Abstract
Melanoma is the most aggressive skin cancer. Its aggressiveness is most commonly attributed to ERK pathway mutations leading to constitutive signaling. Though initial tumor regression results from targeting this pathway, resistance often emerges. Interestingly, interrogation of the NCI-60 database indicates high growth hormone receptor (GHR) expression in melanoma cell lines. To further characterize melanoma, we tested responsiveness to human growth hormone (GH). GH treatment resulted in GHR signaling and increased invasion and migration, which was inhibited by a GHR monoclonal antibody (mAb) antagonist in WM35, SK-MEL 5, SK-MEL 28 and SK-MEL 119 cell lines. We also detected GH in the conditioned medium (CM) of human melanoma cell lines. GHR, JAK2 and STAT5 were basally phosphorylated in these cell lines, consistent with autocrine/paracrine GH production. Together, our results suggest that melanomas are enriched in GHR and produce GH that acts in an autocrine/paracrine manner. We suggest that GHR may constitute a therapeutic target in melanoma.
Keywords: Melanoma, Growth hormone (GH), Growth hormone receptor (GHR), Autocrine, Paracrine
Highlights
-
•
Human melanoma cell lines are enriched in growth hormone receptors (GHR).
-
•
GH treatment of melanoma cell lines activates JAK2, GHR, and STAT5 phosphorylation.
-
•
GH treatment increased melanoma cell invasion and migration.
-
•
GH is detected in human melanoma cell conditioned media.
-
•
An antagonist GHR mAb inhibited melanoma cell invasion and migration.
Implications
The GHR signaling pathway may prove a useful therapeutic target in melanoma cells.
1. Introduction
Invasive melanoma is the most aggressive form of skin cancer [1], with roughly 9700 melanoma-related deaths in the United States in 2017 (https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf). Localized melanoma is surgically curable, but distant metastases result in poorer prognosis; the 5-year survival rate is 98% for localized stage and 18% for distant metastasis. Most melanomas harbor a mutation in the RAS-RAF pathway, with more than half being BRAFV600E [2], with constitutively active RAS-RAF-MEK-ERK signaling and oncogene addiction [3]. Small molecule BRAF inhibitors are useful, but resistance to oncogenic pathway inhibition often emerges [4]. Thus, better understanding of other mechanisms of melanoma progression might foster alternative and/or adjunctive therapeutic approaches.
Growth hormone (GH) is a 22kDa protein hormone largely emanating from the anterior pituitary gland; GH binds to the extracellular domain (ECD) of the cytokine receptor superfamily member, GH receptor (GHR), which is widely displayed in tissues in which GH normally regulates growth and metabolism [5]. GH binding activates the intracellular tyrosine kinase, Janus kinase 2 (JAK2) [6]. GHR/JAK2 activation triggers numerous pathways including the signal transducer and activator of transcription (STAT) pathways to regulate genes including IGF-1 [7,8]. GH can also activate MAP kinase and phosphatidylinositol-3-kinase (PI3K)/Akt. In addition to circulating (endocrine) GH action, there are potentially important roles for locally-produced (autocrine/paracrine) GH [9].
GH is a powerful growth-promoting hormone, which has prompted interest in its potential roles in oncogenesis and cancer behavior [10]. Notably, individuals with an inactivating mutation in the GHR have a markedly reduced risk of developing cancer compared to unaffected relatives, who develop cancer at rates similar to the general population [11]. Furthermore, GH-deficient dwarf rats are resistant to inducible mammary tumor formation [12]. Notably, among 60 human cancer cell lines in the National Cancer Institute drug-screening (NCI-60) panel [13], melanoma cells express higher GHR mRNA than other types of cancers. Recent studies have confirmed that human melanoma cell lines are enriched in GHR and that knockdown of the receptor results in modulation of oncogenic signaling pathways such as JAK2, STAT3 and STAT5, AKT, mTOR and ERK1/2 [[14], [15], [16]].
In this study, we assessed the effects of GH on human melanoma cell lines. GH caused phosphorylation of the GHR, JAK2, and STAT5 and enhanced invasion and migration. Pre-treatment with a GHR-specific antagonist monoclonal antibody (anti-GHRext-mAb) inhibited these GH-induced effects. Melanoma cell conditioned media harbored bioactive and immunoreactive GH. Notably, melanoma cells treated with anti-GHRext-mAb in the absence of added GH evidenced reduced invasion and migration. Our data suggest a role(s) for GH/GHR signaling in melanoma behavior and that melanoma cell-derived GH may exert autocrine/paracrine effects.
2. Materials and methods
2.1. Materials
Common reagents were purchased from Sigma-Aldrich (St. Louis, MO), unless otherwise noted. Cell culture medium and reagents were purchased from Corning (Corning, NY). Fetal bovine serum was purchased from Atlanta Biologicals (Lawrenceville, GA). Recombinant human GH was kindly provided by Eli Lilly (Indianapolis, IN).
2.2. Cell culture and GH treatment
Human malignant melanoma cell lines SK-MEL-5 and SK-MEL-28 were obtained from American Type Culture Collection (ATCC; Manassas, VA). Human WM35 melanoma cell line was obtained from Dr. Meenhard Herlyn (Wistar Institute). Human SK-MEL-119 cell line was obtained from Dr. Alan Houghton, Sloan-Kettering Institute for Cancer Research (New York, NY). SK-MEL 5 and WM35 were cultured in MEM, while SK-MEL 28 and SK-MEL 119 were cultured in RPMI1640 medium (HyClone Laboratories Inc., Logan, UT). Media were supplemented with 10% heat-inactivated fetal bovine serum and 100 U/ml penicillin, 100 μg/ml streptomycin. Cells were maintained at 37 °C and 5% CO2 in a humid environment. The generation and maintenance of 32D-GHR cells has been previously described [17]. 32D-GHR cells were cultured in RPMI 1640 medium supplemented with 7% FBS, 2 mM histidinol, 0.8 mg/ml G418, and 50 μg/ml gentamicin sulfate, 100 U/ml penicillin, and 100 μg/ml streptomycin (all Biofluids, Carlsbad, CA).
