Abstract
Purpose
We aimed to evaluate the clinical characteristics of microsatellite instability in early gastric cancer.
Materials and Methods
The microsatellite instability status of resected early gastric tumors was evaluated using two mononucleotide repeat markers (BAT25 and BAT26) and three dinucleotide repeat markers (D5S346, D2S123, and D17S250). Tumors with instability in two or more markers were defined as microsatellite instability-high (MSI-H) and others were classified as microsatellite stable (MSS).
Results
Overall, 1,156 tumors were included in the analysis, with 85 (7.4%) classified as MSI-H compared with MSS tumors. For MSI-H tumors, there was a significant correlation with the female sex, older age, tumor location in the lower gastric body, intestinal histology, lymphovascular invasion (LVI), and submucosal invasion (P<0.05). There was also a trend toward an association with lymph node (LN) metastasis (P=0.056). In mucosal gastric cancer, there was no significant difference in MSI status in tumors with LN metastasis or tumors with LVI. In submucosal gastric cancer, LVI was more frequently observed in MSI-H than in MSS tumors (38.9% vs. 25.0%, P=0.027), but there was no difference in the presence of LN metastases. The prognosis of MSI-H tumors was similar to that of MSS tumors (log-rank test, P=0.797, the hazard ratio for MSI-H was adjusted by age, sex, pT stage, and the number of metastatic LNs, 0.932; 95% confidence interval, 0.423–2.054; P=0.861).
Conclusions
MSI status was not useful in predicting prognosis in early gastric cancer. However, the frequent presence of LVI in early MSI-H gastric cancer may help guide the appropriate treatment for patients, such as endoscopic treatment or limited LN surgical dissection.
Keywords: Microsatellite instability, Gastric cancer, Prognosis, Lymph nodes
INTRODUCTION
Gastric cancer is a major global health burden, accounting for nearly one million new cases and 720,000 deaths worldwide in 2012 [1]. Gastric cancer is one of the most common cancer types in Korea [2] and with well-established screening practices, the proportion of early gastric cancer is high in countries, such as Korea and Japan [3]. While the prognosis for early gastric cancer is relatively favorable, 5%–20% of these patients have lymph node (LN) metastases at the time of diagnosis [4] and 5%–10% experience cancer recurrence after surgical treatment [5,6]. Variable molecular characteristics are responsible for the diverse clinical features and prognosis [7,8,9] in patients with gastric cancer. Discovering biomarkers related to LN metastasis and prognosis are essential for improving patient care [10].
Microsatellite instability (MSI), which is characterized by an inability to repair microsatellite regions, is a molecular phenotype arising from defects in DNA mismatch repair (MMR) machinery [11,12]. Accordingly, a high level of MSI is associated with an increased mutation rate [13]. MSI is related to inherited disorders caused by MMR gene mutations such as in Lynch syndrome [14], and the sporadic type of MSI cancer is mainly caused by silencing of the mut1 homolog 1 (MLH1) gene, a consequence of CpG island hypermethylation [15]. The MSI phenotype is a molecular subtype that is recognized in gastric cancer [13,16]. The proportion of MSI-H gastric cancers was reported as approximately 5%–37%, and was related to females, older age, intestinal histology, less frequent LN metastasis, and earlier tumor stages [17,18,19,20,21]. In addition, recent post-hoc analyses of randomized controlled trials [22,23] showed that MSI-H tumors are related to a favorable prognosis and MSI status may be a marker for predicting chemotherapy responsiveness for stage II/III gastric cancer. However, the clinical implications of MSI status in early gastric cancer have not been thoroughly evaluated. We hypothesized that MSI status is a predictor for LN metastasis and prognosis in early gastric cancer.
MATERIALS AND METHODS
Data collection
Data were obtained from patients who underwent gastrectomy for gastric cancer with curative intent at Severance Hospital, in the Yonsei University Health System, between January 2005 and June 2010 [24]. MSI status was reported for 2,549 of these patients. Patients with prior gastric surgery or neoadjuvant chemotherapy were excluded (n=32). We also excluded cancers that invaded more than the proper muscle layer (n=1,361) but included cancers with the invasion of the mucosa or submucosa. Gastric adenocarcinoma was histologically confirmed for all included patients. A total of 1,156 patients were included in the final analysis. Information was obtained with the appropriate Institutional Review Board approval, and data were collected without revealing any personal information.
Clinicopathological variables
We retrospectively recorded clinicopathological features, including age, sex, tumor location, tumor size, histological type, Lauren classification, pT stage, number of resected LNs, number of metastatic LNs, lymphovascular invasion (LVI), and MSI status. Additional immunohistochemistry for detecting LN micrometastases was not routinely performed. Patient survival was determined from hospital records, the database of the Korean National Statistical Office, and telephone surveys. The patients were evaluated for 5 years after surgery to monitor for tumor recurrence. Evaluations included a physical examination, laboratory testing, diagnostic imaging, and endoscopy. Patient prognosis was evaluated by disease-free survival (DFS) and the median follow-up period was 72 months, with a range of 1–98 months.
