Abstract
Chagas disease (CD) is a tropical neglected illness, affecting mainly populations of low socioeconomic status in Latin America. An estimated 6 to 8 million people worldwide are infected with Trypanosoma cruzi, the etiological agent of CD. Despite being one of the main global health problems, this disease continues without effective treatment during the chronic phase of the infection. The limitation of therapeutic strategies has been one of the biggest challenges on the fight against CD. Nifurtimox and benznidazole, developed in the 1970s, are still the only commercial options with established efficacy on CD. However, the efficacy of these drugs have a proven efficacy only during early infection and the benefits in the chronic phase are questionable. Consequently, there is a growing need for new pharmacological alternatives, either by optimization of existing drugs or by the formulation of new compounds. In the present study, a literature review of the currently adopted therapy, its concomitant combination with other drugs, and potential future treatments for CD was performed, considering articles published from 2012. The revised articles were selected according to the protocol of treatment: evaluation of drug association, drug repositioning and research of new drugs. As a result of the present revision, it was possible to conclude that the use of benznidazole in combination with other compounds showed better results when compared with its use as a single therapy. The search of new drugs has been the strategy most used in pursuing more effective forms of treatment for CD. However, studies have still focused on basic research, that is, they are still in a pre-clinical stage, using methodologies based on in vitro or in animal studies.
Keywords: Chagas disease, Pharmacological treatment, Drugs association, Drugs repositioning, New drugs
Highlights
-
•
There is no effective treatment for chronic Chagas disease.
-
•
Association of benznidazole with other drugs is more efficient than benznidazole alone.
-
•
Nowadays, most researches focus on the development of new drugs to combat Chagas disease.
-
•
Current studies are still in a pre-clinical stage.
Chagas disease (CD) is an endemic anthropozoonosis caused by the hemoflagellate protozoan Trypanosoma cruzi and transmitted mainly by hematophagous triatomines. CD is the most common fatal infection in Central and South America affecting, in endemic countries, about eight million people and causing the death of approximately 50,000 individuals each year (WHO, 2018). T. cruzi acute infection is usually asymptomatic and tissue damage may be detected decades after the first contact (Pérez-Molina and Molina, 2018). The main clinical alteration during the chronic stage are electrocardiographic abnormalities followed by contraction alterations that can lead to heart failure and, in many cases, to sudden death (Bocchi, 2017). CD cardiopathy results in progressive inability to continue working with a consequent overload of the country health system (Da Nóbrega et al., 2014).
No satisfactory treatment exists for CD, especially for the chronic stage of the disease (Morillo et al., 2015). The high costs associated with research and development of new drugs, coupled with the usually low financial return, results in the absence of new medicines. There is, consequently, an urgent need for novel alternatives and effective treatments for this disease. Several lines of research are presently being developed aiming to this objective, either trying to improve existing therapy or focusing in the development of new drugs. These topics will be reviewed in the present work that also intends to highlight the current perspectives on new approaches to the therapy of CD.
1. Available medicines for Chagas disease
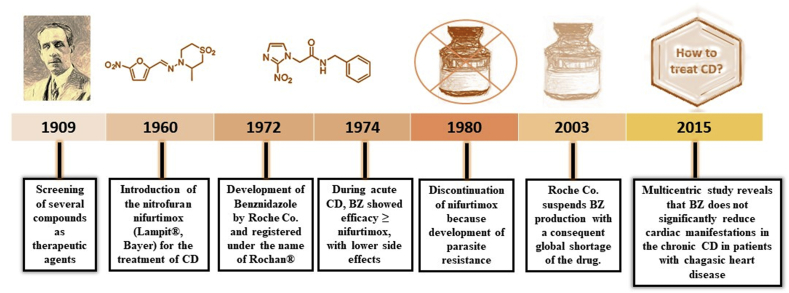
After the first description of the disease, several compounds were tried as therapeutic agents (Fig. 1), such as arsenic, fuchsin, emetic tartrate and mercury chloride (Coura and Castro, 2002; Dias et al., 2009). However, all failed to produce satisfactory results. The antiseptic gentian violet was also used in the past, but it is currently used exclusively in blood banks as a prophylactic agent (Coura and Dias., 2009; Coura and Castro., 2002).
Fig. 1.
Timeline showing the history of Chagas disease treatment.
Since the 1970's, several new compounds were introduced for the treatment of CD. Among them, the antimicrobial nitrofurans, of which the nitrofurfurylidene, known as nifurtimox ((RS)-3-methyl-N-[(1E)-(5-nitro-2-furyl)methylene] thiomorpholin-4-amine 1,1-dioxide) (NF) and produced by the Bayer company under the trade name Lampit®, showed an improved effectiveness. The mechanism of action of this drug is not completely elucidated. Initially, NF was believed to act by oxidative stress, generating free radicals (Sales Junior et al., 2017). However, some studies have showed that its activity depends on a type 1 trypanosomal nitroreductase (NTR), refuting the oxidative stress as the determining factor (Hall et al., 2011; Boiani et al., 2010).
Because of its high toxicity, NF was progressively discontinued and its commercialization was suspended in Brazil, Argentina, Chile and Uruguay (Coura and Castro, 2002) from the 1980's. However, in these countries NF is retained as an option when treatment with BNZ fails, requiring authorization from PAHO or WHO for its use (Dias et al., 2016). Of note, resistance to nitroheterocyclic compounds have been reported (Mejia et al., 2012; Wilkinson et al., 2009), which seems to be associated with the loss of a single copy of the TcNTR gene (Wilkinson et al., 2008). Trying to solve toxicity and resistance limitations, clinical studies have been conducted to change the dose of NF tablet without losing effectiveness reviewed by Sales Junior et al., 2017.
Currently, the only drug available in most Latin American countries is benznidazole (BNZ). Initially produced by the pharmaceutical company Roche (Rochagan® and Radanil®), BNZ is now exclusively manufactured by the Pharmaceutical Laboratory of the State of Pernambuco (Lafepe), Brazil, and by the private laboratory Elea (Abarax®), Argentina. BNZ is the N-benzyl-2-nitro-1-imidazoleacetamide molecule. Different mechanisms of action have been attributed to BNZ. For example, it is suggested that it may act by a reductive stress, involving covalent modifications in DNA, proteins and lipids (Sales Junior et al., 2017). Also, BNZ could be reduced by a type I nitroreductase (NTR) present in the parasite, followed by several reactions that cause the release of dialdehyde glyoxal that has trypanocide effect by forming adducts with guanosine bases in DNA and RNA (Kratz et al., 2018). Furthermore, BNZ may increase the phagocytosis and lysis of the parasite and inhibit its growth by the action of the enzyme fumarate reductase-NADH (Dias et al., 2009; Sobrinho et al., 2007).
Low benefit in the chronic phase of the disease, regional variations in efficacy and emergence of resistant strains are some limitations of the clinical use of BNZ (Sobrinho et al., 2009). In addition, it causes a number of side effects such as rash, epigastric pain pruritus, nausea, abdominal swelling and some severe manifestations as eosinophilia (Oliveira et al., 2017). Recently, the multicenter clinical trial “Benznidazole Evaluation for Interrupting Trypanosomiasis” (BENEFIT) demonstrated that the use of BNZ did not lead to clinical improvements in patients with established Chagas’ cardiomyopathy when compared to the placebo group, even those with New York Heart Association (NYHA) class I or II heart failure, despite a reduction in parasite load (Morillo et al., 2015).
2. Repositioning of therapeutic drugs
Repositioning of established pharmacotherapeutic agents with well-known activity and side effect profiles is considered an effective strategy for the development of new treatments for several diseases, especially for neglected disorders. This repositioning approach is advantageous in view of the cost and time-consuming process required compared to the development of new medicines, since the drugs used in repositioning already have their toxicological and pharmacokinetic profile assessed when used on their previous therapeutic target (Alberca et al., 2016).
Several compounds of different pharmacological classes have already been tested against the T. cruzi (Table 1). Among them, benidipine, a calcium channel blocker usually administered for the treatment of hypertension and angina, and the antibiotic clofazimine were tentatively used against the parasite since they act inhibiting cruzipain, the main lysosomal cysteine protease with an important role in parasite infectivity. The inhibition of cruzipain has been much explored, because it is the most abundant protease of the parasite. Cruzipain has been shown to be crucial for all stage of the parasite life cycle and is involved in the parasite nutrition, invasion of mammalian cells and evasion of the host immune response (Dias et al., 2009; Rogers et al., 2012; Salas-Sarduy et al., 2017). Thus, benidipine and clofazimine were considered promising candidates for the treatment of the disease in view of their capacity to reduce the parasitic burden in the blood and skeletal tissues of infected mice, additionally curtailing the inflammatory effects of the infection. The use of these drugs in combination with current adopted therapy should be further evaluated (Sbaraglini et al., 2016).
Table 1.
