Abstract
Hyperglycemia, an important feature of diabetes, can cause oxidative stress, which is associated with varieties of diabetic complications including erectile dysfunction. Therefore, this study sought to investigate the effect of almond-supplemented diet on some biochemical indices relevant to erection in diabetic male rats. Forty-two male rats were divided into two groups: A (n = 6) and B (n = 36). Diabetes was induced in Group B via injection of a single dose of STZ (50 mg/kg) intraperitoneally and confirmed 72 h after induction. Diabetic rats (blood glucose ≥250 mg/dL) were subsequently divided into six groups (n = 6). Fourteen days after confirmation of diabetes, rats were fed with diets containing almond drupe and seeds (10 and 20% inclusion) for fourteen days. The effects of the diets on blood glucose, sexual behavior, sexual hormones, phosphodiesterase-5 activity, nitric oxide, H2S, and AGEs levels were evaluated. Significant increase in blood glucose level, phosphodiesterase-5 activity, and glycated hemoglobin was observed in diabetic rats. Furthermore, diabetes caused a significant decrease in nitric oxide, H2S, sexual hormones (testosterone, follicle-stimulating hormone and luteinizing hormone) levels, and sexual behavioral indices. However, treatment with diets supplemented with almond drupe and seeds significantly reversed these effects in diabetic rats. Findings in this study revealed that almond-supplemented diets enhance some important biomarkers relevant to erection in diabetic rats. Thus, dietary inclusion of almond (drupe and seeds) could serve as a cheap and readily available nutraceutical in the management of erectile dysfunction associated with diabetes.
Keywords: Biological sciences, Metabolite, Biochemistry, Toxicology, Diet, Pathophysiology, Almond-supplemented diet, Sexual hormones, Phosphodiesterase-5, Sexual behavior
Biological sciences; Metabolite; Biochemistry; Toxicology; Diet; Pathophysiology; Almond-supplemented diet; Sexual hormones; Phosphodiesterase-5; Sexual behavior
1. Introduction
Diabetes is a metabolic syndrome characterized by elevated blood sugar levels (hyperglycemia). Hyperglycemia produces oxidative stress, which is associated with varieties of complications that come along with diabetes which include retinopathy, encephalopathy, cardiovascular diseases and erectile dysfunction (Pop-Busui et al., 2006; Krentz et al., 2007; Capellini et al., 2010). Erectile dysfunction, which means persistence inability to achieve and/or maintain penile erection required for satisfactory sexual performance, can lead to low or loss of self-esteem among men (McMahon, 2014). Overproduction of reactive oxygen species (ROS) associated with hyperglycemia destroys smooth muscle and nerves of the penis and this has been reported to cause erectile dysfunction in diabetics (Cameron and Cotter, 2007; Kizub et al., 2014; Dal and Sigrist, 2016). Besides, prolonged hyperglycemia produces inflammation and reactive species that scavenge nitric oxide (NO), a principal mediator of penile erection. Chronic hyperglycemia had been reported to initiates free radical generation through the formation of advanced glycated end-products (AGEs) (Basta et al., 2004; Jakuš and Rietbrock, 2004; Ramasamy et al., 2005). Advance glycated end-products up-regulate adhesion molecules that mediate vascular damage coupled with stimulation of cytokines on monocytes of the endothelial cells (Schalkwijk, 2005; Singh et al., 2014).
Though there are available pharmacological therapies for erectile dysfunction management which include phosphodiesterase-5 (PDE-5) inhibitors such as sildenafil citrate among others. However, there are limitations to the efficacy of these conventional drugs as they come with associated side effects, and besides, some patients respond poorly to PDE-5 inhibitors (Bella et al., 2007; Foresta et al., 2008). Long-time before now, erectile dysfunction was thought to be psychogenic and/or neuropathic in origin, but a recent shred of shreds of evidence has revealed that the predominant etiology of erectile dysfunction is vascular (Wahed et al., 2018). Thus, lifestyle modification which includes healthy diets has been proposed to improve erectile dysfunction. Previous studies have shown that diet rich in whole grains, fruits, nuts, and vegetables was associated with an enhanced erectile function in subject with metabolic syndrome (Esposito et al., 2010; Liu, 2013). It has been reported that diet rich in fruits, nuts, and vegetables improves endothelial function and reduces inflammation, thereby linking diet, inflammation, vascular function and erectile dysfunction (Johnston, 2009; Schwingshackl and Hoffmann et al., 2014).
Almond (Terminalia catappa) belongs to family Combretaceae and its generic name originated from the Latin word "terminalis”. It is a tree commonly grown in tropical and subtropical regions because of its edible, ornamental and medicinal purposes (Pinelo et al., 2004). Terminalia catappa have found to possess various pharmacological properties, such as anti-stress, anti-oxidant (Subashinee et al., 2002; Pinelo et al., 2004; Oyeleye et al., 2017). Almond fruit is rich in vitamins, amino acids, minerals, phenolic compounds with potential medicinal value in managing certain diseases and ailments (Pinelo et al., 2004). Previous studies have reported various biological activities of almond fruit in vitro; antidiabetic, antihypertensive, and antioxidative properties (Adefegha et al., 2017). Furthermore, previous studies have shown that dietary inclusion of plant-based materials rich in phytochemicals such as phenolic compounds and flavonoids could improve endothelial and vascular function (Schroeter et al., 2006; Alissa and Ferns, 2012). But unfortunately, there are little or no scientific findings to substantiate this claim. However, this study was designed to investigate the possible effect of dietary supplementation of almond (drupe and seed) on sexual functions, sex hormones and other important biochemical parameters relevant to erectile dysfunction in diabetic male rats.
2. Materials and methods
2.1. Chemicals
Para-nitrophenylphenylphosphonate, coomassie blue G, sulfanilamide and streptozotocin (STZ) were obtained from Sigma Chemical Co (St. Louis, MO, USA), bovine serum albumin, nitrate, and vanadium chloride was obtained from Reagen (Colombo, Parana, Brazil). Sildenafil citrate was obtained from Cipla Limited., Mumbai, India, and Estradiol benzoate procured from Organon Limited, Kolkata, India, whereas progesterone was obtained from Cadilla Healthcare Limited, Daman, India.
2.2. Sample collection
Fresh samples of almond (Terminalia catappa) fruits were obtained from the botanical garden, Federal University of Technology, Akure, Nigeria. Authentication was carried out at the Centre for Research and Development, Federal University of Technology, Akure, Nigeria. The fruits were cleansed and separated into drupe and seed which were later air-dried to a constant weight. The dried samples (almond drupe and seed) were pulverized into a fine powder using a manual blender and kept inside the refrigerator for subsequent use.
2.3. HPLC analysis of phenolic compounds and amino acids composition of almond fruit
High performance liquid chromatographic analyses were carried out under gradient conditions using C18 column (4.6 mm × 150 mm) packed with 5 μm diameter particles; the mobile phase was water containing 2% acetic acid (A) and methanol (B), and the composition gradient was: 5% of B until 2 min and changed to obtain 25, 40, 50, 60, 70 and 100% B at 10, 20, 30, 40, 50 and 80 min, respectively, following the method described by Oboh et al. (2017).
2.4. Feed formulation
Diets were formulated according to the method of Akinyemi et al. (2014) and following guideline from an expert (Table 1).
Table 1.
Diet formulation for control and test groups.
| Groups | I | II | III | IV | V | VI | VII |
|---|---|---|---|---|---|---|---|
| Skimmed milk | 37.5 | 37.5 | 37.5 | 34.7 | 31.9 | 34.1 | 30.6 |
| Oil | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| Vitamin premix | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 |
| Corn starch | 48.5 | 48.5 | 48.5 | 41.3 | 34.1 | 41.9 | 35.4 |
| Almond drupe | - | - | - | 10 | 20 | - | - |
| Almond seed | - | - | - | - | - | 10 | 20 |
| Total (g) | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
Note: Skimmed milk = 32% protein; The vitamin premix (mg or IU/g) has the following composition; 3200 IU vitamin A, 600 IU vitamin D3, 2.8 mg vitamin E, 0.6 mg vitamin K3, 0.8 mg vitamin B1, 1 mg vitamin B2, 6 mg niacin, 2.2 mg pantothenic acid, 0.8 mg vitamin B6, 0.004 mg vitamin B12, 0.2 mg folic acid, 0.1 mg biotin H2, 70 mg choline chloride, 0.08 mg cobalt, 1.2 mg copper, 0.4 mg iodine, 8.4 mg iron, 16 mg manganese, 0.08 mg selenium, 12.4 mg zinc, 0.5 mg antioxidant. Group I: (Control) serve as normal rats placed on basal diet; Group II: (STZ) serve as diabetic rats placed on basal diet; Group III: (STZ + SC) serve as diabetic rats placed on basal diet plus sildenafil citrate; Group IV: (STZ +10% AD) serve as diabetic rats placed on diet supplemented with almond drupe (10%); Group V: (STZ +20% AD) serve as diabetic rats placed on diet supplemented with almond drupe (20%); Group VI: (STZ +10% AS) serve as diabetic rats placed on diet supplemented with almond seed (10%) and (STZ +20% AS) serve as diabetic rats placed on diet supplemented with almond seed (20%).
