Abstract
Background
In clinical routine, SUVmax and SUVpeak are most often used to determine the glucose metabolism in tumours by 18F-FDG PET/CT. Both metrics can be further normalised to SUVs in reference regions resulting in a SUV ratio (SUVratio). The aim of the study was to directly compare several widely used SUVs/SUVratios with regard to differentiation between common tumours in paediatric patients; a special focus was put on characteristics of reference region SUVs.
Methods
The final study population consisted of 61 children and adolescents with diagnoses of non-Hodgkin lymphoma (NHL, n = 25), Hodgkin lymphoma (HL, n = 14), and sarcoma (n = 22). SUV metrics included SUVmax and SUVpeak as well as both parameters normalised to liver and mediastinal blood pool, respectively, yielding the SUVratios SUVmax/liver, SUVmax/mediastinum, SUVpeak/liver, and SUVpeak/mediastinum.
Results
The metrics SUVmax, SUVpeak, SUVmax/liver, and SUVpeak/liver all proved to be sensitive for tumour differentiation (p ≤ 0.008); in contrast, SUVmax/mediastinum and SUVpeak/mediastinum revealed to be non-sensitive approaches. Correlation analyses showed inverse associations between reference region SUVs and SUVratios (p < 0.05). Multiple regression analyses demonstrated significant effects of factors as bodyweight and uptake time on reference region SUVs (p < 0.01), and thus indirectly on the corresponding SUVratios.
Conclusions
In the paediatric population, the ability to differentiate between common tumours remarkably varies between SUV metrics. When using SUVratios, the choice of reference region is crucial. Factors potentially influencing reference region SUVs (and thus SUVratios) should be taken into account in order to avoid erroneous conclusions. When not possible, SUVmax and SUVpeak represent less complex, more robust alternatives.
Keywords: FDG PET/CT, SUV, Paediatric oncology, Lymphoma, Sarcoma
Background
Children and adolescents with malignant solid tumours and lymphoma have in general a good prognosis with five-year survival rates exceeding 70% [1]. A fundamental requirement for the successful management of these tumours is the use of appropriate imaging methods for accurate disease detection and characterization. In this context, due to the increased glucose uptake and glycolysis of most cancer cells, metabolic imaging on a combined positron emission tomography/computed tomography (PET/CT) scanner with the radioactive glucose analogue 2-deoxy-2-(18F)fluoro-D-glucose (18F-FDG) is recognised as highly sensitive and specific. For instance, for detection of paediatric Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL), sensitivity and specificity has been reported to be as high as 95.9% and 99.7%, respectively, in a lesion-based regional analysis [2]; in case of paediatric sarcoma, sensitivities and specificities for staging of all lesions have been reported to be 81.8% and 97.5% for bone tumours [3], and 86% and 80% for soft tissue tumours [4].
Different quantitation methods have been proposed to determine the glucose metabolism within a tumour lesion by 18F-FDG PET/CT, each with its specific advantages and disadvantages [5]. For clinical routine and for scientific purposes, the concept of standardized uptake value (SUV) is generally accepted and most often used as SUV quantification is easy to perform and the correlation with more complex approaches is usually good [6–8]. For SUV determination, the tissue concentration of the tracer within a specific region-of-interest (ROI) as measured by the PET scanner is divided by the activity injected and normalised to body mass, lean body mass, or body surface area [5, 9]. Several approaches exist for ROI selection including fixed-dimension regions centered over the part of the tumour with the highest uptake (e.g., a sphere of 12 × 12 mm) resulting in a metric commonly called SUVpeak or simply determining the single hottest voxel within the tumour, the so-called SUVmax [5]. Moreover, the different semi-quantitative SUV measures can be further normalised to SUVs in unaffected regions as blood pool or normal liver in order to reduce variation and thus increase standardization resulting in a SUV ratio (SUVratio) [10].
The ability to reproducibly quantify tumour metabolism has made SUV measures a powerful tool in 18F-FDG PET/CT imaging in oncology. Beside their use in the diagnosis of malignancy, SUV measures may also provide information about different tumor profiles with different aggressiveness and consequently prognosis given that the degree of FDG uptake is usually associated with tumour histology [11]. For instance, in the special case of paediatric oncology, SUVmax has been demonstrated, amongst others, to be predictive of outcomes in Ewing sarcoma (ES) [12, 13], rhabdomyosarcoma (RMS) [14], and HL [15], and SUVpeak was reported to be a useful prognostic biomarker for osteosarcoma (OS) [16]. Furthermore, SUV measures may also be used for therapy monitoring of antineoplastic treatments. In this regard, the principle behind the SUVratio concept, which is used in several approaches, e.g., PERCIST [5], the visual Deauville criteria [17] or a quantitative extension of the Deauville scale referred to as qPET, has been proposed for the assessment of tumour response to therapy by 18F-FDG PET/CT in paediatric HL patients [18].
Despite the wide use of various SUV metrics there exist to our knowledge no studies directly comparing these measures in paediatric oncology. In order to contribute to this topic, we retrospectively analysed 18F-FDG PET/CT data of children and adolescents with the diagnosis of lymphoma and sarcoma, which together account for approximately 25% of all malignancies in the paediatric population [19–21]. In particular, we tested the ability of SUVmax, SUVpeak, as well as of both parameters normalised to the liver and mediastinal blood pool, respectively, to differentiate between these malignancies at initial staging and, in that context, also evaluated these measures’ characteristics in more detail. This involved taking a closer look at possible associations between reference regions SUVs and SUVratios as well as at the potential impact of factors such as bodyweight or uptake time (i.e., the time between PET tracer injection and beginning of imaging) on reference region SUVs and thus on SUVratios.
