Abstract
Lemon myrtle (Backhousia citriodora) is one of the most commercially grown native herbs in Australia. This study aimed to evaluate the effects of different drying methods on phenolic compounds and antioxidant properties of lemon myrtle leaves to identify the most suitable drying conditions. The drying methods include hot air drying, vacuum drying, microwave drying, sun drying, shade drying and freeze drying. The results showed that drying conditions significantly (p < 0.05) affected the retention of total phenolic content (TPC), total flavonoids (TFC), proanthocyanidins, gallic acid, hesperetin, and antioxidant properties of lemon myrtle leaves. The optimal conditions for hot air drying and vacuum drying are 90 °C for 75 min and 90 °C for 120 min, respectively; whereas optimal drying conditions for microwave drying are 960 W for 7 min, and the time required for sun drying and shade drying are 2 days and 12 days, respectively. The freeze dried leaves contained the highest level of TPC, TFC, proanthocyanidins, gallic acid and hesperetin (74.11 ± 2.87 mg GAE/g dw, 87.15 ± 2.70 mg CE/g dw, 123.49 ± 6.12 mg CE/g dw, 53.77 ± 0.22 mg/g dw and 38.99 ± 0.26 mg/g dw, respectively). The freeze dried leaves also contained higher antioxidant capacity as compared to other samples. No significant difference in phenolic compounds and antioxidant capacity was observed between tested other drying methods. Therefore, any of these methods can be selected for dehydration of lemon myrtle leaves for industrial purposes. However, microwave drying can be selected for drying of lemon myrtle leaves for an industrial scale as it was the most time and/or energy efficient technique.
Keywords: Food engineering, Food quality, Food processing, Thermal food processing, Food analysis, Bioactive compound, Antioxidant, Phenolic compound, Chemical food analysis, Drying, Flavonoid, Gallic acid, Hesperetin, Lemon myrtle, Phenolic compound
Food engineering; Food quality; Food processing; Thermal food processing; Food analysis; Bioactive compound; Antioxidant; Phenolic compound; Chemical food analysis; Antioxidant; Drying; Flavonoid; Gallic acid; Hesperetin; Lemon myrtle; Phenolic compound
1. Introduction
Lemon myrtle (Backhousia citriodora), is a member of the genus Backhousia under the family Myrtaceae. It is an Australian native rainforest plant, naturally grown in coastal Queensland from Brisbane to Cairns, in a range of altitudes from 50 to over 800 m above sea level. Naturally it can grow from a large shrub to medium-sized evergreen tree 3–30 m high (Buchaillot et al., 2009). The leaves contain a high level of bioactive compounds and a lemon scented aroma. Due to its strong aroma, lemon myrtle leaf has been used as a herb or flavouring element in cooking for centuries. Lemon myrtle leaf has been used in tea blends, beverages, dairy products, biscuits, breads, confectionery, pasta, syrups, liqueurs, flavoured oils, packaged fish (salmon), dipping and simmer sauces. It can also be used in lemon-flavoured dairy products, such as cheesecakes, ice-cream, and sorbet as a lemon substitute to solve the curdling problem related with lemon fruit acidity (Konczak et al., 2010). Due to its versatility, its demand is increasing and it has become one of the most widely grown native plants in Australia. Lemon myrtle leaf is a rich source of various volatile and non-volatile compounds. Non-volatile compounds such as phenolic acids, flavonoids and proanthocyanidins have been identified in lemon myrtle leaves (Guo et al., 2014; Sakulnarmrat et al., 2013; Sakulnarmrat and Konczak, 2012; Sommano et al., 2013). The leaf extract exhibits high antioxidant activity and anti-microbial properties (Dupont et al., 2006; Sakulnarmrat et al., 2013; Sakulnarmrat and Konczak, 2012; Sommano et al., 2013), revealing its potential application in food, pharmaceuticals and cosmetics industry. Fresh leaves are usually used for the extraction of phytochemicals, however, it is not always feasible in large quantities; hence, drying of fresh leaves for further extraction is usually applied as the first step in industrial scale operations.
Drying is a process to remove moisture from fresh material and reduce its water activity, which inhibits microbial growth and minimize deteriorative biochemical reactions (Buchaillot et al., 2009). It also reduces the weight and volume of the sample thereby reducing storage and transportation costs (Pham et al., 2015; Saifullah et al., 2016; Shrestha et al., 2007). In addition, drying can modify the physical micro structure of plant tissues, which leads to increased extraction yields. For example, freeze drying causes significant changes in the micro structure of the final dried product making it more porous, meaning solvents can easily penetrate the sample and thus extract more phytochemicals (Harnkarnsujarit et al., 2016; Oikonomopoulou et al., 2011). However, drying can have adverse effects on phytochemical and nutritional components, especially heat sensitive compounds (Nadi, 2017; Nguyen et al., 2015, 2018; Nóbrega et al., 2015; Pham et al., 2015). Different drying methods, such as freeze drying, hot air drying, vacuum drying and microwave drying, and drying conditions including time, temperature, microwave power level, and air velocity have link with various energy consumption and significant effect on phytochemicals and antioxidant properties of the samples (Nguyen et al., 2015, 2018; Papoutsis et al., 2017; Vu et al., 2017). Therefore, it is necessary to identify the most suitable drying method and conditions for a specific type of plant sample.
To date, there are no studies investigating the effects of different drying methods and conditions on phytochemicals and antioxidant properties of lemon myrtle leaves for further processing. Therefore, this study aimed to evaluate the effects of different drying techniques, including hot air drying, vacuum drying, microwave drying, sun drying, shade drying and freeze drying on total phenolic content, total flavonoids, proanthocyanidins, antioxidant capacity and two major phenolic compounds gallic acid and hesperetin of lemon myrtle leaves. The energy consumption by different drying techniques, as well as the correlation between phytochemicals and antioxidant capacity of lemon myrtle leaf were investigated.
2. Materials and methods
2.1. Plant sample
Lemon myrtle leaves were collected from trees located at the Central Coast, NSW, Australia (latitude of 33.4°S, longitude of 151.4°E) in November. The leaves were randomly picked from several trees and mixed well. After picking up, the leaves were transported to the laboratory immediately and directly put into dryer or stored at -18 °C; to minimise the degradation of bioactive compounds and antioxidant properties.
2.2. Analytical chemicals
Organic solvents (acetone, methanol, ethanol and acetonitrile) were purchased from Merck (Darmstadt, Germany). Folin-Ciocalteu's reagent, anhydrous sodium carbonate (Na2CO3), sodium nitrite (NaNO2), hydrochloric acid (HCl), formic acid, potassium persulfate (K2S2O8), copper (II) chloride (CuCl2), ferric chloride (FeCl3), sodium acetate (C2H3NaO2), aluminium chloride hexahydrate (AlCl3•6H2O), ammonium acetate (C2H7NO2), 2,2-diphenyl-1-picrylhydrazil (DPPH), 2,2-Azino-bis(3-ethylbenzothiazoline-6-sulphoonic acid) diammonium salt (ABTS), (±)-6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (trolox), 2, 4, 6-tri(2-pyridyl)-s-triazine (TPTZ), neocuproine, gallic acid, hesperetin, and catechin were purchased from Sigma-Aldrich Pty Ltd. (Castle Hill, Sydney, Australia). Vanillin and sodium hydroxide (NaOH) were obtained from Merck (Darmstadt, Germany). All chemicals used in this study were analytical grade.