MSI status definition
To determine the MSI status, DNA was extracted from the paraffin blocks of each patient's tumor specimen for polymerase chain reaction amplification and fragment analysis, as described previously [17]. MSI analysis was performed using the Revised Bethesda Guidelines for two mononucleotides (BAT 25 and BAT 26) and 3 dinucleotide (D2S123, D5S346, and D17S250) microsatellite markers [25]. If the number of unstable markers was 0 or 1, the tumor was clinically defined as microsatellite stable (MSS), while a tumor with 2 or more unstable markers was defined as microsatellite instability-high (MSI-H).
Tumor staging and histological classification
Tumors were staged according to the 7th edition of the International Union Against Cancer classification [26]. Histologically, tumors were classified into two categories, differentiated or undifferentiated types. Differentiated tumors include papillary, well- or moderately-differentiated, and tubular adenocarcinomas, while undifferentiated types include poorly-differentiated tubular adenocarcinoma, signet ring cell carcinoma, and mucinous carcinoma. Another way to classify tumors is by using the Lauren classification system, which classifies tumors into 3 categories: intestinal type, diffuse type, and others (including mixed types) [27].
Statistical methods
Clinical and pathological data were analyzed with IBM SPSS® software, version 23.0 for Windows® (SPSS, Chicago, IL, USA). To analyze continuous variables, we used the independent t-test. Categorical data were analyzed with Pearson's χ2 test and Fisher's exact test. Survival data were analyzed using the Kaplan-Meier method, log-rank test, and Cox's proportional hazards model. Univariable and multivariable analyses in the Cox model were described with hazard ratios (HRs) and their 95% confidence interval (CI). Variables that were statistically significant in univariable analysis were included in multivariable analysis without any selection method. Age and sex were used as adjustment variables for adjusted HRs. DFS was defined as the date from operation to death or recurrence, whichever occurred first. Statistical significance was defined as having a P-value <0.05.
Ethics statement
This study was conducted in accordance with the Ethical Principles for Medical Research Involving Human Subjects, as outlined in the Declaration of Helsinki after the approval of the Institutional Review Board of Severance Hospital, Yonsei University Health System (IRB No. 4-2019-0081).
RESULTS
Clinical characteristics
Among 1,156 patients with early gastric cancer, 85 patients (7.4%) had MSI-H gastric cancer. The MSI-H type of gastric cancer was related to older age (63.4 vs. 56.7 years, P<0.001), female sex (54.1% vs. 32.8%, P<0.001), location in the lower third of the stomach (81.2% vs. 59.7%, P<0.001), a histologically differentiated type (71.8% vs. 50.0%, P<0.001), and intestinal-type dominance by Lauren classification (63.5% vs. 48.2%, P=0.002) in comparison to the MSS type. The depth of invasion for the MSI-H type was deeper than the MSS type, with 63.5% of the MSI-H showing submucosal invasion while only 48.3% was seen with the MSS type (P=0.007). A trend toward more LN metastases in the MSI-H tumors was observed but was not statistically significant (17.6% vs. 10.8%, P=0.056). However, more LVI was observed in the MSI-H type compared to the MSS type (25.9% vs. 13.3%, P=0.001). These data and further details are summarized in Table 1.
Table 1. MSI status varies based on characteristics of enrolled patients.
| Characteristics | Overall (n=1,156) | MSS (n=1,071; 92.65%) | MSI-H (n=85; 7.35%) | P-value | |
|---|---|---|---|---|---|
| Age (years) | 57.8±11.6 | 56.7±11.5 | 63.4±10.7 | <0.001 | |
| Sex | <0.001 | ||||
| Male | 759 (65.7) | 720 (67.2) | 39 (45.9) | ||
| Female | 397 (34.3) | 351 (32.8) | 46 (54.1) | ||
| Location | <0.001 | ||||
| Upper-third | 95 (8.2) | 92 (8.6) | 3 (3.5) | ||
| Middle-third | 352 (30.5) | 339 (31.7) | 13 (15.3) | ||
| Lower-third | 708 (61.3) | 639 (59.7) | 69 (81.2) | ||
| Size (mm) | 26.8±15.6 | 26.7±15.6 | 28.3±15.7 | 0.381 | |
| Histology | <0.001 | ||||
| Differentiated | 588 (51.6) | 527 (50.0) | 61 (71.8) | ||
| Undifferentiated | 552 (48.4) | 528 (50.0) | 24 (28.2) | ||
| Lauren classification | 0.002 | ||||
| Intestinal | 570 (49.3) | 516 (48.2) | 54 (63.5) | ||
| Diffuse | 370 (32.0) | 357 (33.3) | 13 (15.3) | ||
| Others | 147 (12.7) | 186 (17.4) | 18 (21.2) | ||
| pT stage (%) | 0.007 | ||||
| Mucosa | 585 (50.6) | 554 (51.7) | 31 (36.5) | ||
| Submucosa | 571 (49.4) | 517 (48.3) | 54 (63.5) | ||
| No. of resected LN | 37.44±14.49 | 37.58±14.34 | 35.73±16.24 | 0.258 | |
| LN metastasis (%) | 0.056 | ||||
| Yes | 131 (11.3) | 116 (10.8) | 15 (17.6) | ||
| No | 1,025 (88.7) | 955 (89.2) | 70 (82.4) | ||
| No. of metastatic LN | 0.3±1.5 | 0.3±1.5 | 0.4±1.4 | 0.473 | |
| LVI | 0.001 | ||||
| Yes | 992 (85.8) | 142 (13.3) | 22 (25.9) | ||
| No | 164 (14.2) | 929 (86.7) | 63 (74.1) | ||
Values are expressed as mean±standard deviation or number (%).
