Abstract
Purpose
To identify the potential therapeutic role of postoperative radiotherapy (RT) in patients with locally advanced (stage II and stage III) gastric signet ring cell carcinoma (SRC).
Materials and methods
Patients with locally advanced gastric SRC from the Surveillance, Epidemiology, and End Results program database between 2004 and 2012 were included in our study. Univariate and multivariate Cox proportional models were performed, and survival curves were generated to evaluate the prognostic effect of postoperative RT and surgery alone on SRC patients. Propensity score matching (PSM) was used to avoid selection bias among the study cohorts.
Results
We found that patients with postoperative RT had better probability of survival compared with those who did not receive RT (overall survival [OS], P<0.001; cancer-specific survival [CSS], P<0.001). After PSM, analysis of both overall and CSS showed that patients who underwent postoperative RT had better prognosis than those receiving surgery alone in the matched cohort (OS, P=0.00079; CSS, P=0.0036). Multivariate Cox proportional model indicated that postoperative RT had better effect on prognosis compared with surgery alone with respect to both overall (hazard ratio [HR], 0.716; 95% confidence interval [95% CI], 0.590–0.87; P=0.001) and CSS (HR, 0.713; 95% CI, 0.570–0.890; P=0.003).
Conclusions
Postoperative RT had better prognosis compared with surgery alone for both overall and CSS for patients with locally advanced gastric SRC.
Keywords: Signet ring cell carcinoma, Radiotherapy, Gastric cancer, Nomogram, Propensity score
INTRODUCTION
Gastric cancer (GC) is one of the most common malignancies worldwide, and a variety of histological subtypes have been reported recently [1]. Signet ring cell carcinoma (SRC) is a rare adenocarcinoma subtype, which is found commonly in the stomach and occasionally in the breast, ovary, colon, rectum, and gallbladder [2]. SRC exhibits abundant intracellular mucin in more than 50% of tumor cells, presenting a seal ring. The incidence of GC has declined since the advent of Helicobacter pylori eradication treatments. However, the incidence of gastric SRC has been rising in recent years. In fact, SRC currently accounts for approximately 15%–28% of GC, and this percentage is still increasing [3]. On the other hand, prognosis of gastric SRC in early stage has been reported in all studies as equivalent to or better than that of other gastric adenocarcinomas (non-SRC) [4]. However, in advanced GC, prognosis of gastric SRC is more controversial and is commonly considered to be poorer than that of non-SRC. Furthermore, available evidence suggests that gastric SRC is considered to be less chemosensitive than non-SRC [5,6]. In addition, gastric SRC tends to present with advanced tumor stage and lymph node involvement, hence it is characterized by a lower resection rate and a higher recurrence rate [7]. Due to poor patient responses in cytotoxic therapies and a low rate of curative resection, a unique multimodal treatment plan for gastric SRC in advanced stages is urgently required. However, international clinical practice guidelines do not recommend any special treatment for SRC. Postoperative radiotherapy (RT) is the standard treatment for locally advanced GC (stage II/III) [8,9]. In 2 retrospective studies [10,11] and one case report [12], preoperative RT improved survival outcomes of rectal SRC, especially in stage III SRC. To understand whether RT has potential clinical value for SRC, we examined data from a population-based cancer database (Surveillance, Epidemiology, and End Results [SEER]) with the aim to assess the impact of postoperative RT on patients with stomach SRC by using propensity score matching (PSM).
MATERIALS AND METHODS
Ethics statement
We retrieved patients with locally advanced gastric SRC (stage II/III according to the American Joint Committee on Cancer 7th edition) diagnosed between 2004 and 2012 from SEER, which contains data from 18 registries that cover approximately 30% of the United States population. Radiation and chemotherapy information were retrieved individually after acquiring approval from SEER officials. Only patients with stage II or III SRC were included in the study. In addition, we considered age at diagnosis, sex, marital status, race, registry, tumor size, number of lymph nodes examined (NLNE), chemotherapy and radiation for each patient. Patients were grouped into 3 age groups: less than 45 years old, 45 to 74 years and over 75 years old [13]. Race-based classification included White, Black, and Other (American Indian/AK Native, Asian/Pacific Islander). The 18 registries were grouped into 3 classes: East (New Jersey, Metropolitan Atlanta, Connecticut, Rural Georgia, and Greater Georgia), West (Alaska, Hawaii, Los Angeles, New Mexico, Greater California, San Francisco-Oakland SMSA, Seattle, and San Jose-Monterey), and Central (Iowa, Metropolitan Detroit, Utah, Kentucky, and Louisiana), according to geographical location [14]. Tumors were classified in 2 categories according to their size (above or below 5 cm) [13]. Patients were divided in 2 groups, according to the NLNE (less than 15 and over 15 lymph nodes examined). [15]. The selection of cutoffs for continuous variables was based on previous literature on gastric SRC [13]. Patients with no available information on the considered clinical characteristics or survival information were excluded from analysis, which resulted in a final dataset of 1,303 locally advanced (stage II/III) postoperative patients.
PSM
Due to the heterogeneity of the demographic and clinical features of the treatment and control groups, selection bias is prevalent in retrospective studies. PSM was applied to reduce the effect of selection bias in our study. A multivariate logistic model was constructed with all clinical factors including age, race, sex, marital status, registry, tumor stage, tumor size, NLNE, tumor location and chemotherapy to calculate the probability that a patient will undergo RT. All factors included in the aforementioned model were considered for PSM. Propensity score values are between 0 and 1, and patients with similar propensity scores from the treatment group and control group were matched until all patients in the smaller group were matched [16]. These clinicopathological characteristics, including sex, age, NLNE, pathological tumor-node-metastasis (TNM) stage [15], tumor location [17], marital status [18], race, and tumor size [13] were identified as independent prognostic factors for gastric SRC. Therefore, we selected variables based on their clinical significance in the univariate and multivariate analysis. The multivariate logistic model of the matched population was then used to develop a nomogram to predict the survival probability of patients with gastric SRC at 3, 5, and 10 years. The “MatchIt” package in R software was applied for this analysis, and the algorithm of 1:1 nearest neighbor matching was used in the model.
