Abstract
Characterization of mango kernel seed oil extracted using supercritical CO2 (SC–CO2) and conventional solvent (hexane, petroleum ether, ethanol and acetone) extraction techniques was carried out using differential scanning calorimetry (DSC), Fourier-transform infrared spectroscopy (FTIR), Gas chromatography mass spectroscopy (GC-MS) and fluorescence microscope. The extractor and separator temperatures of the SC-CO2 were 60 and 50 °C respectively while the pressure was varied from 35 to 40 MPa. Solvent extractions were maintained at the boiling points of the various solvents. The results indicated that solvent extraction had higher yields (8.02–19.88%) while SC-CO2 had a lower yield (2.5–3.6 %); the yield of conventional solvent extraction increased with decreasing particle sizes. Ethanol extracted oil had lowest enthalpies of endothermic reaction (1.17–2.74 J/g); while other solvents were between 42.54 and 45.64 J/g with SC-CO2 having 37.40 J/g. The melting points for ethanol extracted oil were 7.34 and 35.20 °C; other solvents ranged between 13.39 and 15.15 °C while, SC-CO2 was 35.05 °C. SC-CO2 extracted oil had no crystallization parameter, while conventional solvent extracted oil with the exception of ethanol were between -33.23 and -33.97 J/g. The FTIR showed that CH3, CH2 and COH were the predominant functional groups in hexane, petroleum ether, acetone and SC-CO2-extracted oil; ethanol extracted oil had –OH and CH2. The extracted oil using solvent extraction technique was higher in unsaturated fatty acid (UFA) with the exception of acetone extracted oil. SC-CO2 extracted oil had higher saturated fatty acid (SFA) content (47.01%). The predominant UFA and SFA were oleic acid stearic acid.
Keywords: Food engineering, Food technology, Analytical chemistry in food science, Biochemical composition of food, Chemical characterization of food, Crystallization, DSC, FTIR, GC-MS, Mango kernel seed oil, Solvents extraction
Food engineering; Food technology; Analytical chemistry in food science; Biochemical composition of food; Chemical characterization of food; Crystallization; DSC; FTIR; GC-MS; Mango kernel seed oil; Solvents extraction
1. Introduction
Edible oil from plant origin has continued to receive attention due to their nutritional importance. In particular, oil and fat from plants contained higher contents of unsaturated fatty acids (Tan and Man, 2000). Mango kernels are conventionally disposed off as waste materials in many parts of the world with about one million tons of mango seeds are treated as wastes annually (Karunanithi et al., 2015). In addition to its high unsaturation level coupled with the presence of vitamins and bioactive compounds (Karunanithi et al., 2015), mango kernel seeds oil had been found to possess characteristics similar to cocoa butter in terms of its availability, cost-effectiveness and physicochemical nature (Sagiri et al., 2014).
The Soxhlet extraction is a traditional technique for extracting lipids, and occurs between a solid phase and a liquid phase (solvent). The diffusion of the liquid phase within the solid phase resulted in the extraction of lipids into the liquid phase. Common solvents are petroleum ether, n-hexane, ethanol and acetone. Although ethanol has been found to be promising solvents for lipid extraction because it is cheap and can be produced from fermentation, oil yields and some economic aspects have favoured the selection of hexane which is an inflammable petroleum-derived solvent. However, some disadvantages of soxhlet extraction include toxicity and flammability of the solvents, environmental pollution, laborious and time-consuming procedures and non-selectivity of extraction (especially ethanol solvent) which has led to the use of supercritical fluid extraction techniques (Rai et al., 2016).
Supercritical is the state above critical pressure and temperature (Gadkari et al., 2015). At its supercritical (critical temperature 31.2 °C, critical pressure 7.4 MPa), carbon dioxide is compressible and possesses both gaseous and liquid properties with enhanced solvating power, hence, giving it an edge above conventional liquids (García-Rodríguez et al., 2008). Supercritical fluid extraction (SFE) using supercritical carbon dioxide as a solvent is gaining widespread acceptability as a viable and better alternative to organic solvent extraction technique as it may offer may offer an efficient and extraction process (Gadkari et al.,2015). Faster extraction rate of SFE had also been reported (Rai et al., 2016; Mukhopadhyay, 2000). In addition, SFE has been adopted to mitigate the health, environmental and safety hazards associated with the use of conventional solvent extraction, as well as prevention of contamination of the final products by traces of used solvent (Sahena et al., 2009). In addition, supercritical carbon dioxide is most commonly employed SFE with carbon dioxide as solvent due to its unique properties such as non-flammability, non-toxicity, non-explosiveness, cost efficiency, higher extraction rate among others (Rai et al., 2016).
