Abstract
Background
Short uncemented stems have recently been proposed as an alternative to classic long stems for shoulder arthroplasty. The early results are promising, but bony adaptations of the proximal humerus have been reported. The aim of this study was to quantify these phenomena using the Ascend Flex stem and to determine the risk factors.
Materials and methods
In a retrospective, single-center study, 183 shoulder arthroplasties were evaluated at 2-year follow-up. All patients underwent clinical evaluations preoperatively and at last follow-up. Radiographs were obtained preoperatively, postoperatively, and at last follow-up. Four types of bony adaptations were analyzed: medial cortical narrowing (MCN), medial metaphysis thinning (MMT), lateral metaphysis thinning (LMT), and under-the-baseplate osteolysis. The risk factors were analyzed in a multivariate model.
Results
MCN was found in 72.6% of cases and was severe (>50%) in 4.4%. MMT was found in 46.4% of cases and was severe in 3.3%. LMT was found in 9.8% of cases and was severe in 2.8%. The risk factors for MCN were the distal filling ratio, osteoporosis, and female sex, whereas MMT and LMT were only influenced by stem axis deviation. Under-the-baseplate osteolysis was found in 34.4% of cases. No influence of bony adaptations on the clinical outcomes was observed. We found no complications related to the stem or to stem loosening.
Conclusion
The radiographic evolution was satisfactory at mid-term follow-up. Bony adaptations seemed to be limited phenomena, without any observed consequence. Avoiding excessive filling and axis deviation may limit these phenomena.
Keywords: Shoulder arthroplasty, short stem, uncemented stem, osteolysis, stress shielding, bony adaptations
Shoulder arthroplasty is recognized as the treatment of choice for degenerative arthritis of the shoulder. We have recently observed a significant worldwide increase in the number of shoulder prostheses implanted, even in younger and more active patients, in proportion to the growing confidence we place in these implants. However, with the increasing implantations and the younger average age of the patients, the question of implant survivorship and the difficulties related to revision surgery increasingly become concerns. In a desire to anticipate further difficulties, newly designed short uncemented stems have been proposed as an alternative to classic implants.2,4,5,7,16,19,23 They allow bone preservation (tuberosities and diaphysis) and facilitate stem extraction (or even conversion) in case of revision, and thus, they actually seem to be a valuable option for primary shoulder arthroplasty.
However, it is well known that the use of uncemented stems exposes patients to the problem of humeral bone remodeling, which has been commonly reported with long uncemented stems, and assimilation to a stress-shielding mechanism.8,10,12,13,22 According to the Wolff law,25 stress shielding induces metaphyseal cortical narrowing and osteopenia because of proximal decreasing constraints, as well as densification phenomena (condensation lines and spot welds), which reflect excessive constraints around the distal part of the stem but are not a source of concern. A biomechanical study suggested that the use of short stems could limit proximal stress shielding compared with long stems and thus limit bone remodeling.15 This has been confirmed in vivo by Denard et al,5 who compared the radiologic evolution of similarly designed short and long stems and reported a lower rate of calcar narrowing with the short stem than with the long stem (50% vs. 74%). The results of the first studies on short stems were very promising, reporting good mid-term clinical and radiologic outcomes comparable to those of long stems.2,4,7,16,19,23 However, a high incidence of proximal humeral bony adaptations is still reported, although to a lesser extent, and questions remain about their incidence, severity, determining factors, and possible consequences.
The aims of this study were to quantify the precise incidence of proximal bone remodeling phenomena with a short, uncemented, porous-coated humeral stem (Aequalis Ascend Flex; Tornier SAS, Montbonnot Saint Martin, France) and to identify the determining factors of these phenomena to make some technical recommendations to limit them. Secondarily, we aimed to evaluate the mid-term consequences of proximal bone remodeling.
Materials and methods
Study design
In this retrospective, observational, single-center study, we included all patients who consecutively underwent shoulder replacement with a short uncemented stem (in an anatomic or reverse configuration) between November 2012 and September 2015, performed by 3 senior shoulder surgeons (D.M., F.S., and A.J.) working in the same institution, with a minimum follow-up period of 2 years after surgery. All the prostheses were implanted for degenerative arthritis; however, patients with acute fractures and tumors were excluded because they were treated using classic long stems. We also excluded 3 shoulders in which the medial proximal metaphysis was initially missing or highly remodeled. We assumed that analysis of proximal bone remodeling would not have been possible in these cases (1 case with revision and 2 cases with fracture sequelae).
Patients
A total of 228 shoulders met the inclusion criteria. At a minimum 2-year follow-up, 26 patients were lost to follow-up: 12 did not want to return for clinical and radiographic evaluation, and 7 died. On the basis of telephone interviews, none of these patients had any reported complications or underwent revision surgery related to the arthroplasty. Finally, 183 patients (80.3%) could be evaluated at a mean follow-up of 27.5 ± 6 months (range, 23-47 months). There were 64 men (35%) and 119 women (65%). The mean age was 70 ± 9 years (range, 26-87 years).
Implant design and surgical technique
All the procedures were performed with the patients under general anesthesia in the beach-chair position. Anatomic shoulder arthroplasty was performed in 102 cases (56%) with a mean age of 65 ± 8 years (range, 26-82 years), including 95 total shoulder arthroplasties (TSAs) and 7 hemiarthroplasties (HAs). Reverse shoulder arthroplasty (RSA) was performed in 81 cases (44%) at a mean age of 76 ± 5 years (range, 64-87 years). Prostheses were implanted through a deltopectoral approach in 106 cases (58%, 102 anatomic prostheses and 4 RSAs) and a superior approach in 77 cases (42%, only RSAs).