Repositioning of therapeutic drugs for Chagas disease treatment.
| References | Repositioning of therapeutic drug/original indication | Culture type/animal type | Main Results |
|---|---|---|---|
| Buckner et al., 2012 | Derivatives of Tipifarnib (anti-cancer drug) | Swiss mice | Highly potent against the parasite, but with lesser cure than posaconazole. |
| Bahia et al. (2012) | Fexinidazole (a broad-spectrum antiprotozoal drug) | Swiss mice | Early treatment can reduce heart inflammation associated with the chronic CD. It is well tolerated by the animals, without the occurrence of adverse effects. |
| Apt et al. (2013) | Itraconazole (triazole antifungal agent) | Patients (human) | Possibility of itraconazole to cure the heart disease associated with T. cruzi, which may interrupt or delay the natural evolution of the disease. There were few adverse effects. |
| Fauro et al. (2013) | Clomipramine: tricyclic antidepressant | Albino Swiss mice | It reduced the parasite load, without eliminating the presence of the parasite in the host. There was a relevant reduction of lesions caused by the disease, such as fibrosis. |
| Bahia et al. (2014) | Fexinidazole metabolites (antiparasitic) | Murine macrophages, Swiss mice | The metabolites were effective in treating the disease superior to Fexinidazole and benznidazole, without inducing toxicity in the experimental models. |
| Molina et al. (2014) | Posaconazole (triazole antifungal) | Patients (human) | Posaconazole treatment was not effective, with high percentages of therapeutic failures. |
| Da Silva et al., 2015 | Auranofin (Antirheumatic) | Human fibroblast, Balb/c mice | The drug has trypanocidal activity higher than benznidazole, increasing the survival of treated animals without reducing parasitemia. However, the drug has reduced selectivity, presenting toxicity. |
| Sbaraglini et al. (2016) | Benidipine (Anti-Bacterial Agents) and clofazimine (Calcium Channel Blocker) | C3H/HeN mice | Reduced the parasite load and the resulting infection. |
| Vilar-Pereira et al.,. (2016) | Resveratrol (antioxidant/cardioprotective) | Balb/c mice | The compound is able to improve the cardiac integrity of infected animals, provide risk factors for cardiovascular mortality, restore ejection fraction, and edit the parasitic burden on the heart. |
| Alberca et al. (2016) | Polyamine analogs (organic compound) | ND* | Triclabendazole, sertaconazole and paroxetine displayed inhibitory effects on the proliferation of T. cruzi (epimastigotes) and the uptake of putrescine by the parasite. |
| Rettondin et al., 2016 | Gold (III) complexes with ONS-Tridentate thiosemicarbazones (Lligands) |
LLC-MK2 cells | The compounds had satisfactory trypanocidal activity comparable to that of benznidazole. The activity against the amastigote form was lower. |
| Valle-Reyes et al., 2016 | Nitazoxanide (Anti-Infective Agent Antiprotozoal) | Balb/c mice | The compound presents unsatisfactory results, with worsening of the infection, increased parasitemia and tissue parasitic load. |
| Rojas Márquez et al., 2017 | Rapamycin (Immune Suppressant) | Balb/c mice | Significant increase in NLRP3 expression. Production of higher levels of mitochondrial ROS (mtROS) compared to control cells. |
| Vasconcelo et al., 2017 | N,N dimethylsphingosin (Sphingosine kinase inhibitor) | C57BL/6 mice | Reduction of cardiac inflammation, fibrosis and galectin-3 expression, modulation of inflammatory mediators such as IFNγ and TNFα, improvement in exercise capacity, but without improving cardiac electrical disturbances. |
| Montenote et al., 2017 | M. nigra (Phytotherapeutic compound) | Swiss mice | Decreased parasitemia, and may be the subject of further research for a greater analysis of the mechanisms of action and bioavailability. |
| Horta et al., 2017 | Carvedilol (Adrenergic Blocker) | C57BL/6 mice | For this experimental model, carvedilol therapy was not able to alter levels of circulating parasites, but modulates the pattern of mediators of CCL2 and IL-10 when the VL10 strain of T. cruzi. |
| Reigada et al., 2017 | Isotretinoin (Antiacne) | CHO–K1 cells | Isotretinoin inhibited polyamine transport, also showed strong inhibition of the explanted trypomastigote of infected cells but being significantly less effective at the epimastigote stage. |
| Hernández et al. (2018) | Curcumin (cholagogue, anti-inflammatory) | Human microvascular endothelial cells, C57BL/6 mice | Reduced inflammatory cell infiltration without lowering parasitemia. |
| Ferreira et al. (2018) | Sertraline (Antidepressant) | In silico studies, Balb/c mice | Efficacy against intracellular amastigotes and bloodstream trypomastigotes, with low cytotoxicity to mammalian cells |
| Fracasso et al., 2018 | Resveratrol (antioxidant/cardioprotective) | Swiss mice | Can be use for the treatment of neuroinflammation or neuroprotection during Chagas disease, improving gliogenesis and restoring neural migration. |
| Simões-Silva et al. (2019) | Imatinib (Antineoplastic Agent Immunological Agent) | Mouse fibroblasts | The drug was moderately active against the parasite, with low selectivity index |
ND* not determined.
Other repositioning drugs potentially useful in the ancillary treatment of Chagas disease were clomipramine, sertraline and fexinidazole. Clomipramine, a tricyclic antidepressant, was able to increase the survival of infected mice, reducing parasitemia, although it was not capable of eliminate the T. cruzi. Its beneficial action was initially associated to the inhibition of trypanothione reductase (TR), a unique enzyme that interacts with Flavin-adenine dinucleotide (FAD) and reduces nicotinamide adenine dinucleotide phosphate (NADPH), which is essential to parasite survival (Fauro et al., 2013; Benson et al., 1992). However, this hypothesis diverges from studies showing that the trypanocide effect would only be achieved by almost total inhibition of the TR, what is not obtained with this drug (Krieger et al., 2002; Mendonça et al., 2018). Sertraline is also indicated for the treatment of depression and anxiety, and its use in CD in vitro experiments showed efficacy against intracellular and bloodstream forms, with low cytotoxicity to mammalian cells (Ferreira et al., 2018).
Fexinidazole is a promising broad spectrum antiparasitic agent with clinical trials already taking place. This compound has a nitro group in its composition that is metabolized by the parasite nitroreductases, forming reactive species that inhibits DNA synthesis (Bahia et al., 2012, 2014). Mice treated with fexinidazole at both phases of the infection, and accompanied by bioluminescence, showed a higher cure rate when compared to the groups treated with reference drugs, even after promoting immunosuppression after treatment (Francisco et al., 2016). Mice treated with fexinidazole at both phases of the infection, and accompanied by bioluminescence, showed a higher cure rate when compared to the groups treated with reference drugs, even after promoting immunosuppression after treatment (Francisco et al., 2016). Of interest, fexinidazole has been approved by the European Medianes Agency (EMA) for the treatment of African Trypanosomiasis, in both adults and children (Deeks, 2019), thus demonstrating its therapeutic potential to be applied to CD. Probably, dose and treatment time adjustments may be required for clinical trials (Watson et al., 2019).
Dietary supplements have also been evaluated in CD treatment. Resveratrol, considered a food supplement by health surveillance agencies, has antioxidant and cardioprotective properties, acting in the chronic phase of the disease by non-trypanocidal mechanisms (Vilar-Pereira et al., 2016). Furthermore, resveratrol acts as a neuroprotector during CD, improving gliogenesis and neural migration (Fracasso et al., 2019). Curcumin, a herbal supplement and a natural food coloring extracted from ginger, is a natural phenol that failed in reducing parasitemia, although lowering inflammatory leukocyte infiltration (Hernández et al., 2018).
Like curcumin, several repositioning attempts failed to achieve the expected results. For example, auronofin, a compound derived from gold, generally prescribed for the treatment of rheumatoid arthritis, showed ability to increase the survival of mice infected with the parasite without reducing parasitemia and with reduced selectivity (Da Silva et al., 2016). Nitazoxanide, a broad-spectrum antiparasitic drug that inhibits the parasitic intracellular enzyme pyruvate-ferredoxin oxidoreductase (PFOR) and blocks the ion channel of glutamate chloride, disclosed poor results against the T. cruzi worsening the infection in mice, increasing tissue damage in relation to untreated animals, and exhibiting an increased mortality. Consequently, this compound was considered highly unsafe (Valle-Reyes et al., 2017).
Other drugs comprised in the strategy of therapeutic repositioning belong to the class of sterol biosynthesis inhibitors that are components of the parasitic membranes and essential for the cellular growth (Dias et al., 2009). A 20-year follow-up study of patients treated during 120 days with itraconazole, a synthetic analogue derived from imidazole, showed the drug capacity to interrupt or delay the natural course of CD during the chronic phase, causing regression of some abnormalities on the electrocardiogram and showing few adverse effects (Apt et al., 2013). However, in this research, problems in the control group and poor sensitivity of the used tests to determine cure were observed; so further studies are needed to confirm the findings.
The prospective, randomized clinical trial of CHAGASAZOL evaluated the efficacy and safety of posaconazole, an antifungal that actively inhibits the synthesis of ergosterol, the main sterol of the parasite. Contrary to previous findings in vitro and in experimental models of Chagas disease, which posaconazole showed excellent activity, the drug failed to disclose the same expected efficacy in humans (Molina et al., 2014). The results in the human trial were recapitulated in a study using bioluminescent parasites in a mouse model, which confirmed the low efficacy of posaconazole in both acute and chronic phases of the disease (Francisco et al., 2015). The use of ravuconazole was evaluated in a phase 2 clinical trial in patients in undetermined chronic phase, without cardiac damage. Of interest, it was observed that the prodrug E1224 led to parasite clearance, but with transient response when using low- or short doses regimens (Torrico et al., 2018).
3. Drug association
The concomitant or sequential use of two or more pharmaceutical compounds represents an alternative that is also being explored in CD treatment studies (Table 2). Theoretically, the combination of different compounds allows the reduction of the doses and/or treatment length, resulting in reduction of drug adverse side effects and costs. Synergistic treatment generally improves compounds activity with distinct mechanisms of action, and additionally reduces the drug toxicity and the chance of developing resistance (Ferreira and Andricopulo, 2016; Andrews et al., 2014).
Table 2.