2.5. Experimental animals
Forty-two (42) adult male Wistar albino rats (weighing 180–200 g) were procured from the breeding colony of the Department of Veterinary Physiology and Biochemistry, University of Ibadan, Nigeria. The animals were acclimatized for two weeks and allowed to ad libitum access to water and commercial diet. The handling of experimental animals was in accordance with the institutional guidelines for the care and use of animals and all procedures were approved by the School of Sciences Animal Ethical Committee, Federal University of Technology, Akure, Nigeria (FUTA/SOS/1411). The animals were housed in stainless steel cages and kept in a room where 12 h light/dark cycle and room temperature (25–28 °C) was maintained throughout the period of the experiment.
2.6. Experimental design
Diabetes was induced via intraperitoneal injection of a single dose of streptozotocin (50 mg/kg) in fresh citrate buffer (0.1 M, pH 4.5). Seventy-two (72) hours after streptozotocin injection, diabetes was confirmed using Fine test glucometer and animals with blood glucose ≥250 mg/dL were considered diabetic and used for the experiment. Since erectile dysfunction is one of the complications associated with diabetes, diabetic rats were left alone untreated for two weeks. Thereafter, the animals were randomly divided into seven groups as follows (n = 6): group 1 (normal control) served as normal rats placed on basal diet; group 2 (diabetic control) served as diabetic rats placed on basal diet; group 3 (diabetic + sildenafil citrate) served as diabetic rats placed on basal diet plus sildenafil citrate (5 mg/kg); group 4 (diabetic +10% almond drupe) served as diabetic rats placed on diet supplemented with 10% almond drupe; group 5 (diabetic +20% almond drupe) served as diabetic rats placed on diet supplemented with 20% almond drupe group; 6 (diabetic +10% almond seed) served as diabetic rats placed on diet supplemented with 10% almond seed; and group 7 (diabetic +20% almond seed) served as diabetic rats placed on diet supplemented with 20% almond seed. The rats were placed on their respective diet (Table 1). Treatment lasted for 14 days, after which the animals were subjected to sexual behavioral studies.
2.7. Sexual behavioral procedure
To evaluate the effect of dietary supplementation of almond (drupe and seed) on sexual behavior, estrous female rats were paired with treated male rats. Female rats were made estrous by the administration of estradiol benzoate at a dose of 2 μg/kg body weight and progesterone, 500 μg/kg body weight at 48 and 4 h, respectively before the commencement of sexual behavioral studies. The male rat sexual behavioral study was conducted after the training of the male rats with the sexually receptive females for three consecutive days. Sexual behavior was monitored in a separate room for 1 h in a clear plastic box (60 × 60 × 80 cm) and captured by digital video recording. The behavioral study was carried out early hour in the morning (6:00 a.m.). The sexual behavior parameters examined were the mounting number, mount latency, intromission number, and intromission latency (Guohua et al., 2009; Ademosun et al., 2019).
2.8. Tissue preparation
Twenty-four hours after the last treatment, animals were anesthetized under mild diethylether, and their blood was collected into a plain bottle for serum preparation and cholesterol level determination. The penile tissues were excised, rinsed in cold phosphate buffer and weighed. The penile tissues were chopped with scissors and homogenized in cold phosphate buffer (0.1 M, pH 7.4) using Teflon glass homogenizer. The homogenates were centrifuged at 10,000 rpm for 10 min. The supernatant obtained was kept (-20 °C) for subsequent analyses.
2.9. Biochemical assays
Blood glucose level was determined using Fine test glucometer (OSANG Healthcare Co. Ltd., Korea), phosphodiesterase-5 activity was determined according to Ademosun et al. (2019), hormonal (testosterone, follicle-stimulating hormone, and luteinizing hormone) levels were determined using Accu-Bind ELISA kits (Monobind Inc., California, USA), glycated hemoglobin level was evaluated as previously described (Nayak and Pattabiraman, 1981), nitric oxide level was determined according to Miranda et al. (2001), Hydrogen sulfide level was estimated (Padiya et al., 2014) and total protein content was determined using standard method (Bradford, 1976).
2.10. Data analysis
Data were expressed as mean ± standard error of the mean (SEM) (n = 6). One way analysis of variance was used for statistical analyses followed by post hoc Dunnett's test for comparing the mean. The significant difference was accepted at p < 0.05.
3. Results
In order to identify the major components of almond drupe and seed, HPLC analysis was carried out using standard phenolic acids and flavonoids. The results of the analysis revealed the abundance of gallic acid (tR = 9.97 min; peak 1), catechin (tR = 16.39 min; peak 2), chlorogenic acid (tR = 21.47 min; peak 3), caffeic acid (tR = 25.03 min; peak 4), ellagic acid (tR = 31.20; peak 5), epicatechin (tR = 32.71 min; peak 6), rutin (tR = 39.98 min; peak 7), quercitrin (tR = 44.15 min; peak 8), isoquercitrin (tR = 47.83 min; peak 9), quercetin (tR = 52.64 min; peak 10) and kaempferol (tR = 59.86 min; peak 11) (Table 2). In addition, all the basic amino acids found in protein were present in varying amount in almond fruit (Table 3).
Table 2.
Phenolic composition of almond fruit.
| Compounds | Almond drupe (mg/g) | Almond seed (mg/g) |
|---|---|---|
| Gallic acid | 45.08 ± 0.03b | 14.57 ± 0.02a |
| Catechin | 7.15 ± 0.01a | 6.13 ± 0.01a |
| Chlorogenic acid | 26.89 ± 0.02a | 62.18 ± 0.01b |
| Caffeic acid | 6.31 ± 0.02a | 11.86 ± 0.01b |
| Ellagic acid | 107.53 ± 0.01b | 80.09 ± 0.03a |
| Epicatechin | 7.69 ± 0.03a | 13.90 ± 0.01b |
| Rutin | 45.27 ± 0.02b | 13.24 ± 0.02a |
| Quercitrin | 25.73 ± 0.01a | 23.15 ± 0.01a |
| Isoquercitrin | 44.18 ± 0.01b | 39.68 ± 0.02a |
| Quercetin | 21.94 ± 0.03a | 23.37 ± 0.03a |
| Kaempferol | 62.59 ± 0.02a | 65.02 ± 0.01a |
Values represent mean ± deviation of replicate experiment.
Values with the same superscript alphabet are not significantly different (p < 0.05).
Table 3.
Amino acids composition of almond fruit.
| Amino acids | Samples |
|
|---|---|---|
| Almond Drupe (mg/g) |
Almond Seed (mg/g) |
|
| Gly | 4.243 ± 0.04a | 5.766 ± 0.02b |
| Ala | 2.390 ± 0.01a | 3.224 ± 0.03a |
| Ser | 2.920 ± 0.02a | 3.101 ± 0.05a |
| Pro | 3.813 ± 0.01a | 3.659 ± 0.06a |
| Val | 3.698 ± 0.03a | 3.618 ± 0.01a |
| Thr | 4.371 ± 0.04a | 5.283 ± 0.05a |
| Ile | 3.341 ± 0.01a | 3.906 ± 0.03a |
| Leu | 5.394 ± 0.04a | 5.111 ± 0.06a |
| Asx | 12.158 ± 0.11a | 11.371 ± 0.09a |
| Lys | 4.765 ± 0.01a | 4.276 ± 0.07a |
| Met | 0.903 ± 0.01a | 0.788 ± 0.02a |
| Glx | 15.685 ± 0.43b | 14.061 ± 0.37a |
| Phe | 3.620 ± 0.05a | 3.335 ± 0.07a |
| His | 4.948 ± 0.03a | 4.723 ± 0.01a |
| Arg | 4.761 ± 0.09a | 4.863 ± 0.07a |
| Tyr | 1.967 ± 0.02a | 2.199 ± 0.01a |
| Trp | 0.677 ± 0.01a | 0.878 ± 0.02a |
| Cys | 1.101 ± 0.01a | 0.853 ± 0.01a |
Values represent mean ± deviation of replicate experiment.