The Aequalis Ascend Flex stem was used in every case. It is a short and convertible titanium stem, designed for proximal metaphyseal uncemented fixation, with a proximal porous titanium coat (1-mm press fit). The stem sizes used ranged from size 1 (66-mm length) to size 7 (90-mm length), with a mean size of 3.8 ± 1.3. All the TSAs were associated with a cemented keeled polyethylene glenoid implant (Perform; Tornier SAS). All the RSAs were performed with a classic press-fit, short- or long-pegged glenoid implant (Aequalis Reversed II; Tornier SAS). The bony increased offset-RSA technique, using humeral head autograft as described by Boileau et al,1 was performed in 32 cases (40%).
Clinical evaluation
All patients underwent a preoperative clinical evaluation including range-of-motion assessment and determination of the Constant score (CS).3 The same clinical examination was performed at last follow-up (≥2 years) by a single examiner (L.P.) who had not performed the surgical procedures. Intraoperative and postoperative complications were systematically recorded, as was the need for revision surgery.
Radiologic evaluation
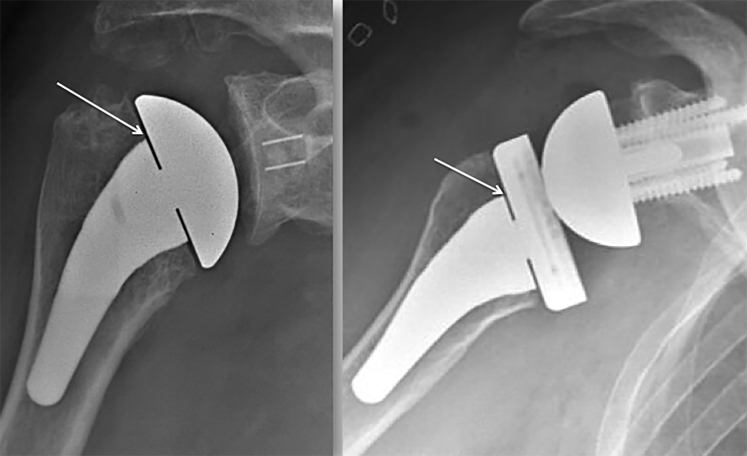
Radiologic analysis was performed by the same independent single examiner (L.P.) who had not performed the surgical procedures. The shoulder radiographic protocol included a strict anteroposterior view and a Lamy view, and radiographs were obtained at 3 time points: before surgery, immediately after surgery, and at minimum 2-year follow-up. When the prosthesis was in place (on immediate postoperative and 2-year follow-up radiographs), the anteroposterior radiograph was accepted only if the Morse taper could be visualized in the free space between the stem and the head (Fig. 1), allowing precise analysis of the proximal metaphysis. Because of the retrospective design of our study, even though radiographs deemed inadequate were routinely obtained again in our center, a few postoperative radiographs were judged acceptable at the time they were obtained, even though the rotation was not perfect, and we had to accept them in our study because we assumed this would not alter our analysis. All the radiographs were obtained in our radiology unit and were accessible for analysis using the same software (OSA PatientViewer; J4Care, Mödling, Austria), which was used for all visual analyses and measurements, ensuring the reliability of measurements from one case to another.
Figure 1.
Standard postoperative radiographs (anteroposterior view, with visible Morse taper [white arrow]).
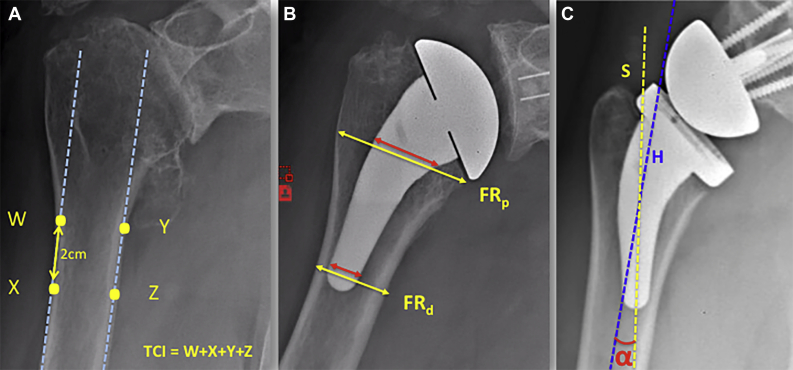
Bone quality was assessed on preoperative radiographs by measuring the Tingart cortical index (TCI), as described by Tingart et al24 (Fig. 2). The proximal filling ratio (pFR) and distal filling ratio (dFR) were measured on immediate postoperative radiographs (Fig. 2). We looked at eventual deviation between the stem axis (as defined by the manufacturer) and the humeral shaft axis, defined by the α angle between these 2 axes on anteroposterior radiographs (Fig. 2) and by the orientation in varus or valgus.
Figure 2.
Radiologic potential risk factors. (A) The Tingart cortical index (TCI) is obtained by summing 4 measurements of diaphyseal cortical thickness (W, X, Y, and Z). The 2 proximal points (medial and lateral) are positioned at the level at which the cortices become parallel ( ). The 2 other points are 2 cm more distal. (B) Proximal filling ratio (FRp) and distal filling ratio (FRd) obtained by calculating the ratio between the stem diameter (
). The 2 other points are 2 cm more distal. (B) Proximal filling ratio (FRp) and distal filling ratio (FRd) obtained by calculating the ratio between the stem diameter ( ) and the diaphysis diameter (
) and the diaphysis diameter ( ). The measured distances are perpendicular to the axis of the humeral shaft. The FRp is measured at the level of the cut, and the FRd is measured just over the tip of the stem. (C) Stem axis deviation obtained by measuring the α angle between the axis of the stem (S,
). The measured distances are perpendicular to the axis of the humeral shaft. The FRp is measured at the level of the cut, and the FRd is measured just over the tip of the stem. (C) Stem axis deviation obtained by measuring the α angle between the axis of the stem (S,  ) and the axis of the humeral shaft (H,
) and the axis of the humeral shaft (H,  ).
