Summary
The bioactivity of biomaterials is closely related to cell response in contact with them. However, shortly after their insertion, materials are soon covered with proteins that constitute the biological fluids, and which render the direct surface recognition by cells almost impossible. The control of protein adsorption at the interface is therefore desirable. Extracellular matrix proteins are of particular interest in this sense, due to their well-known ability to modulate cell behavior. Particularly, fibronectin plays a leading role, being present in both healthy and injured tissues undergoing healing and regeneration. The aim of the present work is to give an overview on fibronectin and on its involvement in the control of cell behavior providing evidence of its pivotal role in the control of cell adhesion, spreading, migration, proliferation and differentiation. A deep insight into methods to enrich biomaterials surface with fibronectin will be then discussed, as well as new cues on the possibility to design tailored platforms able to specifically retain fibronectin from the surrounding extracellular milieu.
Keywords: Fibronectin, Biomaterials, Tissue engineering, Regenerative medicine, Bone regeneration
1. Introduction
Once a biomaterial is inserted in the host site, protein adsorption from biological fluids, e.g. blood plasma, occurs rapidly, mediating the interaction surface-cells. The composition of the adsorbed protein layer at the interface plays a vital role in determining the nature of the tissue-material reciprocal fate, determining crucial characteristics of cell response, including adhesion, spreading, migration, proliferation and differentiation [1]. Particularly, some proteins can stimulate a constructive cells response, thus promoting wound healing and tissue regeneration, only when correctly presented. On the other hand, when in different conformation or modified, proteins may trigger a host immune reaction leading to its removal or isolation. Regrettably, protein adsorption on biomaterials is mostly a haphazard process and it is mainly driven by the chemical and physical characteristics of the material, as by protein availability and reciprocal interactions, which may lead to the adsorption of proteins which do not convey useful stimuli to cells because of an impaired conformation [2,3]. Thus, controlling specific protein adsorption at the interface of biomaterials may represent a viable approach in tissue engineering (TE), to design highly performant scaffolds able to address cell activity in detail [4].
Fibronectin (FBN) is an extracellular matrix (ECM) component that, through binding integrin receptors of the cell surface, acts as a key player of the communication between the intra and the extracellular environment, thus controlling cell behavior. Furthermore, in regenerative dentistry the role of FBN in promoting the attachment of cells to root surface has been shown, as well as FBN probable pivotal role in bone and periodontal regeneration is of considerable interest [5]. Therefore, the modulation of integrin-FBN interaction may offer a promising approach to tailor tissue regenerative responses, i.e. bone and periodontal regeneration [6].
The aim of the present concise narrative review is to focus the research supporting this crucial role of FBN and the methods developed to ameliorate scaffold bioactivity modulating functional FBN availability at the cell-biomaterial interface in TE approaches.
2. Fibronectin as a controller of cell behavior
The ECM is the non-cellular component of tissues and it basically provides physical scaffolding for cells, besides transmitting biochemical and biomechanical stimuli required for tissue morphogenesis and homeostasis. ECM main components are water, proteoglycans and proteins. Proteoglycans fill the major part of the extracellular environment and are the responsible for ECM force-resistance properties [7]. While, proteins are involved in orchestrating cell adhesion and migration. Among them, FBN is an important cell-adhesive ECM protein, which is present also in injured tissues undergoing regeneration. It exists into two main forms: i) the soluble FBN, which is a major component of blood plasma (300 μg/ml) and it is synthetized by hepatocytes and ii) the less-soluble cellular FBN that is synthetized by different types of fibroblasts to be then assembled into the ECMs [8]. FBN plays a key role in cell behaviors, such as adhesion, migration and differentiation, as well as in morphogenesis and wound healing. As such, FBN and its mechanisms are clear candidates for the amelioration of scaffold bioactivity.
2.1. Fibronectin structure
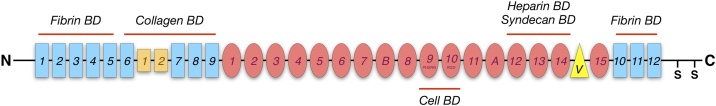
FBN exists in human in more than 20 alternative splicing isoforms. Structurally, FBN is a dimeric high-molecular weight glycoprotein (∼440 kDa), composed by two nearly identical subunits (∼250 kDa) covalently bound by disulfide bonds near their C-term portion (Fig.1) [8]. Each of these subunits consists of three different types of repeats: type I, II and III. Type I repeats are 12 modules of 40 amino acidic residues, structured as stacked ß-sheets, linked by a disulphide bond, and that present a hydrophobic core made up of aromatic conserved residues. Type II repeats (2 modules) are 60 residues long, constituted by perpendicular antiparallel ß-sheets linked together by 2 intra-chain disulphide bonds. Eventually, type III repeats, are 90 residues long and between 15 and 17 modules. These repeats do not possess disulfide bonds and the antiparallel ß-sheets are linked together with flexible loops and stabilized through hydrogen bonding [[9], [10], [11]]. As such, type III repeats are the highest sensitive to possible FBN unfolding [12].
