Highlights
-
•
KHCO3 enhanced the macromolecular polymerization of wheat gluten.
-
•
KHCO3 increased the content of β-sheet structure in gluten.
-
•
Non-redox reaction may induce the increase of free SH group in gluten.
-
•
Adding KHCO3 didn’t adversely affect baking and sensory properties of cookies.
Keywords: Potassium bicarbonate, Sodium reduction, Cookie, Gluten aggregation, Gliadin to glutenin ratio, Secondary structure
Abstract
Baking soda (NaHCO3) has critical technological functions in cookie products. Health concern on excessive sodium consumption is increasing; therefore, it is necessary to explore NaHCO3 alternatives, such as KHCO3, for bakery products. This study investigated the impact of KHCO3 on the technological behaviors of cookie dough and end-uses in comparison with control samples prepared with NaHCO3 and explore the changes of physicochemical and conformation properties of soft wheat gluten during the process. Dough rheological measurements demonstrated that addition of KHCO3 reduced dough stickiness, and adding KHCO3 achieved similar dough and baking performances as using NaHCO3, which were partially attributed to the decrease of gliadin to glutenin ratio, changes of secondary structure, and intensive aggregation of gluten by introducing KHCO3. Cookie sensory attributes were also not adversely affected by using KHCO3. Therefore, partially replacing NaHCO3 with KHCO3 in cookie products can be an effective approach for sodium reduction.
1. Introduction
Sodium (Na) plays an essential role in maintaining normal body function and health. However, excessive sodium consumption can significantly increase blood pressure (Stamler et al., 2018), which has been associated with cardiovascular diseases. Currently, the average intake of sodium is 3,440 mg per day in the U.S., which largely exceeds the amount of 2,300 mg per day recommended by the 2015 Dietary Guidelines for Americans (HSS, 2015). Table salt (NaCl) and baking soda (NaHCO3) in processed foods are the two largest contributors of dietary sodium (Mattes & Donnelly, 1991). Reducing their amount in foods, especially in bakery and other cereal products, accounting for over 30% of dietary sodium sources, could be a crucial step to mitigate the excessive dietary sodium exposure (Angus, 2007). However, baking soda provides critical technological functions, which can impact dough processability and product quality attributes, including their well-known sensory and leavening functions. Therefore, desirable baking soda replacement should provide similar technological functions without negatively impacting dough processing and cookie qualities.
Potassium (K) is another important mineral and one of the key electrolytes for the proper functioning of human body. Diets rich in potassium lower blood pressure and have many health benefits (Geleijnse, Kok, & Grobbee, 2003). Vegetables and fruits have been the major sources of dietary potassium. The minimal daily intake of potassium is recommended to be 4,700 mg (HSS, 2015). However, the current diet of most population contains much more sodium but much less potassium (2,800 mg/day) than the recommended level (HSS, 2015). Therefore, (partially) substitution of sodium salts (e.g., NaCl, NaHCO3) with potassium salts in some food products could bring dual benefits: sodium reduction and potassium supplementation. In a previous research on low sodium cookie, application of KCl and K2CO3 combination (1:1) in three flours resulted in higher texture scores compared with cookies prepared with NaCl only (Bala, Kaur, & Bakshi, 2004). Potassium bicarbonate (KHCO3) has a higher water solubility than NaHCO3 (i.e., 33% vs. 9% at 20 °C), which could be an advantage in formulating some food products. It also has FDA approved Generally Recognized as Safe (GRAS) status and is appropriate for use in foods (FDA, 2016). Therefore, KHCO3 could be a promising chemical leavening agent, especially in reduced-sodium formulas.
Wheat flour dough is regarded as a composite material of a gluten network embedded in other ingredients such as starch. A three-dimensional network of gluten can form via different inter- and intra-molecular linkages, and wheat gluten network are partially responsible for the dough behavior and end-product quality. Gluten structure evolution during dough mixing and development is critical in manipulating dough physical and rheological properties, which determines final product quality. Pareyt, Brijs, and Delcour (2009) observed that spread behavior of cookie was related to the gluten cross-linking. Singh and MacRitchie (2014) reported that the polymerization of gluten network may involve oxidation of sulfhydryl (SH) groups. The alkaline reagent was found to influence the structure and interaction of wheat gluten (Wu, Beta, & Corke, 2006). For example, NaHCO3 was reported to induce gluten aggregation via disulfide (SS) group formation (Shiau & Yeh, 2001). However, to the best of our knowledge, there has been no mechanistic investigation into the impact of KHCO3 on gluten structure, dough texture, and cookies properties. We hypothesized that addition of KHCO3 could induce gluten polymerization and strengthen the structure of gluten through both physical and chemical interactions. Therefore, the objective of this study was to explore the effect of different levels of KHCO3 on the physical and rheological properties and conformational changes of gluten in wheat dough, in comparison with NaHCO3. A typical bakery product, sugar-snap cookie, was produced using KHCO3, and the quality was characterized as well. This study provides the critical knowledge towards fundamentally understanding the functions of KHCO3 in wheat products and benefits the development of working strategies to reduce sodium in bakery products.
