Abstract
Straw is one of the most abundant stock of renewable biomass from crop production. However, its utilization efficiency is still very low. Although co-cultivation of fungi increases the degrading rate, the co-cultivation condition needs to be optimized. To optimize the co-culture condition of Phanerochaete chrysosporium and Trichoderma viride degrading rice straw, we first tested the antagonistic characteristic between the fungi. The results showed that the best co-culture pattern was to first inoculate P. chrysosporium and culture for 4 days, then inoculate T. viride, and co-culture the two fungi for 4 days. The optimum fermentation condition was 14% (w/v) of inoculum concentration, the equivalent inoculation of the fungi, culture temperature at 30 °C, and 1:1.4 for solid-liquid ratio. Under the optimum condition, the degradation ratios of lignin and cellulose were 26.38% and 33.29%, respectively; the soluble carbon content in the culture product was 23.07% (w/v). The results would provide important reference information for the efficient utilization of rice straw to produce more accessible energy resources, such as ethanol and glucose.
Subject terms: Environmental biotechnology, Applied microbiology, Fungi
Introduction
Straw is one of the most abundant stock of renewable biomass by-products in the crop production such as wheat, rice, and corn, and exhibits huge potentiality to be converted to “green energy”1,2. The main components of the straw of most crops are cellulose, hemicellulose, and lignin. Cellulose is the most abundant component in the straw and a renewable resource, as a raw material for fuels, chemicals, and food industries1. Therefore, most studies of biodegradation and reutilization of straws focus on cellulose degradation, and a lot of microbiomes have been identified as cellulose degrading agency, such as Neurospora crassa, Trichoderma viride, and brown-rot fungi3,4. However, the degradation ratio and efficiency of straw cellulose are still very low1.
Chemical constitution analysis shows that lignin forms a matrix surrounding the cellulose in woody cell walls, which protects the hemicellulose and cellulose from microbial depolymerization1,5. Decomposition of the lignin changes the complex structure of lignin and cellulose with hemicellulose, and exposes cellulose and hemicellulose to cellulase. Therefore, in order to efficiently utilize the energy of the straw, a process called delignification is required to break down the lignin structure and provide an access for enzymatic saccharification of the cellulosic component2.
There are various methods to break down the lignin structure in the straw, such as the thermal treatment1, chemical degradation6, and biological degradation7,8. Biological degradation has received extensive attention as it is easily integrated with subsequent processes of cellulose and hemicellulose degradation. Rees et al.9 reported that co-culture with the hydrogen-utilizing acetogenic bacterium Acetitomaculum ruminis increased acetate production of Neocallimastix patriciarum, Neocallimastix sp. L2, or Methanobrevibacter smithii. However, co-culture does not just mechanically integrate two metabolic reactions in the same reactor, but needs to regulate and advance to each other of the co-cultivated microorganisms. Their growth requirement also needs to balance.
White-rot fungi are among the best degrading agents for lignin5. White-rot fungi can produce lignin peroxidase, lemanganese peroxidase, and laccase at low-glucose medium, which are the major oxidoreductases of lignin10,11. In many studies, rice straws were degraded by white-rot fungi and T. viride. Currently, co-cultivation of white-rot fungi and T. viride to degrade rice straw has also been reported12,13. However, co-cultivation patterns and conditions still need to be optimized as the degradation ratios of lignin and cellulose are still very low.
In the present study, we utilized T. viride ACCC30169 and white-rot fungus Phanerochaete chrysosporium ACCC30942 to screen the optimum co-culture condition of rice straw through antagonistic experiment and co-culture experiments. The results would provide important reference information to utilize rice straw to efficiently produce available resources, such as easily available forage and energy.
Results and Discussion
T. viride overgrown than P. chrysosporium in plates
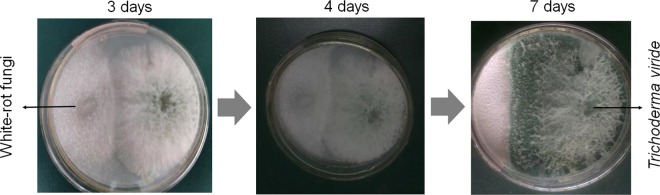
To analyze whether the fungi suit to simultaneous culture, we firstly tested the antagonistic characteristic between the fungi. T. viride overgrown than P. chrysosporium in plates on the fourth day. T. viride overwhelmingly overgrown than P. chrysosporium on the seventh day (Fig. 1). The results implied the two fungi did not suit to co-culture synchronously. As T. viride overgrown than P. chrysosporium, we predicted that successive inoculations of P. chrysosporium and T. viride probably raised the degradation efficiency to rice straw. In addition, considering white-rot fungi, such as P. chrysosporium, are among the best degrading agents for lignin5, we predicted that P. chrysosporium should be firstly inoculated to the culture medium.