A broad understanding of SUV measures with their specific characteristics and potential pitfalls in paediatric patients, which according to common view are not little adults, is in general mandatory for the accurate interpretation of oncologic PET/CT studies in this population with regard to detection and characterization of the tumour in clinical routine as well as prognosis estimation in clinical routine and scientific investigations, respectively. This is also true for interpreting the response to treatment.
Methods
Subjects
All procedures performed in the study involving human participants were in accordance with the ethical standards of the Institutional Review Board and with the 1964 Helsinki Declaration and its later amendments. The study was approved by the local ethics committee.
The electronic database of all PET/CT scans performed between the years 2011 and 2016 at the authors’ University Hospital was searched to identify children and adolescents with the initial diagnosis of NHL, HL, and sarcoma who underwent imaging for initial staging purposes. The search yielded imaging data of 75 patients: NHL, n = 30; HL, n = 20; RMS, n = 8; OS, n = 8; and ES, n = 9. Data were excluded from the analysis in the following cases: multifocal liver involvement (2 NHL; no adequate SUV measurement in healthy hepatic reference tissue possible); lacking of sufficient histological data (2 HL); recurrent disease with primary diagnosis not done at our institution (3 NHL, 4 HL, 1 OS); imaging performed after beginning of chemotherapy (1 ES) or after tumor resection (1 ES). Thus, the final study population consisted of 61 children and adolescents (age range 1–17 years) with the diagnosis of NHL (n = 25), HL (n = 14), RMS (n = 8), OS (n = 7), and ES (n = 7).
18F-FDG PET/CT acquisition
All patients fasted for at least 6 hours and had serum glucose levels < 120 mg/dl. Furosemide and butylscopolamine were administered prior to 18F-FDG for diuresis and to decrease bowel activity. Dosage was 10 mg for each drug for children < 10 years and 20 mg for older children and adolescents. 18F-FDG was given as an intravenous injection adapted to the patient’s weight according to the EANM dosage card. Imaging covered the whole body with the patient in the supine position. The need for sedation and anaesthesia was assessed individually and the appropriate procedure was provided by a dedicated anaesthetics team.
All studies have been performed on one and the same GE Discovery 690 PET/CT system (General Electric, USA) combining a lutetium-yttrium-orthosilicate block detector designed PET and a 64-slice CT.
A diagnostic CT with intravenous contrast agent was performed with the following parameters adapted to patient´s age: < 5 years: 80 kV, 40 mA; 5–12 years: 100 kV, 40–60 mA; 17–18 years: 100 kV, 60 mA. For patients with recent MRI examinations, an unenhanced low dose CT protocol with 100 kV and 10 mA was applied.
PET scans were acquired in a 3D mode (144 × 144 matrix) in a caudocranial direction and corrected for decay and scatter. PET data were reconstructed iteratively by using the CT images for attenuation correction. Combined transaxial images of 18F-FDG PET and CT were reconstructed to a resolution of 128 × 128 and a thickness of 2.5 mm resulting in a voxel size of approx. 75 mm3.
SUV quantitation methods
Parameters for SUV calculation included the injected dose of 18F-FDG and the patient’s body surface area; the latter was calculated based on patient’s height and bodyweight. SUV metrics included SUVmax and SUVpeak as well as both parameters normalised to liver and mediastinal blood pool, respectively, yielding the SUVratios SUVmax/liver, SUVmax/mediastinum, SUVpeak/liver, and SUVpeak/mediastinum. For both, liver and mediastinal blood pool, a mean SUV of all voxels located in a representative target volume was calculated, resulting in the metrics SUVliver and SUVmediastinum. For the hepatic target volume, a spherical ROI with a diameter of 3 cm was placed to the right lobe showing homogeneous FDG uptake [5]; in case of mediastinal blood pool, a spherical ROI of 2 cm in diameter was placed to the right ventricle of the heart. SUV metrics were determined for the patient’s most active tumour lesion (which was chosen after measuring all lesions within the patient).
Analyses were performed on a workstation using the software HERMES Gold, version 4.16 (Hermes Medical Solutions AB, Sweden).
Statistical analyses
Preparatory analyses showed that for several SUV metrics the assumption of homogeneity of variance across tumour groups was violated. Therefore, the more robust Welch ANOVA was calculated separately for SUVmax, SUVpeak, SUVmax/liver, SUVmax/mediastinum , SUVpeak/liver, and SUVpeak/mediastinum. To protect from type 1 error, a Bonferroni correction was performed accepting Welch ANOVA results as statistically significant at p ≤ 0.008. Post hoc analyses were conducted using the Games-Howell test. As preparatory analyses did not show any significant differences within the sarcoma group (p > 0.05), patients with RMS, OS, and ES were combined resulting in total in three tumour entity groups (i.e., NHL, HL, and sarcoma).
For the same reason as mentioned above, Welch ANOVAs with Games-Howell tests were used to test for between-group differences in age, bodyweight, injected dose of 18F-FDG, and uptake time; in this case, Welch ANOVA results were considered statistically significant at Bonferroni-adjusted p ≤ 0.0125. Potential differences in gender distribution between groups were tested with the chi-square test.
A one-way MANOVA was conducted to test for differences in reference region SUVs among tumour entity groups; significant ANOVAs were followed up with Tukey’s HSD post hoc test.
Pearson correlation coefficients were calculated in order to study the associations between SUV metrics in general and the impact of reference region SUVs on tumour lesion SUVratios in particular.
To extract factors that may influence reference regions SUVs, multiple regressions were run including the factors gender, age, weight, dose of 18F-FDG, and uptake time.
Statistical data was computed with SPSS, version 23.0 (IBM, USA).
