Abstract
von Willebrand factor (VWF) and platelets are key mediators of normal hemostasis. At sites of vascular injury, VWF recruits platelets via binding to the platelet receptor glycoprotein Ibα (GPIbα). Over the past decades, it has become clear that many hemostatic factors, including VWF and platelets, are also involved in inflammatory processes, forming intriguing links between hemostasis, thrombosis, and inflammation. The so-called “thrombo-inflammatory” nature of the VWF-platelet axis becomes increasingly recognized in different cardiovascular pathologies, making it a potential therapeutic target to interfere with both thrombosis and inflammation. In this review, we discuss the current evidence for the thrombo-inflammatory activity of VWF with a focus on the VWF-GPIbα axis and discuss its implications in the setting of ischemic stroke.
Keywords: von Willebrand factor, platelet glycoprotein Ibα, thromboinflammation, ischemic stroke, ADAMTS13
von Willebrand Factor In Hemostasis: Recruitment Of Platelets
In this section, we briefly summarize the synthesis, structure, and role of VWF in hemostasis and refer to more extensive reviews for further reading.
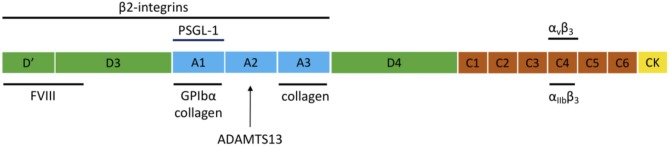
VWF is a large multimeric plasma protein that plays a major role in hemostasis (1–4). First, VWF recruits platelets to sites of vascular injury by forming a bridge between the damaged vessel wall and platelets. Second, VWF also serves as a carrier protein for coagulation factor VIII (FVIII) and hence protects FVIII from degradation, cellular uptake or binding to the surface of activated platelets and endothelial cells (5). VWF is produced exclusively by endothelial cells and megakaryocytes. VWF is synthesized as a pre-pro-VWF that consists of a 22 amino acid signal peptide, a 741 amino acid propeptide (D1-D2) and a mature subunit of 2,050 amino acids (6). The mature subunit is composed of different types of domains arranged in the following order: D′-D3-A1-A2-A3-D4-C1-C2-C3-C4-C5-C6-CK (Figure 1) (1, 7).
Figure 1.
Domain structure of VWF and the main interaction sites. The domain structure of VWF is given and the most important interactions for inflammation and hemostasis are indicated.
After removal of the signal peptide, pro-VWF monomers dimerize in the endoplasmic reticulum through disulfide linkage of the C-terminal CK domains. In the Golgi complex, complete multimerization of the dimers occurs via disulfide linkage of the N-terminal D3 domains, together with additional modifications such as removal of the propeptide, glycosylation, and sulfation. After synthesis, VWF is either constitutively secreted into the blood or is stored in endothelial Weibel-Palade bodies (WPB) and platelet α-granules, from which VWF is locally released via regulated secretion (8). Basolateral release of endothelial VWF leads to accumulation of VWF in the subendothelial matrix, which becomes exposed following damage to the vessel wall. Ultra Large-VWF (UL-VWF) that is released at the apical surface can remain anchored on the surface of endothelial cells and form platelet-decorated strings (9). The structure of VWF is important for its function since several domains are essential for the hemostatic activity of VWF, such as the A1, A3, and C4 domains that mediate binding to GPIbα, collagen, and αIIbβ3, respectively (Figure 1).
The interaction of VWF with platelet GPIbα is crucial for initial platelet adhesion, especially in environments with high hemodynamic shear forces. GPIbα is a subunit of the platelet GPIb-IX-V complex that also contains the GPIbβ, GPIX, and GPV subunits, all of which are type I transmembrane proteins containing leucine-rich repeat domains. Under normal conditions, VWF circulates as a globular protein in which the binding site for GPIbα in the A1 domain is not accessible. However, upon blood vessel damage, VWF binds via its collagen binding sites (mainly in the A1 and A3 domains) to the exposed subendothelial matrix. Immobilization and flow shear forces then result in a conformational activation of the VWF A1 domain, enabling binding of the N-terminal domain of GPIbα (4). This force-induced regulation of the VWF-GPIbα interaction occurs via changes in intramolecular shielding of the VWF A1 domain by neighboring VWF sequences, possibly together with intrinsic changes in the affinity state of the VWF A1 domain itself (10).
The reversible nature of the VWF A1-GPIbα interaction permits platelets to roll and thus decelerate on immobilized VWF, ultimately allowing firm adhesion of platelets to the exposed subendothelial matrix via the platelet collagen receptors GPVI and integrin α2β1. The GPIbα-VWF and GPVI/α2β1-collagen interactions induce downstream intracellular platelet signaling leading to activation of platelet αIIbβ3, which mediates further stable adhesion and aggregation via binding to fibrinogen and VWF.
A central aspect of VWF activity is that larger VWF multimers are more active due to the presence of more monomeric subunits and the higher sensitivity for shear forces. UL-VWF multimers have a molecular weight of >10,000 kD and are highly reactive because the GPIbα binding sites in the VWF A1 domains are continuously exposed. As a result, spontaneous binding of platelets to VWF can occur. UL-VWF is stored in the endothelial WPBs from which it is released via both basal and regulated secretion pathways but also in platelet α-granules from which it is released only after agonist-induced stimulation (6). The local, regulated release of UL-VWF allows fast and confined hemostasis when needed at sites of injury. To prevent accumulation of prothrombotic UL-VWF, however, UL-VWF is cleaved by the VWF cleaving protease ADAMTS13 (A Disintegrin and Metalloprotease with ThromboSpondin type 1 repeats, number 13). Proteolysis of VWF by ADAMTS13 occurs in the VWF A2 domain and is dependent on conformational activation of the A2 domain to expose the cleavage site (11). Digestion of UL-VWF by ADAMTS13 results in smaller, less active VWF multimers (≤10,000 kDa) that adopt a folded conformation in which the platelet binding site in the A1 domain and the ADAMTS13 cleavage site in the A2 domain are cryptic. In the absence of ADAMTS13, spontaneous formation of VWF-platelet complexes leads to thrombotic complications as seen in patients with thrombotic thrombocytopenic purpura (12).
von Willebrand Factor in Inflammation: Recruitment of Leukocytes
Besides its well-established role in hemostasis, VWF is recognized as an effective mediator of inflammatory responses as well. VWF can actively participate in the development of inflammatory processes by recruiting leukocytes at sites of vascular inflammation. Indeed, VWF deficiency or blockade has been shown to reduce leukocyte recruitment in various murine models of inflammation, including cytokine-induced meningitis (13), wound healing (13), atherosclerosis (14), cutaneous inflammation (15, 16), vasculitis (17), and peritonitis (18). When studying the inflammatory effects of VWF, it is important to keep in mind that VWF itself is essential for the formation of WPBs in endothelial cells (19). Alongside VWF, WPBs store also other molecules involved in inflammation and even angiogenesis (e.g., P-selectin, interleukin-6, interleukin-8, Eotaxin-3, Factor H, and angiopoietin-2). Failure of co-storage of inflammatory proteins in the endothelium of VWF-deficient mice can thus also cause defects in inflammation (20). However, recent research provided ample evidence for a direct role of VWF in inflammation, which might potentially be more important than co-storage of inflammatory proteins in the acute-phase response of the vessel wall.