Values with the same superscript alphabet are not significantly different (p < 0.05).
Where Asx = Asparagine + Aspartic acid and Glx = Glutamine + Glutamic acid.
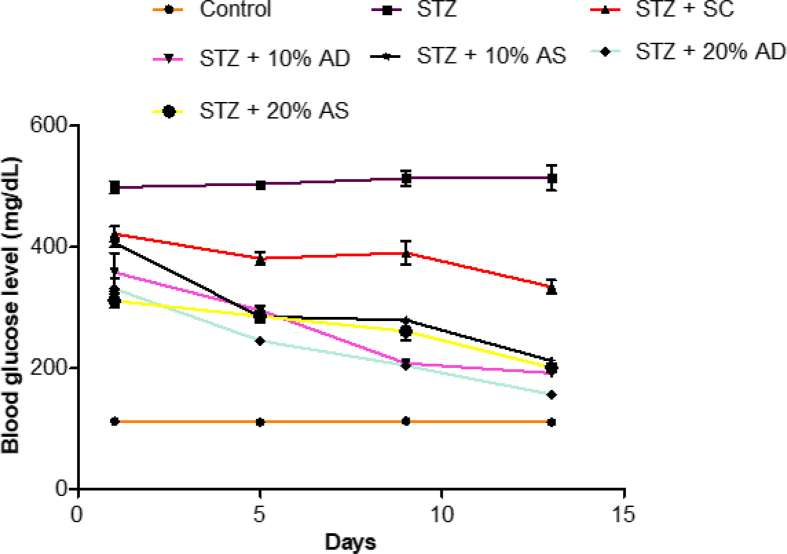
The blood glucose levels of diabetic (STZ-induced) rats showed a significant increase (p < 0.05) when compared with rats in the control group. Treatment with diets supplemented with almond drupe and seed (10 and 20% inclusion) resulted in a significant decrease in blood glucose level in comparison with diabetic rats fed with basal diet. Also, almond-supplemented diet reduced blood glucose level in diabetic rats than sildenafil citrate (Figure 1).
Figure 1.
Effect of Almond drupe (AD) and seed (AS) supplemented diets on blood glucose level in diabetic rats.
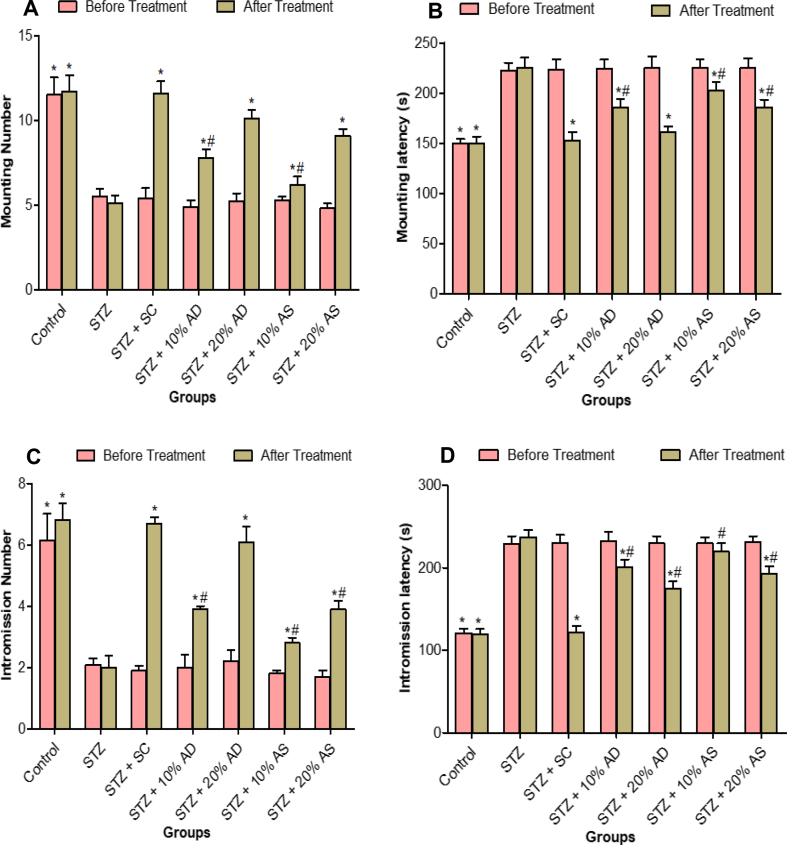
Sexual activity of all rats in different groups (except normal control) was observed to be almost the same immediately after induction of diabetes. However, noticeable changes were observed after 14 days of treatment with almond-supplemented diets. Sexual activities in diabetic rats reduced significantly (p < 0.05) when compared with rats in the normal control group. Almond-supplemented diets and sildenafil citrate increased sexual function in diabetic rats (Figure 2). The effect of sildenafil citrate on sexual function in diabetic rats is significantly higher than that of almond-supplemented diets. It is also important to note that almond drupe improve sexual functions (mounting and intromission numbers) compared to almond seed.
Figure 2.
Effect of Almond drupe (AD) and seed (AS) supplemented diets on sexual behaviour (A) mounting number, (B) mounting latency, (C) intromission number and (D) intromission latency in diabetic rats. Bars represent mean ± standard error of the mean (SEM) (n = 6). *p < 0.05 versus diabetic (STZ) group and #p < 0.05 versus sildenafil citrate (SC).
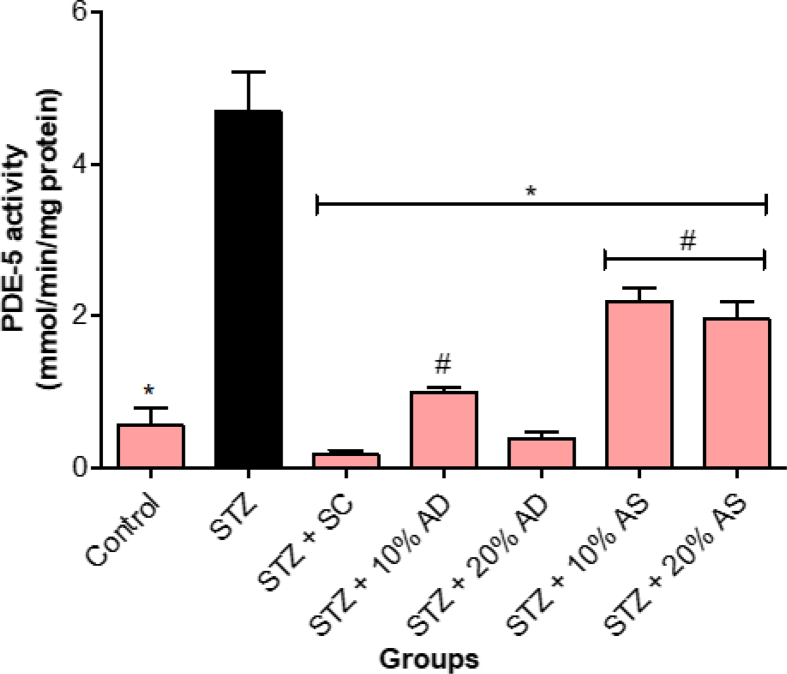
Figure 3 showed a significant increase (p < 0.05) in phosphodiesterase-5 activity in diabetic rats when compared with the control group. However, treatment with almond-supplemented diets and sildenafil citrate significantly lowered phosphodiesterase-5 activity in comparison with diabetic rats. Sildenafil citrate had a better effect on lowering phosphodiesterase-5 activity than almond-supplemented diets (Figure 3).
Figure 3.
Effect of Almond drupe (AD) and seed (AS) supplemented diets on phosphodiesterase-5 activity in STZ-induced diabetic rats. Bars represent mean ± standard error of the mean (SEM) (n = 6). *p < 0.05 versus diabetic (STZ) group and #p < 0.05 versus sildenafil citrate (SC).