Figure 1.
Antagonistic tests between P. chrysosporium and T. viride in PDA solid plate medium.
First inoculation of P. chrysosporium and culture for 4 days, followed by inoculation of T. viride and then co-culture (B4-L) was the optimum co-culture pattern
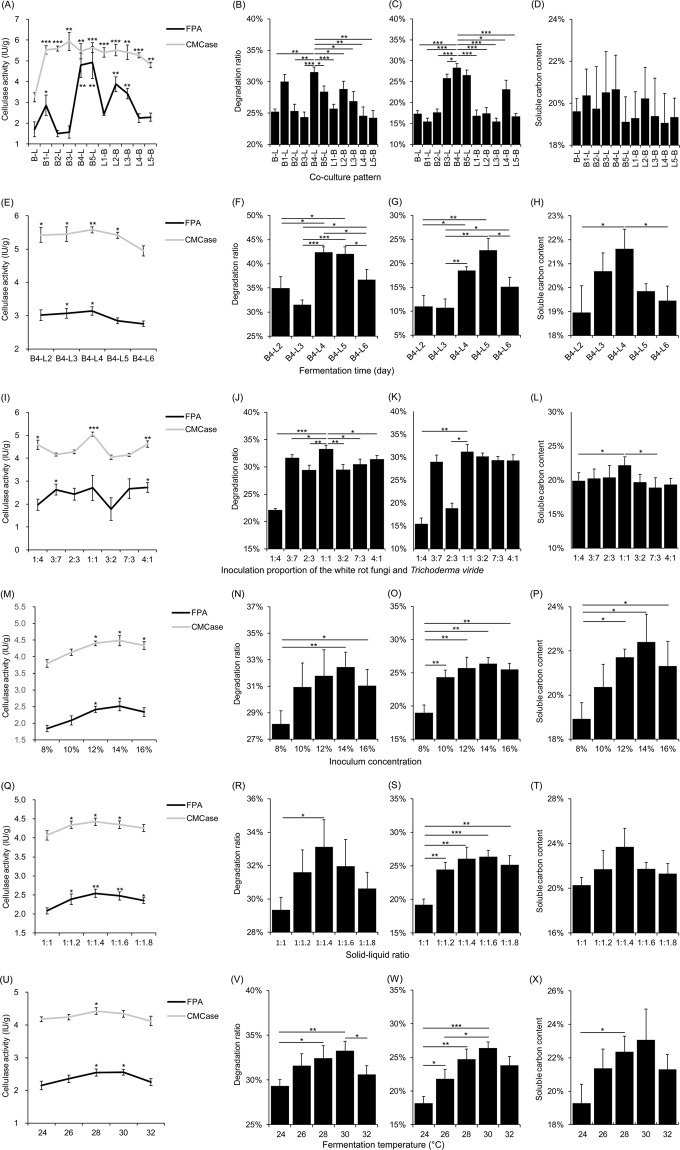
The cellulase activity in co-culture pattern B-L was significantly lower than other treatment groups (Fig. 2A). This result implied that growth of P. chrysosporium was probably competitively depressed by T. viride. However, the cellulase activity of the co-culture patterns inoculated T. virideand first and cultured for 1 to 5 days, and then inoculated P. chrysosporium were also higher than that of the simultaneously inoculated P. chrysosporium and T. viride (Fig. 2A). The cause of this phenomenon is unknown yet. Degradation ratios of lignin (DRL), degradation ratios of cellulose (DRC), and soluble carbon content (SCC) in the culture product of the co-culture pattern B4-L, i.e. first inoculation of P. chrysosporium and culture for 4 days, followed by inoculation of T. viride and then co-culture for 3 days, were the highest (Fig. 2B–D). Therefore, the co-culture pattern B4-L had the greatest ability to transform the rice straw to SCC.
Figure 2.
