Abstract
Background
This study aims to evaluate the use of fluorescent dye Dil and super vital dye acridine orange (AO) in vitro tracking of labeled L. major in the fibroblast cells.
Methods
Dil crystal and AO were used to stain L. major in a co-culture of the fibroblasts with the parasite. AO staining solution was added to 1 × 106 parasites. After 10 min, the stained parasites were centrifuged and washed seven times with phosphate buffered saline (PBS). The stained promastigote was incubated with fibroblasts for 6–8 h. The presence of stained parasites with AO in the fibroblast was assessed using a fluorescence microscope. 1 × 106/mL promastigote of L. major was gently suspended and mixed by Dil staining solution with an ultimate concentration of 0.002 μg/mL and it was kept for 20 min at the room temperature. Subsequently, after washing it in PBS for seven times, it was centrifuged at 3000 rpm for 10 min. The supernatant was removed and the precipitate containing stained promastigote was suspended in fresh DMEM F12 with fibroblasts at 37 °C for 6 h. The presence of stained parasites with Dil in fibroblast was assessed using a fluorescence microscope. Fibroblast characterization was undertaken by a real-time polymerase chain reaction (PCR).
Results
Acridine orange staining assisted in detection of the live parasite in the fibroblast cells. Free promastigote looked green before entering into the fibroblasts after 12 h culture. The parasite entered the cytoplasm of fibroblasts at the beginning of the exposure and gradually entered the nucleus of the fibroblast. The fibroblast nucleus was entirely stained green by AO. The L. major appeared green under the fluorescent microscope. Dil staining revealed that the internalized parasites with red/orange color were localized within the cytoplasm after 6-hours and the nucleus of the fibroblasts after 72-hours following culture. Human fibroblasts were positive at the expression of CD10, CD26, matrix metalloproteinase-1 (MMP-1) and matrix metalloproteinase-3 (MMP-3) and negative for CD106 and integrin alpha 11.
Conclusion
The fluorescent dye Dil staining is a safe, easy to use, inexpensive and fast method for labeling of the Leishmania parasite in the fibroblast cells. Acridine orange staining could be useful for tracing the parasites in the fibroblasts too. In this study, both Dil and AO were compared and considered as suitable vital dyes for identifying labeled Leishmania in the fibroblast in vitro, but Dil was superior to AO with its feature does not transfer from the labeled to unlabeled cells.
Keywords: Immunology, Microbiology, Molecular biology, Infectious disease, Acridine orange, AO, Fluorescent dye, Dil, Fibroblast, In vitro, Leishmania major
Immunology; Microbiology; Molecular biology; Infectious disease; Acridine orange; AO; Fluorescent dye; Dil; Fibroblast; In vitro; Leishmania major
1. Introduction
Leishmaniasis is one of the most important vector-borne worldwide diseases and its annual incidence rate is estimated to be 1 to 1.5 million cases of cutaneous leishmaniasis (CL) and 500,000 cases of visceral leishmaniasis (VL). Annually, 350 million people are at risk and 12 million suffer from the disease. About 90% of the CL occurs in Afghanistan, Saudi Arabia, Algeria, Brazil, Iran, Iraq, Syria, and Sudan [1, 2, 3].
Leishmaniasis is caused by diverse species of the genus Leishmania, a flagellated protozoan that usually infect macrophages of the vertebrate host. During the bite of the infected sand fly vector as a blood meal, parasites enter into the dermis of the vertebrate host [4].
Recent investigations indicate that there is an interaction between Leishmania promastigotes and their host cellular targets of the immune system such as macrophages and dermal dendritic cells, but other cell types including neutrophils, eosinophils and epithelial cells have shown the Leishmania entry in vitro or in vivo [5, 6, 7]. There are several clinical forms of leishmaniasis, including a self-healing CL, a mutilating mucocutaneous disease (MCL) and even a lethal systemic illness [1]. There are some methods in the laboratory to detect the parasite such as microscopic examination of stained smears, immunologic methods, cultivation, histopathology, enzyme-linked immunosorbent assay (ELISA), immunofluorescence assay (IFA) and also the recently used nanoparticles. However, these methods cannot be used for the early detection of multiple extensive and mucosal cutaneous lesions. Polymerase chain reaction (PCR) recently has been used to diagnose CL. It can detect the Leishmania DNA in host lesions with suspected infection [8, 9, 10, 11].
The disease begins at the site of inoculation, with an erythematous papule, often in uncovered sites such as upper limbs, lower extremities and face. The papule enlarges and forms a painless ulcer with a raised border, making 0.5–10 cm in diameter. After healing, a depressed scar remains which makes the main problem of the disease. The rural form of leishmaniasis is due to L. major infection with an incubation period of about 2–4 weeks and it barely surpasses 2 months [12]. Multiple studies have shown that several cell types, besides macrophages such as neutrophils, eosinophils, and epithelial cells, are a potent harbor which can hide the parasites during a chronic phase of the disease. Several studies have shown that the uptake of promastigote or amastigotes of Leishmania mexicana, Leishmania donovani, Leishmania amazonensis, L. major, and Leishmania braziliensis by human and animal fibroblasts provided the evidence that the parasites can hide within these cells without any replication for the prolonged periods [5, 6, 13]. For a better understanding of host-parasite interaction in CL, we should consider the cell surface of L. major. A distinct class of complex glycosylphosphatidylinositols (GPIs), acting as the membrane anchors for cell surface glycoproteins linked to polysaccharide to form the lipophosphoglycans (LPGs). All The glycolipids in L. major belong to a class of glycoinositolphospholipids (GIPLs) which can be metabolically labelled with [3H] inositol and are sensitive to phosphatidylinositol-specific phospholipase C, has covered promastigotes and amastigotes of Leishmania [14, 15]. For parasite lasting in the macrophage phagolysosome compartment, LPG-like molecules are essential [14]. Lipophosphoglycans are anchored to the membrane of the parasite by an unusual lysoalkyl-PI-containing 24 and 26 alkyl chains and hexaglycosyl glycan core. The LPG of L. major consists of a tripartite structure, including a repeating phosphorylated di-tri, and tetrasaccharides phosphoglycan, is built up of at least 8 different oligosaccharides on average 27 repeat units/molecule of –PO4-6Gal (β1-4) Manpα1-and 3 position of glucose, galactose arabinose and mannose and a variably phosphorylated glycan core, and a lysoalkyl-PI lipid moiety [15].