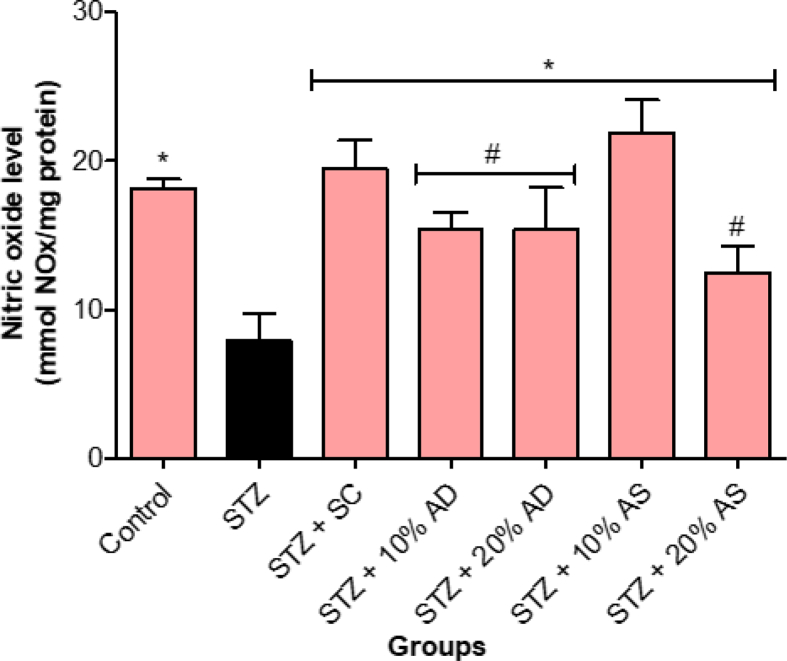
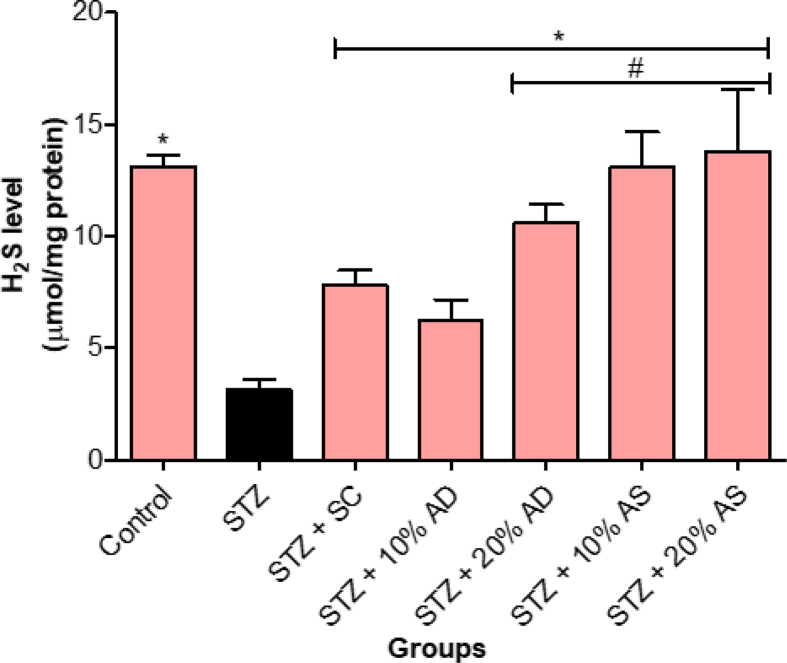
The effect of almond-supplemented diets and sildenafil citrate on the level of gasotransmitters responsible for the initiation of penile erection was assessed in diabetic rats and the results are presented in Figures 4 and 5. Nitric oxide level of diabetic rats decreased significantly (p < 0.05) in comparison with normal control rats. Diets supplemented with almond (drupe and seed) increased the nitric oxide level in diabetic rats (Figure 4). Similarly, the hydrogen sulfide level was significantly decreased in diabetic rats in comparison with normal control rats. However, treatment with almond-supplemented diets (drupe and seed) reversed the hydrogen sulfide level in diabetic rats (Figure 5).
Figure 4.
Effect of Almond drupe (AD) and seed (AS) supplemented diets on nitric oxide level in diabetic rats. Bars represent mean ± standard error of the mean (SEM) (n = 6). *p < 0.05 versus diabetic (STZ) group and #p < 0.05 versus sildenafil citrate (SC).
Figure 5.
Effect of Almond drupe (AD) and seed (AS) supplemented diets on hydrogen sulfide level in diabetic rats. Bars represent mean ± standard error of the mean (SEM) (n = 6). *p < 0.05 versus diabetic (STZ) group and #p < 0.05 versus sildenafil citrate (SC).
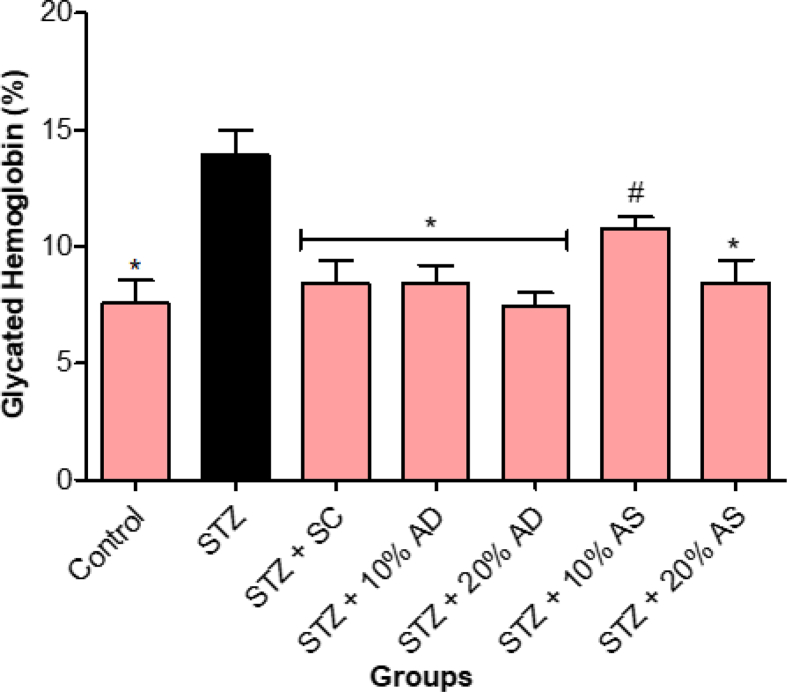
As shown in Figure 6, diabetes increased glycated hemoglobin level in comparison with normal control rats. However, treatment with diets supplemented with almond (drupe and seed) significantly (p < 0.05) reduced glycated hemoglobin level when compared with diabetic rats. Also, sildenafil citrate lowered glycated hemoglobin level in diabetic rats, but the effect was not statistically different from the group treated with almond-supplemented diets.
Figure 6.
Effect of Almond drupe (AD) and seed (AS) supplemented diets on glycated hemoglobin level in diabetic rats. Bars represent mean ± standard error of the mean (SEM) (n = 6). *p < 0.05 versus diabetic (STZ) group and #p < 0.05 versus sildenafil citrate (SC).
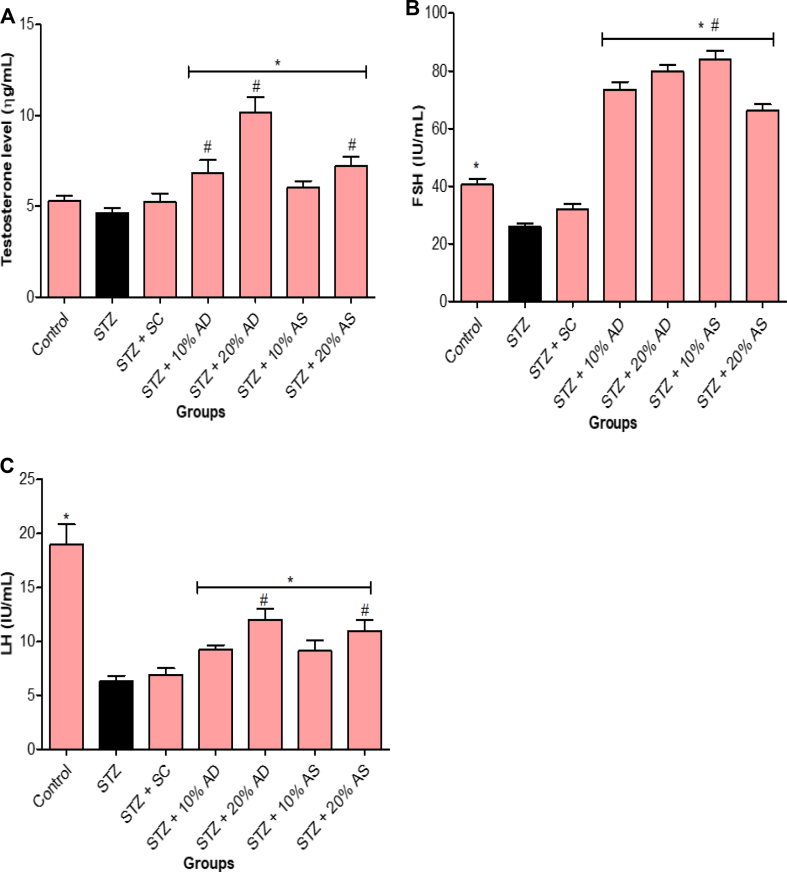
Furthermore, the results from this study showed a significant decrease in sexual hormones (follicle-stimulating hormone and luteinizing hormone) levels except for testosterone that is not significantly different in comparison with diabetic rats. However, treatment with diets supplemented with almond drupe and seed (10 and 20% inclusion) resulted in a significant increase in sexual hormone level (Figure 7). Treatment with sildenafil citrate did not affect the hormone (testosterone, follicle-stimulating hormone, and luteinizing hormone) levels when compared with diabetic rats.