Drug association for Chagas disease treatment.
| References | Drug association/original indication | Culture type/animal type | Main Results |
|---|---|---|---|
| Da Silva et al. (2012) | Itraconazole (Antifungal) and Benznidazole (Antiprotozoal) | Swiss albino mice, | Itraconazole alters the pharmacokinetic profile of benznidazole with accumulation in vivo, which represents a benefit as it provides the necessary dose reduction. |
| Perez-Mazliah et al. (2012) | Allopurinol (Antigout) and Benznidazole (Antiprotozoal) | Patients (human) | The sequential treatment with the drugs was well tolerated by the patients and considered viable. Immunological changes were detected giving benefits to patients with a disease in the chronic phase. |
| Valdez et al., 2012 | C4 (new drug) and Benznidazole (Antiprotozoal) | LLCMK2 and Balb/c mice | The association with C4 provided a reduction in the dose of benznidazole, achieving the same results as the usual dose. |
| Cencig et al., 2012 | Benznidazole and Nifurtimox, Posaconazole or Amphotericin B (Antiprotozoal; Antifungal) | Balb/c mice | The association of benznidazole with other drugs, in short periods, can cause the elimination of the parasite, except the association with amphotericin B. The association with nifurtimox led to the behavioral alteration of treated animals |
| Veiga-Santos et al., 2012 | Amiodarone (Antiarrhythmic) and Posaconazole (Antifungal) | ND* | The compounds interfered in the growth of the epimastigote form, in a dose-dependent manner. In all evolutionary forms, there are structural alterations in the parasite, with the formation of structures that indicate autophagy. |
| Diniz et al. (2013) | Benznidazole (Antiprotozoal) and Posaconazole (Antifungal) | Swiss mice | They presented synergism when administered together, being able to reduce the parasitemia to values inferior to those obtained with administration of the drugs alone. |
| Strauss et al. (2013) | Clomipramine (Antidepressant,Tricyclic) and Benznidazole (Antiprotozoal) | Swiss mice | Prevented cardiac conduction abnormalities in animals. In addition, the association prevented the formation of perivascular necrosis and inflammation in the liver |
| Martins et al. (2015) | Benznidazole (Antiprotozoal) and Itraconazole (Antifungal) | Swiss mice | The association of drugs led to less need for days of treatment, and reduced tissue damage caused by the disease in treated animals. |
| Herrera et al., 2017 | Simvastatin (Antihyperlipidemic) | Balb/c and sV/129 | In the murine model the association increases BNZ activity. Since 5-epi-lipoxin A4 induced by simvastatin treatment could improve the pathophysiological condition of patients. |
| Aguilera et al., 2017 | Benznidazole (Antiprotozoal) and Triosephosphate isomerase inhibitors, 2, 3, and 4. | Balb/c mice | In vivo were analyzed, showing increased BNZ effect, survival, trypomastigote decrease and lower levels of anti-T-cruzi Ig G 34 dpi. |
| Cevey et al., 2017 | Benznidazole (Antiprotozoal) and Fenofibrate (Antihyperlipidemic) | Balb/c mice | There was a reversal of the cardiac dysfunction associated with the inflammatory response, decreasing pro-inflammatory mediators, with potential for clinical trial in humans. |
| Santeliz et al., 2017 | Dipyridamole (blood Modifier Agent) and nifurtimox (Antiprotozoal) | NMRI albino mice | DPY potentiated the Nfx effect.The association of DPY increased the survival rate to 85%, and all tested parameters were significantly improved. |
| Contreras-Ortiz et al., 2017 | Astaxanthin (antioxidant) and/or nifurtimox (Antiprotozoal) | Vero cells, Balb/c mice | The compound alone did not show any effect on the reduction of parasitemia.Test survival in the acute phase and when associated with NFMX interfered negatively in its activity. |
| Peron et al., 2017 | A3K2A3 (compound of class Dibenzylideneacetone) and benznidazole (Antiprotozoal) or Ketoconazole, or Fluconazole (antifungal) | LLCMK2 cells | The evaluation of the association of A3K2A3 with ketoconazole and benznidazole with greater protection of cells. The association with fluconazole was not effective. |
| Lourenço et al. (2018) | Amiodarone hydrochloride (Antiarrhythmic) and/or Benznidazole (Antiprotozoal) |
T. cruzi (Y strain) cultures with drugs. | Drugs combination was effective, but without synergism, since similar results were obtained when the drugs were tested individually |
| Strauss et al. (2018) | Clomipramine (Antidepressant, tricyclic) Benznidazole (Antiprotozoal) |
Mammalian cells, | Synergistic activity in vitro against the clinically relevant life-stages of T. cruzi. Significantly diminished the parasitic load in blood and the mortality rate |
| Providello et al. (2018) | Ascorbic Acid (Genitourinary Agent) Benznidazole (Antiprotozoal) |
Swiss mice | Reduction in parasitemia, in cardiac parasitism and inflammation, and prevention of the hepatic damage. |
| Scarim et al., 2018b, Scarim et al., 2018a | Hydroxymethylnitrofurazone (a nitrofurazone prodrug) Benznidazole (Antiprotozoal) |
Balb/c mice | Amastigote nests were not found in heart, skeletal muscle, liver, kidney, colon, spleen and brain. The histopathological analysis showed fewer tissue lesions and parasite infiltrates |
| Torrico F et al. (2018) | E1224 (a water-soluble ravuconazole prodrug) | Human | E1224 displayed a transient, suppressive effect on parasite clearance, whereas benznidazole showed early and sustained efficacy until 12 months of follow-up. |
| Guedes-da-Silva et al. (2019) | Sterol 14α-Demethylase (Inhibitor VFV) Benznidazole (Antiprotozoal) |
Swiss Webster mice | parasitemia suppression and 100% animal survival Coadministration of Bz + VFV (resulted in 106-fold lower blood parasitism as compared to the monotherapy of Bz) |
| De Araújo et al. (2019) | Imidazole Derivatives and Benznidazole (Antiprotozoal) | MRC -5 cells and Primary cardiac cells | Potent and selective activity against T. cruzi |
| Rial et al., 2019 | Allopurinol (Antigout) and Benznidazole (Antiprotozoal) | C57BL/6J and C3H/HeN mice | absence of electrical abnormalities, significant reductions in antibody titres and parasitic loads |
| Mazzeti et al., 2019 | Allopurinol combined with benzinidazole (Antiprotozoal) or Nifurtimox (Antiprotozoal) | Mammalian cell, Swiss mice | The interactions were synergic. Administration of the drugs in combination increased the cure rate. |
| Rocha et al. (2019) | Levamisole (anthelminthic) Benznidazole (Antiprotozoal) |
Swiss Webster mice | In vivo: The association partially reduced parasitemia and did not increased animal survival |
ND* not determined.
A successful association between itraconazole and BNZ was demonstrated in a murine model. The concomitant administration of both drugs allowed a decrease in BNZ dose by 25% and on time needed to curtail the parasitemia. In addition, mice treated with this drug combination showed lessened lesions in the cardiac tissue and fewer inflammatory cells associated with the chronic phase of the disease (Martins et al., 2015). Itraconazole seems to alter the pharmacokinetic profile of BNZ, increasing its half-life and its volume distribution extent, allowing an increased accumulation of BNZ in the biological systems (Da Silva et al., 2012; Martins et al., 2015).
Diniz et al. (2013), using a seven-day rapid treatment protocol, observed that the combination of BNZ and posaconazole was more effective compared to using each one alone. Additionally, the association allowed reduced doses compared to individual administration.
The combination of BNZ and clomipramine showed a synergic activity against T. cruzi (Strauss et al., 2018), and attenuated necrosis and liver damage, possibly due to the hepato-protective effect of the antidepressant. Effectively, mice treated with the association of these drugs disclosed less renal damage when compared to the group treated with BNZ alone (Strauss et al., 2013). Besides that, they showed reduced parasitemia and mortality rate (Strauss et al., 2018).
In a murine model of chronic Chagas cardiomyopathy the concomitant use of simvastatin, a lipid-lowering medication, and BNZ decreased fibrosis and inflammation. Importantly, both drugs are well-studied, with a well-established safety profile, requiring no further expensive and long-term clinical studies (González-Herrera et al., 2017). Furthermore, the sequential use of BNZ and allopurinol (used in the treatment of gout) was well tolerated in humans, leading to beneficial therapeutic immunological changes during the chronic CD, significant reduction of parasitic loads, absence of electrical abnormalities in the heart, and increased cure rate (Perez-Mazliah et al., 2012; Rial et al., 2018; Mazzeti et al., 2019). Other combinations with BNZ that have shown to be interesting and deserve better investigation are ascorbic acid (Providello et al., 2018), hydroxymethylnitrofurazone (Scarim et al., 2018a,b), and imidazole (De Araújo et al., 2019).
4. New drugs
Despite the high cost and the time required to search new compounds, this is the main strategy to identify novel treatments for CD in recent years (Table 3. These new drugs, of natural or synthetic origin, are being tested but they still in the pre-clinical phase. Most of them show activity against the parasite, however, in many cases, their mechanisms of action have not yet been elucidated. For example, studies with Cordycepin/Pentostatin and with the Piper jericoense plant showed that these compounds have trypanocide effects, but it is not completely known in which pathways of the parasite they are acting (do Carmo et al., 2019; García-Huertaz et al., 2018).
Table 3.