Results
Table 1 summarizes patients’ data regarding age, bodyweight, injected dose of 18F-FDG, and uptake time. No statistically significant differences (p > 0.125 each) between patient groups were present for age (F(2, 35.29) = 4.797), weight (F(2, 35.03) = 4.462), and dose of 18F-FDG (F(2, 34.61) = 1.990). Uptake time, defined as the time between PET tracer injection and beginning of imaging, differed significantly between groups (F(2, 34.84) = 8.381, p = 0.001); it was shorter in HL patients than in those with NHL (− 9.18 min, p = 0.021) and sarcoma (− 18.79 min, p = 0.004). No significant gender differences were present across groups (χ2 = 0.894, p > 0.05).
Table 1.
Subject’s age, bodyweight, injected dose of 18F-FDG, and uptake time: means ± standard deviation
| All patients | NHL | HL | Sarcoma | |
|---|---|---|---|---|
| Age, years |
10.33 ± 4.97 [1–17] |
9.8 ± 5.0 | 13.1 ± 3.7 | 8.7 ± 5.2 |
| Bodyweight, kg |
38.50 ± 20.69 [7.0–89.5] |
36.3 ± 20.3 | 49.9 ± 15.4 | 32.2 ± 21.9 |
| 18F-FDG dose, MBq |
134.97 ± 53.65 [41–246] |
129.8 ± 51.8 | 155.1 ± 40.9 | 124.3 ± 62.0 |
| Uptake time, min |
70.14 ± 16.94 [46–126] |
69.0 ± 12.7 | 59.9 ± 7.4 | 78.7 ± 21.7 |
NHL non-Hodgkin lymphoma, HL Hodgkin lymphoma. Uptake time: time between PET tracer injection and the beginning of imaging in minutes
Most active tumour lesions (i.e. those used for the analysis) included lymph nodes/lymphatic tissue in the neck (n = 2), mediastinum (n = 9), retroperitoneal (n = 6) and intraperitoneal (n = 5) space, and pelvis (n = 3) for NHL; lymph nodes in cervical/supraclavicular (n = 6), axillary (n = 2), and mediastinal (n = 6) regions in case of HL; head and neck region (n = 4), retroperitoneal space (n = 2), upper and lower extremities (1 each) for RMS; humerus (n = 1), femur (n = 3), tibia (n = 2), and fibula (n = 1) for OS; and humerus (n = 1), radius (n = 1), ribs (n = 1), pelvis (n = 2), femur (n = 1), and tibia (n = 1) in case of ES.
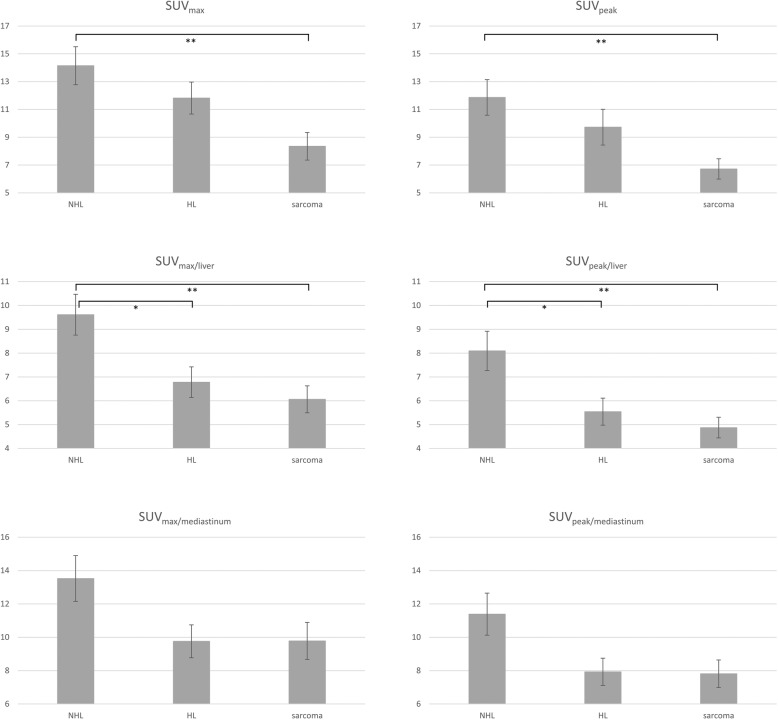
Mean tumour lesion SUV metrics for each tumour entity are summarized in Table 2; Fig. 1 illustrates the results graphically:
Table 2.
SUV metrics: means ± standard deviation
| NHL | HL | Sarcoma | |
|---|---|---|---|
| SUVmax | 14.15 ± 6.85 [11.32–16.98] | 11.82 ± 4.30 [9.33–14.30] | 8.36 ± 4.45 [6.27–10.44] |
| SUVpeak | 11.86 ± 6.42 [9.22–14.51] | 9.72 ± 3.84 [7.51–11.94] | 6.72 ± 3.27 [5.19–8.25] |
| SUVmax/liver | 9.61 ± 4.29 [7.84–11.38] | 6.78 ± 2.40 [5.39–8.17] | 6.06 ± 2.53 [4.88–7.24] |
| SUVmax/mediastinum | 13.52 ± 6.86 [10.69–16.36] | 9.76 ± 3.70 [7.62–11.90] | 9.78 ± 4.98 [7.45–12.11] |
| SUVpeak/liver | 8.09 ± 4.11 [6.40–9.79] | 5.54 ± 2.13 [4.32–6.77] | 4.87 ± 1.94 [3.96–5.78] |
| SUVpeak/mediastinum | 11.39 ± 6.31 [8.78–13.99] | 7.93 ± 3.06 [6.17–9.70] | 7.81 ± 3.69 [6.09–9.54] |
NHL non-Hodgkin lymphoma, HL Hodgkin lymphoma. In brackets: 95% confidence interval
Fig. 1.