2. Materials and methods
2.1. Materials
Soft wheat flour (pastry flour, protein level: 8.05%, moisture level: 13.05%, flour weight basis) was supplied by Mennel Milling Company (Fostoria, OH, USA). Salt, sucrose, dextrose and shortening were purchased from local supermarkets. Chemicals and solvents used were at least analytical grade. Sodium bicarbonate, potassium bicarbonate, Tris hydrochloride (Tris-HCl), urea, sodium sulfite, trifluoroacetic acid (TFA), and acetonitrile were purchased from Fisher Scientific (Fairlawn, NJ, USA). Tetrasodium ethylene diamine tetraacetate (EDTA), wheat gliadin standard (>95%), sodium dodecyl sulfate (SDS), 5,5-dithio-bis-(2-nitrobenzoic acid) (DTNB), and 2-nitro-5-thiosulfobenzoate (NTSB) were obtained from Sigma Aldrich (St. Louis, MO, USA).
2.2. Dough mixing properties
Mixograph (AACCI Method 54–40.02) and farinograph (AACCI Method 54–21.02) (National Manufacturing Co, Lincoln, NE, USA) were used to assess the mixing characteristics of doughs added with different levels of KHCO3. Doughs were prepared by mixing flour, water, and KHCO3 added at 0.5, 1.0, 1.5 and 2.0%, or 2.0% NaHCO3 on flour weight basis (coded as K1, K2, K3, K4 and S1, respectively), as well as a blank control sample without KHCO3 or NaHCO3 (coded as C1). The mixing parameters were collected for each treatment only containing the flour, leavening agent (NaHCO3 or KHCO3), and water.
2.3. Cookie dough physical and rheological properties
All measurements were performed using the same TA-XTplus Texture Analyzer. Dough strength and extensibility were analyzed using an SMS/Keiffer rig, and resistance to extension (force) and extensibility (distance) of dough samples were recorded (Chen, Hu, & Li, 2018). Dough elongational viscosity was tested with a TA25 cylinder probe via a lubricated uniaxial compression study (Miller & Hoseney, 2008). Elongational viscosity (η) was calculated as: , where F is the peak force for deformation, H is the dough height, and R is the dough radius, and Vz is the cross-arm speed. Dough stickiness was measured according to the previous method with an SMS/Chen-Hoseney rig (Chen et al., 2018).
2.4. Protein extractability characterizations
Gluten was extracted from dough for all the treatments according to AACCI Approved Method 38-10.01 (AACC, 2000). Lyophilized gluten samples were ground into fine powder, sealed, and stored in −20 °C freezer until further analysis. Extraction of gliadin and glutenin from lyophilized samples was performed according to Chen et al. (2018). The extracted gluten protein profiles were obtained using reversed phase high performance liquid chromatography (RP-HPLC), using an Aeris XB-C18 column (3.6 μm, 150× 4.6 mm, Phenomenex, Torrance, CA, USA) and HP1100 equipment, with a diode array detector (DAD) operating at 210 nm (Agilent Technologies, Santa Clara, CA, USA). All gliadin or glutenin fractions were filtered with a 0.45 μm membrane (PhenexTM filter membranes, Phenomenex, Torrance, CA, USA) and loaded to the column, and gradient elution was conducted at a flow rate of 0.6 mL/min using two mobile phases composed of deionized water and acetonitrile, respectively, both containing 0.1% trifluoroacetic acid. Acetonitrile content was increased linearly from 24 to 56% between 0 and 30 min. The retention time and spectra of gliadin and glutenin fractions were compared with standards for qualitative analysis, while the quantitative analysis was performed from the respective calibration curves and the linearity ranged from 0.07 to 1.10 mg/ml, in which the correlation coefficient indicated a good linearity (R2 > 0.98).
2.5. Disulfide-sulfhydryl analysis
Disulfide (S—S) and sulfhydryl (SH) contents were measured using a previous method with some modifications (Rombouts, Jansens, Lagrain, Delcour, & Zhu, 2014). Each Sample (30 mg) was mixed with 3 mL Tris-HCl buffer (0.2 M, pH 8.0) containing 8 M urea, 3 mM tetrasodium ethylene diamine tetraacetate (EDTA), and 1% (v/v) SDS for 1 h, and then 0.3 mL Tris-HCl (0.2 M, pH 8.0) buffer containing 10 mM 5,5-dithio-bis-(2-nitrobenzoic acid) (DTNB) was added into the suspension and mixed for one hour. The solution was then centrifuged at 13,600 xg at room temperature for 15 min, followed by measuring the absorbance at 412 nm using a VWR spectrophotometer (UV-6300PC, Radnor, PA, USA). The amount of total SH group was determined according to the report from Thannhauser, Konishi, and Scheraga (1987) with minor modifications. Each sample (10 mg) was mixed with 1 mL Tris-HCl buffer (0.2 M, pH 9.5) containing 0.1 M sodium sulfite, 3 mM EDTA, 1% SDS, and 0.5 mM 2-nitro-5-thiosulfobenzoate (NTSB) for 1 h in dark, followed by centrifuging at 13,600 xg for 15 min. The supernatant was collected and diluted (1:10) by adding Tris-HCl buffer without NTSB, and the absorbance was determined at 412 nm. The concentration of total SH group (Ct) and free SH group (Cf) was obtained according to the equation: , where A is the respective absorbance, ε is the extinction coefficient of 13,600 M−1cm−1, and b is the cell path length. The concentration of total SS group (Css) was calculated using the equation: .