Fig. 1.
Fibronectin. Diagram representing the structure of fibronectin single subunit. Repeats and binding domains (BD) are indicated.
2.2. Fibronectin–integrin recognition
FBN communication with cells occurs through integrin binding. Integrins are the main cell surface receptors that mediate cell-matrix adhesion, some of which are ubiquitously expressed, while others are tissue-specific. Structurally, integrins are heterodimers generated by the coupling of 18-alpha (α) and 8-beta (ß) subunits, which specifically bind different ECM molecules. Each subunit consists of a large extracellular domain with selectivity for ECM ligands, a transmembrane domain and a short cytoplasmic tail. Because integrins lack of intrinsic enzymatic activity, the cytoplasmic tail of the ß subunit is structured to engage intracellular signaling molecules after dimerization and to activate the integrin-mediated transduction pathway [13,14].
There are many different integrins recognizing FBN (Table 1) and each of them extremely depends on FBN structural conformation and on type III residues sensitiveness to unfolding. For example, the classic receptor for FBN is known to be the α5ß1 integrin (FBN-α5ß1 Kd = 8 × 10−7 M) [15]. The α5ß1 recognizes and binds FBN through the interaction with an isolated tri-peptide sequence, the arginine-glycine-aspartic acid (RGD), which is contained in the 10th type III repeat of FBN and that synergizes with a further sequence, the proline-histidine-serine-arginine-asparagine (PHSRN), on the adjacent 9th type III repeat of FBN [16]. In bulk conditions, the RGD cell-binding domain and the recognition sequence PHSRN are separated from 32 Å. This distance results to be extremely important for specific recognition between FBN and α5ß1 integrin. Indeed, if FBN-10th III domain unfolds as an effect of a 10 pN external force application, the RGD loop is pulled away from the PHSRN on the FBN-9th III domain, resulting in a 23 Å removal, which greatly diminishes the ability of α5ß1 integrin to recognize FBN, but which enhances that of the αVß3 integrin isoform [12]. As a results, FBN conformational changes may dramatically drive integrin specificity and pathophysiological cell and tissue responses, including the reactions to grafted biomaterials.
Table 1.
The integrin family of adhesion receptors.
| Cell integrin | FBN and other ECM ligands | Cell expressing integrin |
|---|---|---|
| α3ß1 | Fibronectin, collagen-I, epiligrin, laminin, nidogen, entactin | B-lymphocytes, kidney glomerulus cells |
| α4ß5 | Fibronectin, VCAM-I | Lymphocytes, monocytes, eosinophils, NK-cells, thymocytes |
| α5ß1 | Fibronectin | Bone cells, memory T-cells, monocytes, platelets, fibroblasts |
| α8ß1 | Fibronectin | Not yet identified |
| αVß1 | Fibronectin, vitronectin | Not yet identified |
| αVß3 | Fibronectin, fibrinogem, Von Willebrand’s factor, vitronectin, thrombospondin | Bone cells, endothelial cells, B-cells, platelets, monocytes |
| αIIß3 | Fibronectin fibrinogen, Von Willebrand’s factor, vitronectin | Platelets |
| αVß6 | Fibronectin | Carconoma cells |
2.3. Fibronectin and the control of cell behavior
Precisely, cell-FBN interaction occurs by synergic interplay of proteins at three different level: i) FBN that offers docking points for cells, ii) integrins that allow the recognition of the FBN and iii) intracellular proteins that activate specific transduction pathways to control cell response, including adhesion, spreading, migration, proliferation and differentiation.
2.3.1. Cell adhesion and spreading
Cell adhesion refers both to the mechanisms by which neighboring cells interact, attach or communicate each other by cell junctions (cell–cell adhesion), as to the ability of cells to interact with their surrounding ECM or with an artificial substrate through focal contacts (cell–matrix adhesion) [17].
The sites of cell adhesion with the extracellular environment are called focal adhesions. At this level, after integrin dimerization, a network of 156 components and of more than 690 interactions form the adhesome and lead in the end to cytoskeleton proteins rearrangement. This wide spectrum of proteins may be divided in three categories: i) integrin-binding proteins, ii) adaptors or scaffolding proteins and iii) enzymes [18]. Integrin-binding proteins are directly recruited by the cytoplasmic tail of the integrin ß subunit. Among them, the binding of talin have been established to have a key role in integrin activation and it has been demonstrated that competition for talin binding may severely down-regulate integrin transduction pathway activation. Thus, adaptors or scaffolding proteins (e.g. vinculin, paxillin and α-actinin), link integrin-associated proteins with cytoskeleton components, while enzymes, which are mainly tyrosine-associated kinases (e.g. focal adhesion kinase – FAK, Rho family associated GTPases, Src), contribute to molecular signal transmission [18].