Mean SUVs/SUVratios ± standard error across tumor entities. NHL, non-Hodgkin lymphoma. HL, Hodgkin lymphoma. *p < 0.05; **p < 0.01
Mean SUVs/SUVratios differed statistically significantly between tumour entities for SUVmax, SUVpeak, SUVmax/liver, and SUVpeak/liver, but not for SUVmax/mediastinum and SUVpeak/mediastinum. Table 3 shows details of the Welch ANOVA:
Table 3.
Results of the Welch ANOVA
| SUV metric | Welch‘s F | p value |
|---|---|---|
| SUVmax | F(2, 35.18) = 6.279 | 0.005 |
| SUVpeak | F(2, 33.64) = 6.975 | 0.003 |
| SUVmax/liver | F(2, 35.51) = 5.976 | 0.006 |
| SUVmax/mediastinum | F(2, 36.76) = 2.847 | 0.071 |
| SUVpeak/liver | F(2, 34.34) = 5.882 | 0.006 |
| SUVpeak/mediastinum | F(2, 36.55) = 3.144 | 0.055 |
Uptake was found to be significantly higher in NHL lesions than in sarcoma (p = 0.004 each) for SUVmax (+ 5.79), SUVpeak (+ 5.15), SUVmax/liver (+ 3.55), and SUVpeak/liver (+ 3.22). In addition, significantly higher values were present in NHL compared to HL for SUVmax/liver (+ 2.83, p = 0.031) and SUVpeak/liver (+ 2.55, p = 0.039).
After excluding subjects with multifocal liver involvement, no conditions were identifiable in the study population based on all available information including past medical history and imaging data that could have negatively affected SUV measurements within liver or mediastinal blood pool (e.g. excretory dysfunction due to infiltration/compression of the nephrourinary system with consecutively reduced tracer clearance from blood and body tissues). Table 4 shows the results for mean liver and mediastinal blood pool SUVs for each patient group.
Table 4.
Reference region SUVs: means ± standard deviation
| Reference region SUV | NHL | HL | Sarcoma |
|---|---|---|---|
| SUVliver | 1.58 ± 0.64 | 1.83 ± 0.49 | 1.41 ± 0.40 |
| SUVmediastinum | 1.13 ± 0.37 | 1.31 ± 0.42 | 0.92 ± 0.30 |
NHL non-Hodgkin lymphoma; HL Hodgkin lymphoma
The MANOVA revealed a significant multivariate main effect for tumour group (F(4,110) = 2.63, p = 0.038, Wilk’s Λ = 0.833, partial η2 = 0.09). Significant univariate main effects were obtained for SUVmediastinum (F(2,56) = 4.85, p = 0.011, partial η2 = 0.15) but not for SUVliver (F(2,56) = 2.52, p > 0.05, partial η2 = 0.08). Mean mediastinal blood pool SUV was significantly higher in patients with HL than in those with sarcoma (+ 0.39, p = 0.009). No differences were identifiable between patients with NHL and HL, and NHL and sarcoma (p > 0.05).
Pearson correlations indicating the associations between tumour SUVs/SUVratios and reference region SUVs are shown in Table 5. High correlations existed between SUVmax and SUVpeak as well as between SUVratios. Correlations were usually lower between tumour SUVs and SUVratios. SUVmax and SUVpeak were also associated with reference region SUVs. Accordingly, there was to some extent an inverse correlation between reference region SUVs and SUVratios.
Table 5.
Correlation matrix displaying Pearson correlations across tumour SUVs/SUVratios and reference region SUVs
| SUVmax | SUVpeak | SUVliver | SUVmediastinum | SUVmax/liver | SUVmax/mediastinum | SUVpeak/liver | |
|---|---|---|---|---|---|---|---|
| SUVpeak | 0.986*** | ||||||
| SUVliver | 0.414** | 0.399** | |||||
| SUVmediastinum | 0.300* | 0.299* | 0.864*** | ||||
| SUVmax/liver | 0.684*** | 0.689*** | (0.295)* | (0.310)* | |||
| SUVmax/mediastinum | 0.690*** | 0.672*** | (0.212) | (0.393)** | 0.930*** | ||
| SUVpeak/liver | 0.663*** | 0.696*** | (0.282)* | (0.281)* | 0.984*** | 0.890* | |
| SUVpeak/mediastinum | 0.692*** | 0.704*** | (0.208) | (0.363)** | 0.948*** | 0.981*** | 0.941*** |
*p < 0.05; **p < 0.01; ***p < 0.001. Numbers in parenthesis represent negative associations
Gender, age, weight, dose of 18F-FDG, and uptake time statistically significantly predicted SUVliver (F(5,52) = 25.022, p < 0.0001, R2 = 0.706) and SUVmediastinum (F(5,52) = 26.348, p < 0.0001, R2 = 0.717). In case of SUVliver, female gender (β = 0.218, p = 0.008) and weight (β = 0.687, p < 0.001) added significantly to the prediction; in case of SUVmediastinum, these were weight (β = 0.681, p < 0.001) and uptake time (β = − 0.281, p < 0.001).
Discussion
Our retrospective data suggests that the ability to differentiate between common tumours in paediatric patients remarkably varies between SUV metrics. It also shows that there is an inverse association between reference region SUVs and the corresponding SUVratios, especially when SUVmediastinum is used, indicating that uptake behaviour of reference regions may substantially impact SUVratios.