Statistical analysis
All data were analyzed using the R version 3.5.2. The χ2 test was used to analyze the clinicopathological characteristics of patients undergoing RT before and after PSM. Survival curves were plotted with the Kaplan-Meier method and compared with the log rank test. Univariate and multivariate Cox regression model analyses were performed with the R package “survminer” and “survival”. The 3-, 5-, and 10-year overall survival (OS) rates served as endpoints in the nomogram for multivariate Cox proportional hazards models. All statistical tests were 2-sided and a value of P<0.05 was considered as statistically significant.
RESULTS
Clinical characteristics of the study cohort
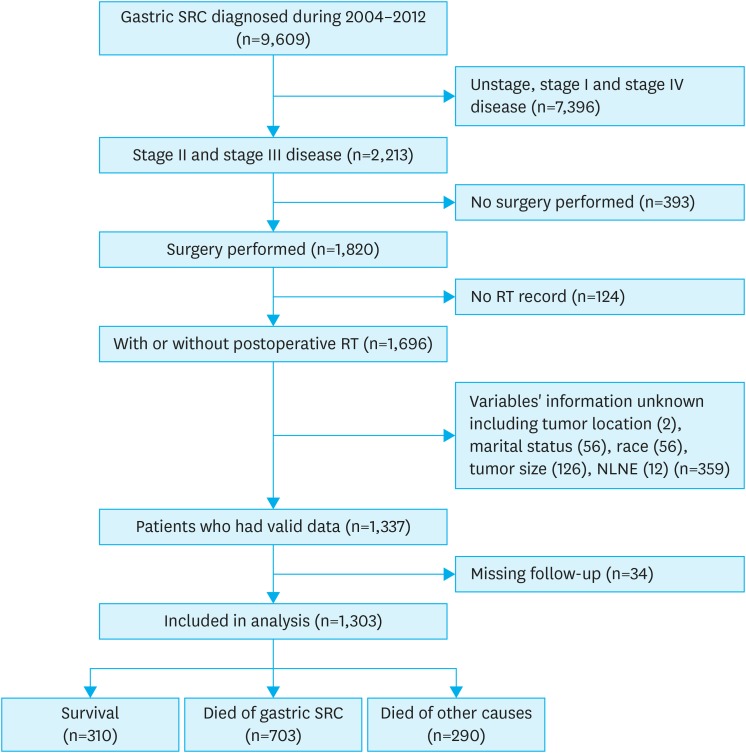
Our patient selection strategy is shown in Fig. 1. A total of 34 patients (2.6%) were excluded based on missing follow-up data. Our final analysis included 1,303 patients who met the inclusion criteria. The median follow-up time was 85 months (range, 1–143 months). The annual distribution of patients and the annual rate of RT use in this study are presented in Supplementary Fig. 1. Of all patients, 64.9% were between 45 and 74 years old when diagnosed and the number of male patients was slightly more than that of females (53.1% vs. 46.9%). Of all patients, 63.6% were married. White patients accounted for 66.3% of the cohort, while the remaining were Black and Other (American Indian/AK Native, Asian/Pacific Islander). A larger proportion of patients came from Western registries compared to Central and East. Tumors were located in the distal third of the stomach in 38% of patients. Tumor diameter was less than 5 cm in 51.2% of patients, while tumors in the remaining patients were greater than or equal to 5 cm. Information on tumor grade was available for 38% of patients, and most of the tumors were well or moderately differentiated (18.8% and 16.8%, respectively). The cohort consisted solely of stage II (43.2%) and stage III (56.8%) patients. Furthermore, more than 15 lymph nodes were removed in 52.6% of the cohort. Finally, 65.9% of the patients had undergone chemotherapy during the treatment process (Table 1).
Fig. 1. Flowchart of study design.
SRC = signet ring cell carcinoma; RT = radiotherapy; NLNE = number of lymph nodes examined.
Table 1. Demographic and clinical characteristics of patients with locally advanced (stage II/III) gastric cancer.
| Characteristics | No. of cases | |
|---|---|---|
| Total | 1,303 (100.0) | |
| Age (yr) | ||
| <45 | 152 (11.7) | |
| 45–74 | 845 (64.9) | |
| >75 | 306 (23.5) | |
| Sex | ||
| Female | 611 (46.9) | |
| Male | 692 (53.1) | |
| Marital status | ||
| Married | 829 (63.6) | |
| Single | 474 (36.4) | |
| Race | ||
| White | 864 (66.3) | |
| Black | 167 (12.8) | |
| Other* | 272 (20.9) | |
| Registry | ||
| Central | 215 (16.5) | |
| East | 327 (25.1) | |
| West | 761 (58.4) | |
| Location | ||
| Proximal third | 237 (18.2) | |
| Mid third | 142 (10.9) | |
| Distal third | 495 (38.0) | |
| Lesser curvature | 196 (15.0) | |
| Greater curvature | 83 (6.4) | |
| Overlapping lesion | 150 (11.5) | |
| Size | ||
| ≤5 cm | 667 (51.2) | |
| >5 cm | 636 (48.8) | |
| NLNE | ||
| <15 | 617 (47.4) | |
| ≥15 | 686 (52.6) | |
| Stage | ||
| II | 563 (43.2) | |
| III | 740 (56.8) | |
| Chemotherapy | ||
| Chemotherapy− | 444 (34.1) | |
| Chemotherapy+ | 859 (65.9) | |
| Radiation | ||
| Radiation− | 700 (53.7) | |
| Radiation+ | 603 (46.3) | |
Values are presented as number (%).
NLNE = number of lymph nodes examined.
*American Indian/AK Native, Asian/Pacific Islander.