Dil staining lipophilic fluorescent stains are a family of dyes such as Dio (green fluorescence), DiI (orange fluorescence), DiR (deep red fluorescent) and DiD (red fluorescence) that are used to label hydrophobic structures and cell membranes, providing a suitable device for labeling live cells and tissues and is suitable for flow cytometric survey and multi-colored imaging. The fluorescence of these dyes is strongly increased when merges into membranes or bounds to lipophilic biomolecules. They are characterized by polarity-dependent fluorescence, short excited-state lifetimes and high extinction coefficients [16]. The fluorescent lipophilic dye dialkyl carbocyanine Dil (1, 1′-dioctadecyl-3, 3, 3′, 3′-tetramethyl indocarbocyanine perchlorate) is a carbocyanine membrane dye contains a hydrophilic head that inserts inside the plasma cell membrane and two lipophilic hydrocarbon side chains into the hydrophobic plasma membrane [17]. Dil labeling does not affect basic evolution, physiological feature, and cell motility. It has been shown that the Dil-labeled neurons remain viable for up to 4 weeks in the culture media and up to 1 year in vivo. In some cases, in the aldehyde-fixed tissue, the labeled cells with Dil can be followed for more than 2 years. Fortunately the transfer of Dil dyes from the labeled to the unlabeled cells isn't possible. Due to the intense fluorescence and high photo stability of the dye, the low toxicity and trend to give highly stable cell labeling, the dye has been usually used for long-term cell tracing, both in cultures and in the living cells. Lipophilic dyes are the best standard to label neurons and internal organelles, such as endoplasmic reticulum and Golgi apparatus. Dil emits its fluorescence in the orange-red and it can be used with standard fluorescein rhodamine optical filter [17].
Another vital dye is acridine orange (AO) that is a cell-permeable and nucleic acid stain which after binding to dsDNA emits green fluorescence (525 nm) and when it binds to RNA or ssDNA (at 650 nm) emits red fluorescence. AO staining is a useful and easy way to distinguish between active and inactive reproducing cells, detecting intracellular pH gradients, measuring apoptosis and proton-pump activity, flow cytometry analysis and fluorescence microscopy research [18]. The AO staining technique can evaluate the vital cells and the rate of dead cells accurately. Therefore, AO staining also is a useful method for the in vitro follow-up of the therapeutic operation, such as leishmaniasis therapy. Apoptotic cells show an intense red fluorescence and a reduced green emission when compared to non-apoptotic interphase cells. Because it is a cationic dye, it emits different colors to help the differentiation of cellular organelles. For example, lysosomes in a low pH environment emit the orange light. The AO staining method requires less time and is more sensitive and used for a better observation of the cell morphology such as Leishmania details against a dark background that is often impossible in Giemsa-stained slides [19]. Regarding the epidemiological and clinical importance of the CL diagnosis, this study for the first time aims to stain L. major promastigote while present in infected fibroblasts by AO and Dil staining methods separately.
2. Materials and methods
2.1. Fibroblast cultivation
Fibroblasts were isolated from human foreskin. The research protocols were approved by ethics committee of Shiraz University of Medical Sciences and informed consent was obtained from all subjects providing samples for our experiments. The tissue samples were washed in PBS and 1% penicillin/streptomycin for 3 times and blood vessels and adipose tissues were removed. The remained tissue was chopped and 0.5% dispase (Gibco, 0295) was added to the sample tissue at 4 °C for 18 h. The epidermal layer was further removed and the remained dermal layer was cut into 1–2 mm3 pieces and then 0.1% collagenase type 1 (Gibco 374) was added to the samples at 37 °C for 4hours. The DMEM F12 and 10% FBS was added to stop the enzymatic reaction, and finally, the product was filtered through a 70 μm cell strainer, centrifuged at 100 g for 10 min and suspended in DMEM F12, 10% FBS, and 1% penicillin/streptomycin.
The fibroblasts were seeded in tissue culture flasks and transferred into 5% CO2 incubator at 37 °C with saturated humidity, while the medium was changed after 3 days until the cell culture became 80% confluent. Later, the fibroblasts were exposed to 0.025% 1x Trypsin–EDTA for further passages. The cell viability was determined using Trypan Blue. Phenotypic characterization of fibroblasts was morphologically assessed to be spindle-shaped and the matrix metalloproteinase1 (MMP1), matrix metalloproteinase 3 (MMP3), integrin alpha 11 (ITGA11), CD106, CD10, and CD26 markers were evaluated by the real-time PCR.
2.2. L. major cultivation
L. major (MRHO/IR/75/ER) reference strain was received from the Department of Parasitology and Mycology, affiliated to Shiraz University of Medical Sciences. Roswell Park Memorial Institute (RPMI) 1640 Medium, containing 10% FBS, 100 IU/mL penicillin and 100 μg/mL streptomycin, was used for cultivation of the parasite. The logarithmic phase of promastigotes transformed to the stationary phase after the sixth day of cultivation and this was the suitable phase for infecting the fibroblasts. The fibroblasts were permitted to adhere (6 h) to the bottom of the six-well cell culture plates in order to co-culture with the parasites (L. major promastigotes in a ratio of 10:1). After 12 h, the culture washed 3 times by PBS buffer to eliminate the remained parasites.