MSI = microsatellite instability; MSS = microsatellite stable; MSI-H = microsatellite instability-high; LN = lymph node; LVI = lymphovascular invasion.
Patient prognosis by MSI status
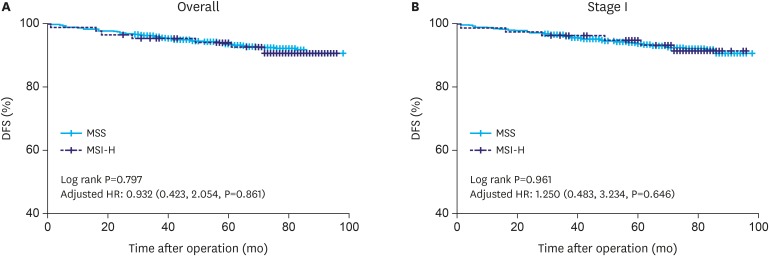
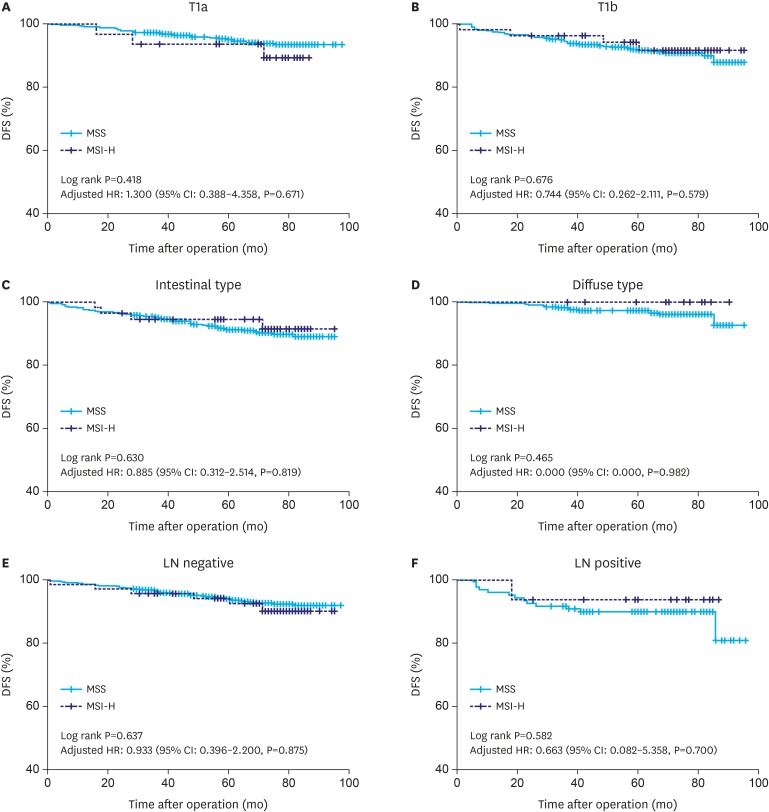
As Fig. 1 depicts, the prognosis of MSI-H tumors did not differ from that of MSS tumors in patients with early gastric cancer (log-rank, P=0.797; age-, sex-, pT stage-, and the number of metastatic LN-adjusted HR was 0.932; 95% CI, 0.423–2.054; P=0.861). MSI status was not a significant prognostic factor in univariable or multivariable Cox analyses (HR, 1.107; 95% CI, 0.511–2.398; P=0.7970 and HR, 0.855; 95% CI, 0.387–1.890; P=0.699, respectively) (Table 2). In the subgroup analysis by pT stage (mucosa and submucosa) (Fig. 2A and B), the Lauren classification (intestinal and diffuse type) (Fig. 2C and D), and the LN metastasis (without and with LN metastasis) (Fig. 2E and F), there were no differences or trends for prognosis by MSI status.
Fig. 1. DFS Kaplan-Meier curves (A) in overall cohort, (B) in stage I.
DFS = disease-free survival; HR = hazard ratio; CI = confidence interval; MSS = microsatellite stable; MSI-H = microsatellite instability-high.