Comparison of covariates with PSM
We found that patients with postoperative RT had significantly better survival probability compared with those who did not receive RT (OS, P<0.001; cancer-specific survival [CSS], P<0.001; Supplementary Fig. 2). However, we observed significant structural differences between the 2 groups. Younger patients were more likely to receive RT after surgery (<45 years old: 15.3% vs. 8.6%; 45–74 years old: 72.5% vs. 58.3%; P<0.001). More patients that underwent postoperative RT were married compared to those that underwent surgery alone (66.8% vs. 60.9%, P=0.029), and they tended to have more lymph nodes removed (56.4% vs. 49.4% had more than 15 lymph nodes removed, P=0.014) and to undergo chemotherapy (95.2% vs. 40.7%, P<0.001). These results indicate that patients undergoing postoperative RT were in better condition than those who did not receive the same treatment in this cohort (Table 2). Subsequently, PSM was used to avoid selection bias and to ensure the reliability of our findings. The distribution of propensity scores is shown in Supplementary Fig. 3. Several clinical factors including age, marital status, race, sex, registry, tumor stage, tumor size, NLNE, tumor location, and chemotherapy were included in the multivariate regression model to match the population with 2 different postoperative procedures. As expected, the 2 groups showed similar structures for all factors considered, suggesting that our method balanced any confounding variates well.
Table 2. Clinical characteristics of the study cohort before and after PSM.
| Characteristics | Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|---|
| Radiation− (n=700) | Radiation+ (n=603) | P-value | Radiation− (n=313) | Radiation+ (n=313) | P-value | ||
| Age (yr) | <0.001 | 0.177 | |||||
| <45 | 60 (8.6) | 92 (15.3) | 45 (14.4) | 44 (14.1) | |||
| 45–74 | 408 (58.3) | 437 (72.5) | 206 (65.8) | 224 (71.6) | |||
| >75 | 232 (33.1) | 74 (12.3) | 62 (19.8) | 45 (14.4) | |||
| Sex | 0.760 | 0.873 | |||||
| Female | 325 (46.4) | 286 (47.4) | 145 (46.3) | 148 (43.3) | |||
| Male | 375 (53.6) | 317 (52.6) | 168 (53.7) | 165 (52.7) | |||
| Marital status | 0.029 | 0.930 | |||||
| Married | 426 (60.9) | 403 (66.8) | 224 (71.6) | 222 (70.9) | |||
| Single | 274 (39.1) | 200 (33.2) | 89 (28.4) | 91 (29.1) | |||
| Race | 0.443 | 0.429 | |||||
| White | 475 (67.9) | 389 (64.5) | 27 (8.6) | 33 (10.5) | |||
| Black | 86 (12.3) | 81 (13.4) | 94 (30.0) | 81 (25.9) | |||
| Other* | 139 (19.9) | 133 (22.1) | 192 (61.3) | 199 (63.6) | |||
| Registry | 0.157 | 0.827 | |||||
| Central | 103 (14.7) | 112 (18.6) | 27 (8.6) | 30 (9.6) | |||
| East | 176 (25.1) | 151 (25.0) | 94 (30.0) | 88 (28.1) | |||
| West | 421 (60.1) | 340 (56.4) | 192 (61.3) | 195 (62.3) | |||
| Location | 0.167 | 0.732 | |||||
| Proximal third | 137 (19.6) | 100 (16.6) | 74 (23.6) | 60 (19.2) | |||
| Mid third | 73 (10.4) | 69 (11.4) | 34 (10.9) | 33 (10.5) | |||
| Distal third | 263 (37.6) | 232 (38.5) | 103 (32.9) | 111 (35.5) | |||
| Lesser curvature | 94 (13.4) | 102 (16.9) | 39 (12.5) | 48 (15.3) | |||
| Greater curvature | 42 (6.0) | 41 (6.8) | 25 (8.0) | 23 (7.3) | |||
| Overlapping lesion | 91 (13.0) | 59 (9.8) | 38 (12.1) | 38 (12.1) | |||
| Size | 0.275 | 0.936 | |||||
| ≤5 cm | 348 (49.7) | 319 (52.9) | 152 (48.6) | 150 (47.9) | |||
| >5 cm | 352 (50.3) | 284 (47.1) | 161 (51.4) | 163 (52.1) | |||
| NLNE | 0.014 | 0.872 | |||||
| <15 | 354 (50.6) | 263 (43.6) | 132 (42.2) | 135 (43.1) | |||
| ≥15 | 346 (49.4) | 340 (56.4) | 181 (57.8) | 178 (56.9) | |||
| Stage | 0.143 | 0.936 | |||||
| II | 316 (45.1) | 263 (41.0) | 143 (45.7) | 141 (45) | |||
| III | 384 (54.9) | 356 (59.0) | 170 (54.3) | 172 (55.0) | |||
| Chemotherapy | <0.001 | 1.000 | |||||
| Chemotherapy− | 415 (59.3) | 29 (4.8) | 29 (8.3) | 29 (8.3) | |||
| Chemotherapy+ | 285 (40.7) | 574 (95.2) | 284 (90.7) | 284 (90.7) | |||
Values are presented as number (%).
PSM = propensity score matching; NLNE = number of lymph nodes examined.
*American Indian/AK Native, Asian/Pacific Islander.
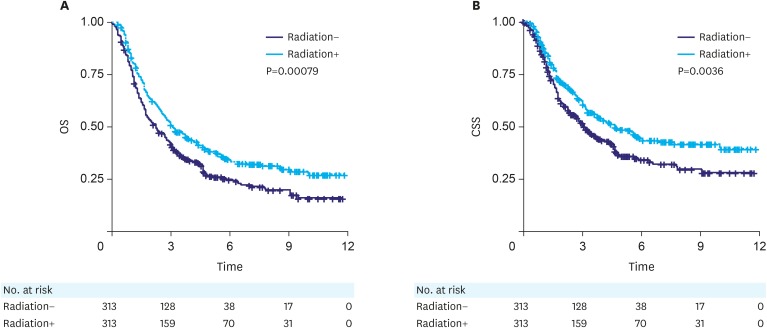
Locally advanced (stage II/III) patients who underwent postoperative RT showed better prognosis than those who received surgery alone
The analysis of both overall and CSS demonstrated that patients who underwent postoperative RT showed better prognosis than those receiving surgery alone in the matched cohort with locally advanced (stage II/III) SRC in the stomach (OS, P=0.00079; CSS, P=0.0036, Fig. 2). The results were similar, even after subgroup analysis of staging (Fig. 3). Subsequently, univariate Cox proportional hazards regression analyses were conducted in the matched cohort for all clinical factors to explore their prognostic effect (Table 3). Both the overall and CSS showed a statistically significant difference between the 2 radiation groups (OS, P=0.001; CSS, P=0.004). Older ages (>75 years old) showed poorer OS probability (P=0.001), and patients who were American Indian/AK Native or Asian/Pacific Islander had better prognosis (OS, P=0.012; CSS, P=0.027). Patients with stage II SRC had significantly better prognosis than those with stage III SRC (OS, P<0.001; CSS, P<0.001). Furthermore, patients with larger tumor size tended to have a lower survival rate (OS, P=0.007; CSS, P<0.001) and patients with >15 lymph nodes examined had significantly poorer prognosis for both overall (P<0.001) and CSS probability (P=0.022). Remarkably, prognosis was similar independent of chemotherapy (OS, P=0.17; CSS, P=0.982). After including these risk factors in the multivariate Cox proportional hazards model (Table 4 and Fig. 4), patients who underwent postoperative RT still had better prognosis compared with those receiving surgery alone, as shown by both overall (HR, 0.778; CI, 0.644–0.94, P=0.009) and CSS (HR, 0.757; CI, 0.607–0.944; P=0.014).