Fermentation effects of P. chrysosporium and T. viride co-culture under different conditions. (A,E,I,M,Q,U) showed the changes of cellulase activities of different treatment groups. (B,F,J,N,R,V) showed the degradation ratios of cellulose; (C,G,K,O,S,W) showed the degradation ratios of lignin; and (D,H,L,P,T,X) showed the soluble carbon contents in the culture product. For panels (A–D), the total culture time was 7 days. The different co-culture patterns were named by the firstly inoculated fungus (B represented P. chrysosporium and L represented T. viride) and cultured days (from 1 to 5), and the secondly inoculated fungus. For instance, B2-L represented that firstly inoculated P. chrysosporium and cultured 2 days, and then inoculated T. viride and co-cultured 5 days. B-L represented that inoculated T. viride and P. chrysosporium synchronously and co-cultured 7 days. For panels (E–H), the optimum co-culture time patterns were named by the firstly cultured 4 days after inoculating P. chrysosporium (B4), and then co-cultured days (from 2 to 6) after inoculating T. viride (L2 to L6). For instance, B4-L3 represented the firstly inoculated 4 days of P. chrysosporium, and then inoculated T. viride and co-cultured 3 days. (A), the statistically significant marker “*” shows the different significances between (B–L) and other co-culture patterns. (B,C), just show the significant differences between B4-L and other co-culture patterns. (E), the statistically significant marker “*” shows the different significances between B4-L6 and other co-culture time patterns. (I), the statistically significant marker “*” shows the different significances between group 3:2 and other groups. (M,Q,U), the statistically significant marker “*” shows the different significances between the first group and other groups. (J) just show the significant differences between group 1:1 and other co-culture groups. *p < 0.05; **P < 0.01; ***p < 0.001.
There are many studies have documented that the degraded ratio and rate of co-culture of fungi were significantly higher than the culture of single fungus9,13. Although we did not compare the digestibility of co-culture of P. chrysosporium and T. viride, and that of single P. chrysosporium or T. viride, the degradation ratio of B5-L significantly lower than that of B4-L (Fig. 2A–D) implied that the digestibility of single P. chrysosporium was probably lower than that of the B4-L.
Firstly inoculated P. chrysosporium and cultured for 4 days, and subsequently inoculated T. viride and then co-cultured for 4 days (B4-L4) was the optimum co-culture time pattern
Although our results showed the optimum co-culture pattern was first inoculation of P. chrysosporium and culture for 4 days, followed by inoculation of T. viride, and then co-culture for 3 days, the optimum co-culture time after the inoculation of T. viride was still unclear. Theoretically, longer co-culture time would increase DRL and DRC. However, increasing co-culture time would cause higher energy consumption, and probably reduced SCC in the culture product. Therefore, it is important to screen the optimum co-culture time according to DRL and DRC, and SCC in culture product. Five groups were established to analyze the optimum co-culture time, i.e. B4-L2, B4-L3, B4-L4, B4-L5, and B4-L6. Three repetitive samples were set in each group. There was no significant difference in cellulase activity among the groups with exception to B4-L6 (Fig. 2E). DRL and DRC of the groups B4-L4 and B4-L5 were higher than other groups (Fig. 2F,G), and SCC in the culture product of the group B4-L4 was higher than other groups (Fig. 2H). The results showed that the best co-culture time pattern was B4-L4, i.e. firstly inoculated P. chrysosporium and cultured for 4 days, and subsequently inoculated T. viride and then co-cultured for 4 days.
Equivalent inoculation of P. chrysosporium and T. viride was the optimum inoculation proportion
Inoculation proportion of co-cultured fungi influences their growth and metabolic activities. To analyze the optimum inoculation proportion of P. chrysosporium (B) and T. viride (L), the degradation efficiencies of seven inoculation proportions, i.e. 1:4, 3:7, 2:3, 1:1, 3:2, 7:3, and 4:1 (B/L), were compared. The cellulase activity was the highest when the inoculation proportion was 1:1. Simultaneously, DRL and DRC were significantly higher than those in other inoculation proportions, and SCC in the culture product was also the highest (Fig. 2I–L). Although P. chrysosporium was firstly inoculated and cultured 4 days, our results showed that because T. viride had an obviously competitive advantage, T. viride could quickly reproduce and participate in the degradation reaction of the rice straw. However, the ratio of P. chrysosporium and T. viride in the final culture product should be further measured in future study.