Figure 7.
Effect of Almond drupe (AD) and seed (AS) supplemented diets on hormonal level (A) testosterone, (B) follicle stimulating hormone and (C) luteinizing hormone in diabetic rats. Bars represent mean ± standard error of the mean (SEM) (n = 6). *p < 0.05 versus diabetic (STZ) group and #p < 0.05 versus sildenafil citrate (SC).
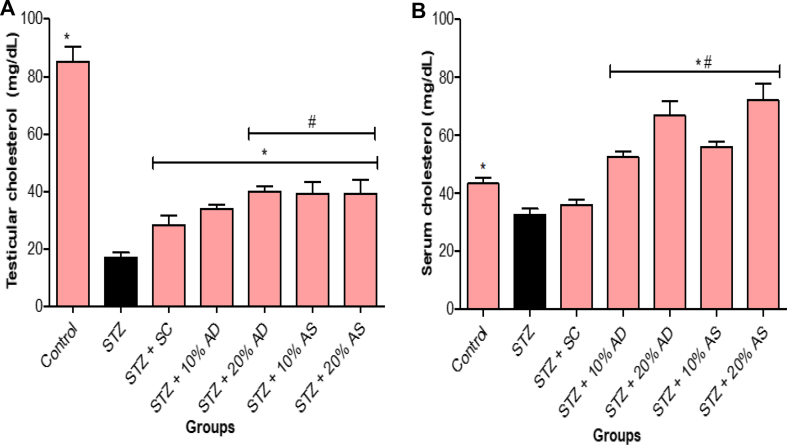
Diabetes caused a significant (p < 0.05) decrease in both serum and testicular cholesterol level when compared with the normal control group. However, dietary supplementation with almond drupe and seed (10 and 20% inclusion) significantly improve the cholesterol level in diabetic rats (Figure 8). Diabetic rats treated with sildenafil citrate showed a slight increase in cholesterol level, but not statistically different from diabetic rats.
Figure 8.
Effect of Almond drupe (AD) and seed (AS) supplemented diets on (A) testicular and (B) serum cholesterol levels in diabetic rats. Bars represent mean ± standard error of the mean (SEM) (n = 6). *p < 0.05 versus diabetic (STZ) group and #p < 0.05 versus sildenafil citrate (SC).
4. Discussion
There is an existing association between diabetes mellitus and the development of erectile dysfunction. This study proved efficacies of almond fruits supplemented diets as a dietary intervention in the management of erectile dysfunction induced by diabetes mellitus. Almond-supplemented diets decreased blood glucose significantly in diabetic rats.
The biological activities of plant and plant foods depend on their phytochemical components. The HPLC quantitative analysis of almond (drupe and seed) revealed an abundance of phenolic acids and flavonoids. The polyphenolics present in almond fruit include; gallic acid, catechin, chlorogenic acid, caffeic acid, ellagic acid, epicatechin, rutin, quercetin, isoquercitrin, quercitrin, and kaempferol. Polyphenolics such as caffeic acid, ellagic acid, gallic acid, quercetin, and isoquercitrin are well-known antioxidants. Besides, ellagic acid, rutin, and quercetin have been reported for their aphrodisiac properties (Goswami et al., 2014; Adefegha et al., 2018). Almond fruits are rich sources of amino acids like glutamine, arginine, tryptophan, tyrosine, and phenylalanine that have been reported to be involved in effective sexual activities of animals (Kayode and Yakubu, 2017). Glutamine and inositol combined to form gamma-aminobutyric acid (GABA), which facilitates orgasm and inhibit excitatory neurotransmitters that cause loss of sexual urge and consequently sexual dysfunction (Rowland et al., 2010). Besides, arginine enhances blood flow throughout the body including penile tissue and it is a precursor for nitric oxide (NO). NO elevates arterial elastic properties, lowers blood pressure and improves erection and sexual performance (Vlachopoulos et al., 2008; Suzuki et al., 2019). Hydroxylation of phenylalanine gives L-tyrosine, a precursor for L-3,4-dihydroxyphenylalanine, dopamine, norepinephrine, and epinephrine, compounds that enhance libido (Kayode and Yakubu, 2017). Amino acids like glycine, glutamic acid and cysteine are precursors of glutathione, a compound that shields cells against oxidative damage which give rise to many diseases including diabetes and its complication such as erectile dysfunction (Sekhar et al., 2011).
Androgen has been reported to play a crucial role in the proper functioning of the entire male sexual activity (Chistiakov et al., 2018; Rastrelli et al., 2018). The gonadotropin-releasing hormone secreted by neurons in the hypothalamus stimulates the release of follicle-stimulating hormone and luteinizing hormone into the circulation (Seal et al., 2000; Ciechanowska et al., 2018). Luteinizing hormone synthesized and released testosterone, the main hormone that acts on androgen receptors to enhance the components of sexual behavior (Corona et al., 2016a, Corona et al., 2016b). Findings in this study revealed that diabetes caused a significant decrease in sexual hormones (testosterone, follicle-stimulating hormone, and luteinizing hormone). However, a decreased level of hormone observed in this study could be associated with a reduced level of testicular cholesterol, a precursor for the synthesis of sexual hormones (Abarikwu and Farombi, 2014). Treatment with diets supplemented with almond fruits (drupe and seed) increased the testosterone level in diabetic rats possibly through improved cholesterol level. Testosterone synchronizes sexual activity by acting both centrally and peripherally to modulate libido and regulate the synthesis and release of nitric oxide synthase (Hull et al., 2007; Chistiakov et al., 2018). The tenacity of almond-supplemented diets to restore the levels of reproductive hormones (testosterone, follicle-stimulating hormone, and luteinizing hormone) may partly account for the enhanced sexual activities exhibited by sexually compromised diabetic rats. Besides the enhanced testosterone level observed in this study, there was a concomitant increase in the level of gonadotropins and this could be a direct impact of almond-supplemented diets on the pituitary gland giving rise to the release of both follicle-stimulating hormone and luteinizing hormone (Ciechanowska et al., 2018).
Nitric oxide, a potent vasodilator, is known to play important physiological roles that include stimulation of sexual activities in animals via the mediation of penile erection (Vincent et al., 2003; Tropea et al., 2018). Findings in this investigation revealed that there was a significant reduction in the level of nitric oxide in diabetic rats when compared with the control group. The reduced level of NO in diabetic rats could be as a result of hyperglycemia which has been reported to generate reactive oxygen species (ROS) (Yaribeygi et al., 2019; Volpe et al., 2018). The ROS generated are capable of reacting with NO to form peroxynitrite (ONOO−) which reduce the concentration and bioavailability of NO. However, treatment with almond-supplemented diets increased NO level which could be attributed to the antioxidant activities and L-arginine content of almond fruits. This is in agreement with the previous report that consumption of polyphenol-rich diets is associated with reduced risk of vascular diseases possibly due to their positive role on normal endothelial function (Abarikwu and Farombi, 2014; Olabiyi et al., 2017). In addition, we examined the effect of almond-supplemented diets on H2S level in diabetic rats. Besides the possible role of H2S in mediating penile erection (Tang et al., 2006; Qiu et al., 2012), it also scavenge reactive oxygen species and enhance endogenous antioxidant system (Whiteman et al., 2004; Yan et al., 2006). Findings in this present study revealed that almond-supplemented diet increased H2S level in diabetic rats, suggesting the possible role of H2S in activating antioxidant defense mechanism in the vascular tissues of diabetic rats.