Liver and mediastinal blood pool SUVs were both predicted by the patient’s weight; in addition, SUVliver was also affected by gender, and SUVmediastinum by uptake time. To the best of our knowledge, the association between SUVs in liver/mediastinal blood pool and these factors has not yet been investigated in children and adolescents. However, prior research on adult patients have repeatedly shown that bodyweight affects SUVs in both reference regions, e.g., the work by Malladi et al. [22] or a study by Groheux et al., who concentrated on the effect of weight on liver only [23]. Malladi et al. also found effects of gender on liver and mediastinal blood pool SUV [22], a finding that in our work on paediatric patients could be replicated for liver only. Regarding the negative impact of uptake time on SUVmediastinum our finding is in line with data reported for adults [22, 24], presumably due to continuing renal excretion of the tracer over time.
Uptake time substantially varied across patient groups. The reason for this unintended result was a different need for sedation and anaesthesia between groups (data not shown here); the HL group involved more of the older children (13.1 ± 3.7 years), whereas the NHL (9.8 ± 5.0 years) and especially the sarcoma group (8.7 ± 5.2 years) included more of the very young patients where need for time-consuming sedation or anaesthesia—and thus probability of scan delays—was higher. Consecutively, uptake time was generally shorter in HL patients (59.9 ± 7.4 min) than in those with NHL (69.0 ± 12.7 min) and sarcoma (78.7 ± 21.7).
Given the considerably shorter uptake time in HL than in sarcoma patients combined with an inverse association between uptake time and mediastinal blood pool SUV, it is not surprising that SUVmediastinum was significantly higher in HL patients than in those with sarcoma. Considering also a generally more pronounced impact of SUVmediastinum on SUVratio, we reason that the between-group differences in uptake time substantially biased both SUVmax/mediastinum and SUVpeak/mediastinum and thus abolished these metrics’ ability to differentiate between tumour entities. But we acknowledge that measuring the SUVmean in a small volume as the right ventricle of a child may be difficult and thus prone to inaccurate measurements, which may have contributed to the low performance of these metrics.
Besides the obvious susceptibility of SUVmax/mediastinum and SUVpeak/mediastinum to variations in uptake time, use of liver as reference tissue may also be critical given the liver SUV’s association with bodyweight and gender; for this reason, the results found for SUVmax/liver and SUVpeak/liver should be interpreted cautiously as they may be biased by between-group differences in these factors, too (even though they were not found to be statistically significant in this study).
In general, our findings suggest that in children and adolescents validity of SUVratios is compromised in cases where the metric is based on reference regions highly sensitive to factors like gender, uptake time and/or bodyweight. Ignoring these factors may thus lead to erroneous study interpretations. This can be the case for SUVratio comparisons between different groups as well as within groups over time in scientific studies when substantial between-group or between-examination differences in these factors exist. In clinical routine, misinterpretations of longitudinal PET/CT studies may happen, which, in worst case, could have adverse effects on patients management, e.g. by negatively impacting the decision on follow-up examinations whether or not to continue a specific therapy regimen; but also at initial staging, the extent of the disease could be over- or underestimated in cases where SUVs in reference tissues are abnormally altered, thus potentially influencing treatment options or prognosis estimation. In the paediatric population, the role of bodyweight may be of particular importance given that relative differences in this factor can be substantial between groups (e.g. when comparing groups of different ages) but also longitudinally within a group or in an individual, as children grow and get heavier over time. It follows that choice of reference regions is crucial.
If it can be ensured that biasing factors are known and adequately controlled for, SUVratios may represent suitable SUV metrics. In all other cases, it seems reasonable that tumour lesion SUVs are preferred over SUVratios in paediatric oncology. Here, the eschewal of a reference tissue apparently makes the SUVmax or SUVpeak more robust as it decreases the degree of complexity. But it should be noted that both SUV metrics also have their disadvantages. Beside some technical issues [5], the concentration of 18F-FDG is known to gradually increase with time within glucose-avid tumour lesions [25], so the factor uptake time should also here be taken into account.
SUVmax and SUVpeak were highly correlated delivering similar results with regard to tumour differentiation, independent of their use solely or in combination with reference regions. This similarity is remarkable as the parameters rely on uptake information derived from substantially different volumes (75 mm3 vs. 1 cm3 in our study). In general, due to its small size, SUVmax is known to be adversely affected by noise and to be sensitive to respiratory motion artifacts that naturally occur during PET acquisition [26, 27]. SUVpeak on the other hand is less affected by these inherent disadvantages of SUVmax and thus preferred by some authors [5]; in contrast, however, SUVpeak is more prone to partial volume effects [5]. We think that the similarity of both values in this study is linked to the population examined. In comparison to adults, paediatric patients are smaller in total volume and so are the volumes of their malignancies (but also their motion range and thus potential motion artifacts). A SUVmax derived from a small volume will more likely capture a representative uptake value within a smaller paediatric tumour lesion and it should be less affected by motion artifacts than in adults. Partial volume effects associated with the SUVpeak, on the other hand, could be more problematic in small paediatric tumours. In conclusion, it seems that in the special case of paediatric oncologic PET imaging, the (dis-)advantages of either SUV metric tend to balance each other, thus delivering similar results, at least with the specifications used in the current study.
However, it has to be noted that SUVs are in general imperfect parameters as they are altered by various factors that can result in considerable variations of accuracy and reproducibility. For instance, technical errors may derive from alterations in the calibration of the PET scanner and the dose calibrator or by paravenous tracer injection, effecting SUVs by ± 10% and up to 50%, respectively; biologic factors as patient motion may result in SUV alterations by up to 30%; finally, data acquisition and processing parameters may lead to further significant variability [28, 29]. Thus, the implementation of standardized scan and good quality control procedures as well as the consideration of sources of variability are mandatory for PET/CT analysis.
There are several limitations of the current study that must be taken into consideration. First, we evaluate the ability of different SUV metrics for differentiation between tumours by implicitly assuming that differences in glucose consumption between these entities exist. To our knowledge, studies illuminating this issue in paediatric patients have not yet been performed. Also, we combine different tumor subtypes into three tumor entity groups. This type of data reduction can be considered problematic as substantial differences in FDG uptake may also exist on the subtype level of a tumor entity.