Table 2. Cox regression analysis of DFS for each patient characteristic.
| Characteristics | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | ||
| Age | 1.064 (1.042–1.087) | <0.001 | 1.065 (1.042–1.088) | <0.001 | |
| Sex | <0.001 | 0.001 | |||
| Male | 1 | 1 | |||
| Female | 0.303 (0.165–0.558) | 0.362 (0.196–0.668) | |||
| Location | 0.865 | - | |||
| Upper body | 1 | - | |||
| Middle body | 1.268 (0.485–3.312) | - | |||
| Lower body | 1.430 (0.573–3.574) | - | |||
| Size (mm) | 1.012 (1.000–1.024) | 0.047 | 1.008 (0.996–1.021) | 0.196 | |
| Histology | 0.035 | 0.397 | |||
| Differentiated | 1 | 1 | |||
| Undifferentiated | 0.620 (0.398–0.966) | 1.311 (0.700–2.455) | |||
| Lauren classification | 0.022 | 0.138 | |||
| Intestinal | 1 | 1 | |||
| Diffuse | 0.404 (0.224–0.729) | 0.563 (0.251–1.263) | |||
| Others | 1.068 (0.459–2.488) | 1.189 (0.645–2.194) | |||
| pT stage | 0.056 | - | |||
| Mucosa | 1 | - | |||
| Submucosa | 1.525 (0.990–2.349) | - | |||
| No. of resected LN | 0.986 (0.970–1.002) | 0.084 | - | - | |
| No. of metastatic LN | 1.172 (1.101–1.248) | <0.001 | 1.158 (1.091–1.229) | <0.001 | |
| LN metastasis | 0.105 | - | |||
| No | 1 | - | |||
| YES | 1.608 (0.906–2.852) | - | |||
| LVI | 0.024 | 0.348 | |||
| No | 1 | 1 | |||
| Yes | 1.799 (1.080–2.997) | 1.292 (0.757–2.206) | |||
| MSI status | 0.797 | 0.699 | |||
| MSS | 1 | 1 | |||
| MSI-H | 1.107 (0.511–2.398) | 0.855 (0.387–1.890) | |||
DFS = disease-free survival; HR = hazard ratio; CI = confidence interval; LN = lymph node; LVI = lymphovascular invasion; MSI = microsatellite instability; MSS = microsatellite stable; MSI-H = microsatellite instability-high.
Fig. 2. Kaplan-Meier curve for histologic type, pT stage, pN stage.
LN = lymph node; DFS = disease-free survival; HR = hazard ratio; CI = confidence interval; MSS = microsatellite stable; MSI-H = microsatellite instability-high.
LN metastasis and LVI by MSI status
As there was a trend and significant association between LN metastasis, LVI, and MSI status, we conducted subgroup analyses. In gastric cancer with mucosal invasion, there was no significant difference between LN metastasis and LVI based on MSI status (P=0.755 and P=0.755, respectively) (Table 3). In submucosal cancer, no significant association was observed between LN metastasis and MSI status concerning overall submucosal invasion, invasion <500 μm, and invasion ≥500 μm (P=0.298, P=0.175, and P=0.287, respectively) (Table 3). The MSI-H type of gastric cancer was significantly related to overall LVI and invasion ≥500 μm (P=0.027 and P=0.019, respectively). However, no statistically significant relationship was noted when the tumor invaded <500 μm (P=0.129) (Table 3). LVI was significantly associated with LN metastasis, regardless of MSI status. 47.4% (55 out of 116 patients) and 9.1% (87 out of 955 patients) of MSS tumors, and 40.9% (9 out of 22 patients) and 9.5% (6 out of 63 patients) of MSI-H tumors were related to LN metastasis with and without LVI, respectively (P≤0.001 and P=0.002, respectively).
Table 3. Comparison of MSI status, LN metastasis, and LVI depth.
| Variables | pT1a (n=585) | pT1b (n=571) | |||||
|---|---|---|---|---|---|---|---|
| MSS (n=554) | MSI-H (n=31) | P-value | MSS (n=517) | MSI-H (n=54) | P-value | ||
| LN metastasis | 0.755 | 0.298 | |||||
| Yes | 13 (2.35) | 1 (3.23) | 13/90 (16.0/20.6)* | 0/14 (0.0/29.2)* | 0.175† | ||
| No | 541 (97.65) | 30 (96.77) | 68/346 (84.0/79.4)* | 6/34 (100.0/70.8)* | 0.287‡ | ||
| LVI | 0.755 | 0.027 | |||||
| Yes | 13 (2.35) | 1 (3.23) | 11/118 (13.6/27.1)* | 3/18 (50.0/37.5)* | 0.129† | ||
| No | 541 (97.65) | 30 (96.77) | 70/318 (86.4/72.9)* | 3/30 (50.0/62.5)* | 0.019‡ | ||
MSI = microsatellite instability; LN = lymph node; LVI = lymphovascular invasion; MSS = microsatellite stable; MSI-H = microsatellite instability-high.
*Sm1 (<500 μm)/Sm2, 3 (≥500 μm); number (%); †P-value of χ2 or Fisher's exact test between MSI status and LN metastasis/LVI on Sm1 stage gastric cancer; ‡P-value of χ2 or Fisher's exact test between MSI status and LN metastasis/LVI on Sm2, 3 stage gastric cancer.