Fourteen percent fungal solution was the optimum inoculum concentration
Inoculum concentration influences the initial growth of fungi and the degradation efficiency. Our results showed that the optimum inoculum concentration was 14% (w/v), with 32.46% of DRC, 26.38% of DRL, and 22.40% of SSC in the culture product (Figs. 2M–P). In addition, although there was no significantly difference, the degradation efficiency of rice straw was slightly lower when the inoculum concentration was 12% or 16% (Figs. 2M–P). Theoretically, if the inoculated fungi directly degraded the rice straw, the higher inoculum concentration could cause the higher degradation efficiency. However, our results showed that when the inoculum concentration was 16%, the degradation efficiency of rice straw, and the SCC was slightly reduced. These results implied that the inoculated fungi did not degrade the rice straw directly, but experienced an unknown regulatory process.
The optimum solid-liquid ratio was 1:1.4
DRL and DRC increased with the decrease of solid-liquid ratio from 1:1 to 1:1.4, but the degradation ratio decreased when the solid-liquid ratio was less than 1:1.4. The degradation ratio was the highest when the solid-liquid ratio was 1:1.4. DRL and DRC were 26.07% and 33.11%, respectively. The SCC in the culture product was 23.70% (Fig. 2Q–T).
The optimum culture temperature was 30 °C
DRL and DRC increased with the increase of culture temperature when the temperature was lower than 30 °C, but they decreased when the temperature was higher than 30 °C (Fig. 2U–X). The optimum temperature was 30 °C. At the temperature, the DRL (26.38%) and DRC (33.29%) were the highest. And the SCC in the culture product was the highest (23.07% (w/v); Fig. 2U–X).
Although there were some reports about the stepwise co-culture of fungi to degrade rice straw14, the DRLs and DRCs were commonly less than 30%, and the culture cycle was commonly more than 10 days. In addition, although some anaerobic fungi from rumen can degrade the rice straw reaching to the digestibility of 50%15, the reaction time and the SCC in the culture product should be considered. Because we did not just degrade the rice straw, we actually using the fungi to transform the rice straw to more accessible energy resources, such as ethanol and glucose. In the present study, under the optimum condition, the DRL and DRC reached to 26.38% and 33.29% during 8 days, respectively.
Conclusions
The best co-culture pattern of the fungi was B4-L4, i.e. firstly inoculated P. chrysosporium and cultured for 4 days, and subsequently inoculated T. viride and co-cultured for 4 days. The optimum co-culture condition was 14% (w/v) of the inoculum concentration, the equivalent inoculation of the fungi, 30 °C of the culture temperature, and 1:1.4 of the solid-liquid ratio. Under the optimum co-culture condition, the DRL and DRC were 26.38% and 33.29%, respectively; and the SCC in the culture product was 23.07% (w/v).
Materials and Methods
Experimental design, microorganism strains, and culture conditions
T. viride ACCC30169 and P. chrysosporium ACCC30942 were provided by microbiology laboratory of College of Bioscience and Biotechnology, Hunan Agricultural University. T. viride exists high ability to degrade cellulose, and P. chrysosporium exists high ability to degrade lignin. The strains stored at 4 °C were inoculated to fresh PDA slant culture-medium (The 20% (w/v) of peeled potato blocks were boiled for 20 min and filtered. 2% (w/v) of glucose, and 2% (w/v) of agar was added to the filtrate and its pH was regulated to 7.2; then the culture medium was steam-sterilized at 121 °C to 25 min using a high-pressure steam sterilizer.), then the inoculations were cultured for 5 days at 28 °C. Subsequently, the spores of T. viride and P. chrysosporium were picked from the PDA slant culture-medium by oese and inoculated to 100 ml of seed liquid medium (The seed liquid medium contains 2% (w/v) of wheat bran, 2% (w/v) of glucose, 2% (w/v) of bean cake powder, 1% (w/v) of sucrose, 0.5% (w/v) of (NH4)2SO4, 0.2% (w/v) of KH2PO4, 0.1% (w/v) of MgSO4·7H2O, and 0.2% (w/v) of yeast powder. Its pH was regulated to 7.2; then the culture medium was steam-sterilized at 121 °C to 25 min using a high-pressure steam sterilizer.) in the 250 ml conical flasks, and cultured for 3 days at 28 °C with 150r/min of shake. Ten percent (V/W) of seed liquid mediums were inoculated to the solid fermentation medium, mixed and cultured for 7 days at 28 °C in a constant temperature incubator with mixed once per 24 hours. The solid fermentation medium contains 85% of straw comminution (The rice straw was provided by the Rice Experimental Field of Human Agricultural University. The rice straw was cut to 2–3 cm of fragments and dried to less than 5% of moisture under 80 °C. Then the dried straw fragments were shattered to less than 10 mesh.), 10% of wheat bran, 2.5% of glucose, 2% of bean cake powder, 0.4% of KH2PO4, and 0.1% of MgSO4·7H2O. Its pH was regulated to 7.2; then the culture medium was steam sterilized at 121 °C to 25 min using a high-pressure steam sterilizer.