2.6. FTIR measurements
Conformation of gluten (secondary structure) was analyzed via collecting Fourier-transform infrared (FTIR) spectra by means of an ATR module on a 400 FTIR Spectrometer (PerkinElmer, Inc., Waltham, MA, US) under the following conditions: 4000–400 cm−1 spectral range, 4 cm−1 resolution, and total of 64 scans. The amide I band (1600–1700 cm−1) components were further interpreted by deconvolution using OriginPro 2016 software (OriginLab, Inc. Northampton, MA, US) and calculated according to the previous method (Georget & Belton, 2006).
2.7. Cookie preparation and characterization
The formula of cookie contained: flour (222.7 g), water (18.3 g), sugar (130.0 g), shortening (64.0 g), sodium chloride (2.1 g), dextrose solution (33.0 g), and leavening agent (KHCO3 of 0, 0.5, 1.0, 1.5, or 2.0%, or 2.0% NaHCO3, fwb). Cookie was baked according to AACCI Approved Method 10–50.05 (AACC, 2000). Cookie weight and diameter were measured using a digital caliper, and spread ratio was calculated. Cookie color was measured by a CIE-LAB color system (XITIAN machine equipment Co., Ltd., Huizhou, China), which provides lightness (L*), redness-greenness (a*) and yellowness-blueness (b*) values. The pH of cookies was tested following AACCI Approved Method 02–52.01 (AACC, 2000). Cookie firmness was measured using the TA-XTPlus Texture Analyzer with a three-point bending probe at test speed of 1.0 mm/sec, strain of 50%, and trigger force of 20 g.
2.8. Sensory test
Sensory analysis of cookie was performed by a panel consisting of 21 members. Participants evaluated a set of selected cookies (i.e., control sample, 1.0% KHCO3, 2.0% KHCO3, and 2.0% NaHCO3) presented in blind condition and random order across subjects (Torri et al., 2016). Consumers rated their liking for appearance, odor, taste, texture, and overall acceptance on a nine-point hedonic scale ranging from 1 (extremely dislike) to 9 (extremely like). They were required to assess the samples avoiding social interaction under white light, and rinse their mouths with water between sample evaluations.
2.9. Statistical analysis
Data were subjected to the analysis of variance (ANOVA) and the mean values and standard deviations were calculated. The dough physical and rheological properties, cookie characterization, gluten physicochemical and structural characterizations (protein extractabilities, disulfide-sulfhydryl, and FTIR analysis) were conducted in at least three replications. A SAS Institute software version 9.4 (Cary, NC, US) was used to perform the statistical analysis via Tukey’s test at a significance being p < 0.05 in all cases.
3. Results and discussion
3.1. Impact of KHCO3 on dough mixing properties
Dough mixing characteristics are summarized in Table 1. The recorded mixograph parameters include optimal water absorption and dough development time, whereas farinograph provides the water absorption, mixing time, and dough stability (Supplementary document, Fig. S1 & S2). In the mixographs, water absorption and mixing time for control dough were 62% and 5.5 mins, respectively. With the increasing levels of KHCO3 in the dough to 2.0%, both water absorption and mixing time gradually increased, and similar effect was observed for the sample added with 2.0% NaHCO3. Salt (i.e., ionic compound) competes with flour macromolecules (e.g., gluten, starch) for water, and therefore, delaying gluten hydration and lengthening mixing time to reach the peak. Similar results were found for doughs added with other types of ionic compounds, such as sodium chloride, sodium dodecyl sulfate, and sodium sulfate (Danno and Hoseney, 1982, He et al., 1992). In the farinographs, the peak time was 8.7 min for the dough with 2.0% KHCO3 compared to the control sample (1.2 min), where the dough stability also increased from 11.6 min to 15.7 min. The dough strengthening mechanism was partially attributed to the effect of ionic compound, where more KHCO3 in the systems may induce protein aggregation and interaction, yielding the stronger gluten network. Overall, KHCO3 exhibited similar effect on dough mixing properties as NaHCO3.
Table 1.
Cookie dough mixing characteristics, and rheological and physical properties with different levels of KHCO3 and 2% NaHCO3.
| Sample* | Mixograph |
Farinograph |
Compression Test |
Stickiness test |
Kieffer Test |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Water Abs (% fwb) | Mix Time (min) | Water Abs (% fwb) | Mix Time (min) | Stability (min) | Elongational viscosity (Pa·s) | Stickiness (g) | Work of adhesion (g·sec) | Cohesiveness (mm) | Resistance to extension (g) | Extensibility (mm) | |
| C1 | 62 | 5.5 | 59.7 | 1.2 | 11.6 | 4894.1 ± 259.0d | 60.7 ± 4.6a | 23.0 ± 5.1a | 8.0 ± 0.5a | 7.7 ± 0.2c | 62.9 ± 6.9ab |
| K1 | 62 | 7.0 | 59.8 | 4.9 | 14.8 | 8061.0 ± 172.4b | 37.9 ± 3.2b | 3.4 ± 1.2b | 2.7 ± 0.8b | 9.1 ± 0.7bc | 66.7 ± 6.5a |
| K2 | 63 | 7.9 | 59.4 | 7.7 | 13.6 | 8384.3 ± 152.5ab | 42.3 ± 3.7b | 6.0 ± 1.9b | 3.3 ± 1.0b | 10.9 ± 1.2ab | 42.8 ± 7.2c |
| K3 | 63 | 8.5 | 59.8 | 9.5 | 14.3 | 9076.5 ± 408.8a | 45.4 ± 0.9b | 6.1 ± 1.3b | 3.4 ± 0.3b | 9.6 ± 1.3abc | 62.0 ± 4.4ab |
| K4 | 64 | 9.2 | 59.8 | 8.7 | 15.7 | 7672.3 ± 323.2b | 44.1 ± 1.3b | 4.6 ± 1.3b | 2.8 ± 1.0b | 12.3 ± 2.1a | 50.4 ± 3.9bc |
| S1 | 64 | 11.9 | 60.1 | 10.3 | 16.9 | 6732.0 ± 261.5c | 44.6 ± 5.1b | 5.1 ± 1.9b | 3.0 ± 0.9b | 12.0 ± 1.5a | 45.4 ± 8.1c |
Means with different superscripts within the same column are significantly different at p < 0.05. Statistics were not run on mixograph and farinograph data as there was only one replicate after achieving optimized water absorbance.