New drugs for treatment of Chagas disease.
| References | New drugs (compound) | Culture type/animal type | Main Results |
|---|---|---|---|
| Ramírez-Macías et al., 2012 | Terpenoid derivatives | Vero cells and Balb/c mice | The trypanocidal activity of the derivatives was slightly higher (1 and 2) with respect to that found for BZN. Reduced the growth capacity of the parasite, its multiplication and differentiation. In addition to induce mitochondrial changes in the epimastigota. |
| Becco et al., 2012 | Casiopeínas® (copper complexes) | T. cruzi (Dm28c strain) against proliferative eukaryotic cells | The compounds tested showed similar results to nifurtimox. |
| Da Silva et al. (2012) | Arylimidamide and its mesylated salt form | cardiac cells, mammalian host cells and Swiss Webster mice | Trypanocidal effect against both relevant forms in mammalian hosts in vivo: The compounds presented significant selectivity, DB1965 shows high activity in acute experimental models. They reduce parasitemia and decrease animal mortality. |
| Santos et al., 2012 | Eugenia jambolana (natural extract) | Murine J774 macrophages | The compound exhibits activity against the relevant epimastigote form, with reduced toxicity. |
| Polanco-Hernández et al., 2012 | B. pulchra, S. yucatanensis, S. villosa, and B. bucidaefolia | Vero cells and Balb/c mice | The leaf extracts of S. yucatanensis and B. pulchra were the most active against and trypomastigotes. Only the S. yucatanensis extract showed significant trypanocidal activity in vivo, reducing 75% of parasitemia. |
| Higa et al., 2013 | Archaea (to act as adjuvant for soluble parasite antigens) | C3H/HeN mice | Immunization of the animals with the vaccine was able to limit the course of infection in terms of parasitemia and mortality. |
| Moreira et al., 2013 | Thiazolidine LPSF SF29 (organic compound) | LLC-MK2 cells | Induced ultrastructural changes, such as flagellar membrane detachment, intense mitochondrial edema, formation of myelin-like figures and appearance of autophagosomes. |
| Lazarin-Bidóia et al. (2013) | Eupomatenoid-5 (isolated from of P. regnellii) | LLCMK2 cells | The compounds possess activity against the three forms of the parasite, involving alterations induced by oxidative stress. |
| Villalta et al. (2013) | VNI (Experimental inhibitor of T. cruzi sterol 14α-demethylase) | Cardiomyocyte tissue culture and Balb/c mice | The compound presents activity in the acute and chronic phase, with reduction of parasitemia in the animals. |
| Soeiro et al. (2013) | VNI and its derivative VNF | Mammalian cell cultures and Swiss mice | Dose-dependent trypanocidal activities against bloodstream trypomastigotes.The compounds have effective activity against the parasite, with trypanocidal potential, low cytotoxicity and reduced parasitemia. |
| Mello et al., 2013 | Nitroimidazole, thiadiazole, megazole (Nitro analogs) | RAW264.7 macrophages | The analogs were reduced in toxicity as a mutagenesis of the analogs was detected at a concentration greater than the lower concentration of megazole. |
| Papadopoulo et al.,. 2013 | Novel 3-nitro-1H-1,2,4-triazole (Based compounds) | Caco-2 cells, L6 cells and Balb/c mice | Among the compounds tested, some showed the ability to reduce parasitemia, two compounds did not present in vivo activity. |
| Jiménez-Coello et al., 2013 | Carica papaya (Seed extract) | Balb/c mice | The compound shows activity only against the trypomastigote form with antioxidant activity. |
| Raviolo et al., 2013 | C6-alkyl (2a-c) and N1-acyl (3a-c) (derivatives of Allopurinol, Allop) |
Mammalian cells (murine splenocytes) and Vero cells | Only 1 of the compounds, called 3c, has activity against the parasite, with better solubility and lipophilicity than allopurinol, in addition to a reduction in cytotoxicity |
| Hargrove et al. (2013) | UDO e UDD (pyridine derivative) | L6 cells and Swiss mice | The compounds are effective against T. cruzi, being selective against the parasite. The heme-heterocycle and apoprotein-ligand interactions may be helpful in minimizing the off-target activity of CYP51 inhibitors and directing the design selective drugs. |
| Fonseca-Berzal et al., 2013 | Tetrahydroquinolines (Organic compound, derivative of quinoline) | Murine fibroblasts and Vero cells | The showing less effectiveness than the reference drug but also accomplishing great selectivity on the intracellular stage. Low toxicity, however the compounds present solubility problems. |
| Adade et al., 2013 | Melittin (antimicrobial peptides) | LLC-MK2 cells | The compound shows activity against the three evolutionary forms of the parasite, causing morphological alterations, with autophagy and apoptosis. |
| Esperandi et al., 2013 | CUB((isolated from P. cubeba) and HNK (obtained from CUB) | Balb/c mice | The compounds tested showed in vivo activity against the parasite, without cytotoxicity. |
| Branquinho et al. (2014) | Lychnopholide Sesquiterpene lactone, isolated from Lychnophora trichocarpha |
Swiss mice | The nanoparticle loaded compounds were able to reduce parasitemia and improve animal survival. However, it was not able to lead to serologic cure. |
| Cazorla et al., 2014 | Attenuated Salmonella. | Vero cells and C3H/HeN | The animals immunized with the multicomponent vaccine showed reduction of the parasitic load and maintenance of the body ozanoe. |
| Adade et al., 2014 | Crovirin (Crude venom extract) | LLC-MK2 cells | The compound inhibited the growth of the amastigote forms of the parasite and caused lysis of the trypomastigote form, presenting low cytotoxicity against the host. |
| Caballero et al., 2014 | Triazolopyrimidine compounds (six newly synthesized transition metal complexes) | J774.2 macrophages and Balb/c mice | Efficacy of the compounds in relation to the reference drugs. A reduction of parasitemia was found with respect to the control, 4 > 3 > 5. with low toxicity towards Vero cells and greater selectivity than for reference drug. |
| Matos et al., 2014 | Tc52 amino-terminal-domain DNA (Carried by Salmonella entérica) | COS-7 cells, Vero cells, Spleen cells and C3H/HeN mice | Immunization with the vaccine was able to protect animals with humoral and mucosal responses, and leading to the prevention of cellular invasion of the parasite. |
| Varela et al., 2014 | Aristeguietia glutinosa (Hydro-Ethanolic Extract and Isolated Active Principles) | Balb/c mice | The fraction of the complete compound exhibits in vitro and in vivo activity against the parasite with a dose-dependent reduction of parasitemia. |
| Carneiro et al., 2014 | H 2 bdtc (Dithiocarbazate complexes) | Spleen cells isolated from C57BL/6 mice and Swiss mice | The compound was able to reduce inflammatory damage caused by the parasite, reduced parasitemia and increased survival of treated animals. |
| Moraes et al., 2014 | Nitroheterocyclic compounds and four ergosterol biosynthesis inhibitors | U2OS cells and LLC-MK2 | The heterocyclic nitro compounds presented superior efficacy to the other compounds tested. Oxaborol AN4169 is a candidate with potential for broad-spectrum activity and for favorable trypanocidal kinetics. |
| Veiga-Santos et al., 2014 | KH-TFMDI (sirtuin inhibitor) | LLC-MK2 cells | It presented an inhibitory effect against the three evolutionary forms of the parasite and induced lysis of the trypomastigote form. It caused alterations in the structure of the parasite, such as loss of stability and autophagy, besides having high selectivity. |
| Olmo et al., 2014 | 5 derived compounds N, N squaramides (amide-type compounds) | Vero cells and Balb/c mice | Four showed greater in vitro activity than benznidazole, reducing the number of amastigotes in infected cells. In vivo, the compound succeeded in eradicating the parasites in most animals. |
| Papadopoulo et al. (2015) | Nitrotriazole-based derivative | L6 cells and Balb/c mice | The 3-nitrotriazole compounds showed potent and selective anti-T. cruzi activity. |
| Cortes et al., 2015 | Four gallic acid derivatives | Vero cells | The compounds showed high selectivity, and two were more potent against the parasite superior to nifurtimox. |
| Meira et al., 2015 | Physalis ozanoe L. (Ethanolic extract) | LLC-MK2 cells and Balb/c mice | The extract inhibited the proliferation of the epimastigote form of the parasite and induced lysis of the trypomastigote form. In vivo analysis, it was able to reduce parasitemia. |
| Montesino et al., 2015 | 58 extracts of medicinal plants | L6 cells | Of the extracts tested only Valeriana officinalis presented activity against T. cruzi, Salvia, Valeriana, Hypericum, Silybum, Arnica, and Curcuma exhibited high activity. |
| Suto et al., 2015 | Komaroviquinona (quinones derived) | Swiss3T3 cells and HT1080 cells | Four of the synthesized compounds showed considerable effect against T. cruzi and had low cytotoxicity. Further studies are in progress. |
| Papadopoulou et al. (2015) | 3-nitrotriazole (Based amides and carbinols) | L6 cells, Caco-2 cells and Balb/c mice | Reduced the parasitemia in the acute experimental model of the disease.The bifunctional compounds were able to completely clear the parasites after 10-day treatment |
| Santos et al., 2015 | Porcelia macrocara (acetylene fatty acid derivatives) | NCTC cells | The derivative 1 exhibited activity against trypomastigotes of T. cruzi ten times more effective than the benznidazole. |
| Palace-Berl et al., 2015 | 5- substituted hydrazides (compounds derived) | LL-24 human fibroblast cells | All of the derivatives, except for one, showed increased trypanocidal activity against the three strains compared to BZD. 62% of the compounds were more active than nifurtimox against the Y strain. |