To obtain the optimum co-culture condition of P. chrysosporium and T. viride co-culture to rice straw, we firstly tested the antagonistic characteristic between the fungi, and then optimized the co-culture conditions through optimizing the co-culture pattern, fermentation time, inoculation proportion of the fungi, inoculum concentration, solid-liquid ratio, and culture temperature.
Antagonistic experiment
The spores of the fungi were picked from the PDA slant culture-medium by oese and inoculated to two poles of PDA solid plate medium. The inverted plates were cultured at 28 °C in a constant temperature incubator and the growth of fungi was observed every day.
Co-culture pattern and time experiments
To screen the optimum co-culture pattern of the fungi to degrade the rice straw, we set eleven combination groups as shown in Table 1. Ten percent of seed culture medium (v/w) was inoculated to solid fermentation medium and mixed, then cultured at 28 °C in a constant temperature incubator. It was stirred once a day in all fermentation experiments.
Table 1.
Experimental co-culture patterns of T. viride and P. chrysosporium.
| Co-culture pattern | Firstly inoculated and cultured days | Secondly inoculated and co-cultured days |
|---|---|---|
| B-L | T. viride and P. chrysosporium synchronously | Co-cultured 7 days |
| B1-L | P. chrysosporium, 1 day | T. viride, co-cultured 6 days |
| B2-L | P. chrysosporium, 2 days | T. viride, co-cultured 5 days |
| B3-L | P. chrysosporium, 3 days | T. viride, co-cultured 4 days |
| B4-L | P. chrysosporium, 4 days | T. viride, co-cultured 3 days |
| B5-L | P. chrysosporium, 5 days | T. viride, co-cultured 2 days |
| L1-B | T. viride, 1 days | P. chrysosporium, co-cultured 6 days |
| L2-B | T. viride, 2 days | P. chrysosporium, co-cultured 5 days |
| L3-B | T. viride, 3 days | P. chrysosporium, co-cultured 4 days |
| L4-B | T. viride, 4 days | P. chrysosporium, co-cultured 3 days |
| L5-B | T. viride, 5 days | P. chrysosporium, co-cultured 2 days |
The total culture time was 7 days. The co-culture patterns were consisted of the firstly inoculated fungus (B represented P. chrysosporium and L represented T. viride) and cultured days (from 1 to 5), and the secondly inoculated fungus. For instance, B2-L represented that firstly inoculated P. chrysosporium and cultured 2 days, and then inoculated T. viride and co-cultured 5 days. B-L represented that inoculated T. viride and P. chrysosporium synchronously and co-cultured 7 days.
To screen the optimum co-culture time of the fungi to degrade the rice straw, the optimum co-culture pattern of the fungi was used, i.e., the culture time of firstly inoculated P. chrysosporium was selected according to the result of the co-culture pattern experiments, and the co-culture time after inoculated T. viride was changed from two to six days. The solid fermentation mediums were cultured at 28 °C in a constant temperature incubator.
Inoculation proportion and inoculum concentration experiments
To screen the optimum inoculation proportion of the fungi culture to degrade the rice straw, the optimum co-culture pattern of the fungi and the optimum co-culture time was used. A series of inoculation proportions of the fungi, i.e. 1:4, 3:7, 2:3, 1:1, 3:2, 7:3, and 4:1 were compared. The solid fermentation mediums were cultured at 28 °C in a constant temperature incubator.
To screen the optimum inoculum concentration of the co-culture to degrade the rice straw, the optimum co-culture pattern, the optimum co-culture time, and the optimum inoculation proportion of the fungi were used. A series of inoculum concentrations (w/v), i.e. 8%, 10%, 12%, 14%, and 16% were compared. The solid fermentation mediums were cultured at 28 °C in a constant temperature incubator.