Fig. 2. (A, B) Both overall and CSS analysis showed that patients with locally advanced (stage II/III) cancer that underwent postoperative radiotherapy had better prognosis than those receiving surgery alone after propensity score matching (OS, P=0.00079; CSS, P=0.0036).
OS = overall survival; CSS = cancer-specific survival.
Fig. 3. (A, B) Both overall and CSS analysis showed that stage II patients that underwent postoperative RT had better prognosis than those receiving surgery alone after PSM (OS, P=0.037; CSS, P=0.036); (C, D) Both overall and CSS analysis showed that stage III patients that underwent postoperative RT had better prognosis than those receiving surgery alone after PSM (OS, P=0.0039; CSS, P=0.022).
OS = overall survival; CSS = cancer-specific survival; RT = radiotherapy; PSM = propensity score matching.
Table 3. Univariate analysis of the matched population for OS and CSS.
| Characteristics | OS | CSS | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | ||
| Age (yr) | |||||||
| <45 | Ref. | Ref. | |||||
| 45–74 | 1.012 | 0.76–1.34 | 0.932 | 0.884 | 0.65–1.20 | 0.428 | |
| >75 | 1.803 | 1.29–2.52 | 0.001 | 1.193 | 0.81–1.76 | 0.374 | |
| Sex | |||||||
| Female | Ref. | Ref. | |||||
| Male | 1.081 | 0.89–1.31 | 0.419 | 1.013 | 0.81–1.26 | 0.905 | |
| Marital status | |||||||
| Married | Ref. | Ref. | |||||
| Single | 1.134 | 0.92–1.39 | 0.232 | 1.044 | 0.82–1.33 | 0.728 | |
| Race | |||||||
| White | Ref. | Ref. | |||||
| Black | 1.255 | 0.96–1.64 | 0.097 | 1.239 | 0.91–1.69 | 0.179 | |
| Other* | 0.720 | 0.56–0.93 | 0.012 | 0.717 | 0.53–0.96 | 0.027 | |
| Registry | |||||||
| Central | Ref. | Ref. | |||||
| East | 0.694 | 0.50–0.97 | 0.033 | 1.239 | 0.91–1.69 | 0.179 | |
| West | 0.769 | 0.57–1.05 | 0.093 | 0.717 | 0.53–0.96 | 0.027 | |
| Location | |||||||
| Proximal third | Ref. | Ref. | |||||
| Mid third | 0.973 | 0.68–1.39 | 0.881 | 0.985 | 0.67–1.45 | 0.938 | |
| Distal third | 0.958 | 0.74–1.24 | 0.740 | 0.786 | 0.59–1.05 | 0.108 | |
| Lesser curvature | 0.794 | 0.57–1.11 | 0.173 | 0.622 | 0.42–0.92 | 0.019 | |
| Greater curvature | 0.963 | 0.65–1.42 | 0.848 | 0.725 | 0.45–1.17 | 0.189 | |
| Overlapping lesion | 1.131 | 0.82–1.57 | 0.458 | 1.204 | 0.85–1.71 | 0.302 | |
| Size | |||||||
| ≤5 cm | Ref. | Ref. | |||||
| >5 cm | 1.298 | 1.07–1.57 | 0.007 | 1.497 | 1.20–1.87 | 0 | |
| NLNE | |||||||
| <15 | Ref. | Ref. | |||||
| ≥15 | 0.704 | 0.58–0.85 | 0 | 0.774 | 0.62–0.96 | 0.022 | |
| Stage | |||||||
| II | Ref. | Ref. | |||||
| III | 1.721 | 1.42–2.09 | 0 | 1.839 | 1.47–2.30 | 0 | |
| Chemotherapy | |||||||
| Chemotherapy− | Ref. | Ref. | |||||
| Chemotherapy+ | 0.802 | 0.59–1.10 | 0.170 | 1.005 | 0.67–1.50 | 0.982 | |
| Radiation | |||||||
| Radiation− | Ref. | Ref. | |||||
| Radiation+ | 0.725 | 0.60–0.88 | 0.001 | 0.724 | 0.58–0.90 | 0.004 | |
Values are presented as number (%).
OS = overall survival; CSS = cancer-specific survival; HR = hazard ratio; CI = confidence interval; NLNE = number of lymph nodes examined.
*American Indian/AK Native, Asian/Pacific Islander.