).
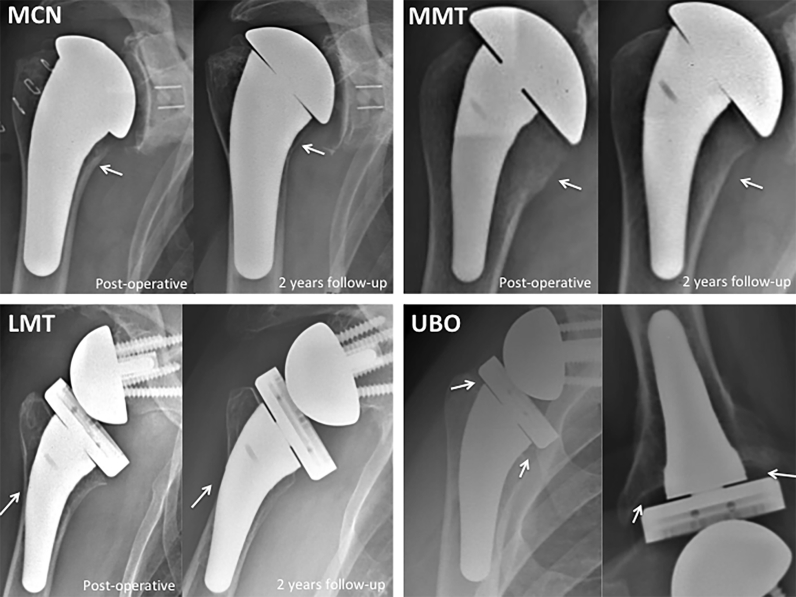
We noted 4 different types of proximal humeral bony adaptations (Fig. 3) that we decided to analyze and quantify at last follow-up:
-
1.
Medial cortical narrowing (MCN) was evaluated by establishing the ratio between the proximal medial cortical thickness on the last follow-up radiograph and that on the immediate postoperative radiograph (cortical thickness ratio) (Figs. 3 and 4). Because osteophyte removal often leads to disappearance of the cortical bone in the proximal few milimeters of the medial humerus, measurement of proximal cortical thickness was arbitrarily performed 1 cm under the cut.
-
2.
Medial metaphysis thinning (MMT) was evaluated by establishing the ratio between the medial metaphysis thickness on the last follow-up radiograph and that on the immediate postoperative radiograph (Figs. 3 and 4).
-
3.
Lateral metaphysis thinning (LMT) was evaluated by establishing the ratio between the lateral metaphysis thickness on the last follow-up radiograph and that on the immediate postoperative radiograph (Figs. 3 and 4).
-
4.
Under-the-baseplate osteolysis (UBO) was defined by the disappearance of the cancellous bone under the baseplate (Fig. 3). When this phenomenon was visualized, we measured its maximum height in millimeters.
Figure 3.
Four types of proximal bone resorption (white arrow): medial cortical narrowing (MCN), medial metaphyseal thinning (MMT), and lateral metaphyseal thinning (LMT) on postoperative view (left) and 2-year follow-up view (right) and under-the-baseplate osteolysis (UBO) at 2-year follow-up on frontal view (left) and axillary view (right).
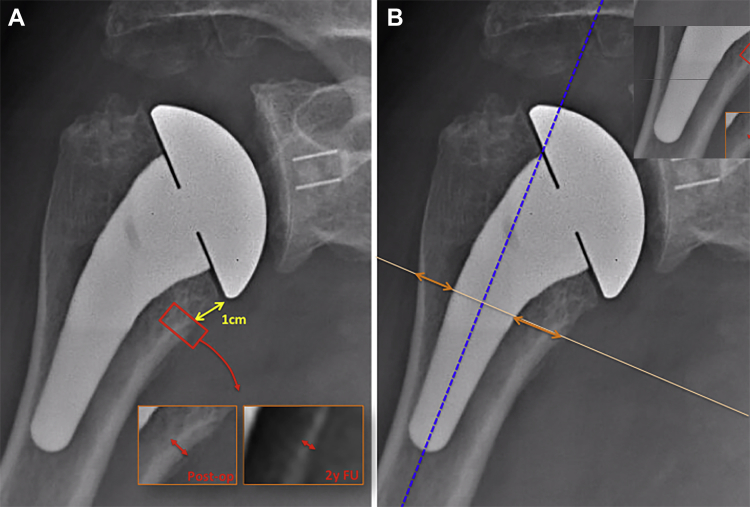
Figure 4.
Quantification of bony adaptations related to stress shielding. (A) Medial cortical narrowing ( ) was evaluated by measuring the cortical thickness 1 cm under the level of the humeral cut (
) was evaluated by measuring the cortical thickness 1 cm under the level of the humeral cut ( ), performed postoperatively (Post-op) and at 2-year follow-up (2y FU). A ratio (2-year follow-up to postoperative) was then calculated for each patient. (B) Medial metaphyseal thinning and lateral metaphyseal thinning were evaluated by measuring the metaphysis thickness (
), performed postoperatively (Post-op) and at 2-year follow-up (2y FU). A ratio (2-year follow-up to postoperative) was then calculated for each patient. (B) Medial metaphyseal thinning and lateral metaphyseal thinning were evaluated by measuring the metaphysis thickness ( ), at the same level as the measurement of medial cortical narrowing, perpendicular to the humeral shaft axis (
), at the same level as the measurement of medial cortical narrowing, perpendicular to the humeral shaft axis ( ). A ratio (2-year follow-up to postoperative) was then calculated for each patient.