When endothelial cells are activated by inflammatory mediators, UL-VWF is rapidly released from endothelial WPBs. As a consequence, increased levels of circulating VWF antigen has become a well-known marker of inflammation and endothelial activation. When secreted into the blood stream, released VWF can also remain anchored on the surface of endothelial cells through binding with P-selectin (21), integrin αVß3 (22), or the glycocalyx (23) and locally form platelet-decorated strings. VWF facilitates inflammatory processes by promoting leukocyte recruitment to inflamed tissues, either directly or indirectly after binding platelets.
An elegant study by Pendu et al. demonstrated that VWF can act as an adhesive surface for neutrophils and monocytes and that the adhesion process of these inflammatory cells involves various interactions that act in a concerted way (24). Direct adhesion of leukocytes occurs via multiple regions within the VWF molecule that interact with PSGL-1 and ß2 integrins on leukocytes (Figure 1). Whereas, PSGL-1 would be involved in initial rolling on VWF, ß2 integrins would be responsible for stable adhesion on VWF. ß2 integrins can interact with two distinct binding sites on VWF that are located in the D′D3 and A1-A2-A3 regions of VWF as well as to the Leu-Leu-Gly motifs found in the VWF D3 and the connecting region between the A1 and A2 domains (24, 25). The binding site for PSGL-1 is located in the VWF A1 domain (24). Similar for binding to GPIbα, the A1 domain needs to be in its active conformation to bind PSGL-1, which shares structural similarities with GPIbα (24).
Apart from binding directly to leukocytes, VWF can also indirectly promote leukocyte recruitment by forming VWF-platelet-leukocyte complexes, with a crucial role for the VWF-GPIbα axis. The exact mechanisms by which VWF-platelet complexes facilitate leukocyte diapedesis are not yet fully understood and might vary between different inflamed tissues. First, activated platelets bind to VWF and can then interact via P-selectin or GPIbα with leukocytes, thus promoting local adhesion of inflammatory cells (26). As such, immobilized VWF can function as a local matrix to recruit both platelets and leukocytes. Whereas, direct interactions between VWF and leukocytes might be sufficient under venous low-shear conditions, it is conceivable that platelets are needed for leukocyte recruitment under arterial high-shear conditions (26). Second, VWF-platelet complexes can regulate vascular permeability, leading to facilitated leukocyte extravasation. Indeed, using a model of thioglycollate-induced peritonitis, Petri et al. showed that leukocyte recruitment to the inflamed peritoneum was dependent on the presence of VWF and platelets and more specifically on the functional availability of GPIbα (18). In this study, the contribution of VWF-platelet complexes could be explained by destabilization of the endothelial barrier function rather than by increased leukocyte rolling and adhesion. The possible mechanisms through which VWF and platelets induce vascular leakage need further study. Endothelial permeability might be regulated by binding of the VWF RGD motif to endothelial αVß3 integrins (22), and platelets can release various soluble factors that influence endothelial junctions (27). A recent study also showed the involvement of microparticles in VWF-mediated vascular leakage (28).
Overall, the VWF A1 domain seems to be central for the participation of VWF in inflammatory processes. This was recently underlined by two studies from the group of Cécile Denis and Peter Lenting showing that specific inhibition of the VWF A1 domain leads to reduced vascular permeability and leukocyte recruitment (17), whereas a gain-of-function mutation in the VWF A1 domain results in increased leukocyte recruitment (16). Also clinically, the presence of an active A1 domain was shown to predict mortality in patients with systemic inflammatory response syndrome (29). Since an active A1 domain is a typical hallmark of UL-VWF in circulation, it might not be surprising that ADAMTS13 can exert anti-inflammatory activity by reducing the activity of VWF. By cleaving VWF, ADAMTS13 can remove VWF strings from the endothelial surface or reduce the size of reactive VWF to less adhesive VWF molecules. As a result, ADAMTS13 is able to attenuate VWF-dependent leukocyte rolling, adhesion, and extravasation under acute inflammatory conditions. The anti-inflammatory properties of ADAMTS13 have been demonstrated in various settings, including peritonitis (30), atherosclerosis (31), colitis (32), myocardial infarction (33–35), cardiac fibrosis (36), and ischemic stroke, as discussed further.
VWF-Glycoprotein Ib Mediated Thromboinflammation in Ischemic Stroke
Ischemic stroke occurs when a blood clot obstructs cerebral blood flow and causes ischemic brain damage. The primary objective in acute ischemic stroke care is achieving fast reperfusion of the occluded blood vessel to limit ischemic brain injury. Yet, sometimes progressive stroke still develops despite reperfusion of the affected brain tissue, a phenomenon attributed to “reperfusion injury” (37, 38). It has become clear that cerebral ischemia/reperfusion injury is a complex pathology that involves crosstalk between both thrombotic and inflammatory pathways, which has lead to the concept of thrombo-inflammation in stroke (39, 40). Given the dual role of VWF and GPIbα in both thrombosis and inflammation, the VWF-GPIbα axis has received quite some attention in the setting of ischemic stroke (41).