2.3. Experimental design
In this study, six different methods were performed to assess the effect of different drying techniques: sun, shade, hot air, vacuum, microwave and freeze drying. After collection, the leaves were immediately put into the drying oven or put on trays for shade drying and sun drying, then the leaves were dried to a constant weight.
Hot air drying: Lemon myrtle leaves were spread on three separate trays with a layer thickness of approximately 0.5 cm. The samples were dried to a constant weight under three different drying temperatures (50 °C, 70 °C and 90 °C) using a hot air convection oven (LABEC Laboratory Equipment Pty Ltd, Marrickville, NSW, Australia).
Vacuum drying: Lemon myrtle leaves were spread on three separate trays with a layer thickness of approximately 0.5 cm. The samples were dried to a constant weight under three different drying temperatures (50 °C, 70 °C and 90 °C) with a constant vacuum pressure of 69 ± 1 kPa using a vacuum oven (Thermoline, Australian Marketing Group, Marrickville, NSW, Australia).
Microwave drying: Lemon myrtle leaves were spread with a thickness of approximately 0.5cm on the turntable glass plate of a microwave oven (Sharp Carousel Inverter 1200W, The Good Guys, Tuggerah, NSW, Australia) and dried to a constant weight under three different microwave out put power levels: 720 W, 960 W and 1200 W. The true microwave power level was determined according to IMPI 2-L test (Buffler, 1993). The true microwave output power levels were 604 W, 814 W, and 1036 W for the microwave out power levels 720 W, 960 W, and 1200 W respectively.
Freeze drying: Lemon myrtle leaves were dipped into liquid nitrogen to facilitate better drying, then spread on trays with a layer thickness of approximately 0.5 cm. The samples were dried to a constant weight for 48 h using a freeze dryer (SP Scientific, Bench Top Pro BTP-3ESE0X, Warminster, Philadelphia, USA).
Shade drying: Lemon myrtle leaves were spread on three separate trays with a layer thickness of approximately 0.5 cm and dried in the shade at ambient laboratory conditions (approximate temperature 25–28 °C) for 12 days.
Sun drying: Lemon myrtle leaves were spread on three separate trays with a layer thickness of approximately 0.5 cm and dried in direct sunlight for 2 days to a constant weight.
After drying, the drying time was recorded. Dried samples were then ground to reduce and homogenise particle size using a commercial blender (John Morris Scientific, Chastwood, NSW, Australia), followed by sieving through a steel mesh sieve with pore size 1.4 mm (EFL, 2000, Endecotts Ltd., London, England). Final moisture content of the ground leaves from different drying conditions was measured using a moisture analyser (AD-4712, Japan). Then all the samples were put into air tight containers with proper labels and stored at -18 °C for further use.
2.4. Energy consumption of drying methods
Energy consumption by hot air oven and vacuum oven at different drying conditions was determined as it has been described by Nguyen et al. (2018) using Eq. (1)
| (1) |
Where, EC is the energy consumption (kWh), Dtemp is the drying temperature used (°C), Mtemp is the maximum temperature (°C) of the drying equipment, MO is the maximum energy output (kW) for the drying equipment and T is the drying time (h).
Energy consumption by freeze dryer and microwave oven at different power levels were estimated as it has been described by Nguyen et al. (2016) using Eq. (2)
| EC = P×t | (2) |
Where, P is the electrical power supplied (kW), and t is the time needed for drying the sample (h).
Sun and shade drying required no electrical input and is considered as 0 kWh.
2.5. Determination of phytochemicals and antioxidant properties
Ground and dried lemon myrtle leaf sample was extracted in 50% acetone in water using ultrasonic assisted extraction technique. 0.25 g of dried sample was transferred into a 50 mL centrifuge tube and 25 mL solvent was then added into the sample. The centrifuge tube containing sample and solvent was sealed with a screw cap and sonicated at 150 W for 30 min at room temperature (28 ± 2 °C) using an ultrasonic bath (Soniclean, 220 V, 50 Hz, 250 W, Soniclean Pty Ltd., Thebarton, Australia). During extraction the tube was vortexed for 2–3 s every 5 min. After sonication the extract was filtered using a nylon syringe filter (0.45 μm) and filtrate was stored at -18 °C for phytochemical and antioxidant analysis.
2.5.1. Phytochemical analysis
Total phenolic contents (TPC): The TPC in the sample extract was measured according to the method described by Škerget et al. (2005). The absorbance was measured at 765 nm using a UV spectrophotometer (Cary 60 Bio, UV-Vis, Malaysia). Gallic acid was used to build up the standard curve and the results were expressed as milligram gallic acid equivalent per gram of dry weight sample (mg GAE/g dw). The calibration curves equation was Y = 0.0126X+ 0.0598 and determination coefficient was R2 = 0.9988. Where, Y was the absorbance of light and X was the concentration of compound.
Total flavonoid contents (TFC): The TFC of lemon myrtle extract was assessed according to a previously reported method Zhishen et al. (1999). Absorbance was measured at 510 nm using a UV spectrophotometer (Cary 60 Bio, UV-Vis, Malaysia). Catechin was used to establish the standard curve and the results were expressed as mg of catechin equivalents per g of dry weight sample (mg CE/g dw). The calibration curves equation was Y = 0.0023X+ 0.0074 and determination coefficient was R2 = 0.9937. Where, Y was the absorbance of light and X was the concentration of compound.
Proanthocyanidins: Proanthocyanidins were measured as described by Li et al. (2006). The absorbance was measured at 500 nm using a UV spectrophotometer (Cary 60 Bio, UV-Vis, Malaysia). Catechin was used to prepare the standard curve and the results were expressed as mg of catechin equivalents per g of dry weight sample (mg CE/g dw). The calibration curves equation was Y = 0.0027X - 0.0066 and determination coefficient was R2 = 0.9976. Where, Y was the absorbance of light and X was the concentration of compound.