Solid-liquid ratio and culture temperature experiments
To screen the optimum solid-liquid ratio of the co-culture to degrade the rice straw, the optimum co-culture pattern, co-culture time, inoculation proportion, and inoculum concentration of the fungi was used. A series of solid-liquid ratios, i.e. 1:1, 1:1.2, 1:1.4, 1:1.6, and 1:1.8 were compared. The solid fermentation mediums were cultured at 28 °C in a constant temperature incubator.
To screen the optimum co-culture temperature of the co-culture to degrade the rice straw, the optimum co-culture pattern, co-culture time, inoculation proportion, inoculum concentration, and the solid-liquid ratio of the fungi was used. A series of culture temperatures, i.e. 24, 26, 28, 30, and 32 °C were compared. The solid fermentation mediums were cultured in constant temperature incubators.
Determination of enzyme activities and polysaccharide contents
Two grams of culture products were accurately weighed and added to 50 ml of distilled water, and then were extracted to crude enzymes at 30 °C with 150 r/min of shake. The filter paper enzyme activity (FPA) and CMC enzyme activity (CMCase) were used to indicate the cellulase activity. Endoglucanase and filter paper enzyme activities in the crude enzyme extracts were determined using 3,5-dinitrosalicylic acid (DNS) method16. Cellulose and lignin contents were determined according to previous reports17,18. DRL and DRC were calculated according to previous reports19,20. SCC was determined referenced to a previous report21.
Data analysis
Results for each parameter are presented as the mean ± standard error (SE) for each group. One-way analysis of variance (ANOVA) was used for testing difference among different treatments, and Tukey’s honestly significant difference (Tukey’s HSD) method was used to the post-hoc test. All statistical analyses were conducted using R version 3.5.1. P-values < 0.05 were considered statistically significant.
Acknowledgements
This work was supported by the Key R & D projects in Hunan Province (No. 2016NK2013). The authors thank anonymous technicians at Guangdong Meilikang Bio-Science Ltd. (China) for assistance with data re-analysis and figure preparation.
Author contributions
K.C., B.X. and S.L. designed the study. K.C., J.T. and Y.C. conducted the experiments. K.C. and J.T. analyzed the data. B.X. and Y.C. wrote the manuscript. S.L. revised the manuscript. All authors approved the manuscript.
Competing interests
Yankun Cao is an employee of Guangdong Meilikang Bio-Science Ltd., China.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kai-Jian Chen, Email: kjchenhnau@163.com.
Bao-Hong Xu, Email: 195453778@qq.com.
Shi-Le Lan, Email: qianyuzhanglu@163.com.
References
- 1.Chen X, Yu J, Zhang Z, Lu C. Study on structure and thermal stability properties of cellulose fibers from rice straw. Carbohydr. Polym. 2011;85:245–250. doi: 10.1016/j.carbpol.2011.02.022. [DOI] [Google Scholar]
- 2.Chang AJ, Fan J, Wen X. Screening of fungi capable of highly selective degradation of lignin in rice straw. Int. Biodeterior. Biodegrad. 2012;72:26–30. doi: 10.1016/j.ibiod.2012.04.013. [DOI] [Google Scholar]
- 3.Phillips CM, Beeson WT, IV, Cate JH, Marletta MA. Cellobiose dehydrogenase and a copper-dependent polysaccharide monooxygenase potentiate cellulose degradation by Neurospora crassa. ACS Chem. Biol. 2011;6:1399–1406. doi: 10.1021/cb200351y. [DOI] [PubMed] [Google Scholar]
- 4.Hassan SHA, et al. Electricity generation from rice straw using a microbial fuel cell. Int. J. Hydrogen. Energy. 2014;39:9490–9496. doi: 10.1016/j.ijhydene.2014.03.259. [DOI] [Google Scholar]
- 5.Have RT, Teunissen PJG. Oxidative mechanisms involved in lignin degradation by white-rot fungi. Chem. Rev. 2001;101:3397–3413. doi: 10.1021/cr000115l. [DOI] [PubMed] [Google Scholar]
- 6.Chen X, et al. Enhancing methane production from rice straw by extrusion pretreatment. Appl. Energy. 2014;122:34–41. doi: 10.1016/j.apenergy.2014.01.076. [DOI] [Google Scholar]