). A ratio (2-year follow-up to postoperative) was then calculated for each patient.
We did not focus on condensation phenomena (spot welds and condensation lines) because they were not considered a source of concern, as recommended by Denard et al.6 The values obtained for MCN, MMT, and LMT were divided into 4 groups according to the calculated ratio: no osteolysis (ratio > 0.9), minor osteolysis (ratio of 0.6-0.9), major osteolysis (ratio of 0.1-0.5), and total disappearance (ratio < 0.1). Bony adaptation was defined by a ratio of 0.9 or less. Severe (or relevant) bony adaptation was defined by a ratio of less than 0.5 (>50% narrowing).
For each type of bony adaptation, we performed a univariate analysis to determine the potential risk factors, including age, sex, pFR, dFR, stem axis deviation, TCI, and type of prosthesis (anatomic or reverse). The relevant risk factors after univariate analysis were then included in a multivariate analysis to determine independent risk factors.
Statistical analysis
Data collection and statistical analysis were performed using EasyMedStat (Neuilly-sur-Seine, France) and RStudio software (Integrated Development for RStudio, Boston, MA, USA). The α risk was set at 5%. The Wilcoxon signed rank test or Student t test was used to compare quantitative variables in different groups. The Spearman correlation coefficient was used to assess the relationship between the different quantitative parameters. After univariate analysis of the risk factors for each type of bony adaptation, only the risk factors with P < .20 were included in the multivariate analysis. Multivariate analysis was performed using a linear regression model. P ≤ .05 was considered statistically significant.
Results
Clinical outcomes
At 2-year follow-up, we observed significant improvements in the CS and all of its items, as well as range of motion (P < .05), for anatomic shoulder arthroplasties and RSAs. The mean CS was 75 ± 8 for HAs and TSAs and 62 ± 11 for RSAs. All the recorded values are presented in Table I.
Table I.
Clinical outcomes
| TSA and HA (n = 102) |
RSA (n = 81) |
|||||
|---|---|---|---|---|---|---|
| Preoperative | Follow-up | P value | Preoperative | Follow-up | P value | |
| CS, points | 32 ± 7 | 75 ± 8 | <.001 | 31 ± 13 | 62 ± 11 | <.001 |
| Pain, points | 4.8 ± 1.5 | 14 ± 1.7 | <.001 | 7.9 ± 12.2 | 13.1 ± 3.2 | <.001 |
| AA, ° | 75 ± 20 | 125 ± 30 | <.001 | 80 ± 28 | 111 ± 27 | <.001 |
| AF, ° | 92 ± 20 | 153 ± 26 | <.001 | 84 ± 36 | 131 ± 21 | <.001 |
| ER, ° | 14 ± 15 | 45 ± 17 | <.001 | 18 ± 22 | 28 ± 20 | <.001 |
TSA, total shoulder arthroplasty; HA, hemiarthroplasty; RSA, reverse shoulder arthroplasty; CS, Constant score; AA, active abduction; AF, active flexion; ER, external rotation.
Data are presented as mean ± standard deviation.
Complications were reported in 18 cases (9.8%). Of these complications, 11 (10.8%) were reported after HA or TSA: 1 dislocation (reduction), 1 traumatic humeral fracture (nonoperative treatment), 4 rotator cuff tears including 2 with early migration of the glenoid component (conversion to RSA), 1 infection (1-stage revision), 3 cases of adhesive capsulitis (resolutive), and 1 minor wound problem. A total of 7 complications (8.6%) were reported after RSA: 1 humeral fracture (stem revision plus open reduction–internal fixation), 1 case of early glenoid loosening (technical error), 3 spine fractures, 1 infection (washout plus insert and glenosphere exchange), and 1 minor wound problem. No complication or revision was directly related to the stem.
Radiologic analysis
Preoperative radiographic analysis
The mean TCI was 3 ± 0.8 mm (range, 1.6-5.1 mm). The TCI was significantly lower in the RSA group than in the TSA and HA group (2.7 mm vs. 3.2 mm, P < .001).
Immediate postoperative radiographic analysis
The mean pFR was 0.58 ± 0.09 (range, 0.36-0.77). It was significantly higher in the RSA group than in the TSA group (0.62 vs. 0.56, P < .001). The mean dFR was 0.52 ± 0.07 (range, 0.35-0.71). No significant difference existed between the TSA group and RSA group (0.52 vs. 0.52, P = .99). The mean stem axis deviation was 4.6° ± 3.4° (range, 0.1°-16.2°). A valgus deviation was found in 120 stems (65.1%), with a mean deviation of 5.9° ± 3.4° (range, 0.2°-16.2°), whereas 63 stems (34.9%) had a varus deviation, with a mean deviation of 2° ± 1.3° (range, 0.1°-5.1°). RSAs were significantly more deviated than TSAs and HAs (5.9° vs. 3.5°, P < .001). Even though the mean axis deviation was negligible (4.6°), it was superior to 10° in 11 cases (6%), always in valgus.
Last follow-up radiographic analysis and evaluation of bony adaptations
The findings of the evaluation of bony adaptations related to a stress-shielding mechanism are summarized in Table II. The mean MCN ratio was 79.6% ± 15.9% (range, 0%-140%), meaning average cortical narrowing of 20.4%. MCN was reported in 133 patients (72.6%) but was considered relevant (>50% narrowing) in only 8 cases (4.4%). MCN was extended at a mean height of 18.1 ± 5 mm (range, 5.6-32.3 mm). The mean MMT ratio was 86.6% ± 16% (range, 0%-145%), meaning average metaphyseal thinning of 13.4%. MMT was reported in 85 cases (46.4%) but was considered relevant (>50% thinning) in only 6 cases (3.3%). The mean LMT ratio was 94.1% ± 19.4% (range, 0%-100%), meaning average metaphyseal thinning of 5.9%. LMT was found in 18 cases (9.8%) but was considered relevant (>50% thinning) in only 5 cases (2.8%). In total, 147 patients (80.3%) had at least 1 of these phenomena at 2-year follow-up, and at least 1 severe adaptation (>50% narrowing) was found in 13 patients (7.1%).