2.5.2. High performance liquid chromatography (HPLC) for gallic acid and hesperetin
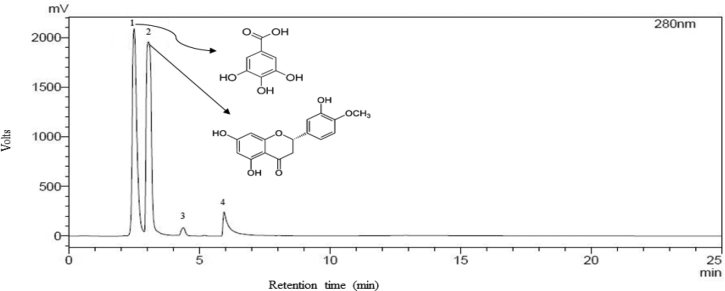
Gallic acid and hesperetin were determined using a HPLC system (CBM-20A, Shimadzu Australia, Rydalmere, NSW, Australia). Two mobile phases were used as gradient solvents, including mobile phase A: 0.2% formic acid in deionized water, and mobile phase B: 100% acetonitrile. Mobile phase flow rate was set to 1 mL/min with the gradient: from 0-5 min 100% (A), from 5 to15 min 30% (B), from15 to 20 min 70% (B), from 20-25 min 100% (B). The injection volume was 20 μL. During operation the column oven temperature was maintained at 25 °C. The column used for this analysis was C18(2) reversed-phase column (Luna 100A° 5 μm, 250 mm × 4.6 mm; Phenomenex Australia Pty., Ltd., Lane Cove, NSW, Australia). An UV-VIS detector was used to detect individual compounds at 280 nm and the chromatogram of the extract is shown in Figure 1. The method was validated by using known standards and the compounds in the extract were identified based on peak retention time. For double confirmation of the identified compounds, a known concentration of the specific standard was added into sample and run through HPLC system. Finally, the sample was analysed using LCMS-2020 (Shimadzu) and the compounds was further confirmed based on molecular mass. Quantification of the compounds was calculated from calibration curve of specific standards. The results were expressed as mg per g of dry weight sample.
Figure 1.
HPLC chromatogram of lemon myrtle leaf extract.
2.5.3. Antioxidant analysis
Ferric-Reducing Antioxidant Power (FRAP) assay: FRAP of lemon myrtle leaf extract was measured according to the method described by Thaipong et al. (2006). The absorbance was measured at 593 nm using a UV spectrophotometer (Cary 60 Bio, UV-Vis, Malaysia). Trolox was used to develop calibration curve and the results were expressed as mM of trolox equivalents per g of dry weight sample (mM TE/g dw). The calibration curves equation was Y = 0.0022X+ 0.1389 and determination coefficient was R2 = 0.9928. Where, Y was the absorbance of light and X was the concentration of compound.
Cupric Ion-Reducing Antioxidant Capacity (CUPRAC) assay: Cupric ion-reducing activity of sample extract was assessed as described by Apak et al. (2004). The absorbance was measured at 450 nm using a UV spectrophotometer (Cary 60 Bio, UV-Vis, Malaysia). Trolox was used to develop calibration curve and the results were expressed as mM of trolox equivalents per g of dry weight sample (mM TE/g dw). The calibration curves equation was Y = 0.001X+ 0.075 and determination coefficient was R2 = 0.9774. Where, Y was the absorbance of light and X was the concentration of compound.
ABTS Radical Scavenging Capacity assay: ABTS radical scavenging capacity of sample extract was measured as described by Thaipong et al. (2006). The absorbance was measured at 734 nm using a UV spectrophotometer (Cary 60 Bio, UV-Vis, Malaysia). Trolox was used to develop calibration curve and the results were expressed as mM of trolox equivalents per g of dry weight sample (mM TE/g dw). The calibration curves equation was Y = 0.0011x - 0.0643 and determination coefficient was R2 = 0.9874. Where, Y was the absorbance of light and X was the concentration of compound.
DPPH Radical Scavenging Capacity assay: DPPH radical scavenging capacity of lemon myrtle extract was evaluated by the method reported by Thaipong et al. (2006). The absorbance was measured at 515 nm using a UV spectrophotometer (Cary 60 Bio, UV-Vis, Malaysia). Trolox was used to develop calibration curve and the results were expressed as mM of trolox equivalents per g of dry weight sample (mM TE/g dw). The calibration curves equation was Y = 0.0011X- 0.0454 and determination coefficient was R2 = 0.9974. Where, Y was the absorbance of light and X was the concentration of compound.
2.6. Statistical analysis
All the experiments were conducted at least in triplicates. JMP (version 13) was used to analyse the data and the results were presented as means ± standard deviations. One-way analysis of variance and Tukey post hoc test were performed to compare different drying conditions and drying methods. Correlation between phytochemicals and antioxidant capacity of sample was analysed using Pearson correlation analysis in SPSS software version 24. The differences were considered at the significance level of p < 0.05.
3. Results and discussion
3.1. Effect of hot air drying on phytochemicals and antioxidant properties of lemon myrtle leaves
In this study, three different hot air drying temperatures were applied to dry lemon myrtle leaves and assess the impact of different drying temperatures on phytochemical recovery. Moisture content in dried samples using 50, 70 and 90 °C drying temperature was 5.29 ± 0.21%, 5.93 ± 0.13%, and 5.04 ± 0.19% respectively. Highest total phenolic content (TPC) was found in the leaves dried at 90 °C for 75 min (Table 1). The TPC was 64.96 ± 3.84 mg GAE/g dw, this value was significantly higher than those of the leaves dried at 70 °C and 50 °C (p < 0.05). No considerable difference in TPC was observed in the leaves dried at 70 °C and 50 °C. Similarly, total flavonoid content (TFC) was also significantly higher in the leaves dried at 90 °C (72.58 ± 2.02 mg CE/g dw) in comparison with those dried at 50 °C and 70 °C (Table 1). It is interesting to note that, levels of proanthocyanidins (Table 1) were not significantly different when the leaves were dried at 50 °C, 70 °C and 90 °C (p > 0.05). Our findings indicate that hot air drying temperatures in the range of 50–90 °C significantly affected retention of TPC and TFC, but not proanthocyanidins. These findings can be explained by the longer times required by lower drying temperatures, thus the leaves had longer exposure to heat, which resulted in degradation of TPC and TFC, which were more unstable than proanthocyanidins. Similar findings were observed by Vu et al. (2017) during drying of banana (Musa cavendish) peels at 80, 100 and 120 °C. They found hot air drying at 120 °C requires shorter drying times and thus retains higher phytochemical content and antioxidant capacity in the dried sample as compared to other drying conditions at lower temperatures.
Table 1.
Effects of hot air drying on phytochemical content and antioxidant properties of lemon myrtle leaf.
| Temperature (°C) | 50 | 70 | 90 |
| Drying time (min) | 315 min | 105 min | 75 min |
|
Final moisture content (%) |
5.29 ± 0.21 |
5.93 ± 0.13 |
5.04 ± 0.19 |
|
Total phytochemical | |||
| TPC (mg GAE/g dw) | 51.63 ± 2.03b | 52.47 ± 1.29b | 64.96 ± 3.84a |
| TFC (mg CE/g dw) | 64.29 ± 4.23b | 64.82 ± 1.25b | 72.58 ± 2.02a |
| Proanthocyanidin (mg CE/g dw) |
79.63 ± 7.47a |
83.73 ± 7.10a |
95.72 ± 6.54a |
|
Antioxidant capacity | |||
| FRAP (mM TE/g dw) | 616.06 ± 35.94b | 622.63 ± 10.74b | 821.15 ± 24.58a |
| CUPRAC (mM TE/g dw) | 4435.06 ± 364.49b | 4674.11 ± 132.16b | 5696.08 ± 113.78a |
| ABTS (mM TE/g dw) | 1258.23 ± 57.83b | 1273.50 ± 66.67b | 1615.48 ± 24.98a |
| DPPH (mM TE/g dw) |
735.24 ± 53.83b |
808.31 ± 35.83b |
963.56 ± 53.18a |
|
Individual compounds | |||
| Gallic acid (mg/g dw) | 41.21 ± 0.86c | 45.21 ± 0.21b | 50.74 ± 0.73a |
| Hesperetin (mg/g dw) | 38.16 ± 0.32ab | 38.47 ± 0.15a | 37.72 ± 0.21b |
The values are the means ± standard deviations for at least triplicate experiments and those in the same row not sharing the same superscript letter are significantly different from each other (p < 0.05). mg GAE/g dw = milligram gallic acid equivalent per gram of sample dry weight, mg CE/g dw = milligram equivalent catechin equivalent per gram of sample dry weight, mM TE/g dw = mM trolox equivalent per gram of sample dry weight, mg/g dw = milligram per gram of sample dry weight.