| Mendoza-Martínez et al., 2015 | Quinazoline derivatives | Vero cells and CD1 mice | 4 of the 9 compounds (44%) presented higher activity than the reference drugs. |
| Wong-Baeza et al., 2015 | Benzyl ester of N-propyl oxamate | Vero cell line and NIH albino mice | The polar NPOx showed no trypanocidal activity. In contrast, the hydrophobic B-NPOx ester exhibited trypanocidal activity in vitro and in vivo. |
| Álvarez et al., 2015 | Amide-containing thiazole | Vero cells and CD-1 mice | Thiazole 4 was active against trypomastigotes and prevented the intracellular growth of amastigotes. Thiazole 4 suppressed parasitemia by modifying the anti- antibodies as the reference drug. |
| Olmo et al., 2015 | Abietic acid derivatives (diterpenoid) | Vero cells and Balb/c mice | Tests in the acute phase inhibited parasitemia more than Bz, and decrease in reactivation of parasitemia was found in the chronic phase after immunosuppression. |
| Neitz et al., 2015 | GNF7198 (xanthine analogue) | C2C12 cells, 3T3 cells, C2C12 cells, CACO 2 cells and Balb/c mice | The xanthine analogs showed in vitro activity against the parasite and were able to reduce parasitemia more than benznidazole in the animal model. |
| Khare et al. (2016) | GNF 6702 (selective inhibitor of the kinetoplastid proteasome) | NIH 3T3 fibroblast cells and Balb/c mice | The compound shows activity in the parasitemia reduction, confirmed after immunosuppression of the animals. |
| Sülsen et al. (2016) | Psilostachyin and Psilostachyin C (Sesquiterpene Lactones) | Action of 2 compounds on T. cruzi | The compounds possess activity against the parasite by different mechanisms of action. The association these compound may be further investigated as a new therapeutic modality for the treatment. |
| Arias et al., 2016 | Nitrofuran derivatives | HeLa cells and mammalian cells | The three compounds were shown to be better inhibitors of trypanothione reductase than Nifurtimox. |
| Fonseca-Berza et al., 2016 | 5-nitroindazole derivatives | Mammalian cells (fibroblasts), Vero cells and cardiac cells | Many series A indazolinones were efficient against different morphological forms of the CL Brener strain of T. cruzi. |
| Farrow et al., 2016 | Adenovirus 48 (Ad48) | HEK293 cells and C57BL/6 mice | Mice that were immunized with the modified vectors were able to induce specific humoral and cellular responses of T. cruzi. Only two compounds named 3 and 5 extracted from plants Dorstenia mannii, and Pentas schimperi respectively, showed activity against T. cruzi in a moderate manner. |
| Sandjo et al., 2016 | Six plant meta bolites | THP-1 cells | |
| Lozano et al., 2016 | Diterpene 5-epi-icetexone (Salvia gilliesii.) | Swiss albino mice | The compound increased the survival of the treated animals, also decreasing parasitemia. |
| Cerny et al., 2016 | Cruzipain and GM-CSF DNAs, | C3H/HeN mice | Therapeutic vaccines were able to modify the Th1 profile response by protecting and sustaining not only Cz but also against a variety of parasite antigens. |
| Pirttimaa et al., 2016 | Abietane diterpenoid | L6 cells | The amide of dehydroabietylamine compound was highly effective against amastigote forms of T. cruzi resident in L6 cells in addition to high selectivity, superior to benznidazole. |
| Calvet. Et al., 2017 | 4-aminopyridyl-based (CYP51 inhibitors) | Swiss mice | Drastic reduction of parasitemia in the two phases of infection. In addition to preventing damage to cardiac tissue caused by inflammation. |
| Guedes-Da-Silva et al., 2017 | Sterol 14α-Demethylase (CYP51) Inhibitors VNI and VFV | Swiss mice | VFV caused a> 99.7% reduction in peak parasitemia, while NIV values ranged from 91 to 100%. |
| Brand et al., 2017 | 5-amino-1,2,3-triazole-4-carboxamides | Vero and HepG2 cells NMRI mice and Balb/c mice | The optimization of the ATC series favored solubility and metabolic stability in oral administration. |
| Cupello et al., 2017 | LQB 123 (Phenyl-t-Butylnitrone Derivative) | Mammalian Cells | Study demonstrated activity against trypomastigotes in the bloodstream, metacyclic trypomastigotes and against epimastigotes and intracellular amastigotes, with a decrease in infection rate and low toxicity. |
| Da Silva et al., 2017 | DB1957, DB1959 and DB1890 (Arylimidamides) | Caco-2 cells and Swiss Webster mice | The phase was found antiparasitic effect. Presented low toxicity, DB1957 allowed 100% animal survival and DB1959 and DB1890B did not reduce the circulating parasitism. |
| Papadopoulou et al., 2017 | Nitrotriazole-Based Compounds | Swiss mice | Compounds demonstrate potent trypanocidal activity comparable or better than that of the reference drug, benznidazole. |
| Villamizar et Al., 2017 | Linalool (Piper aduncum essential oil) | Vero cells | Trypanocidal effect on amastigote and trypomastigote. An interesting candidate for use in the treatment of potentially contaminated RBCs bags at low temperature. |
| Laurella et al., 2017 | sesquiterpene lactones (isolated from Mikania plants species) | RAW 264.7 and Balb/c mice | Deoxymikanolide presented the highest selectivity index for trypomastigotes and amastigotes. Able to decrease parasitemia and weight loss associated with the acute phase. |
| Alexandre et al., 2017 | Ergosterol (basidiomycete Pleurots salmoneostramineus) | Mammalian cells | Permeabilization of the plasma membrane, as well as in the depolarization of mitochondrial membrane potential, leading to the death of the parasite. |
| Branquinho et al., 2017 | Lychnopholide (natural substance) | C57BL/6 mice | Prevent cardiac changes induced in vivo with minimal cardiotoxicity. |
| Meredith et al., 2017 | Aziridinyl 1,4-benzoquinone (ABQ) (Quinone-based compounds) |
THP-1 cells | The ABQs mediate their activities, with features that facilitate the inhibitory effects of antiparasitic growth, could be incorporated into novel and safer compounds. |
| Brandán et al., 2017 | Live attenuated parasites in combination with plasmid pVXVR-mIFN-γ | C57BL/6J mice | Modify the infection-induced immune response, with attenuated parasites, and improve protection against new infections. |
| Burtoloso et al., 2017 | Non-peptidic nitrile-based cysteine protease inhibitors | LLC-MK2 cells Balb/C 3T3 clone A31 cells | 5 and 11 were as potent against T. cruzi as against the recombinant form of cruzipaine, which is generally considered a valid target. |
| Konduri et al. (2017) | Dendritic cells transduced with the adjuvant | HEK293 cells and Balb/c mice | It reduced parasitic loads from 76% to 99%, compared to a variety of different controls, in addition to significantly reducing cardiac changes. |
| Spósito et al., 2017 | Ravuconazole (triazole antifungal) | H9c2 cells and Swiss mice | Results obtained demonstrated a marked improvement in ravuconazole anti-T. cruzi activity when associated with SEDDS. |
| Branquinho et al., 2017 | Lychnopholide (lipophilic sesquiterpene lactone) | ND* | The best performance was obtained with PLA-PEG NC, PCL NC also showed promising results. In addition to improving LYC pharmacokinetic parameters (iv), the NC protected the encapsulated LYC from degradation in mouse plasma. |
| García-Huertaz et al. (2018) | Furofuran lignan (Piper jericoense) | Vero cells and Balb/c mice | Active against all parasite forms and presented lower toxicity than Benznidazole. Reduced parasitemia in acute phase |
| Villanueva-Lizama et al. (2018) | TSA-1 and Tc24 antigens | Patients (human) | Results confirm the ability of both TSA-1 and Tc24 recombinant proteins to recall an immune response induced by native antigens during natural infection. |
| Paixão et al. (2019) | 1,10-phenanthroline 2,2-bipyridine 4-4′-dimethoxy-2-2′-bipyridine |
L929 cells and Balb/c mice | Higher anti-Trypanosoma cruzi activity than benznidazole.Reduction of parasitemia during acute phase. |
| Miana et al. (2019) | N-arylsulfonyl-benzimidazoles | Vero cells | Bioactivity against epimastigote and amastigote forms, with less cytotoxicity than Benzinidazole |
| Martín-Escolano et al. (2019) | Tris(2-aminomethyl)amine | Vero cells and Balb/c mice | Trypanocidal efficacy in acute and chronic Chagas disease, with lower toxicity than benznidazole |
| Sülsen et al. (2019) | Estafietin (Sesquiterpene lactones derivatives) | Vero cells | Trypanocidal activity, Epoxyestafietin was the most active compound against T. cruzi trypomastigotes and amastigotes |
| Bastos et al. (2019) | Natural compounds isolated from cashew nut (Anacardium occidentale, L. Anacardiaceae) | hFIB cells | Inhibition the enzyme sirtuin, with anti-T. cruzi effects similar to those of benznidazole. |
| Martín-Escolano et al. (2019) | AS-48 (Bacteriocin) | Vero cells | Effective against all T. cruzi morphological forms, displaying lower cytotoxicity than Benznidazole. |
| Do Carmo et al. (2019) | Cordycepin Pentostatin |
HeLa cells and swiss mice | Potent trypanocidal effect in vitro. However, reduced curative effect in vivo. |
ND* not determined.
Psilostachin (Psi) and Psilostachin C (PsiC) (sesquiterpene lactones) are natural compounds found in the Asteracea family that have displayed activity against the parasite by different mechanisms of action. Psi possibly interacts with the heme group, and PsiC inhibits the synthesis of ergosterol. Both compounds showed interaction with hemin, a product of hemoglobin digestion essential for parasite survival (Sülsen et al., 2016). Of interest, the sesquiterpene lactone isolated from Lychnophora trichocarpha loaded with nanoparticles was able to reduce parasitemia and increases the lifetime of treated animals (Branquinho et al., 2014).