Sample identification: C1: control, K1: 0.5% KHCO3, K2: 1.0% KHCO3, K3: 1.5% KHCO3, K4: 2.0% KHCO3, and S1: 2.0% NaHCO3.
3.2. Impact of KHCO3 on dough rheological and physical properties
It is well known that dough rheological behavior is correlated with wheat gluten microstructures (McCann & Day, 2013). Kieffer extensibility test showed that the forces to break the doughs increased significantly with high level of KHCO3 addition (2.0% fwb), in comparison with the sample without KHCO3 (p < 0.05), whereas the elongation was not significantly different from the control one (p > 0.05). When it comes to the elongational viscosity, the cookie dough treated with KHCO3 had significantly increased viscosity values (p < 0.05), being similar to the influence of 2.0% NaHCO3. As illustrated in Table 1, adding KHCO3 could mitigate dough stickiness, adhesion and cohesiveness in all the samples containing KHCO3 and 2.0% NaHCO3. The degree of stickiness was significantly reduced (p < 0.05) from 60.7 g (control) to 44.1 g (2.0% KHCO3), which may be due to an obstruction effect of alkaline salt on gluten hydration by interacting with water molecules (Caramanico et al., 2017). Hence, the presence of KHCO3 caused the dough less sticky with higher viscosity, and it was in agreement with the Kieffer test results of the formation of stiffer and less elastic dough compared with the control. This may be caused by more gluten aggregation during dough development and dough formation (Chen et al., 2018).
3.3. Effect of KHCO3 on the structure, physico-chemical and conformation properties of gluten
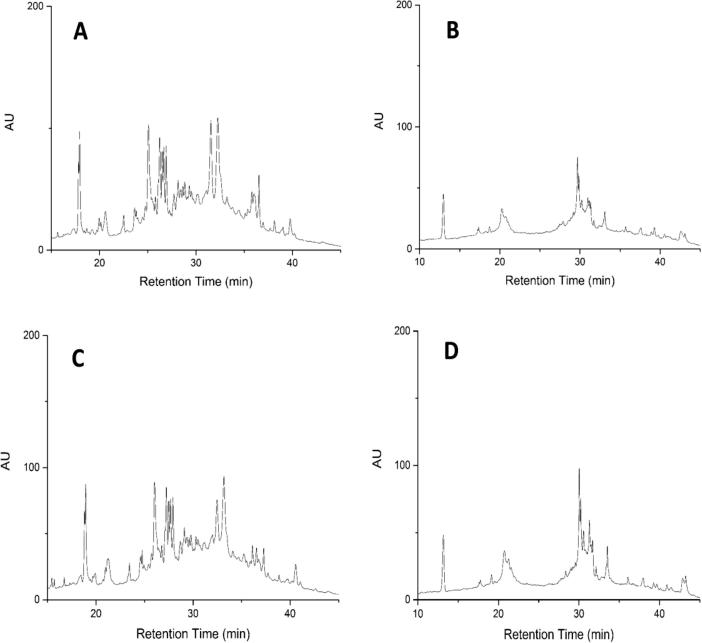
To evaluate the degree of protein polymerization, gluten extractability was determined. The profiles of gluten subunits are presented in Fig. 1. In a recovery test, the gluten samples without NaHCO3 or KHCO3 were added with 1% gliadin standard to obtain an average recovery rate of 88% (n = 3). It is reported that gliadin to glutenin ratio (GTG) contributes to the rheological properties of doughs and the bakery end-use quality (Ukai et al., 2008, Wieser, 2007). As illustrated in Table 2, although there was no significant difference for the GTG ratio between the control and 2.0% NaHCO3 treatment (p > 0.05), the GTG in the treatment of 2.0% KHCO3 addition was 1.50, in comparison with the value of 1.96 for the control, indicating that high level of KHCO3 significantly reduced the ratio (p < 0.05). The addition of KHCO3 possibly enhanced the polymeric interactions between gliadin and glutenin through oxidation of SH groups or SH-SS exchanges (Lagrain, Thewissen, Brijs, & Delcour, 2007). Besides, the change in gluten extractability in the presence of KHCO3 agrees with Wu et al. (2006), who reported that alkali induced gluten aggregation, and this may be ascribed to covalent SS formation and/or hydrogen bonding (Shiau and Yeh, 2001, Tuhumury et al., 2014). Therefore, adding KHCO3 could enhance gluten polymerization through gliadin-glutenin cross-linking, which may contribute to the mechanical and rheological properties of dough and the texture characteristic of cookies.
Fig. 1.