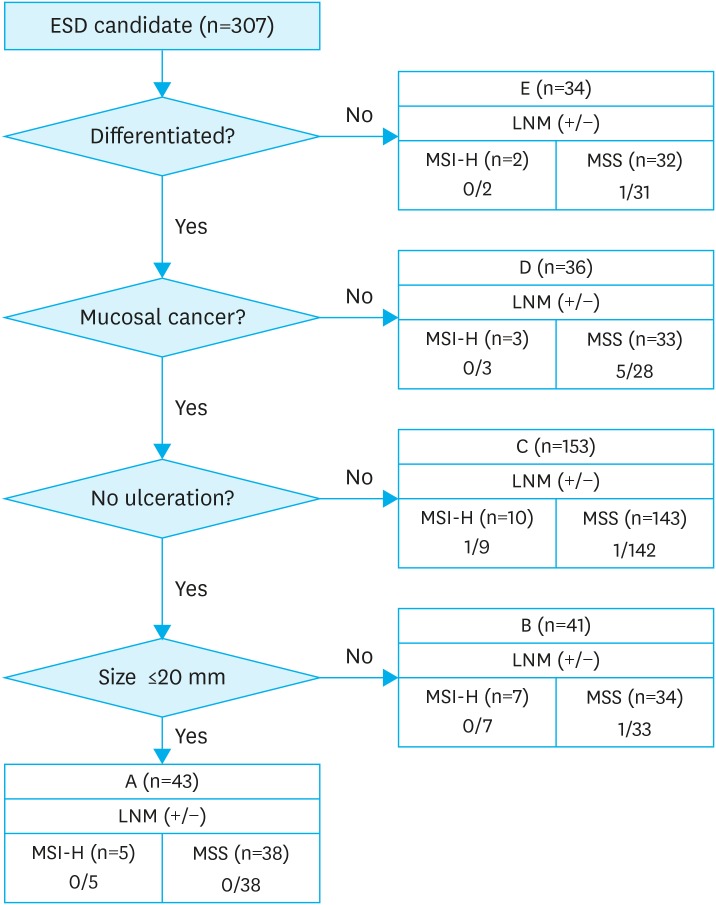
We reviewed LN metastasis by MSI status in 307 patients who were candidates for endoscopic submucosal dissection (ESD) using both absolute and expanded criteria [28]. There were no LN metastases in either MSI-H or MSS types in 43 patients who met the absolute criteria for ESD (Fig. 3). Among 153 patients who satisfied one of the expanded criteria for ESD (size ≤3 cm, differentiated histology, or mucosal invasion with ulcers), there was 1 case of LN metastasis in 10 MSI-H patients, and 1 in 143 MSS patients. In other patients with expanded criteria for ESD, there were no cases of LN metastasis in the MSI-H type of gastric cancer.
Fig. 3. Patient distribution based on extended ESD indication.
A. Tumor size ≤2 cm, histologically of differentiated type, pT1a, UL(−).
B. Tumor size >2 cm, histologically of differentiated type, pT1a, UL(−).
C. Tumor size ≤3 cm, histologically of differentiated type, pT1a, UL(+).
D. Tumor size ≤2 cm, histologically of undifferentiated type, pT1a, UL(−).
E. Tumor size ≤3 cm, histologically of differentiated type, pT1b (Sm1, ≤500 μm from the muscularis mucosae).
ESD = endoscopic submucosal dissection; LNM = lymph node metastasis; MSS = microsatellite stable; MSI-H = microsatellite instability-high; UL = ulcerative lesion.
DISCUSSION
Contrary to our expectations, the present results showed that MSI status was not a prognostic indicator in patients with early gastric cancer. However, MSI-H gastric cancer was related to more aggressive tumors that exhibited deeper local invasion as well as LVI, with a trend towards an increase in LN metastasis.
Through efforts to understand the molecular biology of gastric cancer, clinically applicable biomarkers, such as ones for MSI, have been developed and validated for predicting prognosis and chemotherapeutic responses [7,8,9,22,23,29,30]. Previous studies regarding MSI in gastric cancer have focused on advanced-stage tumors. The MSI-H tumor status is predictive for immune checkpoint inhibitor responses in advanced or recurrent gastric cancer [31]. The MSI-H tumor status also serves as an indicator for the treatment of stage II/III tumors with only surgery since it is associated with a good prognosis, yet a poor response to chemotherapy [22,23]. Since early gastric cancer has a favorable prognosis, with 5-year DFS rates of 90%–95% [6,32], there would only be a small survival difference influenced by MSI status. The possibility of a type II error in the present results seemed to be low because there was no trend toward a different prognosis based on MSI status in the overall or subgroup analyses. Therefore, future biomarker studies regarding MSI status should focus on advanced gastric cancer, rather than early gastric cancer.