Natural chemical constituents isolated from the cashew nut Anacardium occidentale showed trypanocide effects similar to those of benznidazole, by inhibition of T. cruzi sirtuins (Bastos et al., 2019). Furofuran lignan and Eupomatenoid-5, isolated from the leaves of Piper jericoense and Piper regnellii respectively, acted against the three evolutionary forms of the parasite (García-Huertaz et al., 2018; Lazarin-Bidóia et al., 2013). The last decreases the activity of the enzyme trypanothione reductase, resulting in increased amount of oxygen and nitrogen reactive species and the death of T. cruzi. Interestingly, thiazolidine LPSF SF29, a synthetic compound, interferes with the biosynthesis of the substrate of the enzyme trypanothione reductase, also causing mitochondrial dysfunction and parasite death (De B. Moreira et al., 2013).
Several molecules with probable mechanism of action on the parasite sterol biosynthesis were also tested. The enzyme sterol 14-Demethylase (CYP51) is essential for the synthesis of sterols, and its inhibition is used as a pharmacological target. Azole sterol biosynthesis inhibitors, such as, VNI (R)-N-(1-(2,4-dichlorophenyl)-2-(1H-imidazole-1-yl)ethyl)-4-(5-phenyl-1,3,4-oxadi-azol-2-yl)benzamide, inhibitor of protozoan CYP51, and VNF, (R)-4-chloro-N-(1-(2,4-dichlorophenyl)-2-(1H-imidazole-1-yl)ethyl)biphenyl-4-carboxamide, increased the survival of T. cruzi-infected animals. These compounds have low toxicity, absence of mutagenicity and high selectivity index. In the parasite, such compounds cause damage by inducing morphological changes, leading to the formation of vesicles on the membranes, swelling and mitochondrial disorganization. NIV has additionally other important advantages, such as low cost, satisfactory oral bioavailability, disclosing beneficial effect in experimental CD both during the acute and chronic phase. Pyridine-based compounds, identified as pyridine derivatives (S)-(4-chlorophenyl)-1-(4-(4-(trifluoromethyl)phenyl)-piperazin-1-yl)-2-(pyridin-3-yl)ethanone (UDO) and N-[4-(trifluoromethyl)phenyl]-N-[1-[5-(trifluoromethyl)-2-pyridyl]-4-piperidyl]pyridin-3-amine (UDD), are inhibitors of the non-azolic CYP51 enzyme, exhibiting efficiency against T. cruzi similarly to that obtained with posaconazole. Contrastingly, they display an excellent selectivity (Hargrove et al., 2013; Soeiro et al., 2013; Villalta et al., 2013).
Amides and carbinols based on 3-nitrotriazole were developed as bifunctional compounds against T. cruzi. They act by inhibiting the enzyme CYP51 and are also substrates for the nitroreductase type I (NTR) enzyme, thus combining the effects of nitroheterocyclics and inhibitors of ergosterol synthesis against the parasite. They are capable of eliminate the parasite completely within 10 days of treatment in the acute murine model. Moreover, unlike the 2-nitrotriazole derivatives, these compounds have no mutagenic potential (Papadopoulou et al., 2015).
Still regarding synthetic drugs under investigation, the compound GNF6702, proposed by Khare et al. (2016), showed significant activity against T. cruzi by inhibiting the proteasome chymotrypsin-like activity, without any action on human proteasome. Currently, this compound is undergoing pre-clinical toxicological tests.
5. Conclusion
Although CD was discovered more than 100 years ago, the treatment remains inefficient for patients already in the heart phase of the disease. Several strategies have been followed to improve therapeutic treatment against CD: the use of the approved drugs BNZ and NF, the repositioning drugs from other pharmacological classes, the association of different compounds and through the search for more effective new drugs.
From this review, it is possible to conclude that: (a) despite the limited activity of BNZ in the chronic CD, nowadays this is the only treatment available in developing countries, such as Brazil. Thus, is imperative to develop new alternatives drugs in view of the detrimental effects of the infection during the symptomatic chronic phase; (b) the use of BNZ in combination with other compounds has shown advantages compared to its use alone; (c) the search for new pharmacological alternatives has been the most used strategy to find new effective drugs; (d) most of the current studies are either at the stage of animal experimentation or in preclinical phases; (e) the inhibition of the parasite ergosterol biosynthesis has been widely explored as a pharmacological target, especially with the use of antifungal drugs, although poor clinical results.
6. Brief perspective
An innovative line of research, which seeks to use unconventional strategies can be currently observed. For example, the study by Villanueva-Lizama et al. (2018) suggests the possibility of employing vaccines as a potential pharmacological treatment. Using the antigens trypomastigote surface antigen-1 (TSA-1) and flagellar calcium binding protein of 24 kDa (Tc24), researchers showed that corresponding recombinant proteins have the ability to stimulate the immune system, establishing an immune response against these antigens during the infection natural course. In turn, Konduri et al. (2017) produced a vaccine based on dendritic cells, transduced with the dnSHP adenoviral construct and loaded with the Tc24 recombinant protein to reduce or prevent cardiac complications of CD.
In addition, many studies utilize computational approaches to identify potential inhibitors of T. cruzi crucial molecular targets (Scarim et al., 2018a,b). Melo-Filho et al., 2019 used a virtual screening based on quantitative structure-activity relationships (QSAR) model to prospect novel molecules against the T. cruzi in commercial database. This model made possible the identification of seven potent and selective compounds against the parasite, which were validated in in vitro experiments. In another study, the virtual screening approach was used on a protein-based pharmacophore to detect potential targets of the glycolytic pathway of the parasite, enabling the identification of three distinct inhibitors of the glyceraldehyde-3-phosphate dehydrogenase from T. cruzi (Maluf et al., 2013).
Declaration of competing interest
None.
Acknowledgments
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001.
Contributor Information
Vanessa Ribeiro, Email: nessaribbeiro@hotmail.com.
Nayra Dias, Email: nayragda@gmail.com.
Taís Paiva, Email: tais.millene@hotmail.com.
Luciana Hagström-Bex, Email: loubex@hotmail.com.
Nadjar Nitz, Email: nadjarnitz@gmail.com.
Riccardo Pratesi, Email: pratesiunb@gmail.com.
Mariana Hecht, Email: marianahecht@gmail.com.
References
- Albercal L.C., Sbaraglini M.L., Balcazar D. Discovery of novel polyamine analogs with anti-protozoal activity by computer guided drug repositioning. J. Comput. Aided Mol. Des. 2016:305–321. doi: 10.1007/s10822-016-9903-6. [DOI] [PubMed] [Google Scholar]
- Andrews K.T., Fisher G., Skinner-Adams T.S. Drug repurposing and human parasitic protozoan diseases. Int. J. Parasitol.: Drugs Drug Resist. 2014;4(2):95–111. doi: 10.1016/j.ijpddr.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apt W., Arribada A., Zulantay Treatment of Chagas' disease with itraconazole: electrocardiographic and parasitological conditions after 20 years of follow-up. J. Antimicrob. Chemother. 2013:2164–2169. doi: 10.1093/jac/dkt135. [DOI] [PubMed] [Google Scholar]
- Bahia M.T., de Andrade I.M., Martins T.A.F. Fexinidazole: a potential new drug candidate for Chagas disease. PLoS Neglected Trop. Dis. 2012:1–9. doi: 10.1371/journal.pntd.0001870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahia M.T., Nascimento A.F.S., Mazzeti A.L. Antitrypanosomal activity of fexinidazole metabolites, potential new drug candidates for Chagas disease. Antimicrob. Agents Chemother. 2014:4362–4370. doi: 10.1128/AAC.02754-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos M.T., Mannochio Russo H., Silvio Moretti N. Chemical constituents of Anacardium occidentale as inhibitors of Trypanosoma cruzi sirtuins. Molecules. 2019;24(7):1299. doi: 10.3390/molecules24071299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson T.J., McKie J.H., Garforth J. Rationally designed selective inhibitors of trypanothione reductase. Phenothiazines and related tricyclics as lead structures. Biochem. J. 1992;286(1):9–11. doi: 10.1042/bj2860009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchi E.A., Bestetti R.B., Scanavacca M.I. Chronic Chagas heart disease management: from etiology to cardiomyopathy treatment. J. Am. Coll. Cardiol. 2017:1510–1524. doi: 10.1016/j.jacc.2017.08.004. [DOI] [PubMed] [Google Scholar]
- Boiani M., Piacenza L., Hernández P. Mode of action of Nifurtimox and N-oxide-containing heterocycles against Trypanosoma cruzi: is oxidative stress involved? Biochem. Pharmacol. 2010;79(12):1736–1745. doi: 10.1016/j.bcp.2010.02.009. [DOI] [PubMed] [Google Scholar]