Evidence for the involvement of VWF in ischemic brain injury comes from mouse studies showing that absence of VWF is associated with a significant reduction in ischemic stroke brain injury and improved functional outcome (42, 43). The detrimental effects of VWF were later attributed to the specific involvement of the VWF A1 and A3 (but not C4) domains, indicating a key role for the VWF-GPIbα and VWF-collagen interactions (44). Of note, whereas platelet-derived VWF is largely dispensable for normal hemostasis and thrombosis in mice, we showed that it can actively contribute to ischemic brain injury via a mechanism that is GPIbα-dependent (45). In parallel with these studies on VWF, similar research demonstrated that also GPIbα is an important mediator of cerebral ischemia/reperfusion injury. Indeed, mice lacking functional GPIbα also develop smaller brain infarctions together with improved stroke outcome (46, 47), an observation that was extended in a more translational setting using aged and comorbid (atherosclerotic, diabetic, and hypertensive) animals (48). Furthermore, anfibatide, a snake venom-derived GPIbα antagonist that specifically blocks platelet GPIbα binding to VWF had a potent protective effect in mouse models of ischemic stroke (49–52). As mentioned above, UL-VWF can spontaneously bind platelets and its reactivity can cause thrombotic events without proper regulation by ADAMTS13. Accordingly, experimental stroke studies showed that ADAMTS13-deficient mice developed larger brain infarctions and worse neurologic outcomes, whereas infusion of recombinant ADAMTS13 was able to attenuate ischemic brain damage (42, 53–56). Together, these studies highlight the pathophysiological involvement of VWF and GPIbα in cerebral ischemia/reperfusion injury, which can be counterbalanced by blocking the VWF-GPIbα interaction or by reducing the activity of VWF via ADAMTS13.
The precise mechanisms underlying the pathophysiological involvement of the VWF-GPIbα axis in ischemic brain injury are not yet fully elucidated but available data strongly points toward an intricate process that includes both thrombotic and inflammatory pathways. The cerebral microvasculature rapidly responds to brain ischemia leading to endothelial cell activation and endothelial denudation exposing subendothelial matrix components such as collagen. It has been long known that local platelet and leukocyte recruitment can lead to microvascular obstruction within the ischemic territory after occlusion and reperfusion, a process known as the “no-reflow” phenomenon (57–59). Given the fundamental role of VWF and GPIbα in thrombus development at sites of vascular damage, it is not surprising that the VWF-GPIbα axis is responsible for thrombotic events in stroke. In mouse models of cerebral ischemia/reperfusion injury, VWF deficient mice indeed showed less thrombosis in the cerebral microvasculature, as shown by reduced intracerebral fibrin(ogen) deposition in the affected brain tissue of these animals compared to wild-type mice (44, 45, 60). Remarkably, fibrin(ogen) deposition was considerably reduced in the ischemic hemisphere of the VWF deficient mice that were reconstituted with VWF defective in binding to fibrillar collagen or GPIbα compared with controls, again emphasizing the contribution of initial platelet adhesion interactions mediated by VWF (44). By specifically blocking the VWF-GPIbα axis, anfibatide reduced the number of fibrin(ogen)-positive blood vessels and microthrombi in the ischemic hemisphere (49, 51). In line with these results, anti-GPIbα treatment significantly reduced thrombus burden in the cerebral microvasculature, as measured by the number of GPIX-positive platelet aggregates and occluded brain vessels (61). Correspondingly, ADAMTS13 deficient mice showed an increased number of thrombi containing fibrin and VWF in the brain lesions after stroke (53). Recently, analogous observations were made in CD69 deficient mice (62). CD69 was identified as a negative regulator of endothelial VWF release, and in the setting of stroke, its absence resulted in a more severe stroke burden due to increased cerebral thrombosis (62).
Remarkably, whereas thrombus formation requires both platelet adhesion via GPIbα and GPVI and platelet aggregation via αIIbβ3, the latter does not seem to play a major role in acute ischemic stroke injury (44, 46, 48). Hence, platelets and VWF most likely contribute to stroke progression in a way that is not strictly related to thrombus formation. The most plausible explanation is the involvement of a corresponding inflammatory component mediated by the initial interactions between the damaged vessel wall, VWF, and platelets. Ample evidence for such an inflammatory reaction has been gathered in the last decade. Indeed, in mouse models of ischemic stroke, VWF deficiency is associated with reduced neutrophil infiltration in the ischemic hemisphere (55). In addition, expression levels of the pro-inflammatory cytokines IL-6, IL-1ß, and tumor necrosis factor-α are also decreased in the absence of VWF (55, 60). Interestingly, endothelial-derived rather than platelet-derived VWF seems to be the major determinant of these inflammatory effects (60). In line with the high activity of UL-VWF, elevated VWF-mediated inflammation is observed in the injured brain hemisphere of ADAMTS13-deficient mice. Increased myeloperoxidase activity, increased extravasation of neutrophils, and a higher expression of inflammatory cytokines high-mobility group box1, IL-6, and tumor necrosis factor-α were observed in ADAMTS13-deficient mice compared with wild-type controls (53–55). Interestingly, the increased brain damage and worsened neurological outcome observed in ADAMTS13-deficient animals were abrogated when neutrophils were depleted, indicating a causal role of neutrophils in the exacerbation of ischemic brain injury in the absence of ADAMTS13 (55). Blockade of GPIbα similarly led to decreased expression of IL-6, IL-1ß, and tumor necrosis factor-α (50, 61) and was also shown to lower the numbers of infiltrating T-cells and myeloid leukocytes (51, 61). The latter is in accordance with recent data from our group showing that inhibition of the VWF-GPIbα interaction results in significantly decreased recruitment of monocytes, neutrophils, and T-cells in the ischemic brain (63).
In summary, current evidence shows the involvement of the VWF-GPIbα interaction in a vicious circle of thrombotic and inflammatory responses in the ischemic stroke brain. Ischemia leads to endothelial damage, exposure of subendothelial matrix, upregulation of adhesion molecules, and release of UL-VWF. Local accumulation of VWF contributes to intravascular recruitment platelet and leukocytes, which can secrete proinflammatory cytokines that further stimulate inflammation. Aggregates of VWF, platelets, and leukocytes most probably plug brain capillaries, preventing efficient microcirculatory reperfusion. However, many aspects of the spatiotemporal involvement and molecular interactions between VWF and leukocytes in stroke remain to be elucidated. For, example, whether direct interactions between VWF and PSGL-1 and ß2 integrins are involved remains unanswered. Also, the potential effect of VWF and platelets on vascular permeability in the stroke brain needs further study. Initial results indeed indicate that interfering with VWF or GPIb can modulate the cerebrovascular integrity after stroke (51, 64). When blocking the function of GPIbα, it is important to realize that this platelet receptor contributes to arterial thrombosis via additional mechanisms that are independent of its binding to VWF (65). GPIbα also interacts with various other ligands such as thrombin, coagulation factors XI and XII, high molecular weight kininogen, and thrombospondin-1. Hence, further studies are needed to generate a more complete picture of the involvement of GPIbα in ischemic stroke, besides binding to VWF.