- 7.Eggert C, Temp U, Eriksson KL. Laccase is essential for lignin degradation by the white-rot fungus Pycnoporus cinnabarinus. FEBS Lett. 1997;407:89–92. doi: 10.1016/S0014-5793(97)00301-3. [DOI] [PubMed] [Google Scholar]
- 8.Conrad R, Klose M, Lu Y, Chidthaisong A. Methanogenic pathway and archaeal communities in three different anoxic soils amended with rice straw and maize straw. Front. Microbiol. 2012;3:4. doi: 10.3389/fmicb.2012.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rees EMR, Lloyd D, Williams AG. The effects of co-cultivation with the acetogen Acetitomacuhm ruminis on the fermentative metabolism of the rumen fungi Neocallimastix patriciarum and Neocallimastix sp. strain L2. FEMS Microbiol. Lett. 1995;133:175–180. doi: 10.1111/j.1574-6968.1995.tb07880.x. [DOI] [PubMed] [Google Scholar]
- 10.Tekere M, Mswaka AY, Zvarya R, Read JS. Growth, dye degradation and ligninolytic activity studies on Zimbabwean white rot fungi. Enzyme Microb. Technol. 2001;28:420–426. doi: 10.1016/S0141-0229(00)00343-4. [DOI] [PubMed] [Google Scholar]
- 11.Matins LF, Kolling D, Camassola M, Dillon AJP, Ramos LP. Comparison of Penicillium echinulatum and Trichoderma reesei cellulases in relation to their activity against various cellulosic substrates. Bioresour Technol. 2008;99:1417–1424. doi: 10.1016/j.biortech.2007.01.060. [DOI] [PubMed] [Google Scholar]
- 12.Mewada M, Albert S, Pandya B. Enhancement of ligninolytic & xylanolytic enzyme activities in Trichoderma reesei co-cultured with two white rot fungi. Int. J. Biotechnol. Biochem. 2017;13:429–439. [Google Scholar]
- 13.Copete-Pertuz LS, Alandete-Novoa F, Plácido J, Correa-Londoño GA, Mora-Martínez AL. Enhancement of ligninolytic enzymes production and decolourising activity in Leptosphaerulina sp. by co-cultivation with Trichoderma viride and Aspergillus terreus. Sci. Total. Environ. 2019;646:1536–1545. doi: 10.1016/j.scitotenv.2018.07.387. [DOI] [PubMed] [Google Scholar]
- 14.Zhang H, Guo A, He J, Guo L, Wei J. A study on utilizing stage process and the multi-strains solid state fermentation for corn straw. J. Northwest. Univ. 2004;34:691–694. [Google Scholar]
- 15.Khejornsart P, Wanapat M. Diversity of rumen anaerobic fungi and methanogenic archaea in swamp buffalo influenced by various diets. J. Anim. Vet. Adv. 2010;9:3062–3069. doi: 10.3923/javaa.2010.3062.3069. [DOI] [Google Scholar]
- 16.Dashtban M, Maki M, Leung KT, Mao C, Qin W. Cellulase activities in biomass conversion: measurement methods and comparison. Crit. Rev. Biotechnol. 2010;30:302–309. doi: 10.3109/07388551.2010.490938. [DOI] [PubMed] [Google Scholar]
- 17.Klash A, Ncube E, du Toit B, Meincken M. Determination of the cellulose and lignin content on wood fibre surfaces of eucalypts as a function of genotype and site. Eur. J. Forest. Res. 2010;129:741–748. doi: 10.1007/s10342-010-0380-5. [DOI] [Google Scholar]
- 18.Jin X, et al. Determination of hemicellulose, cellulose and lignin content using visible and near infrared spectroscopy in Miscanthus sinensis. Bioresour. Technol. 2017;241:603–609. doi: 10.1016/j.biortech.2017.05.047. [DOI] [PubMed] [Google Scholar]
- 19.Desvaux M, Guedon E, Petitdemange H. Kinetics and metabolism of cellulose degradation at high substrate concentrations in steady-state continuous cultures of Clostridium cellulolyticum on a chemically defined medium. Appl. Environ. Microbiol. 2001;67:3837–3845. doi: 10.1128/AEM.67.9.3837-3845.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pamidipati S, Ahmed A. Degradation of lignin in agricultural residues by locally isolated fungus Neurospora discrete. Appl. Biochem. Biotechnol. 2017;181:1561–1572. doi: 10.1007/s12010-016-2302-6. [DOI] [PubMed] [Google Scholar]
- 21.Zhan X, Zhou L. Colorimetric determination of dissolved organic carbon in soil solution and water environment. China Environ. Sci. 2002;22:433–437. [Google Scholar]