- Branquinho R.T., Mosqueira V.C.F., Oliveira-Silva J.C.V. Sesquiterpene lactone in nanostructured parenteral dosage form is efficacious in experimental Chagas disease. Antimicrob. Agents Chemother. 2014:2067–2075. doi: 10.1128/AAC.00617-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coura J.R., de Castro S.L. A critical review on Chagas disease chemotherapy. Memórias do Inst. Oswaldo Cruz. 2002;97(1):3–24. doi: 10.1590/s0074-02762002000100001. [DOI] [PubMed] [Google Scholar]
- Coura J.R., Dias J.C.P. Epidemiology, control and surveillance of Chagas disease: 100 years after its discovery. Mem. Inst. Oswaldo Cruz. 2009;31:40. doi: 10.1590/s0074-02762009000900006. [DOI] [PubMed] [Google Scholar]
- Da Nóbrega A.A., Araújo W.N., Vasconcelos A.M.N. Mortality due to Chagas disease in Brazil according to a specific cause. Am. J. Trop. Med. Hyg. 2014:528–533. doi: 10.4269/ajtmh.13-0574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva M.T.A., Silva-Jardim I., Portapilla G.B. In vivo and in vitro auranofin activity, against Trypanosoma cruzi: possible new uses for an old drug. Exp. Parasitol. 2016:189–193. doi: 10.1016/j.exppara.2015.05.012. [DOI] [PubMed] [Google Scholar]
- Da Silva R.M., Oliveira L.T., Barcellos N.M.S. Preclinical monitoring of drug association in experimental chemotherapy of Chagas' disease by a new HPLC-UV method. Antimicrob. Agents Chemother. 2012:3444–3448. doi: 10.1128/AAC.05785-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Araújo J.S., Garcia-Rubia A., Sebastian-Perez Imidazole derivatives as promising agents for the treatment of Chagas disease. Antimicrob. Agents Chemother. 2019 doi: 10.1128/AAC.02156-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira T.L.D.B., Barbosa A.F.S., Veiga-Santos P. Effect of thiazolidine LPSF SF29 on the growth and morphology of Trypanosoma cruzi. Int. J. Antimicrob. Agents. 2013;41(2):183–187. doi: 10.1016/j.ijantimicag.2012.09.018. [DOI] [PubMed] [Google Scholar]
- Deeks E.D. Fexinidazole: first global approval. Drugs. 2019 doi: 10.1007/s40265-019-1051-6. [DOI] [PubMed] [Google Scholar]
- Dias L.C., Dessoy M.A., Silva D.J.J. 2009. Chemotherapy of Chagas' Disease: State of the Art and Perspectives for the Development of New Drugs; pp. 2444–2457. [Google Scholar]
- Dias J.C.P., Ramos A.N., Jr., Gontijo E.D. Epidemiol. Serv. Saúde; 2016. Brazilian Consensus on Chagas Disease, 2015; pp. 7–86. [DOI] [PubMed] [Google Scholar]
- de Figueiredo Diniz L., Urbina J.A., de Andrade I.M. Benznidazole and posaconazole in experimental Chagas disease: positive interaction in concomitant and sequential treatments. PLoS Neglected Trop. Dis. 2013:1–9. doi: 10.1371/journal.pntd.0002367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do Carmo G.M., de Sá M.F., Grando T.H. Cordycepin (3′-deoxyadenosine) and pentostatin (deoxycoformycin) against Trypanosoma cruzi. Exp. Parasitol. 2019;199:47–51. doi: 10.1016/j.exppara.2019.02.016. [DOI] [PubMed] [Google Scholar]
- Fauro R., Lo Presti S., Bazan C. Use of clomipramine as chemotherapy of the chronic phase of Chagas disease. Parasitology. 2013:917–927. doi: 10.1017/S0031182013000103. [DOI] [PubMed] [Google Scholar]
- Ferreira D.D., Mesquita J.T., da Costa Silva T.A. Efficacy of sertraline against Trypanosoma cruzi: an in vitro and in silico study. J. Venom. Anim. Toxins Incl. Trop. Dis. 2018;24(1) doi: 10.1186/s40409-018-0165-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira L.G., Andricopulo A.D. Drug repositioning approaches to parasitic diseases: a medicinal chemistry perspective. Drug Discov. Today. 2016;21(10):1699–1710. doi: 10.1016/j.drudis.2016.06.021. [DOI] [PubMed] [Google Scholar]
- Fracasso M., Bottari N.B., da Silva A.D. Effects of resveratrol on the differentiation fate of neural progenitor cells of mouse embryos infected with Trypanosoma cruzi. Microb. Pathog. 2019 doi: 10.1016/j.micpath.2019.04.040. [DOI] [PubMed] [Google Scholar]
- Francisco A.F., Lewis M.D., Jayawardhana S. Limited ability of posaconazole to cure both acute and chronic trypanosoma cruzi infections revealed by highly sensitive in vivo imaging. Antimicrob. Agents Chemother. 2015;59(8):4653–4661. doi: 10.1128/AAC.00520-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francisco A.F., Jayawardhana S., Lewis M.D. Nitroheterocyclic drugs cure experimental Trypanosoma cruzi infections more effectively in the chronic stage than in the acute stage. Sci. Rep. 2016;6(1) doi: 10.1038/srep35351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Huertas P., Olmo F., Sánchez-Moreno M. Activity in vitro and in vivo against Trypanosoma cruzi of a furofuran lignan isolated from Piper jericoense. Exp. Parasitol. 2018;189:34–42. doi: 10.1016/j.exppara.2018.04.009. [DOI] [PubMed] [Google Scholar]
- González-Herrera F., Cramer A., Pimentel P., Castillo C., Liempi A. Simvastatin attenuates endothelial activation through 15-epi-lipoxin A4 production in murine chronic Chagas cardiomyopathy. Antimicrob. Agents Chemother. 2017:1–10. doi: 10.1128/AAC.02137-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedes da Silva F.H., da Gama Jaén Batista D., Da Silva C.F. Successful aspects of the Co-administration of sterol 14α-demethylase inhibitor VFV and benznidazole in experimental mouse models of Chagas disease caused by the drug-resistant strain of Trypanosoma cruzi. ACS Infect. Dis. 2019 doi: 10.1021/acsinfecdis.8b00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall B.S., Bot C., Wilkinson S.R. Nifurtimox activation by trypanosomal type I nitroreductases generates cytotoxic nitrile metabolites. J. Biol. Chem. 2011;286(15):13088–13095. doi: 10.1074/jbc.M111.230847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargrove T.Y., Wawrzak Z., Alexander P.W. Complexes of Trypanosoma cruzi sterol 14α-demethylase (CYP51) with two pyridine-based drug candidates for Chagas disease. J. Biol. Chem. 2013:31602–31615. doi: 10.1074/jbc.M113.497990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández M., Wicz S., Santamaría M.H., Corral R.S. Curcumin exerts anti-inflammatory and vasoprotective effects through amelioration of NFAT-dependent endothelin-1 production in mice with acute Chagas cardiomyopathy. Memórias do Inst. Oswaldo Cruz. 2018;113(9) doi: 10.1590/0074-02760180171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratz J.M., Garcia Bournissen F., Forsyth C.J., Sosa-Estani S. Clinical and pharmacological profile of benznidazole for treatment of Chagas disease. Expert Rev. Clin. Pharmacol. 2018 doi: 10.1080/17512433.2018.1509704. [DOI] [PubMed] [Google Scholar]
- Khare S., Nagle A.S., Biggart A. Proteasome inhibition for treatment of leishmaniasis, Chagas disease and sleeping sickness. Nature. 2016:229–233. doi: 10.1038/nature19339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger S., Schwarz W., Ariyanayagam M.R. Trypanosomes lacking trypanothione reductase are avirulent and show increased sensitivity to oxidative stress. Mol. Microbiol. 2002;35(3):542–552. doi: 10.1046/j.1365-2958.2000.01721.x. [DOI] [PubMed] [Google Scholar]
- Konduri V., Halpert M.M., Liang D. Genetic adjuvantation of a cell-based therapeutic vaccine for amelioration of chagasic cardiomyopathy. Infect. Immun. 2017;85(9) doi: 10.1128/IAI.00127-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarin-Bidóia D., Desoti V.C., Ueda-Nakamura T. Further evidence of the trypanocidal action of eupomatenoid-5: confirmation of involvement of reactive oxygen species and mitochondria owing to a reduction in trypanothione reductase activity. Free Radic. Biol. Med. 2013;60:17–28. doi: 10.1016/j.freeradbiomed.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Lourenço A.M., Faccini C.C., de Jesus Costa C.A. Evaluation of in vitro anti-Trypanosoma cruzi activity of medications benznidazole, amiodarone hydrochloride, and their combination. Rev. Soc. Bras. Med. Trop. 2018;51(1):52–56. doi: 10.1590/0037-8682-0285-2017. [DOI] [PubMed] [Google Scholar]
- Maluf F.V., Andricopulo A.D., Oliva G., Guido R.V. A pharmacophore-based virtual screening approach for the discovery of Trypanosoma cruzi GAPDH inhibitors. Future Med. Chem. 2013;5(17):2019–2035. doi: 10.4155/fmc.13.166. [DOI] [PubMed] [Google Scholar]
- Martín-Escolano R., Cebrián R., Martín-Escolano J. Insights into Chagas treatment based on the potential of bacteriocin AS-48. Int. J. Parasitol.: Drugs Drug Resist. 2019;10:1–8. doi: 10.1016/j.ijpddr.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins T.A.F., Diniz L.F., Mazzeti A.L. Benznidazole/itraconazole combination treatment enhances anti-Trypanosoma cruzi activity in experimental Chagas disease. PLoS One. 2015:1–12. doi: 10.1371/journal.pone.0128707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejia A.M., Hall B.S., Taylor M.C. Benznidazole-resistance in Trypanosoma cruzi is a readily acquired trait that can arise independently in a single population. J. Infect. Dis. 2012;206(2):220–228. doi: 10.1093/infdis/jis331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo-Filho C.C., Braga R.C., Muratov E.N. Discovery of new potent hits against intracellular Trypanosoma cruzi by QSAR-based virtual screening. Eur. J. Med. Chem. 2019 doi: 10.1016/j.ejmech.2018.11.062. [DOI] [PubMed] [Google Scholar]