Differential scanning calorimetry (DSC) and Fourier transform infrared spectroscopy (FTIR) are gaining analytical prominence for authenticating edible oils, hence, prevents adulteration. DSC is increasingly and widely used for thermoanalytical characterization of edible oils (Dollimore, 1996; Cebula and Smith, 1992). It gives information about changes in enthalpy, melting and crystallization temperatures as well as the heat of fusion and crystallization (Sessa, 1996). Melting and crystallization behaviour of edible oils have been described as two important properties for authenticating originality of many food products (Tan and Man, 2000). Fourier-transform infrared (FTIR) technology is a quantitative quality control tool for the food industry (Van de Voort, 1992). It has been used to analyze the mineral structure and organic matrix composition including amide, amine, and carboxylic acid groups in the organic matrix of the whole nacreous layer of shells (Balmain et al., 1999). It has variously been used in the determination of ethanol contents in alcoholic drinks (Gallignani et al., 1994), protein and fat content in raw meat (Dion et al., 1994) and in the study of edible fats and oils (Guillen and Cabo, 1997).
Gas chromatography – mass spectrometry (GC-MS) identifies and quantifies compounds using both the retention times and the relative abundances of the characteristic fragment ions. It is a reliable analytical technique with high accuracy.
Olympus BX51 Trinocular Flouorescence microscope is a multispectral analytical system with a Pixlelteq Spectrocam Multispectral camera capable of high resolution images at eight different wavelengths. It includes a Sentech color camera for conventional color imaging, functioning both as a high end standard microscope with conventional color imaging, as well as multispectral imaging.
Several researches have been carried out, especially on the evaluation of the physicochemical characteristics of mango kernel seeds oil (Akanda et al., 2015; Atta et al., 2016; Kittiphoom and Sutasinee, 2013; Dhara et al., 2010). This study was therefore aimed at characterizing mango kernel seed oil using DSC, FTIR, GC-MS and confocal microscope in order to identify its structural/molecular formula, evaluate its thermodynamic properties, establish its functional properties and microscopy. The use of conventional solvent extraction techniques was compared with supercritical fluid extraction technique.
The search for cocoa butter equivalent in food and pharmaceutical industries has revealed that mango butter is a viable alternative to cocoa butter. Mango butter emulsion gels had been found to have similar physical, thermal and mechanical properties as cocoa butter emulsion gel (Sagiri et al., 2014). Therefore, this study into finding the best solvent vis-à-vis condition for mango kernel seeds oil yield will produce vital information on the best condition for mango kernel oil extraction. Characterization of the oil using GC-MS, FTIR and DSC will help to evaluate the solvent that will produce best quality mango kernel oil.
2. Materials and methods
2.1. Materials
Mango kernel seeds were obtained from main market, Mysore, India. All reagents were of analytical grade. Hammer Mill (Model: CMC = CM- Q = 753 = 97, M = s Cadmach Machinery Company, Ahmedabad, India) and Soxhlet apparatus (6–250ML Soxlet Apparatus, Culture Instruments, Bengalore, India), laboratory sieve shaker (M = s Muhlenbau, Reinbek, Germany) were obtained from Department of Food Engineering, Central Food Technological Research Institute, Mysore, India. All instruments used were located at Central Instruments Facility and Service (CIFS) section, Central Food Technological Research Institute (CFTIR), Mysore, India.
2.2. Sample preparation
The mango seeds were dehulled and sundried to a moisture content of 8% (w/w). It was manually cut to an average size of 6.468 mm using knife, and later milled using an hammer mill to fine particles.
2.3. Oil extraction by soxhlet method
The effect of different solvents (n-hexane, petroleum ether, ethanol and acetone) and time (3, 4 and 5 h) were considered (Table 1). The mango kernel flour size used was 0.402 mm. The mass of mango kernel seeds flour in all cases were 5 g and the extraction temperatures were the boiling temperature of the solvents.
Table 1.
Mango seed kernel oil yield using conventional solvents extraction.
| S/N | Solvent | Yield (%) |
||
|---|---|---|---|---|
| 3 h | 4 h | 5 h | ||
| 1 | Ethanol | 19.86a ± 0.05 | 18.42b ± 0.02 | 16.11c ± 0.02 |
| 2 | Hexane | 9.85a ± 0.05 | 9.15b ± 0.05 | 9.12b ± 0.08 |
| 3 | Acetone | 18.70a ± 0.05 | 18.40b ± 0.10 | 18.30b ± 0.10 |
| 4 | Pet-Ether | 8.75a ± 0.05 | 8.77a ± 0.03 | 8.02b ± 0.03 |
Means of triplicate determinations ± standard deviation. Means with different superscripts on the same column are significantly different at p < 0.05.