Cell spreading is directly connected to cell adhesion and it is fundamental for subsequent proliferation, migration and differentiation, and a lack in cell adhesion/spreading may result in consequent cell apoptosis [19,20].
As we stated above, FBN and many others ECM components, possess adhesive motifs with the ability to promote cell adhesion and spreading. Thus, these molecules may be introduced on biomaterials to modify cell response. For example, the coating of substrates with increasing amount of FBN, leads to an evident promotion of cell adhesion and spreading in term of degree and speed [[21], [22], [23]]. To this purpose, in a recent research we have observed that ameliorating the hydrophilicity of titanium implant surfaces by heating is a viable option to promote FBN adsorption from plasma serum, thus improving the eventual adhesion of osteoblasts [24].
2.3.2. Cell migration
Cell migration is a cellular activity crucial to scaffold colonization, and directly depends on cell adhesion and spreading. It occurs along the substrates through the formation of new focal adhesions and the release of the old ones. Physiologically, the human body has compositional gradients of soluble signals in tissues, which allow the creation of a natural driving force to direct the migration of cells (chemotaxis). Alternatively, substrate-attached factors (haptotaxis) or mechanical cues (durotaxis) may govern cell migration [17].
More specifically, during cell migration processes, gradients guide actin assembly to the cell’s leading edge, thus determining the direction of cell movement. At the migration front, the activation of cell surface receptors, i.e. integrins, generates the activation of the Rho-family GTPases and of the phosphatidylinositol biphosphate (PIP2) pathway, which in turn activate the WASp/Scar proteins and eventually the Arp2/3 complex that guide the formation of new actin filaments branching from the preexisting ones. This process leads to the pushing of cell membrane forward. Simultaneously, at the back side of the cells, myosin II interacts with old actin filaments to enhance local rigidity, preventing lateral filopodia extension and allowing the retraction of the trailing end [25].
The abundance of FBN in the clot is closely related to fibroblasts recruitment during wound healing. As such, FBN introduction on biomaterials may allow the creation of dynamic pathway for cells to move along the scaffold [26]. Accordingly, Nuttelman et al. observed that NIH3T3 fibroblasts migrated faster on poly(vinyl alcohol) hydrogels modified with FBN, if compared to tissue culture plate and to control hydrogels [26]. Similarly, by means of aptamers selected to recognize and bind FBN, we were so far able to improve the migratory capacity of osteoblasts into a hyaluronic acid based matrix [27].
2.3.3. Cell proliferation
Cell proliferation consists in cell number growth as a consequence of cell division. In adult organisms, cell proliferation is generally restricted to cell that replenish tissues. Cell division occurs as eventual stage of the cell cycle, which is broken up into four moments. The G1 phase is the first moment of a cell life and it is mainly characterized by cell growth and development. G1 phase is followed by the S phase, which consists in DNA replication. Thus, G2 phase, which is the gap before to cell division phase, ensures the proper replication of DNA and its packaging prior to cell division (M phase) [28].
The progression of the cell cycle is positively regulated by a family of protein kinases called cyclin-dependent kinases (Cdks), which turn on specific genes promoting growth (cyclines) and off their inhibitors at specific time. Particularly, the transition from G1 to S phase, which is the most critical point in the cell cycle, is positively regulated by Ckd2 and Ckd4, which induce cyclin E and cyclin D1 respectively, via the MAPK/ERK pathway [28]. A few studies have correlated the induction of cyclin D1 by integrin signaling, highlighting that the integrin-dependent phosphorylation of FAK plays a key role in the phosphorylation of ERK and in the induction of cyclin D1.
Thus, the decoration of substrates with FBN may be involved in increasing cell proliferation [29,30]. Noteworthy, it has been observed that FBN α5ß1 integrin activation mediates cell proliferation through the activation of the MAPK/ERK associated to the epithelial growth factor (EGF) receptor [29].
Still by using aptamers selected against FBN, we have also been able to promote the proliferative capacity of cells on chitosan [31]; and, we have further demonstrated that the molding of the cellular behavior was associated with the activation of the integrin pathway due to a massive adsorption of FBN on chitosan surface [32].