For antioxidant capacity, results from all the antioxidant assays (FRAP, CUPRAC, ABTS and DPPH) showed that the sample dried at 90 °C retains significantly (p < 0.05) higher antioxidant properties as compared to those in the samples dried at the two lower temperatures 50 °C and 70 °C (Table 1). However, the antioxidant capacity in the samples dried at 50 °C and 70 °C was insignificantly different for all assays. The antioxidant capacity value of sample dried at 90 °C was 821.15 ± 24.58 mM TE/g dw, 5696.08 ± 113.78 mM TE/g dw, 1615.48 ± 24.98 mM TE/g dw, 963.56 ± 53.18 mM TE/g dw for FRAP, CUPRAC, ABTS and DPPH respectively. Our findings was supported by previous research on drying of red pepper; in which Vega-Gálvez et al. (2009) found samples dried at higher temperatures (i.e. 80 and 90 °C) exhibit higher DPPH racial scavenging activity rather than at low temperatures (i.e. 50, 60 and 70 °C). These can be explained by longer exposure to heat when drying at low temperatures may stimulate degradation of phytochemicals, thus reduce in antioxidant capacity (Garau et al., 2007). In addition, derivatives from certain reactions (i.e. Maillard reaction) may form and accumulate at high temperatures, which might have an effect on antioxidant capacity of the final dried product (Miranda et al., 2009; Vu et al., 2017).
The effects of hot air oven temperature on major individual compounds, gallic acid and hesperetin in lemon myrtle leaves were observed in this study by HPLC analysis (Table 1). The gallic acid retention was considerably different for each drying temperature. The gallic acid content of the sample was higher with increasing temperature, 41.21 ± 0.86 (mg/g dw), 45.21 ± 0.21 (mg/g dw), 50.74 ± 0.73 (mg/g dw) for 50 °C, 70 °C and 90 °C, respectively. Our findings were in agreement with results of a previous study on drying lemon pomace Papoutsis et al. (2017), who found gallic acid retention is higher when oven drying temperature increased from 70 to 110 °C. A different trend was observed with hesperetin. The highest hesperetin content (38.47 ± 0.15 mg/g dw) was obtained in samples dried at 70 °C and that was significantly higher (p < 0.05) in comparison with the sample dried at 90 °C (37.72 ± 0.21 mg/g dw). However, hesperetin levels in samples dried at 50 °C (38.16 ± 0.32 mg/g dw) was not significantly different to the samples dried at 70 °C and 90 °C. These findings were supported by results in a previous study by Mphahlele et al. (2016), who found that oven drying temperatures have different effects on various individual phenolic compounds. These can be explained by different molecular structures of phenolic compounds, which may or may not undergo oxidation, enzymatic degradation/copigmentation reactions at certain drying conditions.
Based on overall results, hot air drying at 90 °C for 75 min was selected as the best conditions for drying lemon myrtle using hot air drying as these conditions retained higher levels of total phytochemical content, antioxidant property and individual compounds. This condition was also used for further comparison with other drying techniques.
3.2. Effect of vacuum drying on phytochemical and antioxidant properties of lemon myrtle leaves
Vacuum drying is a common method used for drying different food products, especially food containing heat sensitive compounds (Methakhup et al., 2005). In this study three drying temperatures 50, 70 and 90 °C was applied under a vacuum pressure of 69 ± 1 kPa and the final moisture content in dried samples was 5.10 ± 0.01%, 5.78 ± 0.11%, and 4.76 ± 0.21% respectively. The highest TPC, TFC and proanthocyanidins were found in lemon myrtle leaves dried at 90 °C for 120 min with 66.08 ± 2.11 (mg GAE/g dw), 75.02 ± 3.54 (mg CE/g dw), 105.14 ± 1.92 (mg CE/g dw), respectively (Table 2). Similarly, leaves dried at 90 °C for 120 min had the highest antioxidant capacities FRAP (851.89 ± 62.29 mM TE/g dw), CUPRAC (5564.08 ± 187.39 mM TE/g dw), ABTS (1567.23 ± 127.16 mM TE/g dw), and DPPH (898.39 ± 105.15 mM TE/g dw). The highest amount of gallic acid was also obtained from the sample dried at 90 °C (51.51 ± 0.89 mg/g dw), whereas the lowest amount of gallic acid was found in sample dried at 50 °C (43.57 ± 0.26 mg/g dw). The amount of hesperetin was higher in samples dried at 70 °C (38.50 ± 0.22 mg/g dw) and 90 °C (38.17 ± 0.04 mg/g dw) in comparison with that in the sample dried at 50 °C (38.00 ± 0.23 mg/g dw). Previous study by Papoutsis et al. (2017) found similar findings when drying lemon pomace using different vacuum drying temperatures (70, 90 and 110 °C) and the higher level of gallic acid was obtained when temperature increased from 70 to 110 °C. These could be explained by faster inactivation of oxidase enzyme (polyphenol oxidase) at higher temperature (Lim and Murtijaya, 2007).
Table 2.
Impact of vacuum drying on phytochemical content and antioxidant properties of lemon myrtle leaf.
| Temperature (°C) | 50 | 70 | 90 |
| Drying time (min) | 330 min | 210 min | 120 min |
|
Final moisture content (%) |
5.1 ± 0.01 |
5.78 ± 0.11 |
4.76 ± 0.21 |
|
Total phytochemical | |||
| TPC (mg GAE/g dw) | 56.27 ± 2.48b | 56.08 ± 0.89b | 66.08 ± 2.11a |
| TFC(mg CE/g dw) | 66.45 ± 1.63b | 63.14 ± 0.39b | 75.02 ± 3.54a |
| Proanthocyanidin (mg CE/g dw) |
95.83 ± 2.51b |
96.24 ± 2.13b |
105.14 ± 1.92a |
|
Antioxidant capacity | |||
| FRAP (mM TE/g dw) | 688.76 ± 6.27b | 688.14 ± 39.62b | 851.89 ± 62.29a |
| CUPRAC (mM TE/g dw) | 4924.48 ± 36.96b | 4868.37 ± 72.68b | 5564.08 ± 187.39a |
| ABTS (mM TE/g dw) | 1357.41 ± 47.57b | 1395.29 ± 34.82ab | 1567.23 ± 127.16a |
| DPPH (mM TE/g dw) |
829.96 ± 50.28a |
780.83 ± 45.07a |
898.39 ± 105.15a |
|
Individual compounds | |||
| Gallic acid (mg/g dw) | 43.57 ± 0.26c | 45.84 ± 0.20b | 51.51 ± 0.89a |
| Hesperetin (mg/g dw) | 38.00 ± 0.23b | 38.50 ± 0.22a | 38.17 ± 0.04ab |
The values are the means ± standard deviations for at least triplicate experiments and those in the same row not sharing the same superscript letter are significantly different from each other (p < 0.05). mg GAE/g dw = milligram gallic acid equivalent per gram of sample dry weight, mg CE/g dw = milligram equivalent catechin equivalent per gram of sample dry weight, mM TE/g dw = mM equivalent trolox equivalent per gram of sample dry weight, mg/g dw = milligram per gram of sample dry weight.