New insights show that already very early during ischemia, neutrophils, and platelets are recruited to the ischemic brain and contribute to microvascular dysfunction (66, 67). Otxoa-de-Amezaga and colleagues recently visualized an early influx of neutrophils to the brain after stroke, predominantly located within the intravascular space already early after reperfusion (68). It would be interesting to further untangle the specific role of VWF during these very early responses in the ischemic tissue to better understand the involvement of VWF in the neurovascular unit.
Translational Aspects
The clinical significance of the VWF-GPIbα interaction in stroke is suggested by an increasing number of human stroke studies showing the pathophysiological involvement of VWF in ischemic stroke (69–74). Furthermore, polymorphisms in the GPIBA gene that lead to enhanced VWF-GPIbα interactions are associated with an increased risk of ischemic stroke in humans (75). Intriguingly, increased VWF activity and/or reduced ADAMTS13 activity are associated not only with higher stroke occurrence, but also with worse long-term stroke outcomes (71, 76–78). Nonetheless, more clinical studies are needed to specifically address the contribution of VWF-mediated thromboinflammatory brain damage during ischemia and reperfusion in ischemic stroke patients. From a clinical perspective, it is promising that the first-generation of VWF-inhibitors is currently enrolled in clinical studies for thrombotic thrombocytopenic purpura, such as a specific inhibitor of the VWF-GPIbα interaction (79) and recombinant ADAMTS13 (80). Notably, we and others have demonstrated that targeting VWF can also promote blood clot dissolution in the setting of ischemic stroke (81–85), which could be of particular relevance to overcome thrombolysis resistance of platelet-rich blood clots in patients (86). Hence, compounds that target VWF could have the attractive potential to promote acute thrombolysis in the occluded blood vessel and attenuate ischemia/reperfusion injury in the microvasculature of the affected brain territory. The safety, especially in terms of bleedings, remains to be further investigated before clinical use. At least in preclinical animal research, targeting VWF via anti-VWF-GPIbα strategies or recombinant ADAMTS13 did not increase the risk of intracranial hemorrhaging in murine stroke models (42, 43, 49), even when combined with tissue-plasminogen activator (87, 88) or when treatment was delayed (56). Of note, ADAMTS13 therapy improved outcomes in murine models of intracerebral hemorrhage in a VWF-dependent way (89–92). More research, preferably also in larger animal models, is needed to bring the concept of blocking VWF-mediated thromboinflammation in stroke closer to the clinic.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. FD is a postdoctoral fellow of the Fonds voor Wetenschappelijk Onderzoek Vlaanderen (FWO, 12U7818N). This work was supported by Fond voor Wetenschappelijk Onderzoek Vlaanderen (G.0A86.13, 1509216N, and G.078517N, to SD), by a research grant from KU Leuven (OT/14/099, to SD), and by a research grant from the Queen Elisabeth Medical Foundation (to SD).
References
- 1.Sadler JE. Biochemistry and genetics of von Willebrand factor. Annu Rev Biochem. (1998) 67:395–424. 10.1146/annurev.biochem.67.1.395 [DOI] [PubMed] [Google Scholar]
- 2.Sadler JE. von Willebrand factor assembly and secretion. J Thromb Haemost. (2009) 7(Suppl. 1):24–7. 10.1111/j.1538-7836.2009.03375.x [DOI] [PubMed] [Google Scholar]
- 3.De Meyer SF, Deckmyn H, Vanhoorelbeke K. von Willebrand factor to the rescue. Blood. (2009) 113:5049–57. 10.1182/blood-2008-10-165621 [DOI] [PubMed] [Google Scholar]
- 4.Springer TA. von Willebrand factor, Jedi knight of the bloodstream. Blood. (2014) 124:1412–25. 10.1182/blood-2014-05-378638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pipe SW, Montgomery RR, Pratt KP, Lenting PJ, Lillicrap D. Life in the shadow of a dominant partner: the FVIII-VWF association and its clinical implications for hemophilia A. Blood. (2016) 128:2007–16. 10.1182/blood-2016-04-713289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lenting PJ, Christophe OD, Denis CV. von Willebrand factor biosynthesis, secretion, and clearance: connecting the far ends. Blood. (2015) 125:2019–28. 10.1182/blood-2014-06-528406 [DOI] [PubMed] [Google Scholar]
- 7.Zhou Y-F, Eng ET, Zhu J, Lu C, Walz T, Springer TA. Sequence and structure relationships within von Willebrand factor. Blood. (2012) 120:449–58. 10.1182/blood-2012-01-405134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mourik M, Eikenboom J. Lifecycle of Weibel-Palade bodies. Hämostaseologie. (2017) 37:13–24. 10.5482/HAMO-16-07-0021 [DOI] [PubMed] [Google Scholar]
- 9.De Ceunynck K, De Meyer SF, Vanhoorelbeke K. Unwinding the von Willebrand factor strings puzzle. Blood. (2013) 121:270–7. 10.1182/blood-2012-07-442285 [DOI] [PubMed] [Google Scholar]
- 10.Löf A, Müller JP, Brehm MA. A biophysical view on von Willebrand factor activation. J Cell Physiol. (2018) 233:799–810. 10.1002/jcp.25887 [DOI] [PubMed] [Google Scholar]
- 11.Crawley JTB, de Groot R, Xiang Y, Luken BM, Lane DA. Unraveling the scissile bond: how ADAMTS13 recognizes and cleaves von Willebrand factor. Blood. (2011) 118:3212–21. 10.1182/blood-2011-02-306597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kremer Hovinga JA, Coppo P, Lämmle B, Moake JL, Miyata T, Vanhoorelbeke K. Thrombotic thrombocytopenic purpura. Nat Rev Dis Primers. (2017) 3:17020. 10.1038/nrdp.2017.20 [DOI] [PubMed] [Google Scholar]