2.4. Supercritical CO2 extraction of mango kernel oil
The instrument (pilot scale unit, NOVA Swiss WERKE AG, EX 1000–1.4-1.2 type, Switzerland model) consist basically the CO2 supply source, the compressors, the heaters, extractor and separator. The sample (200 g) was loaded in to an inserted vessel (1 L capacity) with sintered metal plate on both ends and then inserted extraction vessel. The extraction temperature was set at 60 °C while the separator temperature was set at 50 °C. Extraction was then carried out at two pressures (35 and 40 MPa) for both the extractor and the separator until oil extraction was complete. The yields at the two pressures were calculated. Extraction time was about 15 min, flow rate about 10 g/min, and negligible viscosities.
2.5. Fatty acid analysis
The extracted lipids were esterified and the fatty acid methyl esters (FAME) analyzed using GC-MS (Perkin Elmer, Turbomass Gold, Mass spectrometer) equipped with FID using SPB-1 (poly (dimethysiloxane)) capillary column (30 m × 0.32 mm ID × 0.25 μm film thickness) obtained from CIFS-CFTRI, Mysore, India. The temperature programming is from 150 °C to 280 °C at 5 °C/min. FAMEs were identified by comparing their fragmentation pattern with authentic standards (Sigma) and with NIST 14 library (Dayan et al., 2010).
2.6. FTIR analysis
The extracted oil was characterized using Fourier Tranform-Infrared Spectrometer (Model IFS 25, Bruker, Germany). The spectral range was 4000–400 cm2 while the resolution was 2 cm−1 with high throughput Michelson interferometer controlled by He, Ne Laser. The detector was DTGS with KBr window.
2.7. Thermal profile
The thermal profile of the oil were evaluated using DSC 8000 System (a double-furnace, Power compensation differential scanning calorimeter from M/S. Perkin Elmer) which directly measures the heat flow between two independent furnaces. A precise energy measurement over the temperature range from -40 °C to 70 °C was used.
2.8. Microscopy
Trinocular Flouorescence microscope (Olympus BX51 with Spectrocam model) configured for both transmission and EPI was used. A drop of extracted oil was placed on a clean slide and covered with a microscope slide. Resolutions were adjusted until clear images were obtained. The images were stored in laptop that was preloaded with the Pixelteq and Sentech image capture software.
2.9. Statistical analyses
The Data for the analysis was generated in triplicate and was subjected to one way Analysis of variance (ANOVA), SPSS 19.00 while means was separated using Turkey test at p < 0. Data obtained was subjected to one way Analysis of variance (ANOVA), SPSS 19.00 while means was separated using Duncan multiple range test (DMRT) at p < 0.05.
3. Results and discussion
3.1. Extracted mango seeds oil yield
The results of the mango seeds oil yield as affected by solvent type and extraction time are presented in Table 1. The results indicated that ethanol and acetone had the highest oil yield. Further analyses using FTIR (Figure 1) and DSC (Table 3) revealed that the higher oil yield from acetone and ethanol might be due to impurities or some other compounds extracted with the oil. Hexane and pet-ether had golden yellow coloured oil. The oil yields for n-hexane and pet-ether were between 8.00 and 10.00%. Also, further analyses using FTIR (Figure 1) and DSC (Table 3) indicated that mango kernel seeds oil from n-hexane and pet-ether were of better quality. In addition, optimum yield was observed at 3 h of extraction. After 3 h, the oil yield began to reduce, as also reported by Awolu et al. (2013).