In diabetes, hyperglycemia is well-known to be a major culprit that responsible for almost complications associated with diabetes (Ceriello et al., 2016; Rhee and Kim, 2018; Volpe et al., 2018). As a transporter of oxygen, red blood cells are prone to increased risk of oxidative damage. In this investigation, we observed a redox-disturbance as evident by a significant increase in glycated hemoglobin level in diabetic rats when compared with the control group. This agrees with previous findings (Ramkumar et al., 2014; Sureka et al., 2015; Rhee and Kim, 2018). However, daily intake of almond-supplemented diets confers a protective effect against oxidative damage in red blood cells of diabetic rats by lowering glycated hemoglobin level in diabetic rats. Since mature red blood cells rely on already synthesized proteins, dietary supplementation with antioxidants and samples rich in amino acids could be of importance in maintaining red blood cells structure and functionality (Reeds, 2000). Improved antioxidant status has been reported to be associated with modulation of key biochemical indices in diabetics, such as blood glucose level and glycated hemoglobin. Glycation in diabetics is not limited to hemoglobin alone but also affect other red blood cells and serum proteins, which give rise to accumulation of advanced glycated end-products (AGEs). AGEs negatively affect protein properties structurally and functionally, and ultimately the development of diabetic complications such as erectile dysfunction (Cantero et al., 2007; Forbes and Cooper, 2013).
The significant role of the NO/cGMP signaling pathway in penile erection has been reported (Angulo et al., 2010; Olabiyi et al., 2017). During sexual stimulation, nitric oxide produced from either nNOS or eNOS, or both diffused into the corpora cavernosal of the penile tissue where it activates soluble guanylate cyclase, an enzyme that converts guanosine triphosphate (GTP) to cyclic guanosine monophosphate (cGMP). cGMP is a secondary intracellular messenger that facilitates relaxation of penile smooth muscles, which in turn leads to penile erection (Angulo et al., 2010). cGMP level and concentration in the biological system is controlled by phosphodiesterase-5 (PDE-5), an enzyme that catalyzes the hydrolysis of cGMP. Most approaches available for erectile dysfunction management are PDE-5 inhibitors e.g. sildenafil, tadalafil, etc. Unfortunately, most of these synthetic drugs are expensive and have some associated adverse effects (Corona et al., 2016a, Corona et al., 2016b). However, the use of plant or plant foods has been reported useful in the management of erectile dysfunction and are considered as an alternative to the synthetic drugs because they are cheap, readily available and have little or no side effect (Olabiyi et al., 2017). Findings in this study revealed the elevated activity of PDE-5 in diabetic rats, which was ameliorated by the intake of a diet supplemented with almond fruits. Inhibition of PDE-5 is paramount to erectile dysfunction management as it makes cGMP available for smooth muscle relaxation (Angulo et al., 2010; Olabiyi et al., 2017).
In this study, we examined the influence of dietary supplementation of almond on sexual behavioral activities in diabetic rats. It is interesting to note that the sexual activity of all rats in different groups (except normal control) was observed to be almost the same immediately after the induction of diabetes. However, there was a noticeable improvement in sexual activities following treatment with almond-supplemented diets. Almond-supplemented diets significantly improve sexual activities in diabetic rats as revealed by increased mounting and intromission number with a simultaneous decrease in mounting and intromission latencies. Impaired sexual activities observed in diabetic rats could be due to reduced level of gaseous neurotransmitters (NO and H2S) and hormones (testosterone) and increased activity of PDE-5 and level of advanced glycated end-products (glycated hemoglobin) that have been reported to induce vascular damage and dysfunction (Forbes and Cooper, 2013; Yaribeygi et al., 2019). Improved sexual activities after treatment with almond-supplemented diets could be due to presence of phenolic compounds (such as ellagic acid, rutin and quercetin) and amino acids (such as arginine, phenylalanine and glutamic acid) that have been reported to directly or indirectly take part in stimulation of sexual activities (Goswami et al., 2014; Olabiyi et al., 2017; Adefegha et al., 2018). The enhanced sexual activities observed in this study could be related in part to the increased levels of some pituitary hormones that stimulate the synthesis of dopamine receptor and sexual behavior (Nchegang et al., 2016; Lonstein et al., 2019).
5. Conclusion
Findings in this study revealed that almond-supplemented diets modulate activities and levels of some important biomarkers relevant to erection in diabetic rats. Thus, dietary inclusion of almond (drupe and seeds) could serve as a cheap and readily available nutraceutical in the management of erectile dysfunction associated with diabetes.
Declarations
Author contribution statement
A. Adebayo: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
G. Oboh: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
A. Ademosun: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Abarikwu S.O., Farombi E.O. Quercetin ameliorates atrazine-induced changes in testicular function of rats. Toxicol. Ind. Health. 2014;32(7):1278–1285. doi: 10.1177/0748233714555389. [DOI] [PubMed] [Google Scholar]
- Adefegha S.A., Oboh G., Oyeleye S.I., Ejakpovi I. Erectogenic, antihypertensive, antidiabetic, anti-oxidative properties and phenolic compositions of almond fruit (Terminalia catappa L.) parts (hull and drupe)–in vitro. J. Food Biochem. 2017;41(2) [Google Scholar]
- Adefegha S.A., Oyeleye S.I., Dada F.A., Olasehinde T.A., Oboh G. Modulatory effect of quercetin and its glycosylated form on key enzymes and antioxidant status in rats penile tissue of paroxetine-induced erectile dysfunction. Biomed. Pharmacother. 2018;107:1473–1479. doi: 10.1016/j.biopha.2018.08.128. [DOI] [PubMed] [Google Scholar]
- Ademosun A.O., Adebayo A.A., Oboh G. Anogeissus leiocarpus attenuates paroxetine-induced erectile dysfunction in male rats via enhanced sexual behavior, nitric oxide level and antioxidant status. Biomed. Pharmacother. 2019;111:1029–1035. doi: 10.1016/j.biopha.2019.01.022. [DOI] [PubMed] [Google Scholar]
- Akinyemi A.J., Ademiluyi A.O., Oboh G. Inhibition of angiotensin-1-converting enzyme activity by two varieties of ginger (Zingiber officinale) in rats fed a high cholesterol diet. J. Med. Food. 2014;17:317–323. doi: 10.1089/jmf.2012.0264. [DOI] [PubMed] [Google Scholar]
- Alissa E.M., Ferns G.A. Functional foods and nutraceuticals in the primary prevention of cardiovascular diseases. J. Nutr. Metab. 2012;10:2012. doi: 10.1155/2012/569486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angulo J., González-Corrochano R., Cuevas P., Fernández A., Fuente J.M., Rolo F., Allona A., Sáenz de Tejada I. Diabetes exacerbates the functional deficiency of NO/cGMP pathway associated with erectile dysfunction in human corpus cavernosum and penile arteries. J. Sex. Med. 2010;7(2pt1):758–768. doi: 10.1111/j.1743-6109.2009.01587.x. [DOI] [PubMed] [Google Scholar]