2.3. Labeling of L. major by AO
To prepare the AO staining solution, the stock solution of AO was prepared by dissolving 1 g of AO (Fisher Scientific Co., Pittsburgh, and Pa.) in 100 mL of the distilled water and was stored at 4 °C away from light. To preventing the fibroblast staining, suitable concentrations of dye recommended as non-toxic dose was used [20]. 5 μg/mL, 50 μg/mL and 100 μg/mL of staining solution were added to the (1 × 106) parasites. After 10 min the stained parasites were centrifuged and washed 7 times with PBS. The stained promastigotes were incubated with fibroblasts for 6–8 h. The presence of stained parasites with AO in fibroblast was assessed using a fluorescence microscope (Nikon Eclipse E200) at the magnification: × 200, ×400 and×1000. Live parasites could uptake of acridine orange (green fluorescence) in maximum emission of 525 nm as green color.
2.4. Dil labeling
1 × 106/mL promastigote of L. major was gently suspended and mixed by Dil (Molecular Probes, catalog #D-282) pipetting of 2–3 crystals of the dye to reach an ultimate concentration of 0.002 μg/mL kept for 20 min at the room temperature. Subsequently, after washing it for 7 times with PBS, it was centrifuged at 3000 rpm for 10-minutes. The supernatant was removed and the precipitate containing the stained promastigotes were co cultured with fibroblasts in fresh DMEM F12 and incubated at 37 °C for 6 h.
2.5. Statistical analysis
SPSS software version 2 (SPSS Inc., Chicago, IL) was used for the statistical analysis of the data. T-test was used to compare the data and a Graph Pad was applied to demonstrate the figures.
3. Results
3.1. Characterization of the fibroblast cells
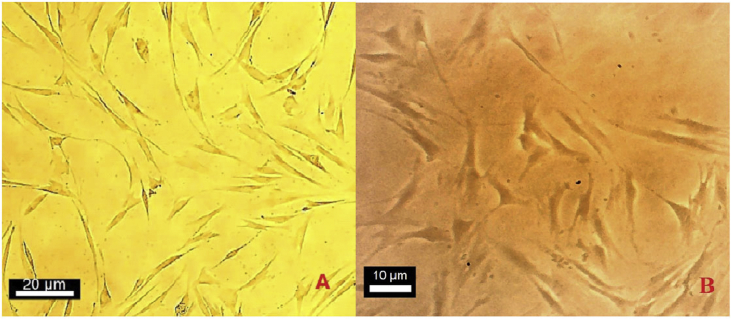
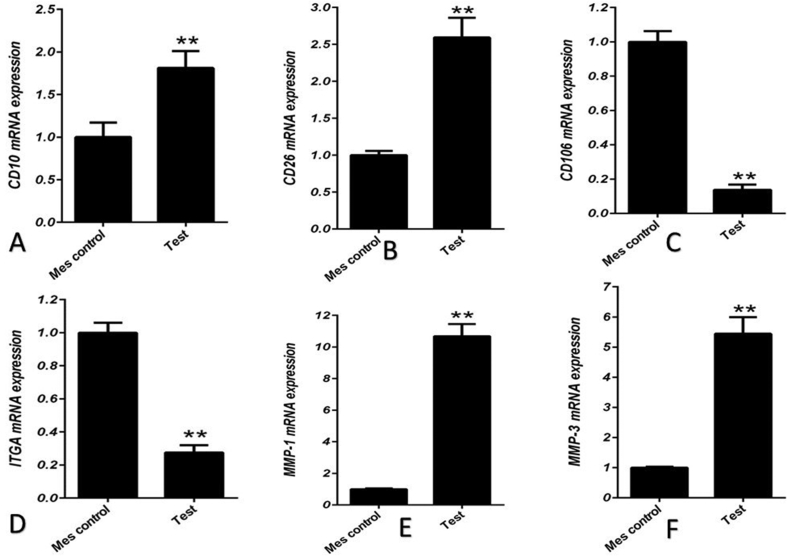
Phenotypic characterization of fibroblasts was morphologically assessed to be large spindle-shaped cells with oval nucleus (Figure 1). For confirming the first step, recommended markers including the MMP1, MMP3, ITAG11, CD106, CD10, and CD26 were evaluated for characterization of the fibroblasts by real-time PCR. The expression of CD10, CD26, MMP1 and MMP3 that are specific for fibroblast was considered. On the other hand the lack of expression of CD106 and ITGA11 that is specific for MSC was noticed [21] (Figure 2).
Figure 1.
Fibroblast spindle shape morphology on day 4.
Figure 2.
Human fibroblasts were positive for expression of CD10 (A), CD26 (B (D), matrix metalloproteinase1 (MMP1) (E), and matrix metalloproteinase 3 (MMP3) (F), Vs negative expression of CD106 (C) and integrin alpha 11 (ITGA11). The expression of CD10 (A), CD26 (B) MMP1 (E) and MMP3 (F) in dermal fibroblasts was shown to be more than mesenchymal stem cells (MSC) vs positive expression of CD106 (C) and ITGA11 in MSC (p ≤ 0.05 and p ≤ 0.01, respectively).
3.2. L. major morphology
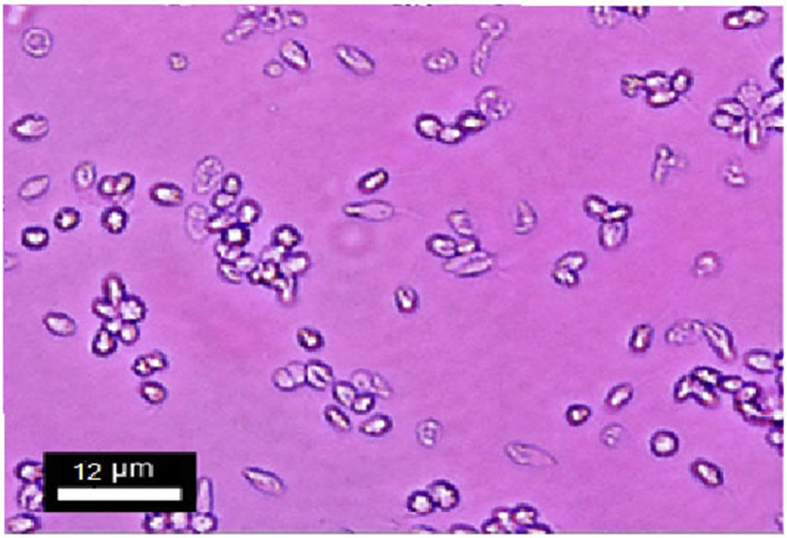
Promastigote is thinly elongated lance-like organism having an anterior kinetoplast and an emergent free flagellum (Figure 3).