Table 4. Multivariate analysis of the matched population for OS and CSS.
| Characteristics | OS | CSS | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | ||
| Age (yr) | |||||||
| <45 | Ref. | Ref. | |||||
| 45–74 | 1.141 | 0.851–1.530 | 0.378 | 0.976 | 0.710–1.342 | 0.882 | |
| >75 | 1.869 | 1.321–2.645 | 0 | 1.344 | 0.898–2.012 | 0.150 | |
| Sex | |||||||
| Female | Ref. | Ref. | |||||
| Male | 1.128 | 0.919–1.386 | 0.250 | 0.974 | 0.765–1.239 | 0.829 | |
| Marital status | |||||||
| Married | Ref. | Ref. | |||||
| Single | 1.042 | 0.834–1.302 | 0.717 | 0.989 | 0.760–1.286 | 0.933 | |
| Race | |||||||
| White | Ref. | Ref. | |||||
| Black | 1.266 | 0.925–1.732 | 0.141 | 1.262 | 0.871–1.830 | 0.218 | |
| Other* | 0.725 | 0.557–0.944 | 0.017 | 0.699 | 0.512–0.955 | 0.025 | |
| Registry | |||||||
| Central | Ref. | Ref. | |||||
| East | 0.776 | 0.551–1.094 | 0.148 | 0.699 | 0.471–1.037 | 0.075 | |
| West | 0.974 | 0.702–1.352 | 0.877 | 0.916 | 0.630–1.333 | 0.646 | |
| Location | |||||||
| Proximal third | Ref. | Ref. | |||||
| Mid third | 0.728 | 0.493–1.075 | 0.111 | 0.695 | 0.447–1.081 | 0.106 | |
| Distal third | 0.801 | 0.613–1.046 | 0.103 | 0.640 | 0.468–0.876 | 0.005 | |
| Lesser curvature | 0.807 | 0.577–1.128 | 0.209 | 0.672 | 0.452–1.001 | 0.050 | |
| Greater curvature | 1.065 | 0.725–1.565 | 0.748 | 0.825 | 0.519–1.311 | 0.415 | |
| Overlapping lesion | 0.896 | 0.645–1.247 | 0.516 | 0.920 | 0.639–1.325 | 0.654 | |
| Size | |||||||
| ≤5 cm | Ref. | Ref. | |||||
| >5 cm | 1.202 | 0.990–1.460 | 0.063 | 1.372 | 1.092–1.724 | 0.007 | |
| NLNE | |||||||
| <15 | Ref. | Ref. | |||||
| ≥15 | 0.711 | 0.587–0.861 | 0 | 0.778 | 0.621–0.975 | 0.029 | |
| Stage | |||||||
| II | Ref. | Ref. | |||||
| III | 1.866 | 1.522–2.287 | 0 | 1.889 | 1.484–2.405 | 0 | |
| Chemotherapy | |||||||
| Chemotherapy− | Ref. | Ref. | |||||
| Chemotherapy+ | 0.935 | 0.674–1.296 | 0.686 | 1.127 | 0.742–1.711 | 0.576 | |
| Radiation | |||||||
| Radiation− | Ref. | Ref. | |||||
| Radiation+ | 0.778 | 0.644–0.940 | 0.009 | 0.757 | 0.607–0.944 | 0.014 | |
OS = overall survival; CSS = cancer-specific survival; HR = hazard ratio; CI = confidence interval; NLNE = number of lymph nodes examined.
*American Indian/AK Native, Asian/Pacific Islander.
Fig. 4. Prognostic nomogram for CSS in patients with locally advanced signet ring cell carcinoma in the stomach. To use this nomogram, first locate the patient's age, then draw a line straight up to the points axis on the top to get the score associated with age. Repeat the process for the other covariates (from Race to Radiation). Total points were calculated by summing the projection points on the scale of each variable to determine the CSS probability of 3-, 5-, and 10- years.
CSS = cancer-specific survival; NLNE = number of lymph nodes examined.
For other observed covariates, patients over 75 years old showed poorer OS than those below 45 years old (HR, 1.869; CI, 1.321–2.645; P<0.001). Stage III patients had poorer prognosis than stage II patients (OS: HR, 1.866; CI, 1.522–2.287; P<0.001; CSS: HR, 1.889; CI, 1.484–2.405; P<0.001). Furthermore, compared with White, Other (American Indian/AK Native or Asian/Pacific Islander) tended to have a better survival (CSS: HR, 0.699; CI, 0.512–0.955; P=0.025). Patients with tumors in the distal third and lesser curvature had better CSS compared with patients with tumors in the proximal third (HR, 0.64; CI, 0.468–0.876; P=0.005 and HR, 0.672; CI, 0.452–1.001; P=0.05). Patients with tumors larger than 5 cm tended to have poor CSS (HR, 1.372; CI, 1.092–1.724; P=0.007). Patients with >15 lymph nodes examined had significantly poorer prognosis for both overall (HR, 0.711; CI, 0.587–0.861; P<0.001) and CSS probability (HR, 0.778; CI, 0.621–0.975; P=0.029).
DISCUSSION
In recent years, although the incidence of GC has decreased, the incidence of gastric SRC is still increasing [19,20]. The incidence of GC is closely related to H. pylori infection; therefore, the decline in the incidence of GC is attributed to H. pylori eradication. However, the link between gastric SRC and H. pylori remains unclear. The World Health Organization classifies gastric SRC as diffuse GC characterized by rapid progression towards gastric adenocarcinoma [21,22,23]. At the molecular level, the expression of E-cadherin encoded by the CDH1 gene is decreased, resulting in lack of adhesion between cells, potentially leading to increased rate of metastasis [23,24]. For a long time, the histological type of SRC has been considered as an independent predictor of poor prognosis [25,26,27], but this view has been questioned in recent years. In fact, progression of SRC is related to the advanced stage at the onset of the disease, and SRC does not show worse prognosis after correction for stage [6]. Several studies have reported that the survival rate of patients with early gastric SRC is better than that of patients with non-SRC. However, a meta-analysis of 35,947 cases of GC (including SRC) in 19 centers showed that the prognosis of advanced SRC was worse than that of non-SRC [24]. In most studies, SRC was found to be more advanced, which may explain poor prognosis [28]. Our results showed a higher proportion (56.8%) of stage III patients with poorer prognosis than stage II patients, which is consistent with previous studies.