Also, it has to be noted that more complex methods to determine a tumour’s metabolic characteristics such as metabolic tumour volume (MTV) and total lesion glycolysis (TLG) were not evaluated. These so-called second-order metrics, also based on the SUV concept, are very interesting as they—in contrast to first order metrics as SUVmax or SUVpeak—try to capture the metabolic information from the entire tumour volume. And indeed, several studies report very promising results regarding the prognostic value of these parameters for various lymphoma types [30, 31] as well as for soft tissue and bone sarcoma [16, 32]. However, studies comparing the value of MTV and TLG with simple SUVmax and SUVpeak metrics as used in the current work do not show a clear advantage of the second-order methods in these tumour types [33–35]. Moreover, to the best of the authors’ knowledge quantitation of tumour metabolism with simple first-order SUV metrics is still most widely used in clinical routine as well as in the scientific world and thus a broader understanding of these measures is of great importance.
For the current work, we used CT scans with and without intravenous contrast agent for attenuation correction of PET data. SUV values calculated from contrast-enhanced CT’s will be slightly higher than SUV values calculated from non-contrast CT's; the difference is usually quite small [36], but may affect SUV based analyses as performed here.
Moreover, our analysis of factors potentially influencing FDG concentration within liver and mediastinal blood pool is not complete; e.g. a well-known factor potentially affecting uptake within these regions, the blood glucose concentration [37, 38], was not taken into consideration in the current study as results of glucose testing were not recorded in the majority of cases, thus no sufficient data was available for further evaluation. Also, the effects of furosemide and butylscopolamine could not be investigated. However, research on adults has demonstrated that furosemide effectively eliminates 18F-FDG activity from the lower urinary tract and thus improves the diagnostic accuracy in abdominopelvic malignancies [39]; interestingly, furosemide was shown to not substantially affect tracer distribution within the mediastinal blood pool and soft tissues, respectively [40]. Butylscopolamine, on the other hand, reduces artefacts coming from bowel peristalsis, thus also improving the accuracy of abdominal 18F-FDG PET reporting [41]. Given that all patients in our study were administered furosemide and butylscopolamine, diagnostic accuracy of the PET data and effects on SUV measurements (including reduction of activity spill-over to lesions adjacent to the urinary system or bowel) should be comparable; some variability, however, may come from different dosages based on patient age (10 mg for each drug for children < 10 years and 20 mg for older children and adolescents).
Finally, we use a retrospective study design and a small sample size that reduces the statistical power. Therefore, our results and the implications made should be replicated and verified in future studies using a prospective setting and larger patient numbers.
Conclusions
Our work shows that the ability to differentiate between common tumours in paediatric patients substantially varies between SUV metrics. In the case of SUVratios, the choice of reference region is crucial as reference region SUVs may be significantly biased by factors like gender, uptake time, and bodyweight. Thus, these factors should be adjusted for. If not possible, we recommend the use of SUVmax and SUVpeak; these metrics represent less complex and more robust—while imperfect—alternatives. Moreover, in the special case of the paediatric population, it apparently does not matter if SUVmax or SUVpeak is used as both parameters deliver very similar results, likely due to overall smaller volumes in these patients compared to adults.
Acknowledgements
Parts of this paper originate from the doctoral thesis of Leonie Grelich and Rebecca Henkel.
Abbreviations
- 18F-FDG
2-deoxy-2-(18F)fluoro-D-glucose
- ES
Ewing sarcoma
- HL
Hodgkin lymphoma
- NHL
non-Hodgkin lymphoma
- OS
Osteosarcoma
- PET/CT
Positron emission tomography/computed tomography
- RMS
Rhabdomyosarcoma
- ROI
Region-of-interest
- SUV
Standardized uptake value
- SUVratio
Standardized uptake value ratio
Authors’ contributions
JB and LG contributed to the conception and design, acquisition of data, analysis and interpretation of data, document writing, and editing. NS and RH contributed to the analysis and interpretation of data, document writing. PB contributed to the intellectual input, drafting, and revising of the manuscript. TP contributed to the conception and design, acquisition of data, document editing, final manuscript approval for submission, and publication. All authors read and approved the final manuscript.
Funding
No funding was obtained in association with this study.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
All procedures performed in the study involving human participants were in accordance with the ethical standards of the Institutional Review Board and with the 1964 Helsinki Declaration and its later amendments. The study was approved by the local ethics committee.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Janusch Blautzik and Leonie Grelich contributed equally to this work.
References
- 1.Noone AM, Howlader N, Krapcho M, Miller D, Brest A, Yu M, et al. SEER Cancer Statistics Review, 1975-2015, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2015/, based on November 2017 SEER data submission, posted to the SEER web site, April 2018.