Figure 3.
The Promastigotes of L. major were thin elongated lance-like in shape on fourth day of culture.
3.3. L. major labeling
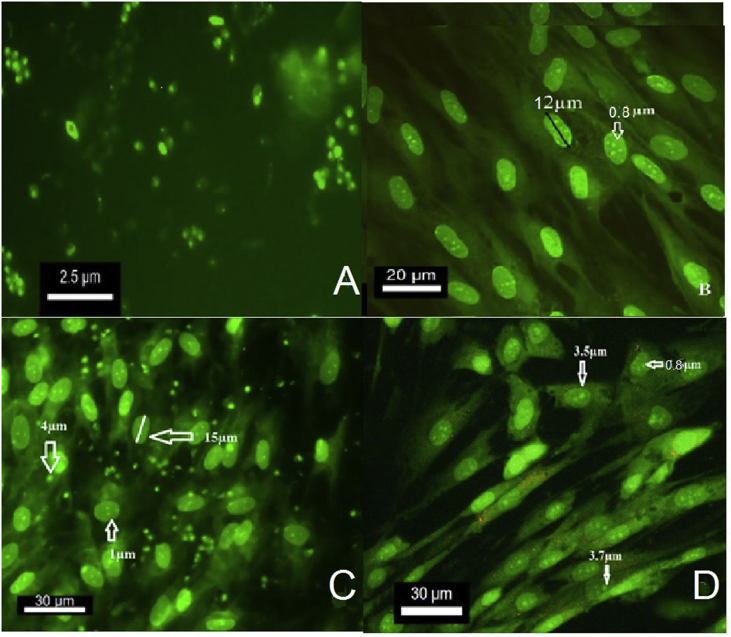
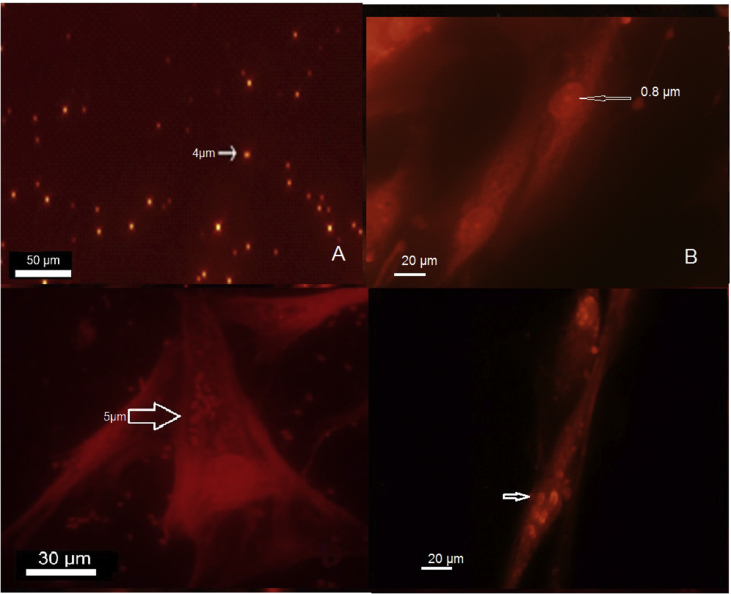
Acridine orange staining denoted to DNA binding dye of the stained free promastigote looked green before entering to the fibroblasts after 12 h culture (Figure 4A). Fluorescence shows that the parasite is alive. The free fibroblasts as a control group stained with AO on the third day of the culture and were compared to the infected fibroblast. It showed a partially green nucleus with 12μm and well green nucleolus with about 0.6–1 μm (medium 0.8 μm) length (Figure 4B). The parasite with 2.5–5 μm size (medium 3 μm) was entered to the cytoplasm of fibroblast at the beginning of the exposure and gradually to its nucleus (Figure 4C). Fibroblast nucleolus entirely stained green by AO, but it could be differentiated from the parasite based on their 1 μm size (Figure 4C and D) and in comparison with the control group (Figure 4B).
Figure 4.
The parasite stained with AO. The L. major parasite before the encounter with fibroblast appears green under fluorescent microscope (Nikon Eclipse E200) in Ex/Em (529nm), fluorescence show that the parasite is alive (A). The free fibroblasts stained with AO on the third day of culture as control group for comparison with infected fibroblast showed a partially green nucleus with 12μm and well green nucleolus with 0.8 μm length (B). The parasite about 3–4.5 μm enters the cytoplasm of fibroblasts at the beginning of the exposure. The fibroblasts are partially green in the presence of stained parasites (C). The parasites (3–4.5 μm) gradually enter the nucleus of the fibroblast and eventually accumulate in the nucleus (D). Fibroblast nucleolus entirely stained green by AO but could be differentiated from the parasite based on their size (C, D), in comparison with the control group (B).
The parasites were stained with Dil and considered by the fluorescence microscopy in Ex/Em, 549/565 nm emission, revealed that the internalized dye was accumulated within the promastigote membrane and the parasites could be seen with shiny red/orange color after 20 min (Figure 5A).The stained free fibroblast used as control (Figure 5B). Subsequent of stained promastigotes and fibroblasts co-culturing and incubation at 37 °C, the parasites with red/orange color were localized within the cytoplasm and nucleus of the fibroblasts after 6 and72 h, respectively. Labeled L. major did not reveal any visible changes in growth kinetics, morphology, or viability in comparison with untreated controls (Figure 5C and D).
Figure 5.