Table II.
Bony adaptations related to stress shielding
| No osteolysis (ratio > 0.9) | Minor osteolysis (ratio of 0.6-0.9) | Considered relevant |
|||
|---|---|---|---|---|---|
| Major osteolysis (ratio of 0.1-0.5) | Complete disappearance (ratio < 0.1) | Major osteolysis and complete disappearance combined | |||
| MCN | 50 (27.3) | 125 (68.3) | 7 (3.8) | 1 (0.5) | 8 (4.4) |
| MMT | 98 (53.6) | 79 (43.2) | 5 (2.7) | 1 (0.5) | 6 (3.2) |
| LMT | 165 (90.2) | 13 (7.1) | 0 (0) | 5 (2.7) | 5 (2.7) |
MCN, medial cortical narrowing; MMT, medial metaphysis thinning; LMT, lateral metaphysis thinning.
Data are presented as number (percentage).
Metaphyseal UBO was found in 63 cases (34.4%), in which the mean osteolysis width was 2.9 ± 1.2 mm (range, 1-6.1 mm). No such osteolysis was seen in the HA group (n = 7). UBO was significantly more frequent in the RSA group than in the TSA group (49.4% vs. 22.5%, P < .0001). The occurrence of UBO was not correlated with the occurrence of MCN (P = .64), MMT (P = .55), or LMT (P = .68). Finally, we found no significant radiolucent lines (≥2 mm width), stem subsidence, or loosening and no mechanical complications related to the humeral stem in our series.
Bone-remodeling risk-factor analysis
The results of the univariate analysis for each type of bony adaptation are presented in Table III. Female sex, a low TCI, and a high dFR were significant risk factors for MCN in the univariate analysis, but the multivariate analysis concluded that none of them was independent.
Table III.
Risk factors for bony adaptations: univariate analysis
| MCN |
MMT |
LMT |
UBO |
|||||
|---|---|---|---|---|---|---|---|---|
| Coef | P value | Coef | P value | Coef | P value | Coef | P value | |
| Age | 0.06 | .38 | –0.02 | .76 | –0.14 | .06 | .02∗ | |
| Female sex | .03∗ | .1 | .15 | .21 | ||||
| pFR | –0.122 | .1 | –0.05 | .51 | –0.15 | .05∗ | 0.34 | <.001∗ |
| dFR | –0.17 | .02∗ | –0.08 | .26 | 0.11 | .14 | 0.13 | .8 |
| TCI | 0.21 | <.01∗ | 0.04 | .55 | 0.26 | <.001∗ | –0.17 | .02∗ |
| Stem axis deviation | 0.08 | .27 | 0.22 | <.01∗ | –0.39 | <.001∗ | 0.24 | <.1 |
| Type of deviation (valgus/varus) | .09 (varus) | .02 (varus)∗ | .02 (valgus)∗ | <.01 (valgus)∗ | ||||
| RSA prosthesis | .41 | .99 | .04∗ | <.001∗ | ||||
MCN, medial cortical narrowing; MMT, medial metaphysis thinning; LMT, lateral metaphysis thinning; UBO, under-the-baseplate osteolysis; Coef, coefficient; pFR, proximal filling ratio; dFR, distal filling ratio; TCI, Tingart cortical index; RSA, reverse shoulder arthroplasty.
Statistically significant (P ≤ .05).
Stem axis deviation in varus was the only risk factor for MMT in univariate and multivariate analyses (P < .001). LMT was significantly associated with a high pFR, a low TCI, stem axis deviation in valgus, and RSA in the univariate analysis, but the multivariate analysis found only stem axis deviation in valgus to be an independent risk factor for LMT (P < .001). Severe LMT (>50%) always occurred with a valgus deviation greater than 10°, and 83% of LMT occurred with valgus deviation greater than 7°. UBO was significantly associated with age, a high pFR, a low TCI, stem axis deviation in valgus, and RSA in the univariate analysis, but in the multivariate analysis, a high pFR and RSA were the only independent risk factors (P < .01).
Consequences of bony adaptations
We found no statistical influence of any type of bony adaptation (MCN, MMT, LMT, or UBO) on the clinical outcomes (CS) for TSA and HA or for RSA (P > .05).
Discussion
In this series, as in the literature,11,19,21,23 the Ascend Flex short stem yielded promising mid-term results, with excellent clinical outcomes and no complications related to the stem. However, proximal bony adaptations related to stress shielding were noted in 80.3% of patients, which might be concerning.