- 13.Denis CV, André P, Saffaripour S, Wagner DD. Defect in regulated secretion of P-selectin affects leukocyte recruitment in von Willebrand factor-deficient mice. Proc Natl Acad Sci USA. (2001) 98:4072–7. 10.1073/pnas.061307098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Methia N, André P, Denis CV, Economopoulos M, Wagner DD. Localized reduction of atherosclerosis in von Willebrand factor–deficient mice. Blood. (2001) 98:1424–8. 10.1182/blood.V98.5.1424 [DOI] [PubMed] [Google Scholar]
- 15.Hillgruber C, Steingräber AK, Pöppelmann B, Denis CV, Ware J, Vestweber D, et al. Blocking Von Willebrand factor for treatment of cutaneous inflammation. J Investig Dermatol. (2013) 134:77–86. 10.1038/jid.2013.292 [DOI] [PubMed] [Google Scholar]
- 16.Adam F, Casari C, Prévost N, Kauskot A, Loubière C, Legendre P, et al. A genetically-engineered von Willebrand disease type 2B mouse model displays defects in hemostasis and inflammation. Sci Rep. (2016) 6:26306. 10.1038/srep26306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aymé G, Adam F, Legendre P, Bazaa A, Proulle V, Denis CV, et al. A novel single-domain antibody against von Willebrand factor A1 domain resolves leukocyte recruitment and vascular leakage during inflammation-brief report. ATVB. (2017) 37:1736–40. 10.1161/ATVBAHA.117.309319 [DOI] [PubMed] [Google Scholar]
- 18.Petri B, Broermann A, Li H, Khandoga AG, Zarbock A, Krombach F, et al. von Willebrand factor promotes leukocyte extravasation. Blood. (2010) 116:4712–9. 10.1182/blood-2010-03-276311 [DOI] [PubMed] [Google Scholar]
- 19.Wagner DD, Saffaripour S, Bonfanti R, Sadler JE, Cramer EM, Chapman B, et al. Induction of specific storage organelles by Von-Willebrand factor propolypeptide. Cell. (1991) 64:403–13. 10.1016/0092-8674(91)90648-I [DOI] [PubMed] [Google Scholar]
- 20.Denis C, Methia N, Frenette PS, Rayburn H, Ullman-Culleré M, Hynes RO, et al. A mouse model of severe von Willebrand disease: defects in hemostasis and thrombosis. Proc Natl Acad Sci USA. (1998) 95:9524–9. 10.1073/pnas.95.16.9524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schneider SW, Nuschele S, Wixforth A, Gorzelanny C, Alexander-Katz A, Netz RR, et al. Shear-induced unfolding triggers adhesion of von Willebrand factor fibers. Proc Natl Acad Sci USA. (2007) 104:7899–903. 10.1073/pnas.0608422104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang J, Roth R, Heuser JE, Sadler JE. Integrin alpha(v)beta(3) on human endothelial cells binds von Willebrand factor strings under fluid shear stress. Blood. (2009) 113:1589–97. 10.1182/blood-2008-05-158584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalagara T, Moutsis T, Yang Y, Pappelbaum KI, Farken A, Cladder-Micus L, et al. The endothelial glycocalyx anchors von Willebrand factor fibers to the vascular endothelium. Blood Adv. (2018) 2:2347–57. 10.1182/bloodadvances.2017013995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pendu R, Terraube V, Christophe OD, Gahmberg CG, de Groot PG, Lenting PJ, et al. P-selectin glycoprotein ligand 1 and β2-integrins cooperate in the adhesion of leukocytes to von Willebrand factor. Blood. (2006) 108:3746–52. 10.1182/blood-2006-03-010322 [DOI] [PubMed] [Google Scholar]
- 25.Koivunen E, Ranta TM, Annila A, Taube S, Uppala A, Jokinen M, et al. Inhibition of beta(2) integrin-mediated leukocyte cell adhesion by leucine-leucine-glycine motif-containing peptides. J Cell Biol. (2001) 153:905–16. 10.1083/jcb.153.5.905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernardo A, Ball C, Nolasco L, Choi H, Moake JL, Dong JF. Platelets adhered to endothelial cell-bound ultra-large von Willebrand factor strings support leukocyte tethering and rolling under high shear stress. J Thromb Haemostasis. (2005) 3:562–70. 10.1111/j.1538-7836.2005.01122.x [DOI] [PubMed] [Google Scholar]
- 27.Gros A, Ollivier V, Ho-Tin-Noé B. Platelets in inflammation: regulation of leukocyte activities and vascular repair. Front Immunol. (2014) 5:678. 10.3389/fimmu.2014.00678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Y, Liu W, Zhou Y, Hilton T, Zhao Z, Wang M, et al. von Willebrand factor enhances microvesicle-induced vascular leakage and coagulopathy in mice with traumatic brain injury. Blood. (2018) 132:1075–84. 10.1182/blood-2018-03-841932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hyseni A, Kemperman H, de Lange DW, Kesecioglu J, de Groot PG, Roest M. Active von Willebrand factor predicts 28-day mortality in patients with systemic inflammatory response syndrome. Blood. (2014) 123:2153–6. 10.1182/blood-2013-08-508093 [DOI] [PubMed] [Google Scholar]
- 30.Chauhan AK, Kisucka J, Brill A, Walsh MT, Walsh MT, Scheiflinger F, et al. ADAMTS13: a new link between thrombosis and inflammation. J Exp Med. (2008) 205:2065–74. 10.1084/jem.20080130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gandhi C, Khan MM, Lentz SR, Chauhan AK. ADAMTS13 reduces vascular inflammation and the development of early atherosclerosis in mice. Blood. (2012) 119:2385–91. 10.1182/blood-2011-09-376202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zitomersky N, Demers M, Martinod K, Gallant M, Cifuni S, Biswas A, et al. ADAMTS13 deficiency worsens colitis and exogenous ADAMTS13 administration decreases colitis severity in mice. TH Open. (2017) 1:e11–23. 10.1055/s-0037-1603927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gandhi C, Motto DG, Jensen M, Lentz SR, Chauhan AK. ADAMTS13 deficiency exacerbates VWF-dependent acute myocardial ischemia/reperfusion injury in mice. Blood. (2012) 120:5224–30. 