The parasite out of fibroblast stained with Dil fluorescence microscopy in Ex/Em, 549/565 nm emission (Nikon Eclipse E200) (A). Free fibroblast stained by Dil with 12μm nucleus and 0.8 μm nucleolus stained red (B). The parasites are seen in the cytoplasm after 6 h of culture as red bodies that show the presence of labeled L. major within the fibroblast cytoplasm (C). They are able to enter the fibroblast nucleus 3 days after exposure and exit in 7th day (D).
4. Discussion
Leishmaniasis is still an important public health problem in many developing countries. Among different forms of the disease, CL seldom causes morbidity, but the lesions may be hard to be treated, taking a long time and usually leaves deep scars on the face or other areas of the body. In Iran, it has been shown that L. major is responsible for most cases of human CL in the rural area and L. tropica is the causative agent of CL in the urban area [3, 22]. Leishmania promastigote infecting the host and phagocytized by skin macrophages can change into amastigote forms, reproduce and release by exploding the cells. These parasites further infect macrophages and the cycle continues until the parasite enters macrophage system such as the bone marrow, liver, and the spleen [23]. Although most analyses concern that the Leishmania invasion can be done by using macrophages as host cells based on their high in vivo infection rates, some studies clearly have shown the infection of other cells including the infection of cultured human skin fibroblasts too [13]. Since fibroblasts are localized in proximity to the parasite inoculation area, they can represent a potential cell target for early infection of Leishmania which can hide some parasite. Since the fibroblasts are not professional phagocytic cells to produce free radicals and are not capable to kill the parasite, therefore, they can contribute to the formation of a chronic form of disease and drug resistance [24]. For this reason, using suitable vital dyes for following and detecting the parasite especially in cell culture is highly recommended. In this study, we consider some vital dyes which confirmed by other scientists in previous researches. Dil and AO as suitable fluorescent dyes for this important purpose were selected. For instance, Dil has been used for parasites to study the lipid utilization in Schistosoma mansoni [25], tracking the tapeworms within intermediate hosts [26], and investigating monogenean infection behaviors [27]. Dil was first introduced as a new fluorescent tracer to label neurons in fixed brain slices of mouse and chicken and it had no discernable effects on the survival of the neurons [16]. Labeling Dil for HDL, LDL, and TLF1 lipoproteins in Trypanosoma brucei showed the uptake of these essential lipids. In 2007 scientists used fluorescence labeling dyes for labeling Leishmania chagasi and oxidant generation by single infected monocytes after short-term fluorescence labeling of a Protozoan [28]. Lipophilic dyes such as Dil tetramethyl indocarbocyanine perchlorate; Vibrant Dil cell labeling solution, DiD-DS dioctadecyl 3,3,3 tetramethylindodicarbocyanine-5,5-disulfonic acid), BODIPY-PC [2-(4,4-difluoro-5,7-dimethyl-4-bora-3a, 4a-diaza-s-indacene-3-pentanoyl)-1- hexadecanoyl-sn-glycero-3-phosphocholine], DiIC1(5) hexamethylindodicarbocyanine iodide) have been used for labeling and detecting of extracellular vesicles and capsule [29]. In 2015 Dil used to stain the mesenchymal stem cells (MSCs) and they showed their exosomes induced proliferation and migration of normal and chronic wound fibroblasts. Mesenchymal stem cells exosomes can activate several signaling pathways that are important in wound healing and can induce the expression of several growth factors [insulin-like growth factor-1 (IGF1), hepatocyte growth factor (HGF), nerve growth factor (NGF), and stromal-derived growth factor-1 (SDF1)] [30]. In vitro using of Dil to labeling and tracing fibroblast cells and delivery of fluorescent dyes into cells providing a well-defined outline of cellular processes [31]. Typical applications of this technique include the study of neuronal morphology during the development and altered development in neurological disorders clarified important points in neurological fields [32]. Dil, as a lipophilic fluorescent dye for labeling cell membranes, has a great affinity to bound to lipophilic biomolecules [16, 17]. Due to the promastigote membrane LPG structure of L. major, we labeled the vital parasites with Dil to identify it inside the characterized fibroblast cells under fluorescent microscopy (Figure 5). The L. major parasite appeared red-orange under fluorescent microscopy in Ex/Em (549–565nm). Therefore, normal fibroblasts did not absorb any orange-red dye while they were indicated in parasites. Dil intercalates into lysophospholipid of the cell membrane and is intensely fluorescent, allowing live cells to be easily visualized under fluorescence microscopy in Ex/Em, 549/565 nm emission (Figure 5). Labeled L. major did not reveal any visible changes in growth kinetics, morphology, or viability in comparison to the untreated controls. This could be a good result for researchers who are interested in the field of vaccine, biology and treatment of the parasite. The nucleus of fibroblast has a size about 10–12 μm and nucleolus 0.6–1 μm and could easily differentiate from the Leishmania amastigote with the size of 2.5–4.5 μm (Figure 5).