Typical RP-HPLC chromatographs of gluten extract separated from soft wheat flour dough as: (A) gliadin in control; (B) glutenin in control; (C) gliadin in sample added with 2% KHCO3; (D) glutenin in sample added with 2% KHCO3 via the Aeris WIDEPORE XB-C18 column (3.6 μm, 150 × 4.6 mm) with UV detection at a wavelength of 210 nm.
Table 2.
Gluten microstructure changes (secondary structure, gluten extractability, and SH and SS groups) induced by KHCO3 or NaHCO3.
| Sample | Secondary structure* |
Gluten extractability |
Concentration of SH and SS groups |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Extended chains (%) | β-Sheet (%) | Random coil (%) | α-helix (%) | β-turn (%) | Gliadin (μg/mg) | Glutenin (μg/mg) | Gliadin to Glutenin | Total SH (nmoles/mg) | Free SH (nmoles/mg) | SS (nmoles/mg) | |
| C1 | 4.74 ± 0.47a | 19.48 ± 0.90b | 27.16 ± 2.10a | 14.30 ± 1.04a | 14.60 ± 1.02a | 234.19 ± 21.15a | 119.86 ± 7.74a | 1.96 ± 0.14a | 7.18 ± 0.52a | 0.83 ± 0.11a | 3.17 ± 0.26a |
| K1 | 4.64 ± 0.53a | 21.19 ± 0.05ab | 18.17 ± 0.72ab | 24.55 ± 2.52a | 20.49 ± 2.99a | 230.80 ± 26.40a | 130.50 ± 12.92a | 1.78 ± 0.21ab | 7.13 ± 0.32a | 0.92 ± 0.04ab | 3.10 ± 0.14a |
| K2 | 5.18 ± 0.62a | 24.08 ± 1.13a | 16.33 ± 1.36b | 19.94 ± 3.84a | 18.26 ± 6.85a | 201.83 ± 6.11a | 133.56 ± 6.41a | 1.51 ± 0.03b | 7.38 ± 0.30a | 1.00 ± 0.15ab | 3.19 ± 0.10a |
| K3 | 4.68 ± 0.70a | 23.59 ± 0.42a | 13.43 ± 2.88b | 18.12 ± 2.43a | 17.09 ± 1.46a | 216.37 ± 19.61a | 115.73 ± 6.05a | 1.87 ± 0.08ab | 8.26 ± 0.85a | 0.92 ± 0.06ab | 3.67 ± 0.42a |
| K4 | 3.12 ± 0.92a | 18.09 ± 0.53b | 17.82 ± 4.11ab | 24.20 ± 0.22a | 12.50 ± 4.28a | 197.66 ± 26.19a | 131.58 ± 11.03a | 1.50 ± 0.08b | 8.30 ± 0.85a | 1.02 ± 0.02b | 3.54 ± 0.22a |
| S1 | 4.57 ± 0.82a | 20.46 ± 0.23ab | 22.04 ± 0.83ab | 20.11 ± 2.09a | 18.01 ± 1.12a | 216.34 ± 18.35a | 132.56 ± 14.43a | 1.64 ± 0.05ab | 7.62 ± 0.04a | 0.89 ± 0.04ab | 3.37 ± 0.03a |
Means with different superscripts within the same column are significantly different at p < 0.05.
Gluten secondary structures deconvolved from amide bands: I. Full assignment is given as extended chains (1600–1615 cm−1), β-Sheet (1624–1640, 1681 cm−1), random coil (1640–1650 cm−1), α-helix (1650–1660 cm−1), β-turn (1660–1670, 1694 cm−1). See Table 1 for sample identification.
As presented in Table 2, the free SH content obviously increased with increasing alkaline concentrations, and the content in 2.0% KHCO3 treatment (1.02 nmoles/mg) was significantly different from the control one (0.83 nmoles/mg) (p < 0.05); however, there were no significant differences of total SH and SS concentration among all the samples (p > 0.05). This observation was not in accordance with the results of Shiau and Yeh (2001) who found the loss of the SH groups since alkali enhanced SH oxidation/SS formation. Nevertheless, Friedman (1999) indicated that free SH groups could also be induced by β-elimination, which corresponded with our result of increase of the SH contents for the sample added with 2.0% KHCO3. Therefore, non-redox reaction (e.g., β-elimination) may induce an observable increase of the free SH when adding 2.0% KHCO3 and account for the aggregation of wheat gluten during dough development, to some extent. It is noteworthy that a significant change of the free SH concentration was not observed in 2.0% NaHCO3 treatment (p > 0.05). The discrepant results might be due to the different ion bonds effect in the system, and other interaction patterns deserve to be further investigated.
To understand the relationship between macroscopic strength of dough and aggregation behavior of wheat gluten, FTIR spectroscopy was used to investigate the differences in their secondary structures that was influenced by different levels of KHCO3 and NaHCO3. In the five sections of amides I bands in FTIR spectra, the peak at 1650–1660 cm−1 is assigned to α-helix, and 1624–1640 and 1681 cm−1 are associated with β-sheet conformation. The band in the region of 1640–1650 cm−1 is assigned to random coil, and 1660–1670 and 1694 cm−1 region is assigned to β-turn conformation, respectively. As shown in Table 2, the proportions of extended chains, a-helix and β-turn structures were not significantly different among all the treatments (p > 0.05), suggesting little impact on the three gluten conformations exerted by KHCO3 and NaHCO3. On the other hand, β-sheet structure was 19.48% in the control and 24.08% in the sample added with 1.0% KHCO3 (significant difference, p < 0.05), which may be ascribed to the decrease of random coil structure (Table 2). The conformation of β-sheet is referred as the characteristic of gluten aggregation (Surewicz & Mantsch, 1988); therefore, higher proportions of β-sheet may demonstrate stronger protein interaction and/or polymerization in the samples added with 1.0 and 1.5% KHCO3 than the control. The increased β-sheet conformation in the samples could explain the more strengthened gluten microstructure in the dough (Table 1); moreover, in comparison with the control sample, the results were also supported by farinograph parameters and dough physical properties with higher dough stability and strength and less stickiness.