10.1182/blood-2012-06-440255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Meyer SF, Savchenko AS, Haas MS, Schatzberg D, Carroll MC, Schiviz A, et al. Protective anti-inflammatory effect of ADAMTS13 on myocardial ischemia/reperfusion injury in mice. Blood. (2012) 120:5217–23. 10.1182/blood-2012-06-439935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Savchenko AS, Borissoff JI, Martinod K, De Meyer SF, Gallant M, Erpenbeck L, et al. VWF-mediated leukocyte recruitment with chromatin decondensation by PAD4 increases myocardial ischemia/reperfusion injury in mice. Blood. (2014) 123:141–8. 10.1182/blood-2013-07-514992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Witsch T, Martinod K, Sorvillo N, Portier I, De Meyer SF, Wagner DD. Recombinant human ADAMTS13 treatment improves myocardial remodeling and functionality after pressure overload injury in mice. J Am Heart Assoc. (2018) 7:1957. 10.1161/JAHA.117.007004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nour M, Scalzo F, Liebeskind DS. Ischemia-reperfusion injury in stroke. Intervent Neurol. (2013) 1:185–99. 10.1159/000353125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eltzschig HK, Eckle T. Ischemia and reperfusion—from mechanism to translation. Nat Med. (2011) 17:1391–401. 10.1038/nm.2507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Meyer SF, Denorme F, Langhauser F, Geuss E, Fluri F, Kleinschnitz C. Thromboinflammation in stroke brain damage. Stroke. (2016) 47:1165–72. 10.1161/STROKEAHA.115.011238 [DOI] [PubMed] [Google Scholar]
- 40.Jackson SP, Darbousset R, Schoenwaelder SM. Thromboinflammation: challenges of therapeutically targeting coagulation and other host defense mechanisms. Blood. (2019) 133:906–18. 10.1182/blood-2018-11-882993 [DOI] [PubMed] [Google Scholar]
- 41.Denorme F, De Meyer SF. The VWF-GPIb axis in ischaemic stroke: lessons from animal models. Thromb Haemost. (2016) 116:597–604. 10.1160/TH16-01-0036 [DOI] [PubMed] [Google Scholar]
- 42.Zhao BQ, Chauhan AK, Canault M, Patten IS, Yang JJ, Dockal M, et al. von Willebrand factor-cleaving protease ADAMTS13 reduces ischemic brain injury in experimental stroke. Blood. (2009) 114:3329–34. 10.1182/blood-2009-03-213264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kleinschnitz C, De Meyer SF, Schwarz T, Austinat M, Vanhoorelbeke K, Nieswandt B, et al. Deficiency of von Willebrand factor protects mice from ischemic stroke. Blood. (2009) 113:3600–3. 10.1182/blood-2008-09-180695 [DOI] [PubMed] [Google Scholar]
- 44.De Meyer SF, Schwarz T, Deckmyn H, Denis CV, Nieswandt B, Stoll G, et al. Binding of von Willebrand factor to collagen and glycoprotein Ibalpha, but not to glycoprotein IIb/IIIa, contributes to ischemic stroke in mice–brief report. ATVB. (2010) 30:1949–51. 10.1161/ATVBAHA.110.208918 [DOI] [PubMed] [Google Scholar]
- 45.Verhenne S, Denorme F, Libbrecht S, Vandenbulcke A, Pareyn I, Deckmyn H, et al. Platelet-derived VWF is not essential for normal thrombosis and hemostasis but fosters ischemic stroke injury in mice. Blood. (2015) 126:1715–22. 10.1182/blood-2015-03-632901 [DOI] [PubMed] [Google Scholar]
- 46.Kleinschnitz C, Pozgajova M, Pham M, Bendszus M, Nieswandt B, Stoll G. Targeting platelets in acute experimental stroke: impact of glycoprotein Ib, VI, and IIb/IIIa blockade on infarct size, functional outcome, and intracranial bleeding. Circulation. (2007) 115:2323–30. 10.1161/CIRCULATIONAHA.107.691279 [DOI] [PubMed] [Google Scholar]
- 47.De Meyer SF, Schwarz T, Schatzberg D, Wagner DD. Platelet glycoprotein Ibα is an important mediator of ischemic stroke in mice. Exp Transl Stroke Med. (2011) 3:9. 10.1186/2040-7378-3-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kraft P, Schuhmann MK, Fluri F, Lorenz K, Zernecke A, Stoll G, et al. Efficacy and safety of platelet glycoprotein receptor blockade in aged and comorbid mice with acute experimental stroke. Stroke. (2015) 46:3502–6. 10.1161/STROKEAHA.115.011114 [DOI] [PubMed] [Google Scholar]
- 49.Li T-T, Fan M-L, Hou S-X, Li X-Y, Barry DM, Jin H, et al. A novel snake venom-derived GPIb antagonist, anfibatide, protects mice from acute experimental ischaemic stroke and reperfusion injury. Br J Pharmacol. (2015) 172:3904–16. 10.1111/bph.13178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luo S-Y, Li R, Le Z-Y, Li Q-L, Chen Z-W. Anfibatide protects against rat cerebral ischemia/reperfusion injury via TLR4/JNK/caspase-3 pathway. Eur J Pharmacol. (2017) 807:127–37. 10.1016/j.ejphar.2017.04.002 [DOI] [PubMed] [Google Scholar]
- 51.Chen C, Li T, Zhao Y, Qian Y, Li X, Dai X, et al. Platelet glycoprotein receptor Ib blockade ameliorates experimental cerebral ischemia-reperfusion injury by strengthening the blood-brain barrier function and anti-thrombo-inflammatory property. Brain Behav Immun. (2018) 69:255–63. 10.1016/j.bbi.2017.11.019 [DOI] [PubMed] [Google Scholar]
- 52.Li J, Kim K, Jeong S-Y, Chiu J, Xiong B, Petukhov PA, et al. Platelet protein disulfide isomerase promotes glycoprotein Ibα-mediated platelet-neutrophil interactions under thromboinflammatory conditions. Circulation. (2018) 139:1300–19. 10.1161/CIRCULATIONAHA.118.036323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fujioka M, Hayakawa K, Mishima K, Kunizawa A, Irie K, Higuchi S, et al. ADAMTS13 gene deletion aggravates ischemic brain damage: a possible neuroprotective role of ADAMTS13 by ameliorating postischemic hypoperfusion. Blood. (2010) 115:1650–3. 10.1182/blood-2009-06-230110 [DOI] [PubMed] [Google Scholar]