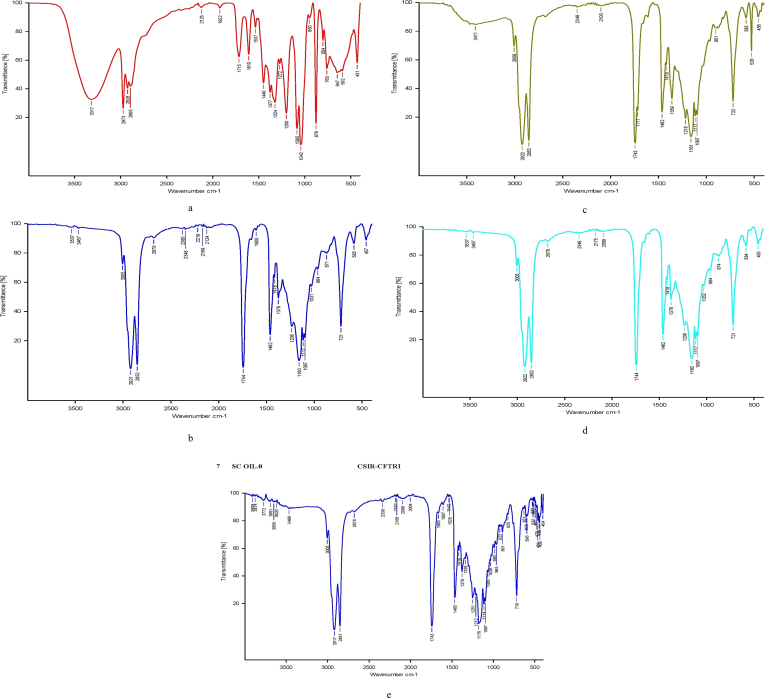
Figure 1.
FTIR spectra for (a) ethanol, (b) n-hexane, (c) acetone, (d) petroleum ether, and (e) SC-CO2 extracted mango kernel seed oil.
Table 3.
Thermal properties of mango kernel Oil Subjected to Different Solvent Extraction.
| Endothermic |
Exothermic |
|||||||
|---|---|---|---|---|---|---|---|---|
| Onset (o C) | PT (o C) | ΔH (J/g) | End (o C) | Onset (o C) | PT (o C) | ΔH (J/g) | End (o C) | |
| Acetone | 0.22 | 13.39 | 45.63 | 17.70 | 10.44 | 7.29 | -32.23 | -4.36 |
| Ethanol | -2.36 | 7.34 | 2.74 | 11.61 | 8.78 | 4.43 | -3.15 | -4.83 |
| 32.06 | 35.20 | 1.16 | 37.98 | |||||
| Hexane | 5.67 | 15.15 | 42.53 | 18.76 | 12.92 | 9.10 | -33.97 | -1.67 |
| Pet-ether | 5.27 | 14.91 | 44.56 | 19.09 | 12.90 | 9.36 | -33.05 | 0.25 |
NB: PT – Peak temperature.
For the supercritical CO2 oil extraction, an increase in the operating pressure from 35 to 40 MPa resulted in about 18% increase in mango kernel oil yield. However, the yields from supercritical CO2 extraction (2.5–3.5%) were lower than that of conventional solvent extraction techniques. A higher yield could be obtained when the pressure is increased; about 95% of the oil yield from n-hexane extraction had been reported by Friedrich et al. (1982) by using a pressure of 69 MPa for the extraction of soybean oil using supercritical CO2 extraction. In fact, Friedrich (1984) reported that the solubility of the lipids in supercritical CO2 dramatically increased by using temperature and pressure exceeding 60 °C and 55 MPa, respectively, contrary to widely acceptable conditions of temperature and pressure lesser than 60 °C and 40 MPa.
Edible oil extracted using supercritical CO2 extraction had been found to be of better quality than hexane-extracted oil; SC-C02 oil were found to have better odor and flavor after four days storage at 60 °C, eliminating degumming and its attendant oil losses without sacrificing oil quality associated with n-hexane oil extraction (Friedrich et al., 1982).
Mango kernel oil yield had been reported to be rich in minerals, high unsaturated fatty acid and phenolic compounds (Nzikou et al., 2010; Kittiphoom and Sutasinee, 2013), hence, the need for research into the extraction of mango kernel oil and more importantly, the necessity of obtaining high yields of mango kernel oil. Nzikou et al. (2010) reported that mango kernel seeds oil contained about 3.2% ash consist mainly calcium, potassium, magnesium and phosphorus. Kittiphoom and Sutasinee (2013) also reported that mango kernel seeds oil was rich in phenolic compounds and nutrients which made them suitable as ingredients for functional or enriched foods. The high unsaponifiable matter in mango kernel oil has also enhanced its use in cosmetic industry (Nzikou et al., 2010).
3.2. GC-MS results
The results of the GC-MS are presented in Table 2. Oleic acid methyl ester, an unsaturated fatty acid, was the predominant fatty acid (44–45%) in the conventional solvent extracted oil with the exception of the oil obtained by using ethanol extraction. Oil extracted using conventional solvents had higher SFA than UFA with the exception of ethanol extracted oil. SC-CO2 oil also had higher SFA than UFA. Nzikou et al. (2010), and Kittiphoom and Sutasinee (2013) had also reported higher UFA in another species of mango kernel oil. The percentages unsaturation of n-hexane, petroleum ether, acetone, ethanol and SC-CO2 extracted oil in this study were 49.67, 48.85, 43.71, 44.34 and 37.588%, respectively, while percentages saturation were 48.44, 48.18, 51, 31.87 and 47.408, respectively. In addition to the nutritional benefits of edible oil with high UFA, vegetable oil with high UFA had also been useful in the production of biodiesel.