2.3.4. Cell differentiation
Eventually, cell differentiation is the process that leads to a cell to reach its specialized and mature phenotype, through the signaling of a defined combinations of transcription factors. Besides growth factors, various kind of ECM-derived proteins have function in regulating cell differentiation [33]. In this sense, it has been shown that active integrin signaling is essential for driving differentiation: e.g. genetic removal of ß1 integrin subunits inhibits the differentiation pathway of the epithelial cells. As such, tailoring the quantity and activity of fibronectin onto substrates could be used to selectively guide cells fate. Fascinatingly, the capacity of FBN to bind multiple integrins by slightly modulating its conformation, represent a design challenge to control specific cell behavior during their commitment. In a work by Martino et al. it was shown the capacity of a structurally stabilized FBN III9*-10 domain to promote osteogenic differentiation both in 2D and in 3D environment if compared to whole FBN or to the less specific FBN III9-10 or FBN III10 fragment. FBN III9*-10 presented a single mutation that switched the Leucine1408 into a Proline on the III9 domain, thus conferring to FBN enhanced affinity for α5ß1 integrin [34]. This example demonstrate how engineered cellular adhesive interaction with the surrounding milieu can lead to cell commitment control.
3. Fibronectin adsorption to biomaterials
As reported in the previous discussion, the adsorption of proteins, particularly of FBN, is of the utmost relevance for tissue-biomaterial interaction. However, body fluids contain a heterogeneity of biomolecules and their adsorption on biomaterials is a complex process. Blood, for instance, consists of more than 150 proteins further supplemented with lipids, carbohydrates and other molecules that compete for their adsorption at the interface of materials. More specifically, when a surface is exposed to blood plasma, certain molecules are preferentially deposited from the bulk and both the affinity of proteins for the surface (e.g. size, charge, conformational stability) and kinetic factors (e.g. size, concentration) contribute to determine the profile of molecules stably adsorbed on the surface. Simply considering the diffusion, molecules that are present in the bulk solution at high concentration and/or with small size are deposited quicker than low concentrated and/or heavier ones (Table 2).
Table 2.
Exchange hierarchy of plasma proteins on surfaces [35].
| Protein | Blood plasma concentration (mg/ml) | Molecular weight (Da) |
|---|---|---|
| Albumin | 40 | 66,000 |
| Immunoglobulin-G | 15 | 150,000 |
| Fibrinogen | 3 | 340,000 |
| Fibronectin | 0,2 | 220,000 |
| Factor XII | 0,015–0,047 | 80,000 |
The strength of the adhesion on the biomaterial plays also a role. Indeed, molecules presenting greater affinity for the surface may induce the detaching of the previously and less affine adsorbed ones. These exchanges start to occur when all the binding sites of the substrate are occupied and continue until the surface is populated with proteins and molecules having strong affinity and interaction for the material. This hierarchical tendency has been called the Vroman effect [36]. It is thus evident that to avoid competitive protein exchange on surfaces, desired proteins, i.e. FBN, could be preferentially immobilized, in order to trigger specific responses. Table 3 summarized the methods that have been proposed to enrich biomaterials interface with FBN highlighting their major issues and anticipates the discussion on the next two paragraphs.
Table 3.
Methods to enrich biomaterial interface with fibronectin.
| Fibronectin source | Way to enrich biomaterial interface with fibronectin | Drawbacks |
|---|---|---|
| Heterologous | Direct immobilization through physical adsorption | Possible host immune response; |
| Possible spontaneous desorption; | ||
| Possible adsorption in an undesired conformation. | ||
| Surface functionalization and consequent covalent immobilization | Possible host immune response; | |
| Possible adsorption in an undesired conformation; | ||
| Possible lost of protein mobility. | ||
| Recombinant fragments | Direct immobilization through physical adsorption | Possible spontaneous desorption; |
| Lack of entire protein availability; | ||
| High costs of production. | ||
| Surface functionalization and consequent covalent immobilization | Lack of entire protein availability; | |
| High costs of production. | ||
| Recombinant cell binding domains | Surface functionalization and consequent covalent immobilization | Lack of specific binding sites interaction. |
| Autologous | Monoclonal antibody immobilization | Possible host immune response; |
| High molecular size; | ||
| High costs of production. | ||
| Aptamer immobilization | High costs of production. |
3.1. Immobilization of heterologous or recombinant fibronectin
The ex vivo decoration of biomaterials with FBN or with its derived fragment or domains has been for years the gold standard strategy to enrich scaffolds with cues to direct control cell response.