Prediction of LN metastasis is a clinically important challenge because limited resections, such as that with ESDs or partial gastrectomies with limited LN dissection, could be possible if there is certainty of no LN metastasis [28,33,34]. However, it is difficult to predict the presence of LN metastasis or LVI before surgery [4,20]. The less aggressive characteristics of MSI-H tumors in advanced gastric cancer suggest the likelihood that the MSI-H status could be inversely correlated to LN metastasis in early gastric cancer. We found a non-significant trend toward more LN metastases with MSI-H tumors. However, subgroup analyses of the depth of tumor invasion (mucosal and submucosal) showed no significant difference with respect to LN metastasis based on MSI status.
The present cohort included only patients with known MSI status and patients who underwent surgery, but patients who were treated by endoscopic resection were not considered. Consequently, there could be a selection bias. In addition, the mean tumor size in this cohort was larger than that in a previous study [4]. Presumably, these larger tumors signify a greater tumor invasion depth for the MSI-H tumors in this study. Since the depth of tumor invasion is strongly related to LN metastasis, one would expect the MSI-H tumors to be associated with more LN metastasis. However, there was no relationship between MSI-H tumors and higher LN metastasis when the tumor satisfied the ESD indications (Fig. 3). Therefore, it is difficult to conclude that the MSI-H phenotype in early gastric cancer is related to LN metastasis.
MSI-H tumors are related to increased immune signaling and a high mutational burden by neoantigens [31,35]. We found a significant positive relationship between LVI and the MSI-H status of gastric cancer in the overall pT1b group and the subgroup that has a depth of invasion ≥500 μm. This finding is not new as the same results have been reported previously [36,37,38]. This result can be explained by the possibility that immune cells are targeting an MSI-H tumor since there are more lymphatic vessels around the tumor, which increase the probability of tumor cell lymphatic invasion. However, the tumor cells can be detected and ultimately killed by the immune cells. The presence of LVI increases the risk of LN metastasis, which is a contraindication for ESD treatment. Therefore, we should be cautious about expanding the indications for ESD in the future when there are MSI-H gastric cancers with submucosal invasion.
Although MSI is one of the well-defined and evaluated biomarkers in gastric cancer, molecular and clinical heterogeneity of MSI-H types have been reported: Intratumoral heterogeneity with both deficient and proficient MMR in a single patient has been reported [31,39] as well as stratified prognosis in MSI-H gastric cancer by expression of immune-related markers [40,41,42], which implies that MSI-H types may be further stratified by additional molecular characteristics. Clinicians and researchers who study MSI-H gastric cancer need to recognize these important issues.
Although this study utilized the largest patient cohort for this subject, it is not without limitations due to the retrospective study design and the low number of deaths and tumor recurrence rates. The biomarkers selected for this population could also be subjected to selection bias. The results of this study need to be validated in other cohorts for confirmation.
In conclusion, MSI status is not a useful prognostic marker in patients with early gastric cancer. Frequent LVI of MSI-H gastric cancers, particularly invasion of the submucosa, could be a sign that careful patient selection for ESD or limited surgical intervention is necessary.
Footnotes
- Conceptualization: C.Y.Y., K.D.G.
- Data curation: K.D.G., A.J.Y., K.H., S.S.J., C.S., S.W.J., R.C.K., C.M., S.T., K.H.I., C.J.H., H.W.J., N.S.H., C.Y.Y.
- Formal analysis: K.D.G., A.J.Y., K.H., S.S.J., C.S., S.W.J., R.C.K., C.M., S.T., K.H.I., C.J.H., H.W.J., N.S.H., C.Y.Y.
- Methodology: C.Y.Y., K.D.G.
- Supervision: C.Y.Y.
- Validation: K.D.G., A.J.Y., K.H., S.S.J., C.S., S.W.J., R.C.K., C.M., S.T., K.H.I., C.J.H., H.W.J., N.S.H., C.Y.Y.
- Visualization: C.Y.Y., K.D.G.
- Writing - original draft: C.Y.Y., K.D.G.