- Basta G., Schmidt A.M., De Caterina R. Advanced glycation end products and vascular inflammation: implications for accelerated atherosclerosis in diabetes. Cardiovasc. Res. 2004;63(4):582–592. doi: 10.1016/j.cardiores.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Bella A.J., DeYoung L.X., Al-Numi M., Brock G.B. Daily administration of phosphodiesterase type 5 inhibitors for urological and nonurological indications. Eur. Urol. 2007;52(4):990–1005. doi: 10.1016/j.eururo.2007.06.048. [DOI] [PubMed] [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cameron N.E., Cotter M.A. Erectile dysfunction and diabetes mellitus: mechanistic considerations from studies in experimental models. Curr. Diabetes Rev. 2007;3(3):149–158. doi: 10.2174/157339907781368977. [DOI] [PubMed] [Google Scholar]
- Cantero A.V., Portero-Otín M., Ayala V., Auge N., Sanson M., Elbaz M., Thiers J.C., Pamplona R., Salvayre R., Nègre-Salvayre A. Methylglyoxal induces advanced glycation end product (AGEs) formation and dysfunction of PDGF receptor-β: implications for diabetic atherosclerosis. FASEB J. 2007;21(12):3096–3106. doi: 10.1096/fj.06-7536com. [DOI] [PubMed] [Google Scholar]
- Capellini K.V., Celotto C.A., Baldo F.C., Olivon C.V., Viaro F., Rodrigues J.A., Evora R.B.P. Diabetes and vascular disease: basic concepts of nitric oxide physiology, endothelial dysfunction, oxidative stress and therapeutic possibilities. Curr. Vasc. Pharmacol. 2010;8(4):526–544. doi: 10.2174/157016110791330834. [DOI] [PubMed] [Google Scholar]
- Ceriello A., Testa R., Genovese S. Clinical implications of oxidative stress and potential role of natural antioxidants in diabetic vascular complications. Nutr. Metab. Cardiovasc. Dis. 2016;26(4):285–292. doi: 10.1016/j.numecd.2016.01.006. [DOI] [PubMed] [Google Scholar]
- Chistiakov D.A., Myasoedova V.A., Melnichenko A.A., Grechko A.V., Orekhov A.N. Role of androgens in cardiovascular pathology. Vasc. Health Risk Manag. 2018;14:283. doi: 10.2147/VHRM.S173259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciechanowska M., Kowalczyk M., Lapot M., Malewski T., Antkowiak B., Brytan M., Winnicka I., Przekop F. Effects of corticotropin releasing hormone and corticotropin releasing hormone antagonist on biosynthesis of gonadotropin releasing hormone and gonadotropin releasing hormone receptor in the hypothalamic-pituitary unit of follicular-phase ewes and contribution of kisspeptin. J. Physiol. Pharmacol. 2018;69(3):451–461. doi: 10.26402/jpp.2018.3.13. [DOI] [PubMed] [Google Scholar]
- Corona G., Isidori A.M., Aversa A., Burnett A.L., Maggi M. Endocrinologic control of men’s sexual desire and arousal/erection. J. Sex. Med. 2016;13(3):317–337. doi: 10.1016/j.jsxm.2016.01.007. [DOI] [PubMed] [Google Scholar]
- Corona G., Rastrelli G., Burri A., Serra E., Gianfrilli D., Mannucci E., Jannini E.A., Maggi M. First-generation phosphodiesterase type 5 inhibitors dropout: a comprehensive review and meta-analysis. Andrology. 2016;4(6):1002–1009. doi: 10.1111/andr.12255. [DOI] [PubMed] [Google Scholar]
- Dal S., Sigrist S. The protective effect of antioxidants consumption on diabetes and vascular complications. Diseases. 2016;4(3):24. doi: 10.3390/diseases4030024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito K., Giugliano F., Maiorino M.I., Giugliano D. Dietary factors, Mediterranean diet and erectile dysfunction. J. Sex. Med. 2010;7(7):2338–2345. doi: 10.1111/j.1743-6109.2010.01842.x. [DOI] [PubMed] [Google Scholar]
- Forbes J.M., Cooper M.E. Mechanisms of diabetic complications. Physiol. Rev. 2013;93(1):137–188. doi: 10.1152/physrev.00045.2011. [DOI] [PubMed] [Google Scholar]
- Foresta C., Caretta N., Zuccarello D., Poletti A., Biagioli A., Caretti L., Galan A. Expression of the PDE5 enzyme on human retinal tissue: new aspects of PDE5 inhibitors ocular side effects. Eye. 2008;22(1):144. doi: 10.1038/sj.eye.6702908. [DOI] [PubMed] [Google Scholar]
- Goswami S.K., Vishwanath M., Gangadarappa S.K., Razdan R., Inamdar M.N. Efficacy of ellagic acid and sildenafil in diabetes-induced sexual dysfunction. Pharmacogn. Mag. 2014;10(3):S581. doi: 10.4103/0973-1296.139790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guohua H., Yanhua L., Rengang M., Dongzhi W., Zhengzhi M., Hua Z. Aphrodisiac properties of Allium tuberosum seeds extract. J. Ethnopharmacol. 2009;122:579–582. doi: 10.1016/j.jep.2009.01.018. [DOI] [PubMed] [Google Scholar]
- Hull E.M., Dominguez J.M., Muschamp J.W. Neurochemistry of male sexual behavior. Handb. Neurochem. Mol. Neurobiol.: Behav. Neurochem. Neuroendocrinol. Mol. Neurobiol. 2007;2007:37–94. [Google Scholar]
- Jakuš V., Rietbrock N. Advanced glycation end-products and the progress of diabetic vascular complications. Physiol. Res. 2004;53(2):131–142. [PubMed] [Google Scholar]
- Johnston C. Functional foods as modifiers of cardiovascular disease. Am. J. Lifestyle Med. 2009;3(1):39S–43S. doi: 10.1177/1559827609332320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayode O.T., Yakubu M.T. Parquetina nigrescens leaves: chemical profile and infl uence on the physical and biochemical indices of sexual activity of male Wistar rats. J. Integr. Med. 2017;15(1):64–76. doi: 10.1016/S2095-4964(17)60318-2. [DOI] [PubMed] [Google Scholar]
- Kizub I.V., Klymenko K.I., Soloviev A.I. Protein kinase C in enhanced vascular tone in diabetes mellitus. Int. J. Cardiol. 2014;174(2):230–242. doi: 10.1016/j.ijcard.2014.04.117. [DOI] [PubMed] [Google Scholar]
- Krentz A.J., Clough G., Byrne C.D. Interactions between microvascular and macrovascular disease in diabetes: pathophysiology and therapeutic implications. Diabetes Obes. Metab. 2007;9(6):781–791. doi: 10.1111/j.1463-1326.2007.00670.x. [DOI] [PubMed] [Google Scholar]
- Liu R.H. Dietary bioactive compounds and their health implications. J. Food Sci. 2013;78(S1):A18–25. doi: 10.1111/1750-3841.12101. [DOI] [PubMed] [Google Scholar]
- Lonstein J.S., Linning-Duffy K., Yan L. Low daytime light intensity disrupts male copulatory behavior, and upregulates medial preoptic area steroid hormone and dopamine receptor expression, in a diurnal rodent model of Seasonal Affective Disorder. Front. Behav. Neurosci. 2019;2019:13. doi: 10.3389/fnbeh.2019.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon C.G. Erectile dysfunction. Intern. Med. J. 2014;44(1):18–26. doi: 10.1111/imj.12325. [DOI] [PubMed] [Google Scholar]
- Miranda K.M., Espay M.G., Wink D.A. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide: Biol. Chem. 2001;5:62–71. doi: 10.1006/niox.2000.0319. [DOI] [PubMed] [Google Scholar]
- Nayak S.S., Pattabiraman T.N. A new colorimetric method for the estimation of glycosylated hemoglobin. Clin. Chim. Acta. 1981;109:267–274. doi: 10.1016/0009-8981(81)90312-0. [DOI] [PubMed] [Google Scholar]
- Nchegang B., Mezui C., Longo F., Nkwengoua Z.E., Amang A.P., Tan P.V. Effects of the aqueous extract of Eremomastax speciosa (Acanthaceae) on sexual behavior in normal male rats. BioMed Res. Int. 2016;2016 doi: 10.1155/2016/9706429. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oboh G., Adebayo A.A., Ademosun A.O., Boligon A.A. In-vitro Inhibition of phosphodiesterase-5 and arginase activities in rat penile tissue by two Nigerianherbs (Hunteria umbellata and Anogeissus leiocarpus) J. Basic Clin. Physiol. Pharmacol. 2017;28:393–401. doi: 10.1515/jbcpp-2016-0143. [DOI] [PubMed] [Google Scholar]
- Olabiyi A.A., Oboh G., Akinyemi A.J., Ademiluyi A.O., Boligon A.A., Anraku de Campos M.M. Tiger nut (Cyperus esculentus L.) supplemented diet modulate key biochemical indices relevant to erectile function in male rats. J. Funct. Foods. 2017;34:152–158. [Google Scholar]