The immobilization of FBN on scaffold surface may occur by physical adsorption or by surface functionalization and consequent covalent immobilization [4]. Even though covalently immobilized FBN is more complex to obtain, it faces and bypasses several issues connected to the physical adsorption, i.e. FBN spontaneous desorption or undesired conformational changes, which in turn lead to FBN loss of function [37,38]. For instance, in a work by Custodio et al. the biological activity of FBN simply adsorbed on chitosan or covalently immobilized via carbodiimide chemistry was compared employing human osteosarcoma SaOs-2 cells [21]. In opposition to bare chitosan, chitosan with adsorbed or immobilized FBN promoted cell adhesion. In the presence of FBN cells were well spread and presented the typical elongated morphology of mature osteoblasts. No differences were detected among the two methods in guiding cell adhesion and spreading, indicating that they were very similar in ameliorating chitosan adhesive properties. On the other hand, proliferation on FBN-immobilized surfaces was clearly enhanced after 7 days if compared to FBN-adsorbed chitosan and to control, suggesting a competitive adsorption of serum proteins contained in the culturing medium. Accordingly, desorption studies revealed that surfaces with immobilized FBN retained more proteins if compared to that with adsorbed FBN. Eventually, this study highlighted the importance of the covalent immobilization as a more desirable method to retain bioactive moieties at scaffold interface.
A clearly limitation linked to the immobilization of entire FBN is the difficulty in completely controlling protein conformational adsorption, which strongly depends on the underlying substrate. For example, Keselowsky et al. have demonstrated that the enrichment of a surface with different functionalities dramatically modulated FBN conformational adsorption and cell response, because of a shift in cell-binding domain availability during FBN adsorption [39]. As such, the immobilization of FBN recombinant fragment or of binding domains, have represented a sought after alternative in the upcoming years. However, since the interaction of numerous FBN specific domains (i.e. PHSRN, IKVAV and RGD) is required for the correct interaction with cell integrins, the anchorage of FBN fragment is more desirable than the immobilization of single binding domains [26,[40], [41], [42]].
3.2. Selective fibronectin binding biomaterials
The enrichment of biomaterials with FBN or with its fragments is mainly limited by the large molecular weight of FBN, which limits its stability and bioavailability. Therefore, the creation of selective fibronectin binding materials is desirable. In this sense, an innovative idea could be the introduction of smaller molecules on scaffold surface, which could be exploited to capture FBN from the extracellular space and to retain it on the surface [[43], [44], [45]]. The advantages of this approach appear evident.
The concept of adding selective binding capabilities to biomaterials was first considered to promote the retention of the bone morphogenetic protein 2 (BMP-2) by grafting monoclonal antibodies (mABs) on scaffolds for bone regeneration [46,47]. A similar approach was later attempted by Oliveira et al. [48]. However, the use of mABs is of course limited by compatibility issues, but also by high costs of production, high size and unability to recognize small molecules. As such, to bypass this problem aptamer may be a viable alternative. Hoffmann et al. were the firsts to pioneer this concept in 2011 by using aptamers to retain endothelial cells from blood on vascular grafts [49]. Similarly, Chen et al. decorated synthetic biomaterials with aptamers to promote the colonization by cells, demonstrating an aptamer dose-dependent response of cell behavior [50]. Both these studies showed the potential of aptamers in designing selective binding biomaterials. Considering these premises, our group has recently demonstrated the possibility to promote the adsorption of serum FBN and to ameliorate osteoblasts colonization by using anti-FBN aptamers to materials thought for bone and periodontal regeneration [27,31,51]. We tested the aptamer modification on two different scaffolds: a hyaluronic acid-based hydrogel (HA) and chitosan-based film (CH). Interestingly, aptamers were able to increase the number of adhering cells in both the cases, which further correlated with the amount of aptamers used for the functionalization. Remarkable, although both aptamer-enriched scaffold showed comparable results in term of biological response, protein adsorption and conformational assays suggested the involvement of different mechanisms. The aptamer-modified HA hydrogel increased quantitatively the amount of adsorbed FBN, enhancing the availability of adhesive proteins for cells [27]. Conversely, aptamer-enriched CH showed a similar amount of adsorbed FBN if compared to its bare counterpart, but its conformation and function were fully preserved only in the presence of aptamers [31,51,52].
4. Conclusions
Protein adsorption on biomaterials plays a pivotal role in the subsequent host reactions, implant integration and tissue regeneration. Therefore, its control is a major goal of advanced biomaterial surfaces. Additionally, the role played by FBN in this arena makes it the optimal candidate for the amelioration of materials surface bioactivity. In this work, we focused on the description of this protein and on its biological role. Furthermore, we gave an overview on research efforts to ameliorate its adsorption at the interface of biomaterials. Different methods have been proposed to this purpose and sFBN-materials seemed to be one of the most promising systems because of the possibility to maximize the available host FBN retaining all of its bioactivity.
Conflict of interests
The authors declare they have no conflict of interests.
Acknowledgement
This study was partially supported by a 2013 grant by the American Academy of Implant Dentistry Foundation.