There are still controversies regarding resection of lymph nodes. The European Society for Medical Oncology recommends that more than 16 lymph nodes should be assessed to avoid misinterpretation of TNM staging [29]. Smith et al. [30] explored the effect of the total number of surgically removed lymph nodes on stage-specific survival rate in 3,814 patients with GC. This study showed that the survival rate increased significantly as the NLNE increased. However, excessive removal of lymph nodes can damage the immune system, leading to a range of other clinical problems, such as increased susceptibility to infection. Our study showed that OS and tumor-specific survival improved when the NLNE in patients with stage II and III gastric SRC was greater than 15
In addition, there are different perspectives regarding other prognostic factors. Lu et al. [31] found that older age and advanced tumor stage were independent predictors of poor OS, while tumor location and chemotherapy regimen were not associated with prognosis. Analysis of SRC subgroups showed that prognosis was not related to sex, stage, and chemotherapy. In contrast, other studies revealed that sex, age, tumor size, location, and TMN staging are important factors that influence prognosis of SRC [28,32]. This inconsistency may be due to staging methods, geographic and medical differences. Our Cox regression analysis and nomogram showed that age, ethnicity, number of lymph node dissection, tumor location and size, and TNM staging were independent prognostic factors for gastric SRC, and were closely related with the 3-, 5-, and 10-year survival rates.
GC usually manifests as late stage disease, and thus adjuvant treatment is regularly recommended before or after surgery. A few landmark trials have shown that over time, the positive effects of adjuvant treatments are becoming more apparent. The Intergroup 0116 (INT 0116) trial was the first major randomized trial to demonstrate a survival benefit for fully resected GC patients with adjuvant chemoradiotherapy (CRT) [33]. The Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) trial showed a positive prognostic effect in patients treated with surgery followed by chemotherapy (epirubicin, cisplatin, and fluorouracil) compared with surgery alone in a Western cohort [34]. INT-0116 and MAGIC were severely criticized for inadequate lymph node dissection in a significant number of eligible patients. Therefore, in these trials adjuvant treatment is considered merely as replacement for inadequate surgery in Western GC patients. However, the results of INT-0116 were confirmed in a non-randomized trial in a cohort of D2-resected Eastern patients [35]. A recent meta-analysis determined that adjuvant RT in resectable GC increased OS and CSS by 20% [36]. The Adjuvant Chemoradiation Therapy in Stomach Cancer (ARTIST) [37] study investigated whether the addition of RT to capecitabine/cisplatin- adjuvant CT after D2 radical surgery is beneficial for the survival of patients with GC. After using RT, the 5-year OS of GC patients was not improved (CT vs. CRT, 73% vs. 75%), but subgroup analysis showed that lymph node-positive and intestinal GC patients may benefit from radiation therapy [38]. Subsequent ARTIST2 [39] mainly aimed to determine whether addition of RT to adjuvant CT improves survival in GC patients with lymph node-positive D2 radical surgery. The authors found that adjuvant CT or CRT improved effectively disease-free survival (DFS) in patients with pathologically-staged II or III, node-positive, D2-resected GC. However, no difference in DFS between CT and CRT (HR, 0.910; P=0.667) was observed. Regarding gastric SRC, ARTIST and INT-0116 showed a low chemo-radiation effect in the diffuse type that included SRC. However, Heger et al. [7] found that although patients with gastric SRC responded poorly to neoadjuvant chemotherapy, they showed better prognosis. Their study showed that radiation therapy can improve prognosis of SRC in locally advanced esophageal cancer. Other studies [10,11] have shown that preoperative RT improved survival of patients with colorectal signet ring cell cancer (especially stage III). However, Asano et al. [40] reported that a 58-year-old woman with mucosa-associated lymphoid tissue (MALT) lymphoma developed gastric SRC after radiation therapy. MALT was related to the occurrence of GC, suggesting that careful consideration should be given to treatment of MALT lymphoma. Similarly, RT has been reported very rarely in gastric SRC. A study of 194 cases of gastric SRC (45%), 124 of which (64%) were operated after RT and 70 (36%) were operated without RT, showed no significant difference in early (pT1) survival rates between the 2 groups [41]. In our study, after PSM calibration, postoperative chemotherapy did not improve patient survival, but postoperative RT significantly improved OS (P=0.001) and tumor-specific survival (P=0.003) in advanced-staged SRC. In most adenocarcinomas, radiosensitivity is related to the degree of cell differentiation; the higher the degree of differentiation, the lower the radiosensitivity. In adenocarcinoma, SRC is usually poorly differentiated. Therefore, RT should be, in theory, more effective for SRC than for non-SRC patients. Moreover, anti-epidermal growth factor receptor (EGFR) drugs improved sensitivity of RT [42] and expression of EGFR in colorectal SRC was negative/low [43]. We speculate that RT should be more effective for SRC than for non-SRC patients. However, only a few studies examining the mechanisms of RT in SRC have been performed. In future studies, we should focus on the mechanisms of RT in SRC, both in vitro and in vivo.
Although the design and analysis of our study is rigorous, it still has several limitations. First, the SEER database lacks important information, such as centralized pathological reviews, quality of surgical operation, details of chemotherapy and RT, pathological response to RT, uniformity in treatment, conditions of local and distant recurrence, and further treatment of complications. Second, it is only a database-based case-control study with inevitably missing data, thus deliberate practice is required. Therefore, multi-center RCT studies with long-term follow-up are required to verify our conclusions.
Footnotes
Funding: This work was supported by the Shanghai Committee of Science and Technology, grant No. 16ZR1427000.
- Conceptualization: C.F.
- Data curation: W.S., W.F.
- Formal analysis: L.H., W.F.
- Funding acquisition: C.F.
- Methodology: W.S., W.F.
- Project administration: W.F., C.Y.
- Supervision: C.Y.
- Validation: C.F.
- Writing - original draft: W.F., L.H.
- Writing - review & editing: C.F.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
SUPPLEMENTARY MATERIALS
(A) Annual distribution of patients undergoing surgery and of patients with postoperative RT. (B) Annual rate of postoperative RT patients relative to all patients.
(A, B) Patients with postoperative RT had significantly better survival probability compared with those who did not receive RT (OS, P<0.001; CSS, P<0.001).
Jittered plot showing matched and unmatched observations and their distribution on the propensity score.