- Mendonça A.A.S., Coelho C.M., Veloso M.P. Relevance of trypanothione reductase inhibitors on trypanosoma cruzi infection: a systematic review, meta-analysis, and in silico integrated approach. Oxidative Med. Cell. Longev. 2018;2018:1–20. doi: 10.1155/2018/8676578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miana G.E., Ribone S.R., Vera D.M.A. Design, synthesis and molecular docking studies of novel N-arylsulfonyl-benzimidazoles with anti-Trypanosoma cruzi activity. Eur. J. Med. Chem. 2019;165:1–10. doi: 10.1016/j.ejmech.2019.01.013. [DOI] [PubMed] [Google Scholar]
- Molina I., Gómez i Prat J., Salvador F. Randomized trial of posaconazole and benznidazole for chronic Chagas' disease. N. Engl. J. Med. 2014:1899–1908. doi: 10.1056/NEJMoa1313122. [DOI] [PubMed] [Google Scholar]
- Morillo C.A., Marin-Neto J.A., Avezum A. Randomized trial of benznidazole for chronic Chagas' cardiomyopathy. N. Engl. J. Med. 2015:1295–1306. doi: 10.1056/NEJMoa1507574. [DOI] [PubMed] [Google Scholar]
- Oliveira M.J., Cucunubá Z.M., Valencia-Hernández Risk factors for treatment interruption and severe adverse effects to benznidazole in adult patients with Chagas disease. 1-13. PLoS One. 2017 doi: 10.1371/journal.pone.0185033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paixão D.A., Lopes C.D., Carneiro Z.A. In vitro anti-Trypanosoma cruzi activity of ternary copper (II) complexes and in vivo evaluation of the most promising complex. Biomed. Pharmacother. 2019;109:157–166. doi: 10.1016/j.biopha.2018.10.057. [DOI] [PubMed] [Google Scholar]
- Papadopoulou M.V., Bloomer W.D., Lepesheva G.I., Rosenzweig H.S., Kaiser M., Aguilera-Venegas B. Novel 3-nitrotriazole-based amides and carbinols as bifunctional antichagasic agents. J. Med. Chem. 2015:1307–1319. doi: 10.1021/jm5015742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Mazliah D.E., Alvarez M.G., Cooley G. Sequential combined treatment with allopurinol and benznidazole in the chronic phase of Trypanosoma cruzi infection: a pilot study. J. Antimicrob. Chemother. 2012:424–437. doi: 10.1093/jac/dks390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Molina J.A., Molina I. Chagas disease. The Lancet. 2018;391(10115):82–94. doi: 10.1016/S0140-6736(17)31612-4. [DOI] [PubMed] [Google Scholar]
- Providello M.V., Carneiro Z.A., Portapilla G.B. Role of ascorbic acid as supporting in the therapeutic of Chagas disease: benefits in the association with low dose of benznidazole. AAC (Antimicrob. Agents Chemother.) 2018 doi: 10.1128/AAC.00514-18. 00514–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rial M.S., Scalise M.L., Alarcón López. Experimental combination therapy using low doses of benznidazole and allopurinol in mouse models of Trypanosoma cruzi chronic infection. Parasitology. 2018:1–9. doi: 10.1017/S0031182018001567. [DOI] [PubMed] [Google Scholar]
- Rocha Simões-Silva M., Brandão Peres R., Britto C. Impact of levamisole in co-administration with benznidazole on experimental Chagas disease. Parasitology. 2019:1–8. doi: 10.1017/S0031182019000374. [DOI] [PubMed] [Google Scholar]
- Rogers K.E., Keränen H., Durrant J.D. Novel cruzain inhibitors for the treatment of Chagas' disease. Chem. Biol. Drug Des. 2012:398–405. doi: 10.1111/j.1747-0285.2012.01416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas-Sarduy S., Landaburu L.U., Karpiak Novel scaffolds for inhibition of Cruzipain identified from high through put screening of anti kinetoplastid chemical boxes. Nature. 2017:1–12. doi: 10.1038/s41598-017-12170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sales Junior P.A., Carneiro C.M., Sánchez-Montalvá A. Experimental and clinical treatment of Chagas disease: a review. Am. J. Trop. Med. Hyg. 2017;97(5):1289–1303. doi: 10.4269/ajtmh.16-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbaraglini M.L., Bellera C.L., Fraccaroli L. Novel cruzipain inhibitors for the chemotherapy of chronic Chagas disease. Int. J. Antimicrob. Agents. 2016:91–95. doi: 10.1016/j.ijantimicag.2016.02.018. [DOI] [PubMed] [Google Scholar]
- Scarim C.B., de Andrade C.R., da Rosa J.A. Hydroxymethylnitrofurazone treatment in indeterminate form of chronic Chagas disease: reduced intensity of tissue parasitism and inflammation-A histopathological study. Int. J. Exp. Pathol. 2018 doi: 10.1111/iep.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarim C.B., Jornada D.H., Chelucci R.C. Current advances in drug discovery for Chagas disease. Eur. J. Med. Chem. 2018;155:824–838. doi: 10.1016/j.ejmech.2018.06.040. [DOI] [PubMed] [Google Scholar]
- Simões-Silva M.R., De Araújo J.S., Peres R.B. Repurposing strategies for Chagas disease therapy: the effect of imatinib and derivatives against Trypanosoma cruzi. Parasitology. 2019:1–7. doi: 10.1017/S0031182019000234. [DOI] [PubMed] [Google Scholar]
- Sobrinho J.L.S., Fontes D.A.F., De Lyra M.A.M. Chagas's disease: 100 years from its Discovery. Rev. Bras. Ciencias Farm. 2009:283–289. [Google Scholar]
- Sobrinho J.L.S., Medeiros F.P.M., de La Roca M.F. Delineamento de alternativas terapêuticas para o tratamento da doença de Chagas. J. Trop. Pathol. 2007:103–118. [Google Scholar]
- de Nazaré Correia Soeiro M., de Souza E.M. In VitroandIn VivoStudies of the antiparasitic activity of sterol 14α-demethylase (CYP51) inhibitor VNI against drug-resistant strains of Trypanosoma cruzi. Antimicrob. Agents Chemother. 2013:4151–4163. doi: 10.1128/AAC.00070-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss M., Lo Presti M.S., Bazán P.C. Clomipramine and benznidazole association for the treatment of acute experimental Trypanosoma cruzi infection. Parasitol. Int. 2013:293–299. doi: 10.1016/j.parint.2013.02.004. [DOI] [PubMed] [Google Scholar]
- Strauss M., Rodrigues J.H.S., Lo Presti M.S. In vitro and in vivo drug combination for the treatment of Trypanosoma cruzi infection: a multivariate approach. Exp. Parasitol. 2018;189:19–27. doi: 10.1016/j.exppara.2018.04.016. [DOI] [PubMed] [Google Scholar]
- Sülsen V.P., Puente V., Papademetrio D. Mode of action of the sesquiterpene lactones psilostachyin and psilostachyin C on Trypanosoma cruzi. PLoS One. 2016:1–14. doi: 10.1371/journal.pone.0150526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sülsen V.P., Lizarraga E.F., Elso O.G. Activity of estafietin and analogues on Trypanosoma cruzi and Leishmania braziliensis. Molecules. 2019:1–13. doi: 10.3390/molecules24071209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrico F., Gascon J., Ortiz L. Treatment of adult chronic indeterminate Chagas disease with benznidazole and three E1224 dosing regimens: a proof-of-concept, randomised, placebo-controlled trial. Lancet Infect. Dis. 2018;18(4):419–430. doi: 10.1016/S1473-3099(17)30538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle-Reyes J.S., Melnikov V., Dobrovinskaya O. Antiprotozoal drug nitazoxanide enhances parasitemia, tissue lesions and mortality caused by Trypanosoma cruzi in murine model. Exp. Parasitol. 2017:44–50. doi: 10.1016/j.exppara.2016.12.013. [DOI] [PubMed] [Google Scholar]
- Vilar-Pereira G., Carneiro V.C., Mata-Santos H. Resveratrol reverses functional Chagas heart disease in mice. PLoS Pathog. 2016:1–19. doi: 10.1371/journal.ppat.1005947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalta F., Dobish M.C., N de P.N., Kleshchenko VNI cures acute and chronic experimental Chagas disease. J. Infect. Dis. 2013;504:511. doi: 10.1093/infdis/jit042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva-Lizama L.E., Cruz-Chan J.V., Aguilar-Cetina A. del C. Trypanosoma cruzi vaccine candidate antigens Tc24 and TSA-1 recall memory immune response associated with HLA-A and -B super types in Chagasic chronic patients from Mexico. PLoS Neglected Trop. Dis. 2018:1–21. doi: 10.1371/journal.pntd.0006240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J.A., Strub-Wourgraft N., Tarral A. A pharmacokinetic-pharmacodynamic assessment of the hepatic and bone-marrow toxicities of the new trypanoside fexinidazole. Antimicrob. Agents Chemother. 2019 doi: 10.1128/AAC.02515-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson S.R., Taylor M.C., Horn D. A mechanism for cross-resistance to nifurtimox and benznidazole in trypanosomes. Proc. Natl. Acad. Sci. 2008;105(13):5022–5027. doi: 10.1073/pnas.0711014105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . WHO; 2018. Chagas Disease (American Trypanosomiasis)http://www.who.int/news-room/fact-sheets/detail/chagas-disease- [Google Scholar]