- Writing - review & editing: K.D.G., A.J.Y., K.H., S.S.J., C.S., S.W.J., R.C.K., C.M., S.T., K.H.I., C.J.H., H.W.J., N.S.H., C.Y.Y.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Jung KW, Won YJ, Kong HJ, Lee ES Community of Population-Based Regional Cancer Registries. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2015. Cancer Res Treat. 2018;50:303–316. doi: 10.4143/crt.2018.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sugano K. Screening of gastric cancer in Asia. Best Pract Res Clin Gastroenterol. 2015;29:895–905. doi: 10.1016/j.bpg.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 4.Nakagawa M, Choi YY, An JY, Chung H, Seo SH, Shin HB, et al. Difficulty of predicting the presence of lymph node metastases in patients with clinical early stage gastric cancer: a case control study. BMC Cancer. 2015;15:943. doi: 10.1186/s12885-015-1940-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai JF, Kim S, Kim K, Li C, Oh SJ, Hyung WJ, et al. Prediction of recurrence of early gastric cancer after curative resection. Ann Surg Oncol. 2009;16:1896–1902. doi: 10.1245/s10434-009-0473-x. [DOI] [PubMed] [Google Scholar]
- 6.Choi YY, An JY, Katai H, Seto Y, Fukagawa T, Okumura Y, et al. A lymph node staging system for gastric cancer: a hybrid type based on topographic and numeric systems. PLoS One. 2016;11:e0149555. doi: 10.1371/journal.pone.0149555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheong JH, Yang HK, Kim H, Kim WH, Kim YW, Kook MC, et al. Predictive test for chemotherapy response in resectable gastric cancer: a multi-cohort, retrospective analysis. Lancet Oncol. 2018;19:629–638. doi: 10.1016/S1470-2045(18)30108-6. [DOI] [PubMed] [Google Scholar]
- 8.Roh CK, Choi YY, Choi S, Seo WJ, Cho M, Jang E, et al. Single patient classifier assay, microsatellite instability, and epstein-barr virus status predict clinical outcomes in stage II/III gastric cancer: results from CLASSIC trial. Yonsei Med J. 2019;60:132–139. doi: 10.3349/ymj.2019.60.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi YY, Jang E, Seo WJ, Son T, Kim HI, Kim H, et al. Modification of the TNM staging system for stage II/III gastric cancer based on a prognostic single patient classifier algorithm. J Gastric Cancer. 2018;18:142–151. doi: 10.5230/jgc.2018.18.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi YY, Cho M, Kwon IG, Son T, Kim HI, Choi SH, et al. Ten thousand consecutive gastrectomies for gastric cancer: perspectives of a master surgeon. Yonsei Med J. 2019;60:235–242. doi: 10.3349/ymj.2019.60.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993;260:816–819. doi: 10.1126/science.8484122. [DOI] [PubMed] [Google Scholar]
- 12.Ionov Y, Peinado MA, Malkhosyan S, Shibata D, Perucho M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature. 1993;363:558–561. doi: 10.1038/363558a0. [DOI] [PubMed] [Google Scholar]
- 13.Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–5257. [PubMed] [Google Scholar]
- 15.Veigl ML, Kasturi L, Olechnowicz J, Ma AH, Lutterbaugh JD, Periyasamy S, et al. Biallelic inactivation of hMLH1 by epigenetic gene silencing, a novel mechanism causing human MSI cancers. Proc Natl Acad Sci U S A. 1998;95:8698–8702. doi: 10.1073/pnas.95.15.8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cristescu R, Lee J, Nebozhyn M, Kim KM, Ting JC, Wong SS, et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. 2015;21:449–456. doi: 10.1038/nm.3850. [DOI] [PubMed] [Google Scholar]
- 17.An JY, Kim H, Cheong JH, Hyung WJ, Kim H, Noh SH. Microsatellite instability in sporadic gastric cancer: its prognostic role and guidance for 5-FU based chemotherapy after R0 resection. Int J Cancer. 2012;131:505–511. doi: 10.1002/ijc.26399. [DOI] [PubMed] [Google Scholar]
- 18.Choi YY, Bae JM, An JY, Kwon IG, Cho I, Shin HB, et al. Is microsatellite instability a prognostic marker in gastric cancer? A systematic review with meta-analysis. J Surg Oncol. 2014;110:129–135. doi: 10.1002/jso.23618. [DOI] [PubMed] [Google Scholar]
- 19.Kim SY, Choi YY, An JY, Shin HB, Jo A, Choi H, et al. The benefit of microsatellite instability is attenuated by chemotherapy in stage II and stage III gastric cancer: Results from a large cohort with subgroup analyses. Int J Cancer. 2015;137:819–825. doi: 10.1002/ijc.29449. [DOI] [PubMed] [Google Scholar]
- 20.Polom K, Marrelli D, Pascale V, Ferrara F, Voglino C, Marini M, et al. The pattern of lymph node metastases in microsatellite unstable gastric cancer. Eur J Surg Oncol. 2017;43:2341–2348. doi: 10.1016/j.ejso.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 21.Polom K, Marano L, Marrelli D, De Luca R, Roviello G, Savelli V, et al. Meta-analysis of microsatellite instability in relation to clinicopathological characteristics and overall survival in gastric cancer. Br J Surg. 2018;105:159–167. doi: 10.1002/bjs.10663. [DOI] [PubMed] [Google Scholar]
- 22.Choi YY, Kim H, Shin SJ, Kim HY, Lee J, Yang HK, et al. Microsatellite instability and programmed cell death-ligand 1 expression in stage II/III gastric cancer: post hoc analysis of the CLASSIC randomized controlled study. Ann Surg. 2019;270:309–316. doi: 10.1097/SLA.0000000000002803. [DOI] [PubMed] [Google Scholar]