- 2.London K, Cross S, Onikul E, Dalla-Pozza L, Howman-Giles R. 18F-FDG PET/CT in paediatric lymphoma: comparison with conventional imaging. Eur J Nucl Med Mol Imaging. 2011;38(2):274–284. doi: 10.1007/s00259-010-1619-6. [DOI] [PubMed] [Google Scholar]
- 3.London K, Stege C, Cross S, Onikul E, Graf N, Kaspers G, et al. 18F-FDG PET/CT compared to conventional imaging modalities in pediatric primary bone tumors. Pediatr Radiol. 2012;42(4):418–430. doi: 10.1007/s00247-011-2278-x. [DOI] [PubMed] [Google Scholar]
- 4.Mody RJ, Bui C, Hutchinson RJ, Yanik GA, Castle VP, Frey KA, et al. FDG PET imaging of childhood sarcomas. Pediatr Blood Cancer. 2010;54(2):222–227. doi: 10.1002/pbc.22307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl 1):122S–150S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krak NC, van der Hoeven JJ, Hoekstra OS, Twisk JW, van der Wall E, Lammertsma AA. Measuring [(18)F]FDG uptake in breast cancer during chemotherapy: comparison of analytical methods. Eur J Nucl Med Mol Imaging. 2003;30(5):674–681. doi: 10.1007/s00259-003-1127-z. [DOI] [PubMed] [Google Scholar]
- 7.Graham MM, Peterson LM, Hayward RM. Comparison of simplified quantitative analyses of FDG uptake. Nucl Med Biol. 2000;27(7):647–655. doi: 10.1016/S0969-8051(00)00143-8. [DOI] [PubMed] [Google Scholar]
- 8.Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42(2):328–354. doi: 10.1007/s00259-014-2961-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zasadny KR, Wahl RL. Standardized uptake values of normal tissues at PET with 2-[fluorine-18]-fluoro-2-deoxy-D-glucose: variations with body weight and a method for correction. Radiology. 1993;189(3):847–850. doi: 10.1148/radiology.189.3.8234714. [DOI] [PubMed] [Google Scholar]
- 10.Boktor RR, Walker G, Stacey R, Gledhill S, Pitman AG. Reference range for intrapatient variability in blood-pool and liver SUV for 18F-FDG PET. J Nucl Med. 2013;54(5):677–682. doi: 10.2967/jnumed.112.108530. [DOI] [PubMed] [Google Scholar]
- 11.Bailly C, Bodet-Milin C, Bourgeois M, Gouard S, Ansquer C, Barbaud M, et al. Exploring Tumor Heterogeneity Using PET Imaging: The Big Picture. Cancers. 2019;31:11(9). doi: 10.3390/cancers11091282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salem U, Amini B, Chuang HH, Daw NC, Wei W, Haygood TM, et al. (18)F-FDG PET/CT as an Indicator of Survival in Ewing Sarcoma of Bone. J Cancer. 2017;8(15):2892–2898. doi: 10.7150/jca.20077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raciborska A, Bilska K, Drabko K, Michalak E, Chaber R, Pogorzala M, et al. Response to chemotherapy estimates by FDG PET is an important prognostic factor in patients with Ewing sarcoma. Clin Trans Oncol. 2016;18(2):189–195. doi: 10.1007/s12094-015-1351-6. [DOI] [PubMed] [Google Scholar]
- 14.Casey DL, Wexler LH, Fox JJ, Dharmarajan KV, Schoder H, Price AN, et al. Predicting outcome in patients with rhabdomyosarcoma: role of [(18)f]fluorodeoxyglucose positron emission tomography. Int J Radiat Oncol Biol Phys. 2014;90(5):1136–1142. doi: 10.1016/j.ijrobp.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Ferrari C, Niccoli Asabella A, Merenda N, Altini C, Fanelli M, Muggeo P, et al. Pediatric Hodgkin Lymphoma: Predictive value of interim 18F-FDG PET/CT in therapy response assessment. Medicine. 2017;96(5):e5973. doi: 10.1097/MD.0000000000005973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Im HJ, Zhang Y, Wu H, Wu J, Daw NC, Navid F, et al. Prognostic Value of Metabolic and Volumetric Parameters of FDG PET in Pediatric Osteosarcoma: A Hypothesis-generating Study. Radiology. 2018;287(1):303–312. doi: 10.1148/radiol.2017162758. [DOI] [PubMed] [Google Scholar]
- 17.Barrington SF, Qian W, Somer EJ, Franceschetto A, Bagni B, Brun E, et al. Concordance between four European centres of PET reporting criteria designed for use in multicentre trials in Hodgkin lymphoma. Eur J Nucl Med Mol Imaging. 2010;37(10):1824–1833. doi: 10.1007/s00259-010-1490-5. [DOI] [PubMed] [Google Scholar]
- 18.Hasenclever D, Kurch L, Mauz-Korholz C, Elsner A, Georgi T, Wallace H, et al. qPET - a quantitative extension of the Deauville scale to assess response in interim FDG-PET scans in lymphoma. Eur J Nucl Med Mol Imaging. 2014;41(7):1301–1308. doi: 10.1007/s00259-014-2715-9. [DOI] [PubMed] [Google Scholar]
- 19.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 20.Amankwah EK, Conley AP, Reed DR. Epidemiology and therapies for metastatic sarcoma. Clin Epidemiol. 2013;5:147–162. doi: 10.2147/CLEP.S28390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baum SH, Fruhwald M, Rahbar K, Wessling J, Schober O, Weckesser M. Contribution of PET/CT to prediction of outcome in children and young adults with rhabdomyosarcoma. J Nucl Med. 2011;52(10):1535–1540. doi: 10.2967/jnumed.110.082511. [DOI] [PubMed] [Google Scholar]
- 22.Malladi A, Viner M, Jackson T, Mercier G, Subramaniam RM. PET/CT mediastinal and liver FDG uptake: effects of biological and procedural factors. J Med Imaging Radiat Oncol. 2013;57(2):169–175. doi: 10.1111/1754-9485.12015. [DOI] [PubMed] [Google Scholar]