3.4. Influence of KHCO3 on macroscopic cookie properties
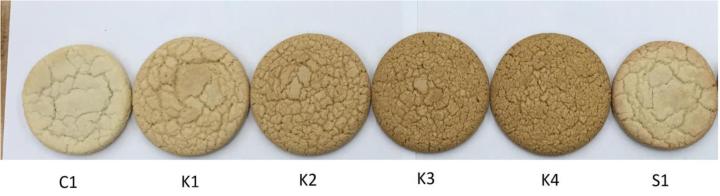
Table 3 summarizes the characteristics of cookies containing the control sample, KHCO3 under different concentrations, and 2.0% NaHCO3. The pH value, W/T spread, hardness, and flexibility of cookie were significantly influenced when adding 0.5–2.0% KHCO3 or 2.0% NaHCO3. The hardness and flexibility of cookie were decreased with the increase of KHCO3 concentration, and adding 2.0% NaHCO3 also resulted in significantly lower hardness and flexibility values compared with the control (p < 0.05). The W/T spread ratio increased to 0.94 with 1.5% KHCO3 addition compared with 0.71 for the control (p < 0.05), and higher spread ratio was considered as a more desirable quality attribute for cookies (Barak, Mudgil, & Khatka, 2014). As shown in Table 3 & Fig. 2, cookies added with KHCO3 were significantly brighter than the control one (p < 0.05); however, all levels of KHCO3 also led to high degree of redness (a*). Moreover, cookies containing KHCO3 or NaHCO3 presented greater yellowness (b*) than the control, where the value of 1.5% KHCO3 treatment (31.60) was significantly (p < 0.05) higher than that of the control (15.87). The strong yellow coloration in the cookie samples may be caused by the formation of flavone-c-diglycosides at alkaline pH (Asenstorfer, Wang, & Mares, 2006). Based on the aforementioned information, in addition to improving the cookie coloration, it can be postulated that the high amount of KHCO3 may induce gluten interaction that contributed to the higher W/T spread ratio and fluffy texture of cookies.
Table 3.
Cookie characteristics with different levels of KHCO3 and 2% NaHCO3.
| Sample | pH | W/T spread ratio | Hardness (g) | Flexibility (mm) | Color |
||
|---|---|---|---|---|---|---|---|
| L* | a* | b* | |||||
| C1 | 6.05 ± 0.01f | 0.71 ± 0.01d | 11258 ± 457a | 1.80 ± 0.22a | 66.98 ± 1.89a | 0.02 ± 0.01d | 15.87 ± 0.53c |
| K1 | 8.15 ± 0.03e | 0.75 ± 0.01c | 9234 ± 519b | 1.39 ± 0.32ab | 63.11 ± 1.38bc | 3.53 ± 0.43c | 22.89 ± 0.98b |
| K2 | 9.14 ± 0.03d | 0.88 ± 0.01b | 7523 ± 772b | 0.85 ± 0.15bc | 59.53 ± 0.95 cd | 6.32 ± 0.51b | 28.28 ± 2.45a |
| K3 | 9.46 ± 0.04b | 0.94 ± 0.01a | 4699 ± 148c | 0.79 ± 0.02c | 57.25 ± 0.15d | 8.01 ± 0.69a | 31.60 ± 1.54a |
| K4 | 9.75 ± 0.02a | 0.89 ± 0.01b | 5384 ± 441c | 0.66 ± 0.02c | 56.68 ± 0.36d | 6.34 ± 0.29b | 28.45 ± 0.55a |
| S1 | 9.26 ± 0.03c | 0.75 ± 0.01c | 7638 ± 573b | 0.71 ± 0.09c | 65.07 ± 0.82ab | 2.39 ± 0.54c | 20.25 ± 0.33b |
abcdefMeans with different superscripts within the same column are significantly different at p < 0.05. See Table 1 for sample identification.
Fig. 2.
Images of cookies of: (C1) control, (K1) 0.5% KHCO3, (K2) 1% KHCO3, (K3) 1.5% KHCO3, (K4) 2.0% KHCO3, and (S1) 2.0% NaHCO3.
Taken together, remarkable differences of rheological and physico-chemical properties in dough and cookies were found in the treatments added with higher concentrations of KHCO3. Hydration and mixing of flour with water result in an 'elastic backbone' of gluten network that is stabilized by covalent and/or non-covalent interactions. The continuous viscoelastic structure warrants the wide utilization of wheats for various products (Domenek, Morel, Redl, & Guilbert, 2003). When adding high amount of KHCO3 into the system, it competes for water molecules and delays flour and gluten hydration, and hydrogen bonds may confer the alternation of the secondary conformation in forming more β-sheet structure, thus it can generally increase the molecular flexibility of samples. In the meantime, the gliadin-glutenin crossing-linking is pronounced via covalent interactions (e.g., β-elimination) in the microstructure. Consequently, gluten aggregation promotes a compact network which may prevent excessive starch swelling or leaching from the structure to such extent that it results in the fluffy texture of cookie after baking.