Table 2.
GC-MC composition of mango kernel oil from different solvents.
| S/N | Compound | % Composition per solvent |
|||
|---|---|---|---|---|---|
| Hexane | Pet-ether | Acetone | Ethanol | ||
| 1 | Palmitic acid (C16:0) | 6.35 | 6.25 | 8.21 | 4.23 |
| 2 | Linolenic acid (C18:2) | 4.75 | 4.69 | - | 2.93 |
| 3 | Oleic acid (C18:1 cis) | 44.93 | 44.07 | 46.34 | 32.65 |
| 4 | Stearic acid (C18:0) | 40.38 | 40.29 | 39.83 | 28.59 |
| 5 | Arachidonic acid (C21H42O2) | 1.70 | 1.63 | 3.06 | - |
| 6 | Trans-9-elaidic acid (C20:0) | - | - | - | 8.13 |
| 7 | Ethyl stearic acid (C18:1 trans) | - | - | - | 7.05 |
| 8 | Others | 1.89 | 2.97 | 4.55 | 16.41 |
| 9 | % saturation | 48.44 | 48.18 | 49.11 | 39.87 |
| 10 | % unsaturation | 49.67 | 48.85 | 46.34 | 43.71 |
Ethanolic extracted oil had ethyl stearic acid (an ethyl ester) at 7.048%. It is a saturated fatty acid commonly found in animal and vegetable fats that is frequently used in cosmetics, candles, soaps, plastics, oil pastels, and for softening rubber. It is used as flavouring agent. In addition, ethanolic extraction had about 8.13% trans-9-elaidic. Trans-9-elaidic has been found to be a major trans-fat found in hydrogenated vegetable oils and has been implicated in heart disease (Tardy et al., 2011).
3.3. Infrared spectrometry
The FTIR spectra for extracted mango kernel seeds oil are shown in Figure 1(a-e). Identification of functional groups and bands corresponding to various stretching and bending vibrations in edible oil samples have been carried out by using the mid-infrared regions (3000–1500 cm−1) of the FTIR spectra (Tariq et al., 2011). N-Hexane and pet-ether extracted mango kernel seed oil had very similar spectra (Figure 1b and d), and coincidentally, the same functional groups with strong and very strong intensities. The functional groups were CH2, CH3 and CO with wavenumbers 2921, 2852, 1744 and 2922, 2852, 1744 cm−1 for hexane and pet-ether extracted mango kernel seed oil respectively. The SC-CO2 extracted oil also had FTIR spectrum (Figure 1e) similar to hexane and pet-ether extracted oil (Figure 2e), and hence, the same functional groups. Acetone extracted mango kernel seed oil FTIR spectrum had minor difference from n-hexane and pet-ether spectra, but with the same functional groups CH2, CH3 and CO with wavenumbers 2922, 2853 and 1743 cm−1. An alkene (CH) with medium intensity was found in n-hexane, pet-ether and acetone extracted mango kernel seeds oil at wavenumbers 3005, 3005 and 3005 cm−1 respectively. On the other hand, ethanol extracted mango kernel seeds oil was almost completely dissimilar to those of n-hexane, pet-ether and acetone. The primary functional groups in ethanol extracted oil were OH and CH2 with wavenumbers 3317 and 2926 cm−1 respectively. This diverse behaviour of ethanol extracted mango kernel seeds oil was also observed in the result of GC-MS where ethanol extracted mango kernel oil had ethyl stearic acid (7.048%) and trans-9-elaidic acid (8.133%) identified with a resultant reduction in oleic and stearic acids.