Based on the overall results, vacuum drying at 90 °C with vacuum pressure of 69 ± 1 kPa for 120 min was recommended as the best conditions for recovery of phytochemicals from lemon myrtle leaves. This condition was applied for further comparison with other drying methods.
3.3. Effect of microwave drying on phytochemical and antioxidant properties of lemon myrtle leaves
Microwave drying has become a popular method for drying food due to faster drying rates, uniform drying and improved quality for some food materials (Punathil and Basak, 2016). Three microwave power levels of 720 W, 960 W and 1200 W (true microwave output 604 W, 814 W and 1036 W respectively) were used to dry lemon myrtle leaves. The final moisture content value in dried samples, and the effects of microwave power on phytochemical content and antioxidant properties are shown in Table 3. The final moisture content 0.4 ± 0.01%, 0.7 ± 0.12% and 0.8 ± 0.09% was in lemon myrtle powdered leaves dried at 720 W, 960 W, and 1200W respectively. Highest TPC and TFC levels were found in samples dried at 960 W and 1200 W. However, there was no significant difference was found for proanthocyanidins at 720 W, 960 W and 1200 W microwave power levels. These findings were in disagreement with a previous study on drying Phyllanthus amarus (Nguyen et al., 2015), which found TPC decreased at microwave power of exceeding 600 W. This could be explained by the difference in sensitivity/degradation of phenolic and flavonoid compounds in the two types of plant materials Phyllanthus amarus and Backhousia citriodora.
Table 3.
Influence of microwave drying on phytochemical content and antioxidant properties of lemon myrtle leaves.
|
Microwave power (W) (real out power) |
720 (604 W) |
960 (814 W) |
1200 (1036W) |
| Irradiation time (min) | 8 min | 7 min | 6 min |
|
Final moisture content (%) |
0.4 ± 0.01 |
0.7 ± 0.12 |
0.8 ± 0.09 |
|
Total phytochemical | |||
| TPC (mg GAE/g dw) | 58.21 ± 0.56b | 65.26 ± 2.60a | 61.17 ± 2.39ab |
| TFC (mg CE/g dw) | 59.22 ± 0.92b | 66.48 ± 3.32a | 65.95 ± 2.38a |
| Proanthocyanidin (mg CE/g dw) |
96.83 ± 8.99a |
96.58 ± 10.94a |
89.34 ± 5.78a |
|
Antioxidant capacity | |||
| FRAP (mM TE/g dw) | 793.57 ± 3.87a | 759.32 ± 19.59b | 771.36 ± 3.47ab |
| CUPRAC (mM TE/g dw) | 4737.57 ± 86.70a | 5074.52 ± 209.03a | 4855.38 ± 236.85a |
| ABTS (mM TE/g dw) | 1414.22 ± 70.91a | 1375.54 ± 62.68a | 1342.50 ± 34.52a |
| DPPH (mM TE/g dw) |
932.28 ± 8.17a |
890.41 ± 31.14a |
923.67 ± 36.45a |
|
Individual compounds | |||
| Gallic acid (mg/g dw) | 47.64 ± 0.34c | 50.66 ± 0.36a | 49.24 ± 0.44b |
| Hesperetin (mg/g dw) | 36.38 ± 0.37a | 36.56 ± 0.32a | 36.21 ± 0.29a |
The values are the means ± standard deviations for at least triplicate experiments and those in the same row not sharing the same superscript letter are significantly different from each other (p < 0.05). mg GAE/g dw = milligram gallic acid equivalent per gram of sample dry weight, mg CE/g dw = milligram equivalent catechin equivalent per gram of sample dry weight, mM TE/g dw = mM equivalent trolox equivalent per gram of sample dry weight, mg/g dw = milligram per gram of sample dry weight.
In case of antioxidant capacity, only FRAP showed a significance influence by different microwave power levels. There was no significant difference for CUPRAC, ABTS and DPPH. The FRAP value was higher at a microwave power of 720 W as compared to that dried at 960 W; however the FRAP value for sample dried at 1200 W was not significantly difference from the sample dried at 720 W and 960 W. These findings were supported by previous findings on drying Phyllanthus amarus (Nguyen et al., 2015). In the case of major individual compounds, the results show that microwave powers had a significant impact on gallic acid, but not hesperitin (Table 3). The sample dried at 960W had the highest amount of gallic acid (50.66 ± 0.36 mg/g dw), followed by leaf dried at 1200 W (Table 3), and the leaf dried at 720 W had the lowest level of gallic acid.
As total phenolic content and gallic acid were high in sample dried at 960 W with 7 min drying time, these conditions are recommended as the best microwave conditions for drying lemon myrtle leaves. These conditions were used for comparison with other drying methods.
3.4. Comparison of different drying methods on the basis of phytochemical and antioxidant property retention in dried lemon myrtle leaves
To identify the most suitable method for drying lemon myrtle leaves, the best conditions of hot air drying, vacuum drying, microwave drying were compared with other drying methods including sun drying, shade drying and freeze drying. The final moisture content in dried samples was varied with drying conditions. The highest and lowest final moisture content was observed in shade dried and microwave dried sample respectively (Table 4). The effects of different drying methods on total phytochemical content, antioxidant capacity and individual compounds are presented in Table 4. Different drying methods had a significant impact on total phytochemical content when drying lemon myrtle leaves. Except for the leaves dried using vacuum drying, freeze dried leaves had a significantly higher level of TPC (74.11 ± 2.87 mg GAE/g dw) in comparison with other drying methods, approximately 14%, 13%, 27% and 21% higher than hot air, microwave, sun and shade drying respectively. However, it is interesting to note that levels of TPC were not significantly different when lemon myrtle leaves were dried using other methods (p > 0.05). The results (Table 4) also showed that the highest level of TFC was observed in the leaves dried using the freeze drying method (87.15 ± 2.70 mg CE/g dw). There was no significant difference in levels of TFC when the leaves were dried using the hot air, vacuum or by sun and shade drying (p > 0.05). Microwave dried lemon myrtle leaves had the lowest level of TFC 66.47 ± 3.22 (mg CE/g dw) and was approximately 23% lower than TFC in freeze dried leaves. The freeze dried leaves also had the highest level of proanthocyanidins (123.49 ± 6.12 mg CE/g dw), however which was not significantly different to the shade dried leaves but significantly higher than other drying methods. Similarly, antioxidant capacity was found to be significantly (p < 0.05) higher in the freeze dried leaves than other methods; with the exception of DPPH in hot air dried samples. The values of FRAP, CUPRAC, ABTS, DPPH of freeze dried leaves were 985.70 ± 10.72 (mM TE/g dw), 6562.33 ± 209.37 (mM TE/g dw), 1806.06 ± 50.81 (mM TE/g dw), and 1158.15 ± 16.35 (mM TE/g dw), respectively (Table 4). The lowest values of FRAP, ABTS and DPPH were from the shade dried sample, with 609.27 ± 10.69 (mM TE/g dw), 1201.76 ± 20.02 (mM TE/g dw), and 878.90 ± 60.63 (mM TE/g dw), accordingly. The lowest CUPRAC value was 5074.52 ± 209.03 (mM TE/g dw) for microwave dried leaves at 960 W.