References
- 1.Hiraguchi Y., Nagahashi K., Shibayama T., Hayashi T., Yano T.A., Kushiro K. Effect of the distribution of adsorbed proteins on cellular adhesion behaviors using surfaces of nanoscale phase-reversed amphiphilic block copolymers. Acta Biomater. 2014;10:2988–2995. doi: 10.1016/j.actbio.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 2.Clause K.C., Barker T.H. Extracellular matrix signaling in morphogenesis and repair. Curr Opin Biotechnol. 2013;24:830–833. doi: 10.1016/j.copbio.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morris A.H., Kyriakides T.R. Matricellular proteins and biomaterials. Matrix Biol. 2014;37:183–191. doi: 10.1016/j.matbio.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parisi L., Toffoli A., Ghiacci G., Macaluso G. Tailoring the interface of biomaterials to design effective scaffolds. J Funct Biomater. 2018;9:E50. doi: 10.3390/jfb9030050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pearson B.S., Klebe R.J., Boyan B.D., Moskowicz D. Comments on the clinical-application of fibronectin in dentistry. J Dent Res. 1988;67:515–517. doi: 10.1177/00220345880670021701. [DOI] [PubMed] [Google Scholar]
- 6.Bachman H., Nicosia J., Dysart M., Barker T.H. Utilizing fibronectin integrin-binding specificity to control cellular responses. Adv Wound Care. 2015;4:501–511. doi: 10.1089/wound.2014.0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frantz C., Stewart K.M., Weaver V.M. The extracellular matrix at a glance. J Cell Sci. 2010;123:4195–4200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pankov R., Yamada K.M. Fibronectin at a glance. J Cell Sci. 2002;115:3861–3863. doi: 10.1242/jcs.00059. [DOI] [PubMed] [Google Scholar]
- 9.Baron M., Norman D., Willis A., Campbell I.D. Structure of the fibronectin type 1 module. Nature. 1990;345:642–646. doi: 10.1038/345642a0. [DOI] [PubMed] [Google Scholar]
- 10.Baron M., Main A.L., Driscoll P.C., Mardon H.J., Boyd J., Campbell I.D. H-1-NMR assignment and secondary structure of the cell-adhesion type-III module of fibronectin. Biochemistry. 1992;31:2068–2073. doi: 10.1021/bi00122a025. [DOI] [PubMed] [Google Scholar]
- 11.Pickford A.R., Potts J.R., Bright J.R., Phan I., Campbell D. Solution structure of a type 2 module from fibronectin: implications for the structure and function of the gelatin-binding domain. Structure. 1997;5:359–370. doi: 10.1016/s0969-2126(97)00193-7. [DOI] [PubMed] [Google Scholar]
- 12.Krammer A., Craig D., Thomas W.E., Schulten K., Vogel V. A structural model for force regulated integrin binding to fibronectin’s RGD-synergy site. Matrix Biol. 2002;21:139–147. doi: 10.1016/s0945-053x(01)00197-4. [DOI] [PubMed] [Google Scholar]
- 13.Liu S.C., Calderwood D.A., Ginsberg M.H. Integrin cytoplasmic domain-binding proteins. J Cell Sci. 2000;113:3563–3571. doi: 10.1242/jcs.113.20.3563. [DOI] [PubMed] [Google Scholar]
- 14.Mitra S.K., Hanson D.A., Schlaepfer D.D. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 15.Akiyama S.K., Nagata K., Yamada K.M. Cell-surface receptors for extracellular-matrix components. Biochim Biophys Acta. 1990;1031:91–110. doi: 10.1016/0304-4157(90)90004-v. [DOI] [PubMed] [Google Scholar]
- 16.Mardon H.J., Grant K.E. The role of the 9th and 10th type-III domains of human fibronectin in cell-adhesion. FEBS Lett. 1994;340:197–201. doi: 10.1016/0014-5793(94)80137-1. [DOI] [PubMed] [Google Scholar]
- 17.Alberts B. Garland Science; 2002. Molecular biology of the cell. [Google Scholar]
- 18.Horton E.R., Humphries J.D., James J., Jones M.C., Askari J.A., Humphries M.J. The integrin adhesome network at a glance. J Cell Sci. 2016;129:4159–4163. doi: 10.1242/jcs.192054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lagonegro P., Trevisi G., Nasi L., Parisi L., Manfredi E., Lumetti S. Osteoblasts preferentially adhere to peaks on micro-structured titanium. Dent Mater J. 2018;37:278–285. doi: 10.4012/dmj.2017-008. [DOI] [PubMed] [Google Scholar]
- 20.Galli C., Parisi L., Elviri L., Bianchera A., Smerieri A., Lagonegro P. Chitosan scaffold modified with d-(+) raffinose and enriched with thiol-modified gelatin for improved osteoblast adhesion. Biomed Mater. 2016;11 doi: 10.1088/1748-6041/11/1/015004. [DOI] [PubMed] [Google Scholar]