- Oyeleye S.I., Adebayo A.A., Ogunsuyi O.B., Dada F.A., Oboh G. Phenolic profile and enzyme inhibitory activities of almond (Terminalia catappa) leaf and stem bark. Int. J. Food Prop. 2017;20(3):S2810–S2821. [Google Scholar]
- Padiya R., Chowdhury D., Borkar R., Srinivas R., Pal Bhadra M. Garlic attenuates cardiac oxidative stress via activation of PI3K/AKT/Nrf2-Keap1 pathway in fructose-fed diabetic rat. PLoS One. 2014;9(5) doi: 10.1371/journal.pone.0094228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinelo M., Rubilar M., Sineiro J., Nunez M.J. Phenolics from almond hulls (prunus amygdalus) and pine sawdust (pinus pinaster) Food Chem. 2004;85:267–273. [Google Scholar]
- Pop-Busui R., Sima A., Stevens M. Diabetic neuropathy and oxidative stress. Diabetes Metabol. Res. Rev. 2006;22(4):257–273. doi: 10.1002/dmrr.625. [DOI] [PubMed] [Google Scholar]
- Qiu X., Villalta J., Lin G., Lue T.F. Role of hydrogen sulfide in the physiology of penile erection. J. Androl. 2012;33:529–535. doi: 10.2164/jandrol.111.014936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy R., Vannucci S.J., Yan S.S., Herold K., Yan S.F., Schmidt A.M. Advanced glycation end products and RAGE: a common thread in aging, diabetes, neurodegeneration, and inflammation. Glycobiology. 2005;15(7):16R–28R. doi: 10.1093/glycob/cwi053. [DOI] [PubMed] [Google Scholar]
- Ramkumar K.M., Vijayakumar R.S., Vanitha P., Suganya N., Manjula C., Rajaguru P., Sivasubramanian S., Gunasekaran P. Protective effect of gallic acid on alloxan-induced oxidative stress and osmotic fragility in rats. Hum. Exp. Toxicol. 2014;33:638–649. doi: 10.1177/0960327113504792. [DOI] [PubMed] [Google Scholar]
- Rastrelli G., Corona G., Maggi M. Testosterone and sexual function in men. Maturitas. 2018;112:46–52. doi: 10.1016/j.maturitas.2018.04.004. [DOI] [PubMed] [Google Scholar]
- Reeds P.J. Dispensable and indispensable amino acids for humans. J. Nutr. 2000;130(7) doi: 10.1093/jn/130.7.1835S. 1835S-40S. [DOI] [PubMed] [Google Scholar]
- Rhee S.Y., Kim Y.S. The role of advanced glycation end products in diabetic vascular complications. Diabetes Metabol. J. 2018;42(3):188–195. doi: 10.4093/dmj.2017.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland D., McMahon C.G., Abdo C., Chen J., Jannini E., Waldinger M.D., Ahn T.Y. Disorders of orgasm and ejaculation in men. J. Sex. Med. 2010;7(4):1668–1686. doi: 10.1111/j.1743-6109.2010.01782.x. [DOI] [PubMed] [Google Scholar]
- Schalkwijk C. Vascular complications in diabetes mellitus: the role of endothelial dysfunction. Clin. Sci. 2005;109(2):143–159. doi: 10.1042/CS20050025. [DOI] [PubMed] [Google Scholar]
- Schroeter H., Heiss C., Balzer J., Kleinbongard P., Keen C.L., Hollenberg N.K., Sies H., Kwik-Uribe C., Schmitz H.H., Kelm M. (–)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc. Natl. Acad. Sci. 2006;103(4):1024–1029. doi: 10.1073/pnas.0510168103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwingshackl L., Hoffmann G. Mediterranean dietary pattern, inflammation and endothelial function: a systematic review and meta-analysis of intervention trials. Nutr. Metab. Cardiovasc. Dis. 2014;24(9):929–939. doi: 10.1016/j.numecd.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Seal L.J., Small C.J., Kim M.S., Stanley S.A., Taheri S., Ghatei M.A., Bloom S.R. Prolactin releasing peptide (PrRP) stimulates luteinizing hormone (LH) and follicle stimulating hormone (FSH) via a hypothalamic mechanism in male rats. Endocrinology. 2000;141(5):1909–1912. doi: 10.1210/endo.141.5.7528. [DOI] [PubMed] [Google Scholar]
- Sekhar R.V., Patel S.G., Guthikonda A.P., Reid M., Balasubramanyam A., Taffet G.E., Jahoor F. Deficient synthesis of glutathione underlies oxidative stress in aging and can be corrected by dietary cysteine and glycine supplementation–. Am. J. Clin. Nutr. 2011;94(3):847–853. doi: 10.3945/ajcn.110.003483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V.P., Bali A., Singh N., Jaggi A.S. Advanced glycation end products and diabetic complications. KOREAN J. PHYSIOL. PHARMACOL. 2014;18(1):1–4. doi: 10.4196/kjpp.2014.18.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subashinee S.K.W., Siriwardhana S.F. Anti-radical activity of extracts of almond and its by-products. J. Am. Oil Chem. Soc. 2002;79:903–908. [Google Scholar]
- Sureka C., Ramesh T., Begum V.H. Attenuation of erythrocyte membrane oxidative stress by Sesbania grandiflora in streptozotocin-induced diabetic rats. Biochem. Cell Biol. 2015;93:385–395. doi: 10.1139/bcb-2015-0039. [DOI] [PubMed] [Google Scholar]
- Suzuki I., Sakuraba K., Horiike T., Kishi T., Yabe J., Suzuki T., Morita M., Nishimura A., Suzuki Y.A. Combination of oral L-citrulline and L-arginine improved 10-min full-power cycling test performance in male collegiate soccer players: a randomized crossover trial. Eur. J. Appl. Physiol. 2019;119(5):1075–1084. doi: 10.1007/s00421-019-04097-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C., Li X., Du J. Hydrogen sulfide as a new endogenous gaseous transmitter in the cardiovascular system. Curr. Vasc. Pharmacol. 2006;4:17–22. doi: 10.2174/157016106775203144. [DOI] [PubMed] [Google Scholar]
- Tropea T., Wareing M., Greenwood S.L., Feelisch M., Sibley C.P., Cottrell E.C. Nitrite mediated vasorelaxation in human chorionic plate vessels is enhanced by hypoxia and dependent on the NO-sGC-cGMP pathway. Nitric Oxide. 2018;80:82–88. doi: 10.1016/j.niox.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent M.A., Montagnani M., Quon M.J. Molecular and physiologic actions of insulin related to production of nitric oxide in vascular endothelium. Curr. Diabetes Rep. 2003;3(4):279–288. doi: 10.1007/s11892-003-0018-9. [DOI] [PubMed] [Google Scholar]
- Vlachopoulos C., Aznaouridis K., Ioakeimidis N., Rokkas K., Tsekoura D., Vasiliadou C., Stefanadi E., Askitis A., Stefanadis C. Arterial function and intima-media thickness in hypertensive patients with erectile dysfunction. J. Hypertens. 2008;26(9):1829–1836. doi: 10.1097/HJH.0b013e3283050886. [DOI] [PubMed] [Google Scholar]
- Volpe C.M., Villar-Delfino P.H., dos Anjos P.M., Nogueira-Machado J.A. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018;9(2):119. doi: 10.1038/s41419-017-0135-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahed A.S., Amer M.A., Mohamed N.M., Mobasher M.I., Mamdouh H., Din S.F., ElSheemy M.S. Serum Arginase II level can be a novel indicator for erectile dysfunction in patients with vasculogenic erectile dysfunction: a comparative study. Int. Urol. Nephrol. 2018;50(8):1389–1395. doi: 10.1007/s11255-018-1921-y. [DOI] [PubMed] [Google Scholar]
- Whiteman M., Armstrong J.S., Chu S.H., Jia-Ling S., Wong B.S., Cheung N.S., Halliwell B., Moore P.K. The novel neuromodulator hydrogen sulfide: an endogenous peroxynitrite ‘scavenger’? J. Neurochem. 2004;90(3):765–768. doi: 10.1111/j.1471-4159.2004.02617.x. [DOI] [PubMed] [Google Scholar]
- Yan S.K., Chang T., Wang H., Wu L., Wang R., Meng Q.H. Effects of hydrogen sulfide on homocysteine-induced oxidative stress in vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 2006;351(2):485–491. doi: 10.1016/j.bbrc.2006.10.058. [DOI] [PubMed] [Google Scholar]
- Yaribeygi H., Atkin S.L., Sahebkar A. A review of the molecular mechanisms of hyperglycemia-induced free radical generation leading to oxidative stress. J. Cell. Physiol. 2019;234(2):1300–1312. doi: 10.1002/jcp.27164. [DOI] [PubMed] [Google Scholar]