References
- 1.Fléjou JF. WHO classification of digestive tumors: the fourth edition. Ann Pathol. 2011;31:S27–S31. doi: 10.1016/j.annpat.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Hass HG, Smith U, Jäger C, Schäffer M, Wellhäuber U, Hehr T, et al. Signet ring cell carcinoma of the stomach is significantly associated with poor prognosis and diffuse gastric cancer (Lauren's): single-center experience of 160 cases. Onkologie. 2011;34:682–686. doi: 10.1159/000334545. [DOI] [PubMed] [Google Scholar]
- 3.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 4.Ha TK, An JY, Youn HK, Noh JH, Sohn TS, Kim S. Indication for endoscopic mucosal resection in early signet ring cell gastric cancer. Ann Surg Oncol. 2008;15:508–513. doi: 10.1245/s10434-007-9660-9. [DOI] [PubMed] [Google Scholar]
- 5.Kim JP, Kim SC, Yang HK. Prognostic significance of signet ring cell carcinoma of the stomach. Surg Oncol. 1994;3:221–227. doi: 10.1016/0960-7404(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 6.Li C, Kim S, Lai JF, Hyung WJ, Choi WH, Choi SH, et al. Advanced gastric carcinoma with signet ring cell histology. Oncology. 2007;72:64–68. doi: 10.1159/000111096. [DOI] [PubMed] [Google Scholar]
- 7.Heger U, Blank S, Wiecha C, Langer R, Weichert W, Lordick F, et al. Is preoperative chemotherapy followed by surgery the appropriate treatment for signet ring cell containing adenocarcinomas of the esophagogastric junction and stomach? Ann Surg Oncol. 2014;21:1739–1748. doi: 10.1245/s10434-013-3462-z. [DOI] [PubMed] [Google Scholar]
- 8.Ajani JA, D'Amico TA, Almhanna K, Bentrem DJ, Chao J, Das P, et al. Gastric cancer, version 3.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016;14:1286–1312. doi: 10.6004/jnccn.2016.0137. [DOI] [PubMed] [Google Scholar]
- 9.Smyth EC, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D. Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v38–v49. doi: 10.1093/annonc/mdw350. [DOI] [PubMed] [Google Scholar]
- 10.Ling CR, Wang R, Wang MJ, Ping J, Zhuang W. Prognosis and value of preoperative radiotherapy in locally advanced rectal signet-ring cell carcinoma. Sci Rep. 2017;7:45334. doi: 10.1038/srep45334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu SG, Zhang WW, Sun JY, He ZY, Su GQ, Li FY. Preoperative radiotherapy improves survival in rectal signet-ring cell carcinoma-a population-based study. Radiat Oncol. 2017;12:141. doi: 10.1186/s13014-017-0874-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bratland A, Vetrhus T, Grøholt KK, Ree AH. Preoperative radiotherapy in rectal signet-ring cell carcinoma - magnetic resonance imaging and treatment outcome: report of six cases. Acta Oncol. 2010;49:42–49. doi: 10.3109/02841860903081897. [DOI] [PubMed] [Google Scholar]
- 13.Ren J, Niu G, Wang X, Song T, Hu Z, Ke C. Effect of age on prognosis of gastric signet-ring cell carcinoma: a SEER database analysis. Med Sci Monit. 2018;24:8524–8532. doi: 10.12659/MSM.911766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X, Wang S, Zhao S, Sun Y, Yang G. Postoperative chemotherapy had no prognostic effect on early-staged young ovarian cancer with unilateral resection. Cancer Med. 2018;7:5488–5496. doi: 10.1002/cam4.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang H, Zhang H, Tian L, Zhang X, Xue Y. The difference in clinic-pathological features between signet ring cell carcinoma and gastric mucinous adenocarcinoma. Tumour Biol. 2013;34:2625–2631. doi: 10.1007/s13277-013-0812-1. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Z. Propensity score method: a non-parametric technique to reduce model dependence. Ann Transl Med. 2017;5:7. doi: 10.21037/atm.2016.08.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yokota T, Kunii Y, Teshima S, Yamada Y, Saito T, Kikuchi S, et al. Signet ring cell carcinoma of the stomach: a clinicopathological comparison with the other histological types. Tohoku J Exp Med. 1998;186:121–130. doi: 10.1620/tjem.186.121. [DOI] [PubMed] [Google Scholar]
- 18.Zhou R, Yan S, Li J. Influence of marital status on the survival of patients with gastric cancer. J Gastroenterol Hepatol. 2016;31:768–775. doi: 10.1111/jgh.13217. [DOI] [PubMed] [Google Scholar]
- 19.Henson DE, Dittus C, Younes M, Nguyen H, Albores-Saavedra J. Differential trends in the intestinal and diffuse types of gastric carcinoma in the United States, 1973–2000: increase in the signet ring cell type. Arch Pathol Lab Med. 2004;128:765–770. doi: 10.5858/2004-128-765-DTITIA. [DOI] [PubMed] [Google Scholar]
- 20.Bamboat ZM, Tang LH, Vinuela E, Kuk D, Gonen M, Shah MA, et al. Stage-stratified prognosis of signet ring cell histology in patients undergoing curative resection for gastric adenocarcinoma. Ann Surg Oncol. 2014;21:1678–1685. doi: 10.1245/s10434-013-3466-8. [DOI] [PubMed] [Google Scholar]
- 21.Yashiro M, Chung YS, Nishimura S, Inoue T, Sowa M. Establishment of two new scirrhous gastric cancer cell lines: analysis of factors associated with disseminated metastasis. Br J Cancer. 1995;72:1200–1210. doi: 10.1038/bjc.1995.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kunz PL, Gubens M, Fisher GA, Ford JM, Lichtensztajn DY, Clarke CA. Long-term survivors of gastric cancer: a California population-based study. J Clin Oncol. 2012;30:3507–3515. doi: 10.1200/JCO.2011.35.8028. [DOI] [PubMed] [Google Scholar]
- 23.Chiurillo MA. Role of the Wnt/β-catenin pathway in gastric cancer: an in-depth literature review. World J Exp Med. 2015;5:84–102. doi: 10.5493/wjem.v5.i2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piessen G, Messager M, Leteurtre E, Jean-Pierre T, Mariette C. Signet ring cell histology is an independent predictor of poor prognosis in gastric adenocarcinoma regardless of tumoral clinical presentation. Ann Surg. 2009;250:878–887. doi: 10.1097/SLA.0b013e3181b21c7b. [DOI] [PubMed] [Google Scholar]