- 54.Fujioka M, Nakano T, Hayakawa K, Irie K, Akitake Y, Sakamoto Y, et al. ADAMTS13 gene deletion enhances plasma high-mobility group box1 elevation and neuroinflammation in brain ischemia-reperfusion injury. Neurol Sci. (2012) 33:1107–15. 10.1007/s10072-011-0913-9 [DOI] [PubMed] [Google Scholar]
- 55.Khan MM, Motto DG, Lentz SR, Chauhan AK. ADAMTS13 reduces VWF-mediated acute inflammation following focal cerebral ischemia in mice. J Thromb Haemost. (2012) 10:1665–71. 10.1111/j.1538-7836.2012.04822.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nakano T, Irie K, Hayakawa K, Sano K, Nakamura Y, Tanaka M, et al. Delayed treatment with ADAMTS13 ameliorates cerebral ischemic injury without hemorrhagic complication. Brain Res. (2015) 1624:330–5. 10.1016/j.brainres.2015.07.027 [DOI] [PubMed] [Google Scholar]
- 57.del Zoppo GJ, Schmid-Schönbein GW, Mori E, Copeland BR, Chang CM. Polymorphonuclear leukocytes occlude capillaries following middle cerebral artery occlusion and reperfusion in baboons. Stroke. (1991) 22:1276–83. 10.1161/01.STR.22.10.1276 [DOI] [PubMed] [Google Scholar]
- 58.Mori E, del Zoppo GJ, Chambers JD, Copeland BR, Arfors KE. Inhibition of polymorphonuclear leukocyte adherence suppresses no-reflow after focal cerebral ischemia in baboons. Stroke. (1992) 23:712–8. 10.1161/01.STR.23.5.712 [DOI] [PubMed] [Google Scholar]
- 59.Garcia JH, Liu KF, Yoshida Y, Lian J, Chen S, del Zoppo GJ. Influx of leukocytes and platelets in an evolving brain infarct. Am J Pathol. (1994) 144:188–99. [PMC free article] [PubMed] [Google Scholar]
- 60.Dhanesha N, Prakash P, Doddapattar P, Khanna I, Pollpeter MJ, Nayak MK, et al. Endothelial cell-derived von willebrand factor is the major determinant that mediates von Willebrand factor-dependent acute ischemic stroke by promoting postischemic thrombo-inflammation. ATVB. (2016) 36:1829–37. 10.1161/ATVBAHA.116.307660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schuhmann MK, Guthmann J, Stoll G, Nieswandt B, Kraft P, Kleinschnitz C. Blocking of platelet glycoprotein receptor Ib reduces “thrombo-inflammation” in mice with acute ischemic stroke. J Neuroinflammation. (2017) 14:18. 10.1186/s12974-017-0792-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brait VH, Miró-Mur F, Perez-de-Puig I, Notario L, Hurtado B, Pedragosa J, et al. CD69 Plays a beneficial role in ischemic stroke by dampening endothelial activation. Circ Res. (2019) 124:279–91. 10.1161/CIRCRESAHA.118.313818 [DOI] [PubMed] [Google Scholar]
- 63.Denorme F, Martinod K, Vandenbulcke A, Lenting PJ, Denis CV, Deckmyn H. The VWF-GPIb interaction mediates thrombo-inflammation in experimental stroke via recruitment of monocytes, neutrophils and T-cells to the brain. RPTH. (2019) 3(Suppl. 1):1–891. 10.1002/rth2.12229 [DOI] [Google Scholar]
- 64.Xu H, Cao Y, Yang X, Cai P, Kang L, Zhu X, et al. ADAMTS13 controls vascular remodeling by modifying VWF reactivity during stroke recovery. Blood. (2017) 130:11–22. 10.1182/blood-2016-10-747089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bergmeier W, Goerge T, Ruggeri ZM, Ware J, Bergmeier W, Cifuni SM, et al. The role of platelet adhesion receptor GPIb far exceeds that of its main ligand, von Willebrand factor, in arterial thrombosis. Proc Natl Acad Sci USA. (2006) 103:16900–5. 10.1073/pnas.0608207103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Desilles J-P, Loyau S, Syvannarath V, Gonzalez-Valcarcel J, Cantier M, Louedec L, et al. Alteplase reduces downstream microvascular thrombosis and improves the benefit of large artery recanalization in stroke. Stroke. (2015) 46:3241–8. 10.1161/STROKEAHA.115.010721 [DOI] [PubMed] [Google Scholar]
- 67.Desilles J-P, Syvannarath V, Di Meglio L, Ducroux C, Boisseau W, Louedec L, et al. Downstream microvascular thrombosis in cortical venules is an early response to proximal cerebral arterial occlusion. J Am Heart Assoc. (2018) 7:e007804–6. 10.1161/JAHA.117.007804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Otxoa-de-Amezaga A, Gallizioli M, Pedragosa J, Justicia C, Miró-Mur F, Salas-Perdomo A, et al. Location of neutrophils in different compartments of the damaged mouse brain after severe ischemia/reperfusion. Stroke. (2019) 50:1548–57. 10.1161/STROKEAHA.118.023837 [DOI] [PubMed] [Google Scholar]
- 69.Bongers TN, de Maat MPM, van Goor M-LPJ, Bhagwanbali V, van Vliet HHDM, Gómez García EB, et al. High von Willebrand factor levels increase the risk of first ischemic stroke: influence of ADAMTS13, inflammation, and genetic variability. Stroke. (2006) 37:2672–7. 10.1161/01.STR.0000244767.39962.f7 [DOI] [PubMed] [Google Scholar]
- 70.Williams SR, Hsu F-C, Keene KL, Chen W-M, Dzhivhuho G, Rowles JL, et al. Genetic drivers of von Willebrand factor levels in an ischemic stroke population and association with risk for recurrent stroke. Stroke. (2017) 48:1444–50. 10.1161/STROKEAHA.116.015677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sonneveld MAH, de Maat MPM, Portegies MLP, Kavousi M, Hofman A, Turecek PL, et al. Low ADAMTS13 activity is associated with an increased risk of ischemic stroke. Blood. (2015) 126:2739–46. 10.1182/blood-2015-05-643338 [DOI] [PubMed] [Google Scholar]
- 72.Wieberdink RG, van Schie MC, Koudstaal PJ, Hofman A, Witteman JCM, de Maat MPM, et al. High von Willebrand factor levels increase the risk of stroke: the Rotterdam study. Stroke. (2010) 41:2151–6. 10.1161/STROKEAHA.110.586289 [DOI] [PubMed] [Google Scholar]