- 23.Groheux D, Delord M, Rubello D, Colletti PM, Nguyen ML, Hindie E. Variation of liver SUV on (18)FDG-PET/CT studies in women with breast cancer. Clin Nuclear Med. 2013;38(6):422–425. doi: 10.1097/RLU.0b013e3182872f0e. [DOI] [PubMed] [Google Scholar]
- 24.Chin BB, Green ED, Turkington TG, Hawk TC, Coleman RE. Increasing uptake time in FDG-PET: standardized uptake values in normal tissues at 1 versus 3 h. Mol Imaging Biol. 2009;11(2):118–122. doi: 10.1007/s11307-008-0177-9. [DOI] [PubMed] [Google Scholar]
- 25.Hamberg LM, Hunter GJ, Alpert NM, Choi NC, Babich JW, Fischman AJ. The dose uptake ratio as an index of glucose metabolism: useful parameter or oversimplification? J Nucl Med. 1994;35(8):1308–1312. [PubMed] [Google Scholar]
- 26.Mansor S, Pfaehler E, Heijtel D, Lodge MA, Boellaard R, Yaqub M. Impact of PET/CT system, reconstruction protocol, data analysis method, and repositioning on PET/CT precision: An experimental evaluation using an oncology and brain phantom. Med Phys. 2017;44(12):6413–6424. doi: 10.1002/mp.12623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lucignani G. SUV and segmentation: pressing challenges in tumour assessment and treatment. Eur J Nucl Med Mol Imaging. 2009;36(4):715–720. doi: 10.1007/s00259-009-1085-1. [DOI] [PubMed] [Google Scholar]
- 28.Boellaard R. Standards for PET image acquisition and quantitative data analysis. J Nucl Med. 2009;50(Suppl 1):11S–20S. doi: 10.2967/jnumed.108.057182. [DOI] [PubMed] [Google Scholar]
- 29.Aide N, Lasnon C, Veit-Haibach P, Sera T, Sattler B, Boellaard R. EANM/EARL harmonization strategies in PET quantification: from daily practice to multicentre oncological studies. Eur J Nucl Med Mol Imaging. 2017;44(Suppl 1):17–31. doi: 10.1007/s00259-017-3740-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo B, Tan X, Ke Q, Cen H. Prognostic value of baseline metabolic tumor volume and total lesion glycolysis in patients with lymphoma: A meta-analysis. PLoS One. 2019;14(1):e0210224. doi: 10.1371/journal.pone.0210224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albano D, Bosio G, Pagani C, Re A, Tucci A, Giubbini R, et al. Prognostic role of baseline 18F-FDG PET/CT metabolic parameters in Burkitt lymphoma. Eur J Nucl Med Mol Imaging. 2019;46(1):87–96. doi: 10.1007/s00259-018-4173-2. [DOI] [PubMed] [Google Scholar]
- 32.Li YJ, Dai YL, Cheng YS, Zhang WB, Tu CQ. Positron emission tomography (18)F-fluorodeoxyglucose uptake and prognosis in patients with bone and soft tissue sarcoma: A meta-analysis. Eur J Surg Oncol. 2016;42(8):1103–1114. doi: 10.1016/j.ejso.2016.04.056. [DOI] [PubMed] [Google Scholar]
- 33.Hussien AE, Furth C, Schonberger S, Hundsdoerfer P, Steffen IG, Amthauer H, et al. FDG-PET Response Prediction in Pediatric Hodgkin's Lymphoma: Impact of Metabolically Defined Tumor Volumes and Individualized SUV Measurements on the Positive Predictive Value. Cancers. 2015;7(1):287–304. doi: 10.3390/cancers7010287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jamet B, Carlier T, Campion L, Bompas E, Girault S, Borrely F, et al. Initial FDG-PET/CT predicts survival in adults Ewing sarcoma family of tumors. Oncotarget. 2017;8(44):77050–77060. doi: 10.18632/oncotarget.20335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hong SP, Lee SE, Choi YL, Seo SW, Sung KS, Koo HH, et al. Prognostic value of 18F-FDG PET/CT in patients with soft tissue sarcoma: comparisons between metabolic parameters. Skeletal radiology. 2014;43(5):641–648. doi: 10.1007/s00256-014-1832-7. [DOI] [PubMed] [Google Scholar]
- 36.Berthelsen AK, Holm S, Loft A, Klausen TL, Andersen F, Hojgaard L. PET/CT with intravenous contrast can be used for PET attenuation correction in cancer patients. Eur J Nucl Med Mol Imaging. 2005;32(10):1167–1175. doi: 10.1007/s00259-005-1784-1. [DOI] [PubMed] [Google Scholar]
- 37.Sprinz C, Zanon M, Altmayer S, Watte G, Irion K, Marchiori E, et al. Effects of blood glucose level on 18F fluorodeoxyglucose (18F-FDG) uptake for PET/CT in normal organs: an analysis on 5623 patients. Sci Rep. 2018;8(1):2126. doi: 10.1038/s41598-018-20529-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sprinz C, Altmayer S, Zanon M, Watte G, Irion K, Marchiori E, et al. Effects of blood glucose level on 18F-FDG uptake for PET/CT in normal organs: A systematic review. PLoS One. 2018;13(2):e0193140. doi: 10.1371/journal.pone.0193140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kamel EM, Jichlinski P, Prior JO, Meuwly JY, Delaloye JF, Vaucher L, et al. Forced diuresis improves the diagnostic accuracy of 18F-FDG PET in abdominopelvic malignancies. J Nucl Med. 2006;47(11):1803–1807. [PubMed] [Google Scholar]
- 40.Ceriani L, Suriano S, Ruberto T, Giovanella L. Could different hydration protocols affect the quality of 18F-FDG PET/CT images? J Nucl Med Technol. 2011;39(2):77–82. doi: 10.2967/jnmt.110.081265. [DOI] [PubMed] [Google Scholar]
- 41.Emmott J, Sanghera B, Chambers J, Wong WL. The effects of N-butylscopolamine on bowel uptake: an 18F-FDG PET study. Nucl Med Commun. 2008;29(1):11–16. doi: 10.1097/MNM.0b013e3282f1d706. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.