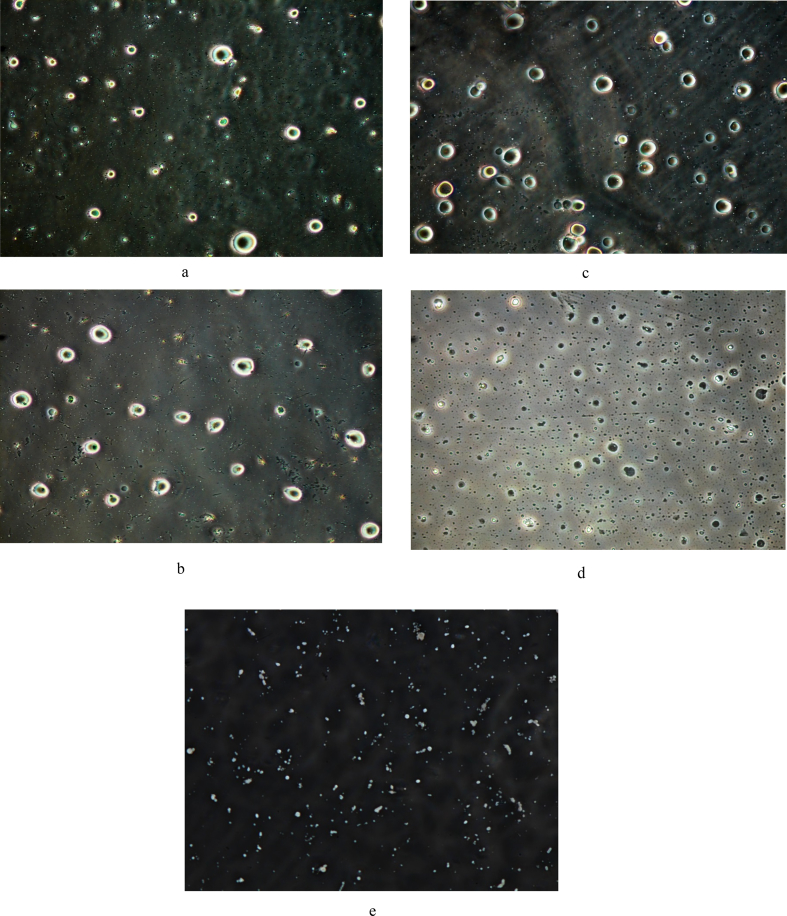
Figure 2.
Microscopic images (×100) of (a) acetone, (b) ethanol, (c) n-hexane, (d) petroleum ether, and (e) SC-CO2 extracted mango kernel seed oil.
3.4. Thermal profile
Melting and crystallization behaviour of edible oils and fats have been shown to be two of the important properties for functionality in many prepared food products (Tan and Man, 2000). The thermal profiles are presented in Table 3. Following the same pattern observed in FTIR, n-hexane and pet-ether extracted mango kernel seeds oil had similar thermal profile, closely followed by acetone extracted oil. Ethanol extracted oil also had very different thermal profile. The enthalpy for the melting (endothermic) reaction of all samples with the exception of ethanol extracted oil ranges from 40.32 J/g to 45.636 J/g, while the enthalpy for crystallization (exothermic) reaction for all sample (except ethanol extracted sample) ranges from -33.977 to -32.231 J/g. Endothermic parameters were highest for SC-CO2 extracted oil. This might not be unconnected to its saturation state at room temperature. Unlike oil extracted by conventional solvent extraction which were liquid at room temperature because they were primarily unsaturated, the SC-CO2 oil was solid at room temperature which is an indication that it would have higher melting points than oil obtained from conventional solvent extraction. The crystallization temperature for pet-ether extracted oil was 9.36 °C; hexane extracted oil was 9.10 °C, while acetone extracted oil was 7.29 °C. The onset and offset temperature (marking beginning of crystallization and end of melting respectively) were 10.44 & 17.70, 12.92 & 18.76, and 12.90 & 19.09 °C for acetone, n-hexane and petroleum ether extracted oils respectively. It will be observed that SC-CO2 had no crystallization parameter up to 40 °C when the oil was at solid state, hence, the non-appearance of exothermic reaction parameters. The extraction of the SC-CO2 oil using pressurized CO2 might be responsible for the behaviour of SC-CO2 oil. DSC shows purity of compounds through its precise melting and crystallization temperatures (Tolstorebrov et al., 2014). Melting temperature had been shown to be the most reliable indicator of thermostability; the higher the melting temperature, the higher the stability; therefore, SC-CO2 oil could be described as the most stable oil. On the other hand, the high melting temperature observed for ethanol extracted oil could be as a result of impurities which can be observed from two peaks reported for ethanol extracted. In addition to displaying polymorphism, ethanol extracted oil had broad melting points. A broad melting point at lower temperature is an indication of the presence of impure compounds.