AO was the second vital dye used in this study. Evaluation of AO staining in 1980 to detect the microorganisms in the blood cultures showed that it is a rapid and inexpensive alternative to conventional subculture techniques for the detection of bacteria in blood cultures [33]. At neutral pH, AO can be used as a vital stain, while the viable bacteria stain green and nonviable ones stain red-orange. When a neutral staining solution of AO was applied, both microorganisms and human cells stained red-orange. At an acid pH, human leukocytes and epithelial cells stain as pale apple green, whereas bacteria stains red-orange. This staining method has been an inexpensive, rapid, and quite sensitive procedure to detect the bacteria in densities as low as 1 × 104 colony-forming units per mL. AO has been used to stain various parasites such as Plasmodium [34, 35], Pneumocystis carinii [36], Blastocystis hominis [2], and intracellular bacteria in culture or clinical specimen [37]. It used for staining fibroblasts to detect any apoptosis and parasite viability, while viable organisms looked green fluorescence [38]. AO alone could provide simultaneous information on cell viability as well as nuclear morphology [38]. The AO method required less time and it was more sensitive under lower magnification than the Giemsa staining method. Therefore, several studies have demonstrated that AO can be successfully used to stain diverse type of Leishmania such as L. donovani [39], L. major [40], L. infantum [41], L. tropica [42], and L. mexicana [43] to differentiate dead or live promastigotes of Leishmania species. Parasites such as T. gondi, Cryptosporidium and Eimeria sporozoite [44], Entamoeba histolytica trophozoites [45], Gram-negative bacteria such as Acinetobacter spp., Bacteroides fragilis, Citrobacter diversus, Enterobacter cloacae and Escherichia coli gram-positive bacteria such as Bacillus subtilis and Staphylococcus aureus [46], and fungi such as Candida albicans [47] have been previously stained by AO. It has also been used to stain the fibroblasts [48]. According to mentioned studies, our study was performed to stain L. major by AO and our findings documented this parasite within the fibroblast in comparison whit the control groups (Figure 4). In this study, it has also been shown that the fibroblasts also stained by AO, despite multiple washing of stained parasite by PBS buffer. As the results of Dil, the size of parasite and fibroblast nucleus and nucleolus is an important index for tracing the Leishmania in the fibroblast especially in comparison with the control group (Stained fibroblast free of the parasite) (Figure 4). In summing up the advantages and disadvantages of the two vital staining systems, the following are significant:
The acridine orange dye can be very effective because also vital staining of promastigotes it can evaluate the mobility of parasites in high quality due to the high contrast between the well-stained parasite and the nearly unstained background that this is well colorimetric diversity can be detected with little skill. But this dye has some negative aspect including is more expensive than other dyes, needs a fluorescent microscope, the probability of parasite loss during masking of the parasite by the stain in daylight and our study stained parasite could effect on the host like fibroblast. This dye could attach to DNA &RNA of both parasites and host cells and lead to some problems in the differentiation of parasite from the host cell. We moderately solved this problem by using a low concentration of AO.
Dil is composed of vigorous fluorescent lipophilic combination, which can quickly penetrate in bilayer lipids of the cells plasma membrane, extremely durable, non-toxic and can be used to detect live and fixed cells using a fluorescence microscope [17]. Dil labeling does not affect basic evolution, physiological feature and cell motility. The most important advantage of Dil in comparison with AO that we consider in this study is that Dil dyes do not transfer from labeled to unlabeled cells. Due to intense fluorescence and high photo stability of the dye, low toxicity and the trend to give highly stable cell labeling, the dye has been usually used for long term cell tracing both in cultures and in living cells. Dil emits its fluorescence in the orange red and it can be used with standard fluoresce in rhodamine optical filter [17]. Labeling of cells with Dil does not impact cells physiological functions, grow and viability.
5. Conclusion
Despite introducing the macrophages as the main target of Leishmania Spp., some studies clearly have shown the infection of other cells including the cultured human skin fibroblasts too. Since the fibroblasts are localized in the proximity to the parasite inoculation area, they can represent a potential cell target for early infection of Leishmania which can hide some parasites. Due to the phospholipid structure of the promastigote of L. major lipophilic fluorescent stain, Dil is used as a safe, easy to use, inexpensive and fast method to label Leishmania parasite, trace it in the fibroblast cells, and compare with AO. In this study, both Dil and AO considered as suitable vital dyes to identify labeled Leishmania in fibroblast in vitro, but Dil was superior to AO with its feature does not transfer from the labeled to unlabeled cells.
Declarations
Author contribution statement
Narjes Yektaeian, Mozhdeh Sepaskhah, Shahrokh Zare, Iman Jamhiri: Performed the experiments.
Davood Mehrabani: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Gholamreza Hatam: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was extracted from a part of the Ph.D. thesis of Narjes Yektaeian which was financially supported by the office of Vice-chancellor for research of Shiraz University of Medical Sciences Shiraz, Iran (Grant No: 12766).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Desjeux P. Leishmaniasis. Public health aspects and control. Clin. Dermatol. 1996;14(5):417–423. doi: 10.1016/0738-081x(96)00057-0. [DOI] [PubMed] [Google Scholar]