Table 4.
Effects of different drying method on the extractable phytochemicals and antioxidant capacity.
| Drying methods |
||||||
|---|---|---|---|---|---|---|
| HA 90 °C (1.25hr) | VO 90 °C (2hr) | MW 960W (814 W) (0.12hr) | Freeze drying (48hr) | Sun drying (2 days) | Shade drying (12 days) | |
| Final moisture content (%) | 5.04 ± 0.19 | 4.76 ± 0.21 | 0.7 ± 0.12 | 3.5 ± 0.31 | 7.03 ± 0.23 | 9.05 ± 0.17 |
| Energy consumption (kWh) |
1.013 |
0.48 |
0.097 |
165.33 |
0 |
0 |
| Total phytochemical groups | ||||||
| TPC (mg GAE/g dw) | 64.96 ± 3.84b | 66.08 ± 2.11ab | 65.26 ± 2.60b | 74.11 ± 2.87a | 58.32 ± 4.56b | 61.04 ± 1.13b |
| TFC (mg CE/g dw) | 72.58 ± 2.02bc | 75.02 ± 3.54b | 66.48 ± 3.32c | 87.15 ± 2.7a | 68.54 ± 2.9bc | 69.75 ± 2.11bc |
| Proanthocyanidin (mg TE/g dw) |
95.72 ± 6.54b |
105.14 ± 1.92b |
96.58 ± 10.94b |
123.49 ± 6.12a |
100.77 ± 5.21b |
109.01 ± 5.96ab |
| Antioxidant capacity | ||||||
| FRAP (mM TE/g dw) | 821.15 ± 24.58bc | 851.89 ± 62.29b | 759.32 ± 19.59cd | 985.7 ± 10.72a | 711.16 ± 29.13d | 609.28 ± 10.69e |
| CUPRAC (mM TE/g dw) | 5696.08 ± 113.78b | 5564.08 ± 187.39bc | 5074.52 ± 209.03d | 6562.33 ± 209.37a | 5335.41 ± 89.44bcd | 5128.46 ± 162.7cd |
| ABTS (mM TE/g dw) | 1615.48 ± 24.98b | 1567.23 ± 127.16bc | 1375.54 ± 62.68de | 1806.06 ± 50.81a | 1405.56 ± 46.17cd | 1201.76 ± 20.02e |
| DPPH (mM TE/g dw) |
963.56 ± 53.18ab |
898.39 ± 105.15bc |
890.41 ± 31.14bc |
1158.15 ± 16.35a |
819.31 ± 63.04bc |
878.9 ± 60.63c |
| Individual compounds | ||||||
| Gallic acid (mg/g dw) | 50.74 ± 0.73b | 51.51 ± 0.89b | 50.66 ± 0.36b | 53.77 ± 0.22a | 46.73 ± 0.27c | 46.14 ± 0.71c |
| Hesperetin (mg/g dw) | 37.72 ± 0.21c | 38.17 ± 0.04bc | 36.56 ± 0.32d | 38.99 ± 0.26b | 37.49 ± 0.50c | 39.85 ± 0.33a |
The values are the mean ± standard deviation for at least triplicate experiments and those in the same row not sharing the same superscript letter are significantly different from each other p < 0.05.
The impact of different drying techniques on gallic acid and hesperetin is revealed in Table 4. Results showed that freeze dried leaves had the highest level of gallic acid (53.77 ± 0.22 mg/g dw), followed by the leaves dried by vacuum drying, hot air drying and microwave drying. Gallic acid content in freeze dried sample was approximately 5%, 4%, 6%, 15% and 15% higher than the samples dried using hot air, vacuum, microwave, and sun and shade drying, respectively. The leaves dried by sun and shade had the lowest levels of gallic acid. The leaves dried by shade had the highest level of hesperetin (39.85 ± 0.33 mg/g dw), whereas the leaves dried by at 960W microwave had the lowest level of hesperetin content. Hesperetin content in shade dried lemon myrtle leaves was 2% and 9% higher as compared to freeze dried and microwave dried samples accordingly.
Our findings were in agreement with previous studies on pomegranate peel and persimmon, which reported that freeze drying retained higher TPC, TFC and antioxidant capacity than other drying methods, such as vacuum and hot air (Mphahlele et al., 2016; Karaman et al., 2014). However, our findings are different to the results of other studies on Kappaphycus alvarezi and Scaevola spinescens, which found that samples dried by natural drying methods including shade drying and sun drying had significantly lower levels of bioactive compounds and antioxidant capacity than those dried by hot air and vacuum drying (Ling et al., 2015; Nguyen et al., 2018). The differences can be explained by the stability of TPC, TFC and proanthocyanidins in lemon myrtle, which appear to be more stable than those of other plant materials when exposure to low temperature for a longer time.
Energy consumption is one of the critical factors for selection of suitable drying methods as it is linked to the cost of drying. The energy consumption was calculated for the different drying methods to estimate the differences in power required to dry lemon myrtle leaves by each mechanical drying method (Table 4). Among the different drying methods, freeze drying required the most energy followed by hot air, vacuum, and microwave drying. Freeze drying consumed energy approximately 163, 344 and 1704 times higher than hot air, vacuum, and microwave, respectively. On the contrary, microwave drying method consumes minimal energy and had the shortest drying time. Natural drying methods (sun drying and shade drying) do not require electricity for drying but these methods need a longer time for drying (2 and 12 days, respectively).
The time consumption for drying of sample by different mechanical technique was following the order of freeze drying > hot air drying > vacuum drying > microwave drying. But, in theory, vacuum drying enables shorter drying time compare to hot air drying due to the rate of evaporation increase (at a fixed temperature) since the boiling point of water is reduced. However, in some cases when drying fresh materials with high moisture content like lemon myrtle, fan forced hot air oven takes less time as compared to vacuum because the high moisture in the drying chamber is removed quicker by a fan than the vacuum system, resulting in shorter drying time. Similar findings were reported by Papoutsis et al. (2017), Vu et al. (2017), Kröncke et al. (2018) and Ozcan-sinir et al. (2019) for various plant materials.