- 21.Custodio C.A., Alves C.M., Reis R.L., Mano J.F. Immobilization of fibronectin in chitosan substrates improves cell adhesion and proliferation. J Tissue Eng Regen Med. 2010;4:316–323. doi: 10.1002/term.248. [DOI] [PubMed] [Google Scholar]
- 22.Toffoli A., Parisi L., Bianchi M., Rivara F., Manfredi E., Lumetti S. A novel dental implant surface treatment which selectively enhance fibronectin adsorption. J Osseointegr. 2017;9:100–101. [Google Scholar]
- 23.Parisi L., Toffoli A., Bianchi M., Lagonegro P., Rivara F., Manfredi E. Hyper-hydrophilic titanium surfaces improve osteoblast attachment by enhancing fibronectin adsorption at the interface. Clin Oral Implant Res. 2017;28:73. [Google Scholar]
- 24.Toffoli A., Parisi L., Bianchi M.G., Lumetti S., Bussolati O., Macaluso G.M. Thermal treatment to increase titanium wettability induces selective proteins adsorption from blood serum thus affecting osteoblasts adhesion. Mater Sci Eng C. 2020;C107 doi: 10.1016/j.msec.2019.110250. [DOI] [PubMed] [Google Scholar]
- 25.Jin T., Xu X.H., Hereld D. Chemotaxis, chemokine receptors and human disease. Cytokine. 2008;44:1–8. doi: 10.1016/j.cyto.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nuttelman C.R., Mortisen D.J., Henry S.M., Anseth K.S. Attachment of fibronectin to poly(vinyl alcohol) hydrogels promotes NIH3T3 cell adhesion, proliferation, and migration. J Biomed Mater Res. 2001;57:217–223. doi: 10.1002/1097-4636(200111)57:2<217::aid-jbm1161>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 27.Galli C., Parisi L., Piergianni M., Smerieri A., Passeri G., Guizzardi S. Improved scaffold biocompatibility through anti-fibronectin aptamer functionalization. Acta Biomater. 2016;42:147–156. doi: 10.1016/j.actbio.2016.07.035. [DOI] [PubMed] [Google Scholar]
- 28.Alberts B. Garland Science; New York: 2002. Molecular biology of the cell. [Google Scholar]
- 29.Roovers K., Assoian R.K. Integrating the MAP kinase signal into the G1 phase cell cycle machinery. Bioessays. 2000;22:818–826. doi: 10.1002/1521-1878(200009)22:9<818::AID-BIES7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 30.Roovers K., Davey G., Zhu X.Y., Bottazzi M.E., Assoian R.K. Alpha 5 beta 1 integrin controls cyclin D1 expression by sustaining mitogen-activated protein kinase activity in growth factor-treated cells. Mol Biol Cell. 1999;10:3197–3204. doi: 10.1091/mbc.10.10.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parisi L., Galli C., Bianchera A., Lagonegro P., Elviri L., Smerieri A. Anti-fibronectin aptamers improve the colonization of chitosan films modified with d-(+) raffinose by murine osteoblastic cells. J Mater Sci Mater Med. 2017;28 doi: 10.1007/s10856-017-5931-6. [DOI] [PubMed] [Google Scholar]
- 32.Parisi L., Toffoli A., Bianchi M.G., Bergonzi C., Bianchera A., Bettini R. Functional fibronectin adsorption on aptamer-doped chitosan modulates cell morphology by integrin-mediated pathway. Materials (Basel) 2019;12 doi: 10.3390/ma12050812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lumetti S., Calciolari E., Parisi L., Toffoli A., Mazzotta S., Ferrillo S. Study of GSK3 beta inhibitors SB415286 and SB216763 to improve osteoblastic differentiation on microstructured titanium. J Biol Regul Homeost Agents. 2017;31:579–587. [PubMed] [Google Scholar]
- 34.Martino M.M., Mochizuki M., Rothenfluh D.A., Rempel S.A., Hubbell J.A., Barker T.H. Controlling integrin specificity and stem cell differentiation in 2D and 3D environments through regulation of fibronectin domain stability. Biomaterials. 2009;30:1089–1097. doi: 10.1016/j.biomaterials.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dee K., Puleo D., Bizios R. Wiley; 2002. An introduction to tissue–biomaterials interactions; pp. 37–52. [Google Scholar]
- 36.Hirsh S.L., McKenzie D.R., Nosworthy N.J., Denman J.A., Sezerman O.U., Bilek M.M.M. The Vroman effect: competitive protein exchange with dynamic multilayer protein aggregates. Colloids Surf B Biointerfaces. 2013;103:395–404. doi: 10.1016/j.colsurfb.2012.10.039. [DOI] [PubMed] [Google Scholar]