3.5. Microscopy
The microscope images of the extracted oil using acetone (A), ethanol (E), n-hexane (H), pet-ether (P) and SC-CO2 are shown in Figure 2 (a-e). SC-CO2 extracted oil had the highest concentration of oil droplets, followed by n-hexane oil, pet-ether extracted oil, and then ethanol and acetone extracted oil. This result verifies the earlier observation that the high oil yields obtained for ethanol and acetone contained some impurities like water and fibre. The SC-CO2 oil had the best quality judging from the clarity of the oil droplets. In addition, SC-CO2 had very tiny droplets which signified that the extracted oil had been reduced in size making SC-CO2 suitable in nanotechnology. SC-CO2 is one of the process utilized in the production of nanoparticles. The advantage of this is that the oil produced via SC-CO2 will be easily absorbed by the body. Oil extracted using conventional solvents were found to possess very large particles. Therefore, in addition to producing pure oil, SC-CO2 also produced oil that is easily absorbed by the body system. Nanoparticle production and utilization is a growing area of science and technology. SC-CO2 extraction will therefore play a vital role in its realization. Mango kernel oil had been reported to contain bioactive compounds; reduction to nanoparticles via SC-CO2 extraction will boost utilization of mango kernel oil using SFE technique.
4. Conclusions
The characterization of mango kernel seeds oil revealed that it has higher unsaturated fatty acid consisting primarily oleic acid in most instances with the exception of the use of ethanol solvent and SC-CO2 techniques. The melting points, which is a parameter used to identify purity of compounds were also identified using DSC. The functional groups present were majorly CH3, CH2 and COH, while ethanol extraction had CH2 and OH; these will assist in authenticating mango kernel oil. Although SC-CO2 had lower yield, the microscopy analysis showed that it had greater oil concentration as well as oil that were very small (probably micro or nano) in size. This would give an edge to SC-CO2 extracted oil in terms of utilization. Ethanol may not be a good solvent for extracting oil from oil seeds because the characterization of ethanol extracted oil showed that it had extraneous compounds as indicated by the thermal profile, FTIR and microscopy.
Declarations
Author contribution statement
Olugbenga Awolu: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Balaraman Manohar: Conceived and designed the experiments.
Funding statement
This work was supported by TWAS-CSIR.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Awolu, O.O. acknowledges The World Academic of Science (TWAS) and Council of Scientific and Industrial Research (CSIR), India for the award of postdoctoral fellowship for this research at Central Food Technological Research Institute (CFTRI), Mysore, India.
References
- Akanda M.J.H., Sarker M.Z.I., Norulaini N., Ferdosh S., Rahman M.M., Omar A.M. Optimization of supercritical carbon dioxide extraction parameters of cocoa butter analogy fat from mango seed kernel oil using response surface methodology. J. Food Sci. Technol. 2015;52(1):319–326. [Google Scholar]
- Atta M.A., Irman J., Muhammad A., Muhammad I., Athar M., Muhammad N., Muhammad A. Chemical characteristics of mango (Mangifera indica L.) kernel oil and palm oil blends for probable use as vanaspati. J. Oil Palm Res. 2016;28(3):344–352. [Google Scholar]
- Awolu O.O., Obafaye R.O., Ayodele B.S. Optimization of solvent extraction of oil from neem (Azadirachta indica) and its characterizations. J. Sci. Res. Rep. 2013;2(1):304–314. [Google Scholar]
- Balmain J., Hannoyer B., Lopez E. Fourier transform infrared spectroscopy (FTIR) and X-ray diffraction analyses of mineral and organic matrix during heating of mother of pearl (nacre) from the shell of the Mollusc pinctada maxima. J. Biomed. Mater. Res. 1999;48(5):749–754. doi: 10.1002/(sici)1097-4636(1999)48:5<749::aid-jbm22>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Cebula D.J., Smith K.W. Differential scanning calorimetry of confectionery fats. Part II—effects of blends and minor components. J. Am. Oil Chem. Soc. 1992;69:992–998. [Google Scholar]
- Dayan C., Kumudha A., Sarada R., Ravishankar G.A. Isolation, characterization and outdoor cultivation of green microalgae Botryococcus sp. Sci. Res. Essays. 2010;5(17):2497–2505. [Google Scholar]