Bony adaptations have been largely reported in previous publications about short stems, with rates ranging from 3% to 93%, but with different stems and heterogeneous criteria. To date, 5 publications have focused on the second-generation Ascend Flex stem (with porous coating),11,14,19,21,23 with reported rates of visible calcar osteolysis ranging from 3% to 42%. These osteolysis phenomena are usually described using a classic 5-zone system, without quantitative analysis (only qualitative) and without differentiation between the different types of adaptations.2,4,5,7,11,16, 17, 18, 19, 20, 21,23 The authors classically reported the presence or absence of several types of bone remodeling (cortical narrowing, spot welds, condensation lines, and so on) in each of the 5 zones around the stem and then classified each patient as having “low adaptation” or “high adaptation.” In our study, we decided to describe these phenomena differently, by converting the 5-zone system into a 3-type system (MCN, MMT, and LMT), using ratios to evaluate bone narrowing. This allowed us to quantify these phenomena precisely and therefore to perform a risk-factor analysis for each. By use of our system, bony adaptations were more frequently observed (80.3%) than in previously published articles about the Ascend Flex stem, probably because of the quantitative methodology, which detected even a very slight bone narrowing that could have been considered normal in a simple quantitative and subjective analysis. However, our quantitative analysis also demonstrated that severe forms (>50%) were quite rare (7.1%). This rate seemed to be lower than the rates reported with the first-generation stem (without a proximal mantle of porous titanium).2,4,11,17,18,20,23 In fact, the addition of a porous coating to the second-generation stem (Ascend Flex) allowed better bony ingrowth and metaphyseal fixation. This aimed to reduce the occurrence of radiolucent lines and early loosening, as well as to limit stress-shielding phenomena by increasing metaphyseal stress. These improvements have been confirmed by 2 studies comparing the 2 generations of this stem,11,23 and our study findings were in accordance with all of those findings.
It has been reported in the literature that proximal bony adaptations were related to stress shielding, with distal stability of the stem leading to a decrease in metaphyseal constraints. Logically, this bony adaptation are constantly correlated to a high filling ratio for long stems as well as for short stems.5,8,12,14,17,20,22 Some authors have also reported a significant influence of diaphyseal cortical contact of the stem,14,20 but age, sex, dominant side, axis deviation, and any other parameters were not found to be risk factors. To our knowledge, this has always been determined by univariate analysis, after a qualitative assessment, and without consideration of the localization of remodeling. Considering that proximal bone remodeling may not be only 1 entity but may comprise several entities with different risk factors, we decided to differentiate them, quantify them with ratios, and analyze all the potential risk factors in a multivariate model. We found that MCN was correlated with a high dFR, low bone quality, and female sex, but these were not independent factors. In fact, a direct correlation may exist between the low bone quality, more frequent in female patients, and the temptation to fill the humerus with the stem to obtain primary stability. We assume that MCN should be considered a consequence of stress shielding owing to distal fixation of the stem, which is more likely to occur with larger stems implanted in osteoporotic bone in female patients to achieve stability. On the other hand, stem axis deviation was the only independent risk factor for MMT (varus deviation) and LMT (valgus deviation). This finding suggested that MMT and LMT might not be related to a classic stress-shielding mechanism but rather to an abnormal distribution of metaphyseal stresses, when the stem is not aligned with the humeral shaft axis. It was impossible to draw a biomechanical conclusion based on our radiologic data, but these data highlighted the importance of correct stem alignment.
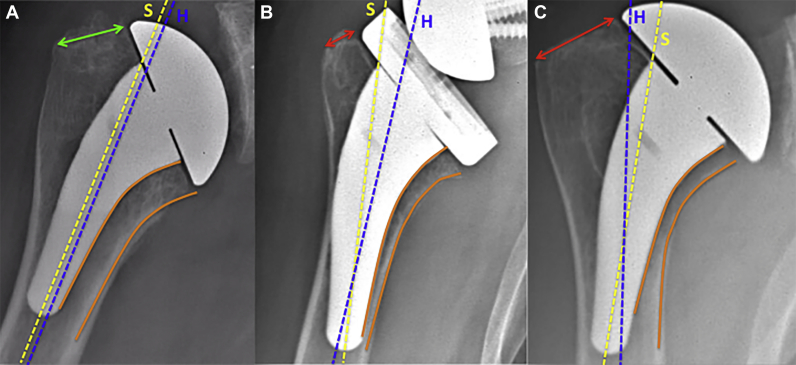
Our findings have led to some technical recommendations related to humeral filling and the stem axis to limit these types of bone remodeling when using a short uncemented stem. Regarding the filling ratio, the primary stability of the stem should be metaphyseal, without any diaphyseal contact. To do so, we recommend stopping the broaching at the first size with rotational stability without reaching the maximum size (with diaphyseal contact), determined by using the sounders available in the standard instrumentation or by using 3-dimensional planning. In the case of poor metaphyseal bone quality, the rotational stability of the broach may only be obtained by reaching this maximum size and, thus, diaphyseal stability. In these cases, we should consider cementation of a polished stem rather than press-fit fixation. In addition, to ensure correct stem alignment, the humeral axis can be monitored during the surgical procedure by using the sounders. Moreover, we assume that it is mandatory to perform the humeral cut at the correct height (at the anatomic neck) so that the curvature of the remaining proximal humerus will fit the curvature and offset of the stem. A cut that is too high will lead to varus deviation, whereas a cut that is too low will lead to valgus deviation (Fig. 5).
Figure 5.
Influence of humeral cut height on axis deviation of stem. (A) Cut at anatomic neck. The remaining neck is adequate ( ). The curvature of the stem is parallel to the medial cortex of the humerus (
). The curvature of the stem is parallel to the medial cortex of the humerus ( ), allowing perfect alignment of the stem with the humerus. (B) Cut that is too low. The remaining neck is too short (
), allowing perfect alignment of the stem with the humerus. (B) Cut that is too low. The remaining neck is too short ( ), and the curved design of the stem leads to valgus positioning. (C) Cut that is too high. The remaining neck is too long (
), and the curved design of the stem leads to valgus positioning. (C) Cut that is too high. The remaining neck is too long ( ), and the curvature of the stem cannot follow the curvature of the calcar, leading to varus positioning. S, axis of the stem (
), and the curvature of the stem cannot follow the curvature of the calcar, leading to varus positioning. S, axis of the stem ( ). H, axis of the humeral shaft (
). H, axis of the humeral shaft ( ).
).