3.5. Cookie sensory test
Consumers’ acceptability tests were conducted to compare the organoleptic characteristics of cookies with or without KHCO3 and NaHCO3. Cookie sensory attributes were affected by both the addition of KHCO3 and NaHCO3 (Table 4). The sample added with 1.0% KHCO3 showed the highest overall acceptance compared with other treatments, although the samples containing 2.0% NaHCO3 or 2.0% KHCO3 did have significantly higher texture score than the control (p < 0.05), in agreement with the other cookie macroscopic parameters described previously. The sensory attribute scores for cookies with KHCO3 were similar as that with NaHCO3. Texture perception from consumers confirmed the instrumental results that the blank control sample has the lowest score. Our results demonstrated that KHCO3 was comparable with NaHCO3 as an effective leaving agent for cookie production, without adversely influencing the organoleptic characteristics of cookies.
Table 4.
Sensory liking attributes of cookies.
| Sample | Appearance | Odor | Taste | Texture | Overall acceptance |
|---|---|---|---|---|---|
| C1 | 6.3 ± 0.7a | 5.8 ± 0.3a | 6.0 ± 0.7a | 4.9 ± 0.9a | 5.4 ± 0.2a |
| K2 | 6.8 ± 0.4a | 7.0 ± 0.4b | 7.3 ± 0.7a | 6.1 ± 0.8ab | 6.8 ± 0.3b |
| K4 | 6.5 ± 0.8a | 6.7 ± 0.5ab | 6.1 ± 1.1a | 6.7 ± 0.7b | 6.4 ± 0.3ab |
| S1 | 6.5 ± 0.9a | 6.5 ± 0.3ab | 6.4 ± 1.0a | 6.6 ± 1.1b | 6.4 ± 0.2ab |
Different superscript letters indicate a significant difference (p < 0.05) among attributes in the same column. See Table 1 for sample identification.
4. Conclusion
In the present study, cookie dough systems were obtained by adding KCHO3 or NaHCO3, and their corresponding dough and end-use qualities were compared. Overall, the inclusion of high level of KHCO3 aggravated the macromolecular aggregation via non-redox reactions rather than SH oxidation. In addition, it altered gluten secondary structure, which contributed to the changes of the dough viscoelastic properties and cookie qualities. The elongational viscosity and dough strength were improved, and the stickiness degree was mitigated with KHCO3 addition; a proper addition level (e.g., 1.0% fwb) of KHCO3 did improve cookie texture and positively impact its coloration. Based on the organoleptic evaluation, replacement of NaHCO3 with KHCO3 is a feasible approach aiming for sodium reduced diet without compromising the acceptance of the cookie product. Although more studies are still needed to address the mechanism of gluten aggregation, these results have indicated that adding KHCO3 in dough system could be a proposing alternative method of developing high quality cookies and achieving sodium reduction in cereal-based products.
CRediT authorship contribution statement
Gengjun Chen: Methodology, Formal analysis, Investigation. Ruijia Hu: Investigation. Yonghui Li: Conceptualization, Methodology, Formal analysis, Resources, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This is contribution no. 19-302-J from the Kansas Agricultural Experimental Station. This project is supported by the USDA National Institute of Food and Agriculture Hatch project KS17HA1008 and the Department of Grain Science and Industry, Kansas State University.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2019.100075.
Contributor Information
Gengjun Chen, Email: gengjunc@ksu.edu.
Ruijia Hu, Email: ruijia@ksu.edu.
Yonghui Li, Email: yonghui@ksu.edu.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- AACC International (2000). Approved methods of the AACC (10th ed.). St. Paul, Minnesota, USA.
- Angus F. Woodhead Publishing; 2007. Dietary salt intake: Sources and targets for reduction. Reducing salt in foods: Practical strategies; pp. 3–17. [Google Scholar]
- Asenstorfer R.E., Wang Y., Mares D.J. Chemical structure of flavonoid compounds in wheat (Triticum aestivum L.) flour that contribute to the yellow colour of Asian alkaline noodles. Journal of Cereal Science. 2006;43:108–109. [Google Scholar]
- Bala R., Kaur A., Bakshi A.K. Studies on preparation of low sodium cookies. Journal of Food Science and Technology-Mysore. 2004;41(6):668–671. [Google Scholar]
- Barak S., Mudgil D., Khatka B. Effect of composition of gluten proteins and dough rheological properties on the cookie-making quality. British Food Journal. 2014;115(4):564–574. [Google Scholar]
- Caramanico R., Barbiroli A., Marengo M., Fessas D., Bonomi F., Lucisano M.…Marti A. Interplay between starch and proteins in waxy wheat. Journal of Cereal Science. 2017;75:198–204. [Google Scholar]
- Chen G., Hu R., Li Y. Potassium chloride affects gluten microstructures and dough/bread characteristics similarly as sodium chloride. Journal of Cereal Science. 2018;82:155–163. [Google Scholar]
- Danno G., Hoseney R.C. Effect of Sodium Chloride and Sodium Dodecyl Sulfate on Mixograph Properties. Cereal Chemistry. 1982;59(3):202–204. [Google Scholar]