Overall, it is suggested that freeze drying is the most suitable for drying lemon myrtle leaf for the maximum recovery of phytochemicals, and for further identification and quantification of bioactive compounds for research purposes or production of high value extracts of lemon myrtle; however due to the high running costs of freeze drying, more economical methods of hot air drying (90 °C, 1.25 h), vacuum drying (90 °C, 2 h) and microwave drying (960 W, 0.12 h) could be applied for further extraction and isolation of bioactive compounds from lemon myrtle leaf in a large scale. Natural drying methods including shade drying (12 days) or sun drying (2 days) should also be considered as a ‘green’ alternative for drying lemon myrtle leaves, with the benefit of maximal hesperitin recovery and consider as economic drying option for large scale production. Depending on availability of equipment and labour, any of these methods can be suitable for drying lemon myrtle leaves.
3.5. Correlation between phytochemical and antioxidant properties using different method
The correlation between phytochemicals and antioxidant properties was analysed and the outcomes can be seen in Table 5. Correlation coefficient values showed a significant (p < 0.05) and strong correlation between phenolic compounds and antioxidant properties. The Pearson correlation coefficient r value of 0.82, 0.85, 0.74, and 0.83 was for FRAP, CUPRAC, ABTS, and DPPH respectively, which revealing that phenolic compounds are the major contributor to lemon myrtle antioxidant capacity. The two secondary metabolites of phenolic compounds including flavonoids and proanthocyanidins also had strong correlation with antioxidant capacity measured by CUPRAC (r-value was 0.91 and 0.81 for TFC and Pro.A respectively) (Table 5). The antioxidant capacity measured by other assays (i.e. FRAP, ABTS and DPPH) showed moderate but significant correlation with flavonoids and proanthocyanidins (r value range from 0.59 to 0.75 and p value < 0.05); which indicates that these secondary metabolites were also contributors to antioxidant capacity in lemon myrtle leaves. Similar results were observed by Pham et al. (2015), they found a strong correlation between total phenolic and flavonoid content and antioxidant properties (DPPH, ABTS and FRAP) of Helicteres hirsuta Lour. leaves. Vuong et al. (2013) also reported similar findings when they dried papaya leaf extracts. Overall, phenolic compounds and their secondary metabolites, flavonoids and proanthocyanidins play an important role in the antioxidant capacity of the lemon myrtle leaf and thus they should be further isolated and identified for understanding their potential biological properties and health benefits.
Table 5.
Correlation between phytochemical and antioxidant capacity.
| Phytochemicals | Antioxidant capacity |
|||||||
|---|---|---|---|---|---|---|---|---|
| FRAP |
CUPRAC |
ABTS |
DPPH |
|||||
| r | p-value | r | p-value | r | p-value | r | p-value | |
| TPC | 0.82 | 0.000 | 0.85 | 0.000 | 0.74 | 0.000 | 0.83 | 0.000 |
| TFC | 0.69 | 0.000 | 0.91 | 0.000 | 0.74 | 0.000 | 0.75 | 0.000 |
| Proanthocyanidin | 0.59 | 0.000 | 0.81 | 0.000 | 0.60 | 0.000 | 0.65 | 0.000 |
4. Conclusion
Drying conditions significantly affect the retention of TPC, TFC, proanthocyanidins, gallic acid, hesperetin, and antioxidant properties (measured by FRAP, DPPH, CUPRAC and ABTS) of lemon myrtle leaves. Among the six drying techniques tested, freeze drying retained the highest phytochemical and antioxidant properties, however, it has the highest energy consumption. For hot air drying and vacuum drying, shorter drying times at high temperatures retained higher phytochemical levels and antioxidant properties as compared to low temperatures with longer drying times. The best conditions for hot air drying and vacuum drying are 90 °C for 75 min and 90 °C for 120 min at vacuum pressure of 69 ± 1 kPa, respectively, and use considerably less electricity than freeze drying. Microwave power and radiation time also affected phenolic compounds and antioxidant capacity giving the best recovery by microwave drying at 960 W for 7min. Shade drying and sun drying requires longer times for drying lemon myrtle leaves with 12 and 2 days, respectively, resulted in similar phytochemical properties to hot air drying, vacuum drying and microwave drying with no energy (kWh) input. For optimal recovery of phytochemicals from lemon myrtle leaves, it is recommended to choose the proposed drying conditions that have been identified for each method. Overall, microwave drying can be applied for industrial purposes for drying lemon myrtle leaves as it consumes least time and energy.
Declarations
Author contribution statement
Md Saifullah: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Rebecca McCullum: Conceived and designed the experiments; Wrote the paper.
Adam McCluskey: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Quan Vuong: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work is supported by an Australian Government Research Training Program (RTP) scholarship awarded to the first author.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
This project is funded by an Australian Government Research Training Program (RTP) scholarship awarded to the first author.
Contributor Information
Md Saifullah, Email: md.saifullah@uon.edu.au.
Quan Vuong, Email: vanquan.vuong@newcastle.edu.au.