- 2.Alvar J., Velez I.D., Bern C., Herrero M., Desjeux P., Cano J. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7(5) doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ardehali S., Moattari A., Hatam G., Hosseini S., Sharifi I. Characterization of Leishmania isolated in Iran: 1. Serotyping with species specific monoclonal antibodies. Acta Trop. 2000;75(3):301–307. doi: 10.1016/s0001-706x(00)00064-4. [DOI] [PubMed] [Google Scholar]
- 4.Rogers M.E., Chance M.L., Bates P.A. The role of promastigote secretory gel in the origin and transmission of the infective stage of Leishmania mexicana by the sandfly Lutzomyia longipalpis. Parasitology. 2002;124(Pt 5):495–507. doi: 10.1017/s0031182002001439. [DOI] [PubMed] [Google Scholar]
- 5.Grimaldi G., Jr., Soares M.J., Moriearty P.L. Tissue eosinophilia and Leishmania mexicana mexicana eosinophil interactions in murine cutaneous leishmaniasis. Parasite Immunol. 1984;6(5):397–408. doi: 10.1111/j.1365-3024.1984.tb00811.x. [DOI] [PubMed] [Google Scholar]
- 6.Pearson R.D., Steigbigel R.T. Phagocytosis and killing of the protozoan Leishmania donovani by human polymorphonuclear leukocytes. J. Immunol. 1981;127(4):1438–1443. [PubMed] [Google Scholar]
- 7.Kaye P., Scott P. Leishmaniasis: complexity at the host-pathogen interface. Nat. Rev. Microbiol. 2011;9(8):604–615. doi: 10.1038/nrmicro2608. [DOI] [PubMed] [Google Scholar]
- 8.Goto H., Lindoso J.A.L. Current diagnosis and treatment of cutaneous and mucocutaneous leishmaniasis. Expert Rev. Anti-infect. Ther. 2010;8(4):419–433. doi: 10.1586/eri.10.19. [DOI] [PubMed] [Google Scholar]
- 9.Shirian S., Oryan A., Hatam G.-R., Panahi S., Daneshbod Y. Comparison of conventional, molecular, and immunohistochemical methods in diagnosis of typical and atypical cutaneous leishmaniasis. Arch. Pathol. Lab Med. 2014;138(2):235–240. doi: 10.5858/arpa.2013-0098-OA. [DOI] [PubMed] [Google Scholar]
- 10.Moradi M., Sattarahmady N., Rahi A., Hatam G., Sorkhabadi S.R., Heli H. A label-free, PCR-free and signal-on electrochemical DNA biosensor for Leishmania major based on gold nanoleaves. Talanta. 2016;161:48–53. doi: 10.1016/j.talanta.2016.08.030. [DOI] [PubMed] [Google Scholar]
- 11.Heli H., Sattarahmady N., Hatam G., Reisi F., Vais R.D. An electrochemical genosensor for Leishmania major detection based on dual effect of immobilization and electrocatalysis of cobalt-zinc ferrite quantum dots. Talanta. 2016;156:172–179. doi: 10.1016/j.talanta.2016.04.065. [DOI] [PubMed] [Google Scholar]
- 12.Clem A. A current perspective on leishmaniasis. J. Glob. Infect. Dis. 2010;2(2):124. doi: 10.4103/0974-777X.62863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang K.P. Leishmania infection of human skin fibroblasts in vitro: absence of phagolysosomal fusion after induced phagocytosis of promastigotes, and their intracellular transformation. Am. J. Trop. Med. Hyg. 1978;27(6):1084–1096. doi: 10.4269/ajtmh.1978.27.1084. [DOI] [PubMed] [Google Scholar]
- 14.Handman E., Goding J. The Leishmania receptor for macrophages is a lipid-containing glycoconjugate. EMBO J. 1985;4(2):329–336. doi: 10.1002/j.1460-2075.1985.tb03633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McConville M.J., Bacic A. The glycoinositolphospholipid profiles of two Leishmania major strains that differ in lipophosphoglycan expression. Mol. Biochem. Parasitol. 1990;38(1):57–67. doi: 10.1016/0166-6851(90)90205-z. [DOI] [PubMed] [Google Scholar]
- 16.Honig M.G., Hume R.I. Dil and diO: versatile fluorescent dyes for neuronal labelling and pathway tracing. Trends Neurosci. 1989;12(9):40–41. 333-5. [PubMed] [Google Scholar]
- 17.Bruce L.L., Christensen M.A., Fritzsch B. Electron microscopic differentiation of directly and transneuronally transported DiI and applications for studies of synaptogenesis. J. Neurosci. Methods. 1997;73(1):107–112. doi: 10.1016/s0165-0270(96)02218-2. [DOI] [PubMed] [Google Scholar]
- 18.Darzynkiewicz Z. Differential staining of DNA and RNA in intact cells and isolated cell nuclei with acridine orange. Methods Cell Biol. 1990;33:285–298. doi: 10.1016/s0091-679x(08)60532-4. [DOI] [PubMed] [Google Scholar]
- 19.Bhakdi S.C.S.P., Chimma P., Rungruang T., Chuncharunee A., Neumann H.P., Malasit P., Pattanapanyasat K. Re-evaluating acridine orange for rapid flow cytometric enumeration of parasitemia in malaria-infected rodents. Cytometry Part A: The Journal of the International Society for Analytical Cytology. 2007 Sep;71(19):662–667. doi: 10.1002/cyto.a.20406. [DOI] [PubMed] [Google Scholar]
- 20.Liu K., Liu P-c, Liu R., Wu X. Dual AO/EB staining to detect apoptosis in osteosarcoma cells compared with flow cytometry. Med. Sci. Mon. Basic Res. 2015;21:15. doi: 10.12659/MSMBR.893327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halfon S., Abramov N., Grinblat B., Ginis I. Markers distinguishing mesenchymal stem cells from fibroblasts are downregulated with passaging. Stem Cells Dev. 2010;20(1):53–66. doi: 10.1089/scd.2010.0040. [DOI] [PubMed] [Google Scholar]
- 22.Yemisen M., Ulas Y., Celik H., Aksoy N. Epidemiological and clinical characteristics of 7172 patients with cutaneous leishmaniasis in Sanliurfa, between 2001 and 2008. Int. J. Dermatol. 2012;51(3):300–304. doi: 10.1111/j.1365-4632.2011.05059.x. [DOI] [PubMed] [Google Scholar]
- 23.Handman E., Bullen D.V. Interaction of Leishmania with the host macrophage. Trends Parasitol. 2002;18(8):332–334. doi: 10.1016/s1471-4922(02)02352-8. [DOI] [PubMed] [Google Scholar]
- 24.Bogdan C., Röllinghoff M., Diefenbach A. Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Curr. Opin. Immunol. 2000;12(1):64–76. doi: 10.1016/s0952-7915(99)00052-7. [DOI] [PubMed] [Google Scholar]
- 25.Furlong S.T., Thibault K.S., Morbelli L.M., Quinn J.J., Rogers R.A. Uptake and compartmentalization of fluorescent lipid analogs in larval Schistosoma mansoni. J. Lipid Res. 1995;36(1):1–12. [PubMed] [Google Scholar]