- 25.Liu X, Cai H, Sheng W, Yu L, Long Z, Shi Y, et al. Clinicopathological characteristics and survival outcomes of primary signet ring cell carcinoma in the stomach: retrospective analysis of single center database. PLoS One. 2015;10:e0144420. doi: 10.1371/journal.pone.0144420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwon KJ, Shim KN, Song EM, Choi JY, Kim SE, Jung HK, et al. Clinicopathological characteristics and prognosis of signet ring cell carcinoma of the stomach. Gastric Cancer. 2014;17:43–53. doi: 10.1007/s10120-013-0234-1. [DOI] [PubMed] [Google Scholar]
- 27.Kunisaki C, Shimada H, Nomura M, Matsuda G, Otsuka Y, Akiyama H. Therapeutic strategy for signet ring cell carcinoma of the stomach. Br J Surg. 2004;91:1319–1324. doi: 10.1002/bjs.4637. [DOI] [PubMed] [Google Scholar]
- 28.Kwon KJ, Shim KN, Song EM, Choi JY, Kim SE, Jung HK, et al. Clinicopathological characteristics and prognosis of signet ring cell carcinoma of the stomach. Gastric Cancer. 2014;17:43–53. doi: 10.1007/s10120-013-0234-1. [DOI] [PubMed] [Google Scholar]
- 29.Ajani JA, Winter K, Okawara GS, Donohue JH, Pisters PW, Crane CH, et al. Phase II trial of preoperative chemoradiation in patients with localized gastric adenocarcinoma (RTOG 9904): quality of combined modality therapy and pathologic response. J Clin Oncol. 2006;24:3953–3958. doi: 10.1200/JCO.2006.06.4840. [DOI] [PubMed] [Google Scholar]
- 30.Smith DD, Schwarz RR, Schwarz RE. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: data from a large US-population database. J Clin Oncol. 2005;23:7114–7124. doi: 10.1200/JCO.2005.14.621. [DOI] [PubMed] [Google Scholar]
- 31.Lu M, Yang Z, Feng Q, Yu M, Zhang Y, Mao C, et al. The characteristics and prognostic value of signet ring cell histology in gastric cancer: a retrospective cohort study of 2199 consecutive patients. Medicine (Baltimore) 2016;95:e4052. doi: 10.1097/MD.0000000000004052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu X, Cai H, Sheng W, Yu L, Long Z, Shi Y, et al. Clinicopathological characteristics and survival outcomes of primary signet ring cell carcinoma in the stomach: retrospective analysis of single center database. PLoS One. 2015;10:e0144420. doi: 10.1371/journal.pone.0144420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smalley SR, Benedetti JK, Haller DG, Hundahl SA, Estes NC, Ajani JA, et al. Updated analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol. 2012;30:2327–2333. doi: 10.1200/JCO.2011.36.7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 35.Kim S, Lim DH, Lee J, Kang WK, MacDonald JS, Park CH, et al. An observational study suggesting clinical benefit for adjuvant postoperative chemoradiation in a population of over 500 cases after gastric resection with D2 nodal dissection for adenocarcinoma of the stomach. Int J Radiat Oncol Biol Phys. 2005;63:1279–1285. doi: 10.1016/j.ijrobp.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 36.Ohri N, Garg MK, Aparo S, Kaubisch A, Tome W, Kennedy TJ, et al. Who benefits from adjuvant radiation therapy for gastric cancer? A meta-analysis. Int J Radiat Oncol Biol Phys. 2013;86:330–335. doi: 10.1016/j.ijrobp.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park SH, Sohn TS, Lee J, Lim DH, Hong ME, Kim KM, et al. Phase III trial to compare adjuvant chemotherapy with capecitabine and cisplatin versus concurrent chemoradiotherapy in gastric cancer: final report of the adjuvant chemoradiotherapy in stomach tumors trial, including survival and subset analyses. J Clin Oncol. 2015;33:3130–3136. doi: 10.1200/JCO.2014.58.3930. [DOI] [PubMed] [Google Scholar]
- 38.Kamat AM. Commentary on “Phase II trial of cetuximab with or without paclitaxel in patients with advanced urothelial tract carcinoma.” Wong YN, Litwin S, Vaughn D, Cohen S, Plimack ER, Lee J, Song W, Dabrow M, Brody M, Tuttle H, Hudes G, University of Pennsylvania, Philadelphia, PA: J Clin Oncol 2012;30(28):3545-51 [Epub 2012 Aug 27] Urol Oncol. 2013;31:719. doi: 10.1200/JCO.2012.41.9572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park SH, Zang DY, Han B, Ji JH, Kim TG, Oh SY, et al. ARTIST 2: interim results of a phase III trial involving adjuvant chemotherapy and/or chemoradiotherapy after D2-gastrectomy in stage II/III gastric cancer (GC) J Clin Oncol. 2019;37:4001. [Google Scholar]
- 40.Asano N, Iijima K, Terai S, Uno K, Endo H, Koike T, et al. Signet ring cell gastric cancer occurring after radiation therapy for Helicobacter pylori-uninfected mucosa-associated lymphoid tissue lymphoma. Case Rep Gastroenterol. 2011;5:325–329. doi: 10.1159/000329559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Skoropad VIu, Berdov BA, Loktionova OV, Mardynskiĭ IuS, Titova LN. Comparative analysis of long-term results of combined and surgical treatment at the patients with signet ring cell carcinoma of stomach. Khirurgiia (Mosk) 2008:13–17. [PubMed] [Google Scholar]
- 42.Gee JM, Nicholson RI. Expanding the therapeutic repertoire of epidermal growth factor receptor blockade: radiosensitization. Breast Cancer Res. 2003;5:126–129. doi: 10.1186/bcr584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Foda AA, Aziz AA, Mohamed MA. Colorectal signet ring cell carcinoma: influence of EGFR, E-cadherin and MMP-13 expression on clinicopathological features and prognosis. Ann Diagn Pathol. 2018;32:41–46. doi: 10.1016/j.anndiagpath.2017.10.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Annual distribution of patients undergoing surgery and of patients with postoperative RT. (B) Annual rate of postoperative RT patients relative to all patients.
(A, B) Patients with postoperative RT had significantly better survival probability compared with those who did not receive RT (OS, P<0.001; CSS, P<0.001).
Jittered plot showing matched and unmatched observations and their distribution on the propensity score.