- 73.McCabe DJH, Murphy SJX, Starke R, Harrison P, Brown MM, Sidhu PS, et al. Relationship between ADAMTS13 activity, von Willebrand factor antigen levels and platelet function in the early and late phases after TIA or ischaemic stroke. J Neurol Sci. (2015) 348:35–40. 10.1016/j.jns.2014.10.035 [DOI] [PubMed] [Google Scholar]
- 74.Sonneveld MAH, de Maat MPM, Leebeek FWG. Von Willebrand factor and ADAMTS13 in arterial thrombosis: a systematic review and meta-analysis. Blood Rev. (2014) 28:167–78. 10.1016/j.blre.2014.04.003 [DOI] [PubMed] [Google Scholar]
- 75.Baker RI, Eikelboom J, Lofthouse E, Staples N, Afshar-Kharghan V, Lopez JA, et al. Platelet glycoprotein Ibalpha Kozak polymorphism is associated with an increased risk of ischemic stroke. Blood. (2001) 98:36–40. 10.1182/blood.V98.1.36 [DOI] [PubMed] [Google Scholar]
- 76.Tóth NK, Székely EG, Czuriga-Kovács KR, Sarkady F, Nagy O, Lánczi LI, et al. Elevated factor VIII and von Willebrand factor levels predict unfavorable outcome in stroke patients treated with intravenous thrombolysis. Front Neurol. (2018) 8:e38. 10.3389/fneur.2017.00721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Prochazka V, Jonszta T, Czerny D, Krajca J, Roubec M, Macak J, et al. The role of von Willebrand factor, ADAMTS13, and cerebral artery thrombus composition in patient outcome following mechanical thrombectomy for acute ischemic stroke. Med Sci Monit. (2018) 24:3929–45. 10.12659/MSM.908441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schuppner R, Dirks M, Grosse GM, Böckmann M, Goetz F, Pasedag T, et al. ADAMTS-13 activity predicts outcome in acute ischaemic stroke patients undergoing endovascular treatment. Thromb Haemost. (2018) 118:758–67. 10.1055/s-0038-1637732 [DOI] [PubMed] [Google Scholar]
- 79.Peyvandi F, Scully M, Kremer Hovinga JA, Cataland S, Knöbl P, Wu H, et al. Caplacizumab for acquired thrombotic thrombocytopenic purpura. N Engl J Med. (2016) 374:511–22. 10.1056/NEJMoa1505533 [DOI] [PubMed] [Google Scholar]
- 80.Scully M, Knöbl P, Kentouche K, Rice L, Windyga J, Schneppenheim R, et al. Recombinant ADAMTS-13: first-in-human pharmacokinetics and safety in congenital thrombotic thrombocytopenic purpura. Blood. (2017) 130:2055–63. 10.1182/blood-2017-06-788026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lapchak PA, Doyan S, Fan X, Woods CM. Synergistic effect of AJW200, a von Willebrand factor neutralizing antibody with low dose (0.9 mg/mg) thrombolytic therapy following embolic stroke in rabbits. J Neurol Neurophysiol. (2013) 4:1–5. 10.4172/2155-9562.1000146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Momi S, Tantucci M, Van Roy M, Ulrichts H, Ricci G, Gresele P. Reperfusion of cerebral artery thrombosis by the GPIb-VWF blockade with the Nanobody ALX-0081 reduces brain infarct size in guinea pigs. Blood. (2013) 121:5088–97. 10.1182/blood-2012-11-464545 [DOI] [PubMed] [Google Scholar]
- 83.Le Behot A, Gauberti M, Martinez De Lizarrondo S, Montagne A, Lemarchand E, Repesse Y, et al. GpIb-VWF blockade restores vessel patency by dissolving platelet aggregates formed under very high shear rate in mice. Blood. (2014) 123:3354–63. 10.1182/blood-2013-12-543074 [DOI] [PubMed] [Google Scholar]
- 84.Denorme F, Langhauser F, Desender L, Vandenbulcke A, Rottensteiner H, Plaimauer B, et al. ADAMTS13-mediated thrombolysis of t-PA-resistant occlusions in ischemic stroke in mice. Blood. (2016) 127:2337–45. 10.1182/blood-2015-08-662650 [DOI] [PubMed] [Google Scholar]
- 85.Martinez de Lizarrondo S, Gakuba C, Herbig BA, Repesse Y, Ali C, Denis CV, et al. Potent thrombolytic effect of N-acetylcysteine on arterial thrombi. Circulation. (2017) 136:646–60. 10.1161/CIRCULATIONAHA.117.027290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Staessens S, Denorme F, François O, Desender L, Dewaele T, Vanacker P, et al. Structural analysis of ischemic stroke thrombi: histological indications for therapy resistance. Haematologica. (2019). 10.3324/haematol.2019.219881 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schuhmann MK, Kraft P, Bieber M, Haarmann A, Homola GA, Pham M, et al. Influence of thrombolysis on the safety and efficacy of blocking platelet adhesion or secretory activity in acute ischemic stroke in mice. Transl Stroke Res. (2018) 372:1–6. 10.1007/s12975-017-0606-7 [DOI] [PubMed] [Google Scholar]
- 88.Wang L, Fan W, Cai P, Fan M, Zhu X, Dai Y, et al. Recombinant ADAMTS13 reduces tissue plasminogen activator-induced hemorrhage after stroke in mice. Ann Neurol. (2012) 73:189–98. 10.1002/ana.23762 [DOI] [PubMed] [Google Scholar]
- 89.Muroi C, Fujioka M, Mishima K, Irie K, Fujimura Y, Nakano T, et al. Effect of ADAMTS-13 on cerebrovascular microthrombosis and neuronal injury after experimental subarachnoid hemorrhage. J Thromb Haemostasis. (2014) 12:505–14. 10.1111/jth.12511 [DOI] [PubMed] [Google Scholar]
- 90.Vergouwen MDI, Knaup VL, Roelofs JJTH, de Boer OJ, Meijers JCM. Effect of recombinant ADAMTS-13 on microthrombosis and brain injury after experimental subarachnoid hemorrhage. J Thromb Haemostasis. (2014) 12:943–7. 10.1111/jth.12574 [DOI] [PubMed] [Google Scholar]
- 91.Cai P, Luo H, Xu H, Zhu X, Xu W, Dai Y, et al. Recombinant ADAMTS 13 attenuates brain injury after intracerebral hemorrhage. Stroke. (2015) 46:2647–53. 10.1161/STROKEAHA.115.009526 [DOI] [PubMed] [Google Scholar]
- 92.Zhu X, Cao Y, Wei L, Cai P, Xu H, Luo H, et al. von Willebrand factor contributes to poor outcome in a mouse model of intracerebral haemorrhage. Sci Rep. (2016) 6:35901. 10.1038/srep35901 [DOI] [PMC free article] [PubMed] [Google Scholar]