- 23.Smyth EC, Wotherspoon A, Peckitt C, Gonzalez D, Hulkki-Wilson S, Eltahir Z, et al. Mismatch repair deficiency, microsatellite instability, and survival: an exploratory analysis of the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) trial. JAMA Oncol. 2017;3:1197–1203. doi: 10.1001/jamaoncol.2016.6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guideline Committee of the Korean Gastric Cancer Association (KGCA), Development Working Group & Review Panel. Korean Practice Guideline for Gastric Cancer 2018: an evidence-based, multi-disciplinary approach. J Gastric Cancer. 2019;19:1–48. doi: 10.5230/jgc.2019.19.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Rüschoff J, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96:261–268. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sobin LH, Compton CC. TNM seventh edition: what's new, what's changed: communication from the International Union Against Cancer and the American Joint Committee on Cancer. Cancer. 2010;116:5336–5339. doi: 10.1002/cncr.25537. [DOI] [PubMed] [Google Scholar]
- 27.Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a Histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 28.Gotoda T, Iwasaki M, Kusano C, Seewald S, Oda I. Endoscopic resection of early gastric cancer treated by guideline and expanded National Cancer Centre criteria. Br J Surg. 2010;97:868–871. doi: 10.1002/bjs.7033. [DOI] [PubMed] [Google Scholar]
- 29.Gambardella V, Cervantes A. Precision medicine in the adjuvant treatment of gastric cancer. Lancet Oncol. 2018;19:583–584. doi: 10.1016/S1470-2045(18)30131-1. [DOI] [PubMed] [Google Scholar]
- 30.Choi YY, Cheong JH. Comment on “To Treat, or Not to Treat, That is the Question Biomarker-guided Adjuvant Chemotherapy for Stage II and III Gastric Cancer”. Ann Surg. 2019;270:e40–e41. doi: 10.1097/SLA.0000000000003102. [DOI] [PubMed] [Google Scholar]
- 31.Kim ST, Cristescu R, Bass AJ, Kim KM, Odegaard JI, Kim K, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018;24:1449–1458. doi: 10.1038/s41591-018-0101-z. [DOI] [PubMed] [Google Scholar]
- 32.Kim HH, Han SU, Kim MC, Kim W, Lee HJ, Ryu SW, et al. Effect of laparoscopic distal gastrectomy vs open distal gastrectomy on long-term survival among patients with stage I gastric cancer: the KLASS-01 randomized clinical trial. JAMA Oncol. 2019;5:506–513. doi: 10.1001/jamaoncol.2018.6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.An JY, Min JS, Lee YJ, Jeong SH, Hur H, Han SU, et al. Safety of laparoscopic sentinel basin dissection in patients with gastric cancer: an analysis from the SENORITA Prospective Multicenter Quality Control Trial. J Gastric Cancer. 2018;18:30–36. doi: 10.5230/jgc.2018.18.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ryu KW, Kim YW, Min JS, Yoon HM, An JY, Eom BW, et al. Results of interim analysis of the multicenter randomized phase III SENORITA trial of laparoscopic sentinel node oriented, stomach-preserving surgery versus laparoscopic standard gastrectomy with lymph node dissection in early gastric cancer. J Clin Oncol. 2017;35:4028. [Google Scholar]
- 35.Kim TM, Laird PW, Park PJ. The landscape of microsatellite instability in colorectal and endometrial cancer genomes. Cell. 2013;155:858–868. doi: 10.1016/j.cell.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park JH, Kim EK, Kim YH, Kim JH, Bae YS, Lee YC, et al. Epstein-Barr virus positivity, not mismatch repair-deficiency, is a favorable risk factor for lymph node metastasis in submucosa-invasive early gastric cancer. Gastric Cancer. 2016;19:1041–1051. doi: 10.1007/s10120-015-0565-1. [DOI] [PubMed] [Google Scholar]
- 37.Sugimoto R, Sugai T, Habano W, Endoh M, Eizuka M, Yamamoto E, et al. Clinicopathological and molecular alterations in early gastric cancers with the microsatellite instability-high phenotype. Int J Cancer. 2016;138:1689–1697. doi: 10.1002/ijc.29916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jahng J, Youn YH, Kim KH, Yu J, Lee YC, Hyung WJ, et al. Endoscopic and clinicopathologic characteristics of early gastric cancer with high microsatellite instability. World J Gastroenterol. 2012;18:3571–3577. doi: 10.3748/wjg.v18.i27.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mathiak M, Warneke VS, Behrens HM, Haag J, Böger C, Krüger S, et al. Clinicopathologic characteristics of microsatellite instable gastric carcinomas revisited: urgent need for standardization. Appl Immunohistochem Mol Morphol. 2017;25:12–24. doi: 10.1097/PAI.0000000000000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cho J, Lee J, Bang H, Kim ST, Park SH, An JY, et al. Programmed cell death-ligand 1 expression predicts survival in patients with gastric carcinoma with microsatellite instability. Oncotarget. 2017;8:13320–13328. doi: 10.18632/oncotarget.14519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Böger C, Behrens HM, Mathiak M, Krüger S, Kalthoff H, Röcken C. PD-L1 is an independent prognostic predictor in gastric cancer of Western patients. Oncotarget. 2016;7:24269–24283. doi: 10.18632/oncotarget.8169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim KJ, Yang HK, Kim WH, Kang GH. Combined prognostic effect of PD-L1 expression and immunoscore in microsatellite-unstable advanced gastric cancers. Oncotarget. 2017;8:58887–58902. doi: 10.18632/oncotarget.19439. [DOI] [PMC free article] [PubMed] [Google Scholar]