. doi: 10.1007/s12350-017-1079-5. [DOI] [PubMed] [Google Scholar]
- 29.Zeintl J, Vija AH, Yahil A, Hornegger J, Kuwert T. Quantitative accuracy of clinical 99mTc SPECT/CT using ordered-subset expectation maximization with 3-dimensional resolution recovery, attenuation, and scatter correction. J Nucl Med. 2010;51:921–928. doi: 10.2967/jnumed.109.071571. [DOI] [PubMed] [Google Scholar]
- 30.Burow RD, Pond M, Schafer AW, Becker L. “Circumferential profiles:” a new method for computer analysis of thallium-201 myocardial perfusion images. J Nucl Med. 1979;20:771–777. [PubMed] [Google Scholar]
- 31.Meade RC, et al. Quantitative methods in the evaluation of thallium-201 myocardial perfusion images. J Nucl Med. 1978;19:1175–1178. [PubMed] [Google Scholar]
- 32.Cerqueira MD, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart a statement for healthcare professionals from the cardiac imaging committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 33.Steffen DA, et al. “Apical thinning”: Relations between myocardial wall thickness and apical left ventricular tracer uptake as assessed with positron emission tomography myocardial perfusion imaging. J Nucl Cardiol. 2018 doi: 10.1007/s12350-018-1397-2. [DOI] [PubMed] [Google Scholar]
- 34.Segars, W. P. & Tsui, B. M. W. Study of the efficacy of respiratory gating in myocardial SPECT using the new 4-D NCAT phantom. Ieee Transactions on Nuclear Science49, 675–679, doi:Pii S0018-9499(02)06187-7, 10.1109/Tns.2002.1039548 (2002).
- 35.Okuda K, Nakajima K, Kikuchi A, Onoguchi M, Hashimoto M. Cardiac and Respiratory Motion-induced Artifact in Myocardial Perfusion SPECT: 4D Digital Anthropomorphic Phantom Study. Ann Nucl Cardiol. 2017;3:88–93. doi: 10.17996/anc.17-00005. [DOI] [Google Scholar]
- 36.Zhang D, Yang BH, Wu NY, Mok GS. Respiratory average CT for attenuation correction in myocardial perfusion SPECT/CT. Ann Nucl Med. 2017;31:172–180. doi: 10.1007/s12149-016-1144-1. [DOI] [PubMed] [Google Scholar]
- 37.Akhter N, et al. Regional wall thickening in gated myocardial perfusion SPECT in a Japanese population: effect of sex, radiotracer, rotation angles and frame rates. Eur J Nucl Med Mol Imaging. 2008;35:1608–1615. doi: 10.1007/s00259-008-0804-3. [DOI] [PubMed] [Google Scholar]
- 38.Bateman TM, et al. Multicenter investigation comparing a highly efficient half-time stress-only attenuation correction approach against standard rest-stress Tc-99m SPECT imaging. J Nucl Cardiol. 2009;16:726–735. doi: 10.1007/s12350-009-9109-6. [DOI] [PubMed] [Google Scholar]
- 39.DePuey EG, et al. A comparison of the image quality of full-time myocardial perfusion SPECT vs wide beam reconstruction half-time and half-dose SPECT. J Nucl Cardiol. 2011;18:273–280. doi: 10.1007/s12350-011-9340-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.