We also found disappearance of the metaphyseal bone under the metallic baseplate (UBO), occurring in 34.4% of cases. We decided to analyze this phenomenon separately from the 3 other phenomena because it has not usually been described in the literature when analyzing the stress-shielding phenomenon. Moreover, it was not statistically associated with the aforementioned bony adaptations (MCN, MMT, and LMT) and thus may not be related to stress shielding. Despite the lack of precise radiographic analysis of the glenoid in our study, we assume that this type of osteolysis could be related to the presence of polyethylene implants and polyethylene wear, as suggested by Raiss et al13 and Jullion et al,9 who already described this phenomenon. In our series, it was significantly more frequent with RSA than with TSA and was never found with HA, which could support this hypothesis. The high rate of UBO in our series, at this short follow-up, could be explained by the straight design of the humeral baseplate allowing earlier detection of slight osteolysis compared with classic stems with bulky inlay metaphyses. With these stems, proximal osteolysis could be identified on standard radiographs only when it is already extended and involves the tuberosities. In our series, the mean width was only 2.9 mm, without any extent to the tuberosities.
In our series, none of the proximal humeral osteolysis phenomena had any influence on clinical outcomes at 2-year follow-up. The same findings have been unanimously reported in the literature.4,5,17,19,20,22 Long-term studies are lacking to confirm that clinical outcomes are maintained over time and to evaluate the progression of these bony adaptations over time. In a series of 106 TSAs with long stems, Spormann et al22 reported a 17% rate of calcar full resorption at a minimum 5-year follow-up. They observed that 86% of these osteolysis events were already visible at 1-year follow-up and 100% were visible at 2-year follow-up; no further case was identified between 2- and 5-year follow-up. The mean height of osteolysis was measured and progressed significantly between 6 and 12 months but remained stable after 12 months, with a tendency toward regression at 5 years (not significant). Schnetzke et al19 reported the same findings with the first generation of the Ascend short stem (Ascend Monolithic; Tornier SAS), without any clinical consequences at 5-year follow-up. Their results are reassuring regarding the risks related to proximal bone disappearance, but it remains necessary to continue the follow-up of our patients to confirm that these phenomena are not evolutive after 2 years with the Ascend Flex implant (second generation).
Our study had some limitations, including the retrospective design, the lack of glenoid-side analysis, and the limited follow-up period of 2 years. However, it also has strengths, such as the large number of cases that associated TSA and RSA in equivalent proportions, making it the largest series published to date about the Aequalis Ascend Flex stem. We also have proposed a quantitative analysis of bone remodeling, with a precise analysis of the risk factors with a multivariate model. Finally, this was a single-center study, with a standardized radiologic protocol performed in the same center for each patient, allowing precision and reliability of all measurements.
Conclusion
The radiographic evolution of the Ascend Flex short stem was satisfactory at mid-term follow-up and was associated with excellent clinical outcomes and a low rate of complications that never related to the stem. Bony adaptations seemed to be limited phenomena, without any observed consequence at follow-up. A high filling ratio and axis deviation of the stem must be avoided to limit bone remodeling. Cementation should be considered in patients with osteoporosis. Further studies with longer follow-up periods will be needed to monitor the progression and potential consequences of these bony adaptations over time.
Disclaimer
François Sirveaux receives royalties from Tornier-Wright and is a consultant for Tornier-Wright Medical.
Daniel Mole receives royalties from Tornier-Wright Medical.
Adrien Jacquot receives consultant fees from Tornier-Wright Medical.
The other authors, their immediate families, and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Footnotes
The Institutional Review Board of the ethical committee of Hôpital Privé Jean Mermoz and Centre Orthopédique Santy approved this project (study no. 20.15.2).
References
- 1.Boileau P., Moineau G., Roussanne Y., O'Shea K. Bony increased-offset reversed shoulder arthroplasty: minimizing scapular impingement while maximizing glenoid fixation. Clin Orthop Relat Res. 2011;469:2558–2567. doi: 10.1007/s11999-011-1775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casagrande D.J., Parks D.L., Torngren T., Schrumpf M.A., Harmsen S.M., Norris T.R. Radiographic evaluation of short-stem press-fit total shoulder arthroplasty: short-term follow-up. J Shoulder Elbow Surg. 2016;25:1163–1169. doi: 10.1016/j.jse.2015.11.067. [DOI] [PubMed] [Google Scholar]
- 3.Constant C.R., Murley A.H. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987:160–164. [PubMed] [Google Scholar]
- 4.Denard P.J., Noyes M.P., Walker J.B., Shishani Y., Gobezie R., Romeo A.A. Radiographic changes differ between two different short press-fit humeral stem designs in total shoulder arthroplasty. J Shoulder Elbow Surg. 2018;27:217–223. doi: 10.1016/j.jse.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Denard P.J., Noyes M.P., Walker J.B., Shishani Y., Gobezie R., Romeo A.A. Proximal stress shielding is decreased with a short stem compared with a traditional-length stem in total shoulder arthroplasty. J Shoulder Elbow Surg. 2018;27:53–58. doi: 10.1016/j.jse.2017.06.042. [DOI] [PubMed] [Google Scholar]