- 37.Garcia A.J., Vega M.D., Boettiger D. Modulation of cell proliferation and differentiation through fibronectin conformational changes. Mol Biol Cell. 1998;9 doi: 10.1091/mbc.10.3.785. 168A-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vallieres K., Chevallier P., Sarra-Bournett C., Turgeon S., Laroche G. AFM imaging of immobilized fibronectin: does the surface conjugation scheme affect the protein orientation/conformation? Langmuir. 2007;23:9745–9751. doi: 10.1021/la701323q. [DOI] [PubMed] [Google Scholar]
- 39.Keselowsky B.G., Collard D.M., Garcia A.J. Surface chemistry modulates fibronectin conformation and directs integrin binding and specificity to control cell adhesion. J Biomed Mater Res A. 2003;66A:247–259. doi: 10.1002/jbm.a.10537. [DOI] [PubMed] [Google Scholar]
- 40.Arnold M., Cavalcanti-Adam E.A., Glass R., Blummel J., Eck W., Kantlehner M. Activation of integrin function by nanopatterned adhesive interfaces. ChemPhysChem. 2004;5:383–388. doi: 10.1002/cphc.200301014. [DOI] [PubMed] [Google Scholar]
- 41.Jongpaiboonkit L., King W.J., Murphy W.L. Screening for 3D environments that support human mesenchymal stem cell viability using hydrogel arrays. Tissue Eng A. 2009;15:343–353. doi: 10.1089/ten.tea.2008.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao M., Sotomayor M., Villa E., Lee E.H., Schulten K. Molecular mechanisms of cellular mechanics. Phys Chem Chem Phys. 2006;8:3692–3706. doi: 10.1039/b606019f. [DOI] [PubMed] [Google Scholar]
- 43.Parisi L., Piergianni M., Smerieri A., Graiani G., Ghiacci G., Lumetti S. Aptamers improve cell biocompatibility of implantable biomaterials. Minerva Stomatol. 2015;64:151. [Google Scholar]
- 44.Parisi L., Galli C., Neri A., Toffoli A., Calciolari E., Manfredi E. Aptamers improve the bioactivity of biomaterials. Aptamers. 2017;1:3–12. [Google Scholar]
- 45.Parisi L., Rivara F., Costa C., Galli C., Lumetti S., Manfredi E. Aptamer-enriched hydrogel promotes new bone deposition in a rat periodontal fenestration model. J Osseointegr. 2017;9:144. [Google Scholar]
- 46.Freire M.O., You H.K., Kook J.K., Choi J.H., Zadeh H.H. Antibody-mediated osseous regeneration: a novel strategy for bioengineering bone by immobilized anti-bone morphogenetic protein-2 antibodies. Tissue Eng A. 2011;17:2911–2918. doi: 10.1089/ten.tea.2010.0584. [DOI] [PubMed] [Google Scholar]
- 47.Ansari S., Moshaverinia A., Pi S.H., Han A., Abdelhamid A.I., Zadeh H.H. Functionalization of scaffolds with chimeric anti-BMP-2 monoclonal antibodies for osseous regeneration. Biomaterials. 2013;34:10191–10198. doi: 10.1016/j.biomaterials.2013.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oliveira C., Costa-Pinto A.R., Reis R.L., Martins A., Neves N.M. Biofunctional nanofibrous substrate comprising immobilized antibodies and selective binding of autologous growth factors. Biomacromolecules. 2014;15:2196–2205. doi: 10.1021/bm500346s. [DOI] [PubMed] [Google Scholar]
- 49.Hoffmann J., Paul A., Harwardt M., Groll J., Reeswinkel T., Klee D. Immobilized DNA aptamers used as potent attractors for porcine endothelial precursor cells. J Biomed Mater Res A. 2008;84:614–621. doi: 10.1002/jbm.a.31309. [DOI] [PubMed] [Google Scholar]
- 50.Chen N., Zhang Z., Soontornworajit B., Zhou J., Wang Y. Cell adhesion on an artificial extracellular matrix using aptamer-functionalized PEG hydrogels. Biomaterials. 2012;33:1353–1362. doi: 10.1016/j.biomaterials.2011.10.062. [DOI] [PubMed] [Google Scholar]
- 51.Saccani M., Parisi L., Bergonzi C., Bianchera A., Galli C., Macaluso G.M. Surface modification of chitosan films with a fibronectin fragment-DNA aptamer complex to enhance osteoblastic cell activity: a mass spectrometry approach probing evidence on protein behavior. Rapid Commun Mass Spectrom. 2018;33:336–342. doi: 10.1002/rcm.8335. [DOI] [PubMed] [Google Scholar]
- 52.Galli C., Parisi L., Elviri L., Saccani M., Ghezzi B., Lumetti S. DNA aptamers improve fibronectin bioavailability and osteoblast responses to chitosan scaffolds. Clin Oral Implant Res. 2017;28:81. [Google Scholar]