- Domenek S., Morel M.H., Redl A., Guilbert S. Rheological investigation of swollen gluten polymer networks: Effects of process parameters on cross-link density. Macromolecular Symposia. 2003;200:137–145. [Google Scholar]
- Friedman M. Chemistry, biochemistry, nutrition, and microbiology of lysinoalanine, lanthionine, and histidinoalanine in food and other proteins. Journal of Agricultural and Food Chemistry. 1999;47(4):1295–1319. doi: 10.1021/jf981000+. [DOI] [PubMed] [Google Scholar]
- Geleijnse J.M., Kok F.J., Grobbee D.E. Blood pressure response to changes in sodium and potassium intake: A metaregression analysis of randomised trials. Journal of Human Hypertension. 2003;17(7):471–480. doi: 10.1038/sj.jhh.1001575. [DOI] [PubMed] [Google Scholar]
- Georget D.M.R., Belton P.S. Effects of temperature and water content on the secondary structure of wheat gluten studied by FTIR spectroscopy. Biomacromolecules. 2006;7(2):469–475. doi: 10.1021/bm050667j. [DOI] [PubMed] [Google Scholar]
- He H., Roach R.R., Hoseney R.C. Effect of Nonchaotropic Salts on Flour Bread-Making Properties. Cereal Chemistry. 1992;69(4):366–371. [Google Scholar]
- Lagrain B., Thewissen B.G., Brijs K., Delcour J.A. Impact of redox agents on the extractability of gluten proteins during bread making. Journal of Agricultural and Food Chemistry. 2007;55(13):5320–5325. doi: 10.1021/jf070639n. [DOI] [PubMed] [Google Scholar]
- Mattes R.D., Donnelly D. Relative contributions of dietary-sodium. Journal of the American College of Nutrition. 1991;10(4):383–393. doi: 10.1080/07315724.1991.10718167. [DOI] [PubMed] [Google Scholar]
- McCann T.H., Day L. Effect of sodium chloride on gluten network formation, dough microstructure and rheology in relation to breadmaking. Journal of Cereal Science. 2013;57(3):444–452. [Google Scholar]
- Miller R.A., Hoseney R.C. Role of salt in baking. Cereal Foods World. 2008;53(1):4–6. [Google Scholar]
- Pareyt B., Brijs K., Delcour J.A. Sugar-Snap Cookie Dough Setting: The Impact of Sucrose on Gluten Functionality. Journal of Agricultural and Food Chemistry. 2009;57(17):7814–7818. doi: 10.1021/jf9010774. [DOI] [PubMed] [Google Scholar]
- Rombouts I., Jansens K.J.A., Lagrain B., Delcour J.A., Zhu K.X. The impact of salt and alkali on gluten polymerization and quality of fresh wheat noodles. Journal of Cereal Science. 2014;60(3):507–513. [Google Scholar]
- Shiau S.Y., Yeh A.I. Effects of alkali and acid on dough rheological properties and characteristics of extruded noodles. Journal of Cereal Science. 2001;33(1):27–37. [Google Scholar]
- Singh H., MacRitchie F. Changes in proteins induced by heating gluten dispersions at high temperature. Journal of Cereal Science. 2014;39(2):297–301. [Google Scholar]
- Stamler J., Chan Q., Daviglus M.L., Dyer A.R., Van Horn L., Garside D.B.…Elliott P. Relation of dietary sodium (Salt) to blood pressure and its possible modulation by other dietary factors. Hypertension. 2018;71(4):631–637. doi: 10.1161/HYPERTENSIONAHA.117.09928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surewicz W.K., Mantsch H.H. New insight into protein secondary structure from resolution-enhanced infrared spectra. Biochimica et Biophysica Acta (BBA)-Protein Structure and Molecular Enzymology. 1988;952:115–130. doi: 10.1016/0167-4838(88)90107-0. [DOI] [PubMed] [Google Scholar]
- Thannhauser T.W., Konishi Y., Scheraga H.A. Analysis for disulfide bonds in peptides and proteins. Methods in Enzymology. 1987;143:115–119. doi: 10.1016/0076-6879(87)43020-6. [DOI] [PubMed] [Google Scholar]
- Torri L., Piochi M., Marchiani R., Zeppa G., Dinnella C., Monteleone E. A sensory- and consumer-based approach to optimize cheese enrichment with grape skin powders. Journal of Dairy Science. 2016;99:194–204. doi: 10.3168/jds.2015-9922. [DOI] [PubMed] [Google Scholar]
- Tuhumury H.C.D., Small D.M., Day L. The effect of sodium chloride on gluten network formation and rheology. Journal of Cereal Science. 2014;60(1):229–237. [Google Scholar]
- Ukai T., Matsumura Y., Urade R. Disaggregation and reaggregation of gluten proteins by sodium chloride. Journal of Agricultural and Food Chemistry. 2008;56(3):1122–1130. doi: 10.1021/jf0725676. [DOI] [PubMed] [Google Scholar]
- United Stated Department of Health and Human Services (HHS). (2015). 2015-2020 Dietary Guidelines for Americans. (https://health.gov/dietaryguidelines/2015/resources/2015-2020_Dietary_Guidelines.pdf).
- United States Food and Drug Administration (FDA). (2016). (http://www.fda.gov/Food/Ingredients PackagingLabeling/GRAS/).
- Wieser H. Chemistry of gluten proteins. Food Microbiology. 2007;24(2):115–119. doi: 10.1016/j.fm.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Wu J.P., Beta T., Corke H. Effects of salt and alkaline reagents on dynamic rheological properties of raw oriental wheat noodles. Cereal Chemistry. 2006;83(2):211–217. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.