- 6.Denard P.J., Raiss P., Gobezie R., Edwards T.B., Lederman E. Stress shielding of the humerus in press-fit anatomic shoulder arthroplasty: review and recommendations for evaluation. J Shoulder Elbow Surg. 2018;27:1139–1147. doi: 10.1016/j.jse.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 7.Giuseffi S.A., Streubel P., Sperling J., Sanchez-Sotelo J. Short-stem uncemented primary reverse shoulder arthroplasty: clinical and radiological outcomes. Bone Joint J. 2014;96-B:526–529. doi: 10.1302/0301-620X.96B3.32702. [DOI] [PubMed] [Google Scholar]
- 8.Inoue K., Suenaga N., Oizumi N., Yamaguchi H., Miyoshi N., Taniguchi N. Humeral bone resorption after anatomic shoulder arthroplasty using an uncemented stem. J Shoulder Elbow Surg. 2017;26:1984–1989. doi: 10.1016/j.jse.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Jullion S., Jacquot A., Mole D. Shoulder concepts. Reverse shoulder arthroplasty. Sauramps Medical; Montpellier: 2016. Long-term results of reverse shoulder arthroplasty. Problems and complications related to the glenoid; pp. 467–471. [Google Scholar]
- 10.Melis B., DeFranco M., Lädermann A., Molé D., Favard L., Nérot C. An evaluation of the radiological changes around the Grammont reverse geometry shoulder arthroplasty after eight to 12 years. J Bone Joint Surg Br. 2011;93:1240–1246. doi: 10.1302/0301-620X.93B9.25926. [DOI] [PubMed] [Google Scholar]
- 11.Morwood M.P., Johnston P.S., Garrigues G.E. Proximal ingrowth coating decreases risk of loosening following uncemented shoulder arthroplasty using mini-stem humeral components and lesser tuberosity osteotomy. J Shoulder Elbow Surg. 2017;26:1246–1252. doi: 10.1016/j.jse.2016.11.041. [DOI] [PubMed] [Google Scholar]
- 12.Nagels J., Stokdijk M., Rozing P.M. Stress shielding and bone resorption in shoulder arthroplasty. J Shoulder Elbow Surg. 2003;12:35–39. doi: 10.1067/mse.2003.22. [DOI] [PubMed] [Google Scholar]
- 13.Raiss P., Edwards T.B., Deutsch A., Shah A., Bruckner T., Loew M. Radiographic changes around humeral components in shoulder arthroplasty. J Bone Joint Surg Am. 2014;96:e54. doi: 10.2106/JBJS.M.00378. [DOI] [PubMed] [Google Scholar]
- 14.Raiss P., Schnetzke M., Wittmann T., Kilian C.M., Edwards T.B., Denard P.J. Postoperative radiographic findings of an uncemented convertible short stem for anatomic and reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2019;28:715–723. doi: 10.1016/j.jse.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 15.Razfar N., Reeves J.M., Langohr D.G., Willing R., Athwal G.S., Johnson J.A. Comparison of proximal humeral bone stresses between stemless, short stem, and standard stem length: a finite element analysis. J Shoulder Elbow Surg. 2016;25:1076–1083. doi: 10.1016/j.jse.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 16.Romeo A.A., Thorsness R.J., Sumner S.A., Gobezie R., Lederman E.S., Denard P.J. Short-term clinical outcome of an anatomic short-stem humeral component in total shoulder arthroplasty. J Shoulder Elbow Surg. 2018;27:70–74. doi: 10.1016/j.jse.2017.05.026. [DOI] [PubMed] [Google Scholar]
- 17.Schnetzke M., Coda S., Raiss P., Walch G., Loew M. Radiologic bone adaptations on a cementless short-stem shoulder prosthesis. J Shoulder Elbow Surg. 2016;25:650–657. doi: 10.1016/j.jse.2015.08.044. [DOI] [PubMed] [Google Scholar]
- 18.Schnetzke M., Coda S., Walch G., Loew M. Clinical and radiological results of a cementless short stem shoulder prosthesis at minimum follow-up of two years. Int Orthop. 2015;39:1351–1357. doi: 10.1007/s00264-015-2770-2. [DOI] [PubMed] [Google Scholar]
- 19.Schnetzke M., Preis A., Coda S., Raiss P., Loew M. Anatomical and reverse shoulder replacement with a convertible, uncemented short-stem shoulder prosthesis: first clinical and radiological results. Arch Orthop Trauma Surg. 2017;137:679–684. doi: 10.1007/s00402-017-2673-3. [DOI] [PubMed] [Google Scholar]
- 20.Schnetzke M., Rick S., Raiss P., Walch G., Loew M. Mid-term results of anatomical total shoulder arthroplasty for primary osteoarthritis using a short-stemmed cementless humeral component. Bone Joint J. 2018 01;100-B:603–609. doi: 10.1302/0301-620X.100B5.BJJ-2017-1102.R2. [DOI] [PubMed] [Google Scholar]
- 21.Schnetzke M., Wittmann T., Raiss P., Walch G. Short-term results of a second generation anatomic short-stem shoulder prosthesis in primary osteoarthritis. Arch Orthop Trauma Surg. 2019;139:149–154. doi: 10.1007/s00402-018-3039-1. [DOI] [PubMed] [Google Scholar]
- 22.Spormann C., Durchholz H., Audigé L., Flury M., Schwyzer H.-K., Simmen B.R. Patterns of proximal humeral bone resorption after total shoulder arthroplasty with an uncemented rectangular stem. J Shoulder Elbow Surg. 2014;23:1028–1035. doi: 10.1016/j.jse.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 23.Szerlip B.W., Morris B.J., Laughlin M.S., Kilian C.M., Edwards T.B. Clinical and radiographic outcomes after total shoulder arthroplasty with an anatomic press-fit short stem. J Shoulder Elbow Surg. 2018;27:10–16. doi: 10.1016/j.jse.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 24.Tingart M.J., Apreleva M., von Stechow D., Zurakowski D., Warner J.J. The cortical thickness of the proximal humeral diaphysis predicts bone mineral density of the proximal humerus. J Bone Joint Surg Br. 2003;85:611–617. doi: 10.1302/0301-620x.85b4.12843. [DOI] [PubMed] [Google Scholar]
- 25.Wolff J. Springer-Verlag; Heidelberg: 1986. The law of bone remodelling. [Google Scholar]