References
- Apak R., Güçlü K., Özyürek M., Karademir S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of Neocuproine: CUPRAC method. J. Agric. Food Chem. 2004;52(26):7970–7981. doi: 10.1021/jf048741x. [DOI] [PubMed] [Google Scholar]
- Buchaillot A., Caffin N., Bhandari B. Drying of lemon myrtle (Backhousia citriodora) leaves: retention of volatiles and color. Dry. Technol. 2009;27:445–450. [Google Scholar]
- Buffler C.R. Avi Book; New York: 1993. Microwave Cooking and Processing: Engineering Fundamentals for the Food Scientist. [Google Scholar]
- Dupont S., Caffin N., Bhandari B., Dykes G.A. In vitro antibacterial activity of Australian native herb extracts against food-related bacteria. Food Control. 2006;17 [Google Scholar]
- Garau M.C., Simal S., Rosselló C., Femenia A. Effect of air-drying temperature on physico-chemical properties of dietary fibre and antioxidant capacity of orange (Citrus aurantium v. Canoneta) by-products. Food Chem. 2007;104:1014–1024. [Google Scholar]
- Guo Y., Sakulnarmrat K., Konczak I. Anti-inflammatory potential of native Australian herbs polyphenols. Toxicol. Reports. 2014;1:385–390. doi: 10.1016/j.toxrep.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnkarnsujarit N., Kawai K., Watanabe M., Suzuki T. Effects of freezing on microstructure and rehydration properties of freeze-dried soybean curd. J. Food Eng. 2016;184:10–20. [Google Scholar]
- Karaman S., Toker O.S., Çam M., Hayta M., Doğan M., Kayacier A. Bioactive and physicochemical properties of persimmon as affected by drying methods. Dry. Technol. 2014;32:258–267. [Google Scholar]
- Konczak I., Zabaras D., Dunstan M., Aguas P. Antioxidant capacity and phenolic compounds in commercially grown native Australian herbs and spices. Food Chem. 2010;122:260–266. [Google Scholar]
- Kröncke N., Böschen V., Woyzichovski J., Demtröder S., Benning R. Comparison of suitable drying processes for mealworms (Tenebrio molitor) Innov. Food Sci. Emerg. Technol. 2018;50:20–25. [Google Scholar]
- Li Y., Guo C., Yang J., Wei J., Xu J., Cheng S. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem. 2006;96:254–260. [Google Scholar]
- Ling A.L.M., Yasir S., Matanjun P., Bakar M.F.A. Effect of different drying techniques on the phytochemical content and antioxidant activity of Kappaphycus alvarezii. J. Appl. Phycol. 2015;27:1717–1723. [Google Scholar]
- Lim Y.Y., Murtijaya J. Antioxidant properties of Phyllanthus amarus extracts as affected by different drying methods. LWT - Food Sci. Technol. 2007;40:1664–1669. [Google Scholar]
- Methakhup S., Chiewchan N., Devahastin S. Effects of drying methods and conditions on drying kinetics and quality of Indian gooseberry flake. LWT - Food Sci. Technol. 2005;3:579–587. [Google Scholar]
- Miranda M., Maureira H., Rodríguez K., Vega-Gálvez A. Influence of temperature on the drying kinetics, physicochemical properties, and antioxidant capacity of Aloe Vera (Aloe Barbadensis Miller) gel. J. Food Eng. 2009;91:297–304. [Google Scholar]
- Mphahlele R.R., Fawole O.A., Makunga N.P., Opara U.L. Effect of drying on the bioactive compounds, antioxidant, antibacterial and antityrosinase activities of pomegranate peel. BMC Complement Altern. Med. 2016;16:143. doi: 10.1186/s12906-016-1132-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadi F. Bioactive compound retention in Echium amoenum Fisch. & C. A. Mey. petals: effect of fluidized bed drying conditions. Int. J. Food Prop. 2017;20:2249–2260. [Google Scholar]
- Nguyen K.Q., Vuong Q.V., Nguyen M.H., Roach P.D. The effects of drying conditions on bioactive compounds and antioxidant activity of the Australian maroon bush, Scaevola spinescens. J. Food Process. Preserv. 2018;42(10) [Google Scholar]
- Nguyen V.T., Pham N.M.Q., Vuong Q.V., Bowyer M.C., van Altena I.A., Scarlett C.J. Phytochemical retention and antioxidant capacity of xao tam phan (Paramignya trimera) root as prepared by different drying methods. Dry. Technol. 2016;34:324–334. [Google Scholar]
- Nguyen V.T., Vuong Q., Bowyer M.C., Van Altena I.A., Scarlett C.J. Effects of different drying methods on bioactive compound yield and antioxidant capacity of Phyllanthus amarus. Dry. Technol. 2015;33:1006–1017. [Google Scholar]
- Nóbrega E.M., Oliveira E.L., Genovese M.I., Correia R.T.P. The impact of hot air drying on the physical-chemical characteristics, bioactive compounds and antioxidant activity of acerola (malphigia emarginata) residue. J. Food Process. Preserv. 2015;39:131–141. [Google Scholar]
- Oikonomopoulou V.P., Krokida M.K., Karathanos V.T. The influence of freeze drying conditions on microstructural changes of food products. Procedia Food Sci. 2011;1:647–654. [Google Scholar]
- Ozcan-sinir G., Ozkan-karabacak A., Tamer C.E., Copur O.U. The effect of hot air, vacuum and microwave drying on drying characteristics, rehydration capacity, color, total phenolic content and antioxidant capacity of Kumquat (Citrus japonica) Food Sci. Technol. 2019;39:475–484. [Google Scholar]
- Papoutsis K., Pristijono P., Golding J.B., Stathopoulos C.E., Bowyer M.C., Scarlett C.J., Vuong Q.V. Effect of vacuum-drying, hot air-drying and freeze-drying on polyphenols and antioxidant capacity of lemon (Citrus limon) pomace aqueous extracts. Int. J. Food Sci. Technol. 2017;52:880–887. [Google Scholar]
- Pham H., Nguyen V., Vuong Q., Bowyer M., Scarlett C. Effect of extraction solvents and drying methods on the physicochemical and antioxidant properties of Helicteres hirsuta Lour. Leaves. Technol. 2015;3:285. [Google Scholar]
- Punathil L., Basak T. Reference Module in Food Science. Elsevier; 2016. Microwave processing of frozen and packaged food materials: experimental. [Google Scholar]
- Saifullah M., Yusof Y.A., Chin N.L., Aziz M.G. Physicochemical and flow properties of fruit powder and their effect on the dissolution of fast dissolving fruit powder tablets. Powder Technol. 2016;301:396–404. [Google Scholar]
- Sakulnarmrat K., Fenech M., Thomas P., Konczak I. Cytoprotective and pro-apoptotic activities of native Australian herbs polyphenolic-rich extracts. Food Chem. 2013;136:9–17. doi: 10.1016/j.foodchem.2012.07.089. [DOI] [PubMed] [Google Scholar]
- Sakulnarmrat K., Konczak I. Composition of native Australian herbs polyphenolic-rich fractions and in vitro inhibitory activities against key enzymes relevant to metabolic syndrome. Food Chem. 2012;134:1011–1019. doi: 10.1016/j.foodchem.2012.02.217. [DOI] [PubMed] [Google Scholar]
- Shrestha A.K., Ua-arak T., Adhikari B.P., Howes T., Bhandari B.R. Glass transition behavior of spray dried orange juice powder measured by differential scanning calorimetry (DSC) and thermal mechanical compression test (TMCT) Int. J. Food Prop. 2007;10:661–673. [Google Scholar]
- Škerget M., Kotnik P., Hadolin M., Hraš A.R., Simonič M., Knez Ž. Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem. 2005;89:191–198. [Google Scholar]
- Sommano S., Caffin N., Kerven G. Screening for antioxidant activity, phenolic content, and flavonoids from Australian native food plants. Int. J. Food Prop. 2013;16:1394–1406. [Google Scholar]
- Thaipong K., Boonprakob U., Crosby K., Cisneros-Zevallos L., Hawkins Byrne D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006;19:669–675. [Google Scholar]
- Vega-Gálvez A., Di Scala K., Rodríguez K., Lemus-Mondaca R., Miranda M., López J., Perez-Won M. Effect of air-drying temperature on physico-chemical properties, antioxidant capacity, colour and total phenolic content of red pepper (Capsicum annuum, L. var. Hungarian) Food Chem. 2009;117:647–653. [Google Scholar]
- Vu H.T., Scarlett C.J., Vuong Q.V. Effects of drying conditions on physicochemical and antioxidant properties of banana (Musa cavendish) peels. Dry. Technol. 2017;35:1141–1151. [Google Scholar]
- Vuong Q.V., Hirun S., Roach P.D., Bowyer M.C., Phillips P.A., Scarlett C.J. Effect of extraction conditions on total phenolic compounds and antioxidant activities of Carica papaya leaf aqueous extracts. J. Herb. Med. 2013;3:104–111. [Google Scholar]
- Zhishen J., Mengcheng T., Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559. [Google Scholar]