- 26.Kurtz J., van der Veen I.T., Christen M. Fluorescent vital labeling to track cestodes in a copepod intermediate host. Exp. Parasitol. 2002;100(1):36–43. doi: 10.1006/expr.2001.4681. [DOI] [PubMed] [Google Scholar]
- 27.Glennon J.J., Tang R., Buhro W.E., Loomis R.A. Synchronous photoluminescence intermittency (blinking) along whole semiconductor quantum wires. Nano Lett. 2007;7(11):3290–3295. doi: 10.1021/nl0714583. [DOI] [PubMed] [Google Scholar]
- 28.Chang H.K., Thalhofer C., Duerkop B.A., Mehling J.S., Verma S., Gollob K.J. Oxidant generation by single infected monocytes after short-term fluorescence labeling of a protozoan parasite. Infect. Immun. 2007;75(2):1017–1024. doi: 10.1128/IAI.00914-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicola A.M., Frases S., Casadevall A. Lipophilic dye staining of Cryptococcus neoformans extracellular vesicles and capsule. Eukaryot. Cell. 2009;8(9):1373–1380. doi: 10.1128/EC.00044-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shabbir A., Cox A., Rodriguez-Menocal L., Salgado M., Badiavas E.V. Mesenchymal stem cell exosomes induce proliferation and migration of normal and chronic wound fibroblasts, and enhance angiogenesis in vitro. Stem Cells Dev. 2015;24(14):1635–1647. doi: 10.1089/scd.2014.0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y., Zhang R., Feng Y., Zhang C. Application of carbocyanines fluorescent dye for tracing fetal fibroblast transplant: experiment with rats. Zhonghua Yixue Zazhi. 2009;89(14):986–989. [PubMed] [Google Scholar]
- 32.Li M., Cui Z., Niu Y., Liu B., Fan W., Yu D. Synaptogenesis in the developing mouse visual cortex. Brain Res. Bull. 2010;81(1):107–113. doi: 10.1016/j.brainresbull.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 33.McCarthy L., Senne J. Evaluation of acridine orange stain for detection of microorganisms in blood cultures. J. Clin. Microbiol. 1980;11(3):281–285. doi: 10.1128/jcm.11.3.281-285.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hansen C.M. CRC press; 2002. Hansen Solubility Parameters: a User's Handbook. [Google Scholar]
- 35.Keiser J., Utzinger J., Premji Z., Yamagata Y., Singer B.H. Acridine Orange for malaria diagnosis: its diagnostic performance, its promotion and implementation in Tanzania, and the implications for malaria control. Ann. Trop. Med. Parasitol. 2002;96(7):643–654. doi: 10.1179/000349802125001834. [DOI] [PubMed] [Google Scholar]
- 36.Thomson R.B., Jr., Smith T.F. Acridine orange staining of Pneumocystis carinii. J. Clin. Microbiol. 1982;16(1):191–192. doi: 10.1128/jcm.16.1.191-192.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heinzen R.A., Scidmore M.A., Rockey D.D., Hackstadt T. Differential interaction with endocytic and exocytic pathways distinguish parasitophorous vacuoles of Coxiella burnetii and Chlamydia trachomatis. Infect. Immun. 1996;64(3):796–809. doi: 10.1128/iai.64.3.796-809.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee C.H., Shah B., Moioli E.K., Mao J.J. CTGF directs fibroblast differentiation from human mesenchymal stem/stromal cells and defines connective tissue healing in a rodent injury model. J. Clin. Investig. 2010;120(9):3340–3349. doi: 10.1172/JCI43230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Channon J.Y., Roberts M.B., Blackwell J.M. A study of the differential respiratory burst activity elicited by promastigotes and amastigotes of Leishmania donovani in murine resident peritoneal macrophages. Immunology. 1984;53(2):345–355. [PMC free article] [PubMed] [Google Scholar]
- 40.Blank C., Fuchs H., Rappersberger K., Rollinghoff M., Moll H. Parasitism of epidermal Langerhans cells in experimental cutaneous leishmaniasis with Leishmania major. J. Infect. Dis. 1993;167(2):418–425. doi: 10.1093/infdis/167.2.418. [DOI] [PubMed] [Google Scholar]
- 41.Tolomeo M., Grimaudo S., Di Cristina A., Roberti M., Pizzirani D., Meli M. Pterostilbene and 3′-hydroxypterostilbene are effective apoptosis-inducing agents in MDR and BCR-ABL-expressing leukemia cells. Int. J. Biochem. Cell Biol. 2005;37(8):1709–1726. doi: 10.1016/j.biocel.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 42.Koru O., Toksoy F., Acikel C.H., Tunca Y.M., Baysallar M., Guclu A.U. In vitro antimicrobial activity of propolis samples from different geographical origins against certain oral pathogens. Anaerobe. 2007;13(3-4):140–145. doi: 10.1016/j.anaerobe.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 43.Torrentera F.A., Glaichenhaus N., Laman J.D., Carlier Y. T-cell responses to immunodominant LACK antigen do not play a critical role in determining susceptibility of BALB/c mice toLeishmania mexicana. Infect. Immun. 2001;69(1):617–621. doi: 10.1128/IAI.69.1.617-621.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goldstein L., Brown S. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu. Rev. Biochem. 1977;46(1):897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- 45.Roman M., Martin-Orozco E., Goodman J.S., Nguyen M.-D., Sato Y., Ronaghy A. Immunostimulatory DNA sequences function as T helper-1-promoting adjuvants. Nat. Med. 1997;3(8):849. doi: 10.1038/nm0897-849. [DOI] [PubMed] [Google Scholar]
- 46.Anderson E. Fluorescence microscopy in the study of nucleic acids.[ii] visual observation of deoxyribonucleic acid changes in bacteria during growth of bacteriophage. Nature. 1957;180:1336–1338. doi: 10.1038/1801336a0. [DOI] [PubMed] [Google Scholar]
- 47.Zufferey J., Rime B., Francioli P., Bille J. Simple method for rapid diagnosis of catheter-associated infection by direct acridine orange staining of catheter tips. J. Clin. Microbiol. 1988;26(2):175–177. doi: 10.1128/jcm.26.2.175-177.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aldridge D.R., Arends M.J., Radford I.R. Increasing the susceptibility of the rat 208F fibroblast cell line to radiation-induced apoptosis does not alter its clonogenic survival dose-response. Br. J. Canc. 1995;71(3):571–577. doi: 10.1038/bjc.1995.111. [DOI] [PMC free article] [PubMed] [Google Scholar]