- Dhara R., Bhattacharyya D.K., Ghosh M. Analysis of sterol and other components present in unsaponifiable matters of mahua, sal and mango kernel oil. J. Oleo Sci. 2010;59(4):169–176. doi: 10.5650/jos.59.169. [DOI] [PubMed] [Google Scholar]
- Dion B., Ruzbie M., Van de Voort F.R., Ismail A.A., Blais J.S. Determination of protein and fat in meat by transform infrared spectrometry. Analyst. 1994;119:1765È1771. [Google Scholar]
- Dollimore D. Thermal analysis. Anal. Chem. 1996;68(10):63R–71R. [Google Scholar]
- Friedrich J.P. U.S. Patent No. 4,466,923. U.S. Patent and Trademark Office; Washington, DC: 1984. [Google Scholar]
- Friedrich J.P., List G.R., Heakin A.J. Petroleum-free extraction of oil from soybeans with supercritical CO2. J. Am. Oil Chem. Soc. 1982;59(7):288–292. [Google Scholar]
- Gadkari P.V., Balarman M., Kadimi U.S. Polyphenols from fresh frozen tea leaves (Camellia assamica L.) by supercritical carbon dioxide extraction with ethanol entrainer-application of response surface methodology. J. Food Sci. Technol. 2015;52(2):720–730. doi: 10.1007/s13197-013-1085-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallignani M., Garrigues S., De la Guardia M. Derivative Fourier transform infrared spectrometric determination of ethanol in alcoholic beverages. Anal. Chim. Acta. 1994;287:275–283. [Google Scholar]
- García-Rodríguez D., Carro-Díaz A.M., Lorenzo-Ferreira R.A. Supercritical fluid extraction of polyhalogenated pollutants from aquaculture and marine environmental samples: a review. J. Sep. Sci. 2008;31(8):1333–1345. doi: 10.1002/jssc.200700637. [DOI] [PubMed] [Google Scholar]
- Guillen M.D., Cabo N. Infrared spectroscopy in the study of edible oils and fats. J. Sci. Food Agric. 1997;75(1):1–11. [Google Scholar]
- Karunanithi B., Bogeshwaran K., Tripuraneni M., Krishna Reddy S. Extraction of mango seed oil from mango kernel. Int. J. Eng. Res. Dev. 2015;11(11):32–41. [Google Scholar]
- Kittiphoom S., Sutasinee S. Mango seed kernel oil and its physicochemical properties. Int. Food Res. J. 2013;20(3):1145–1149. [Google Scholar]
- Mukhopadhyay M. CRC Press; Boca Raton, FL: 2000. Natural Extracts Using Supercritical Carbon Dioxide. [Google Scholar]
- Nzikou J.M., Kimbonguila A., Matos L., Loumouamou B., Pambou-Tobi N.P.G., Ndangui C.B., Desobry S. Extraction and characteristics of seed kernel oil from mango (Mangifera indica) Res. J. Environ. Earth Sci. 2010;2(1):31–35. [Google Scholar]
- Rai A., Mohanty B., Bhargava R. Supercritical extraction of sunflower oil: a central composite design for extraction variables. Food Chem. 2016;192:647–659. doi: 10.1016/j.foodchem.2015.07.070. [DOI] [PubMed] [Google Scholar]
- Sagiri S.S., Sharma V., Basak P., Pal K. Mango butter emulsion gels as cocoa butter equivalents: physical, thermal, and mechanical analyses. J. Agric. Food Chem. 2014;62(47):11357–11368. doi: 10.1021/jf502658y. [DOI] [PubMed] [Google Scholar]
- Sahena F., Zaidul I.S.M., Jinap S., Karim A.A., Abbas K.A., Norulaini N.A.N., Omar A.K.M. Application of supercritical CO2 in lipid extraction – a review. J. Food Eng. 2009;95:240–253. [Google Scholar]
- Sessa D.J. Derivation of a cocoa butter equivalent from jojoba transesterified ester via a differential scanning calorimetry index. J. Sci. Food Agric. 1996;72:295–298. [Google Scholar]
- Tan C.P., Man Y.C. Differential scanning calorimetric analysis of edible oils: comparison of thermal properties and chemical composition. J. Am. Oil Chem. Soc. 2000;77(2):143–155. [Google Scholar]
- Tardy A.L., Morio B., Chardigny J.M., Malpuech-Brugere C. Ruminant and industrial sources of trans-fat and cardiovascular and diabetic diseases. Nutr. Res. Rev. 2011;24(1):111–117. doi: 10.1017/S0954422411000011. [DOI] [PubMed] [Google Scholar]
- Tariq M., Ali S., Ahmad F., Ahmad M., Zafar M., Khalid N., Khan M.A. Identification, FTIR, NMR (1 H and 13 C) and GC/MS studies of fatty acid methyl esters in biodiesel from rocket seed oil. Fuel Process. Technol. 2011;92(3):336–341. [Google Scholar]
- Tolstorebrov I., Eikevik T.M., Bantle M. A DSC determination of phase transitions and liquid fraction in fish oils and mixtures of triacylglycerides. Food Res. Int. 2014;58:132–140. [Google Scholar]
- Van de Voort F.R. Fourier transform infrared spectroscopy applied to food analysis. Food Res. Int. 1992;25(5):397–403. [Google Scholar]