2.3. Cell starvation, cell stimulation, and protein extraction
Serum starvation was accomplished by substitution of 0.5% (wt/vol) bovine serum albumin (fraction V; Roche Molecular Biochemicals) for serum culture media for 16–20 h prior to experiments. Stimulations were performed at 37 °C in starvation medium and were terminated by washing the cells once with and then harvesting by scraping in ice-cold PBS in the presence of 0.4 mM sodium orthovanadate. Pelleted cells were collected by brief centrifugation and solubilized for 30 min at 4 °C in lysis buffer [1% (vol/vol) Triton X-100, 150 mM NaCl, 10% (vol/vol) glycerol, 50 mM Tris-HCl (pH 8.0), 100 mM NaF, 2 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate, 10 mM benzamidine, and 10 μg/mL aprotinin]. After centrifugation at 15,000×g for 15 min at 4 °C, detergent extracts were electrophoresed under reducing conditions.
2.4. Immunoprecipitation, electrophoresis, and immunoblotting
Immunoprecipitation of proteins from detergent cell extract was accomplished as previously described [18]. For analysis of detergent cell extracts, proteins resolved by SDS-PAGE were transferred to Hybond ECL nitrocellulose membranes (Amersham Biosciences). The membranes were blocked with a buffer of 20 mM Tris-HCl (pH 7.6), 150 mM NaCl, and 0.1% (vol/vol) Tween 20 containing 2% (wt/vol) BSA and incubated with primary antibodies for 18 h at 4 °C. After three washes, the membranes were incubated with appropriate secondary antibodies (1:7500 dilution) and washed. Bound antibodies were detected with SuperSignal chemiluminescent substrate (Pierce Chemical Co). Membrane stripping was according to the manufacturer's suggestions (Amersham Biosciences).
2.5. Antibodies
Polyclonal anti-STAT5 (sc-835) was purchased from Santa Cruz Biotechnology, Inc. Polyclonal antiphospho-STAT5 (Tyr694, #9351) was purchased from Cell Signaling Technology. Monoclonal anti-phosphotyrosine antibody, 4G10, was obtained from Upstate Biotechnology. Polyclonal anti-GHR (anti-GHRcyt-AL47) against the intracellular domain of GH receptor [19] and anti-JAK2 (anti-JAK2AL33) [19] were previously described. Anti-GHRext-mAb, a mouse monoclonal antibody against rabbit GHR residues 1–246, has been previously described [20]. Anti-GHRcyt-mAb is a mouse monoclonal antibody against human GHR residues 271–620 and has been previously described [21].
2.6. GH bioassay
32D-GHR cells were harvested by centrifugation and resuspended in fresh RPMI-1640 medium with the FBS replaced by 0.1% BSA. Viable cells were plated into 96-well plates at 1 × 104 per well/100 μl in RPMI-1640 and incubated for 6 h at 37 °C in either: vehicle control (binding buffer), hGH (0.0005ng/mL-0.5 ng/mL), or 50% diluted conditioned medium from melanoma cell lines. After incubation for 48 h, cell viability was assessed using the CellTiter 96® Non-Radioactive Cell Proliferation Assay (Promega Corporation Cat.#G4000 (Madison, WI)). Tetrazolium (3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide) (MTT) was added to each well and cells were incubated at 37 °C for 3 h and detergent solubilized. Absorbance was detected at 570 nm with a microplate reader.
2.7. hGH ELISA
hGH was assayed by an enzyme-linked immunosorbent assay (ELISA; Roche, Indianapolis, IN) according to the manufacturer's instructions.
2.8. Matrigel invasion
Viable cells (20,000/0.5 mL/chamber) were seeded onto Corning Biocoat Matrigel invasion chambers (6.5 mm, 8.0 μm pore size; Corning, Acton, MA, USA) in serum-containing media with or without specified treatment. Growth medium (750 μL) containing 10 μg/mL fibronectin was added to the lower well for each chamber. After 16 h, invaded cells on the lower surface of membranes were fixed with chilled 4% paraformaldehyde and then stained by 0.5% crystal violet. Membranes were then washed, mounted and imaged using a Zeiss Axiovert 200 M (20x) (Carl Zeiss, Jena, Germany). Total cells were quantified in eight different fields using ImageJ software.
2.9. Transwell migration assay
Melanoma cells (4000 per well) in complete culture medium were seeded onto a gelatin coated filter of the transwell (6.5 mm, 8.0 μm pore size; Corning, Acton, MA, USA) and allowed to migrate for 16 h. Cells were fixed with 4% paraformaldehyde and stained by 0.5% crystal violet. Membranes were washed, mounted and imaged using Zeiss Axiovert 200 M (20x) (Carl Zeiss, Jena, Germany). Total cells were quantified in eight different fields using ImageJ software.
2.10. Scratch assay
Melanoma cells (1 × 106 per well) were plated in monolayer in six well plates, scratched by a 1 ml pipette tip (T0 hr), and treated with GH (500 ng/mL), anti-GHRext-mAb, or anti-GHRcyt-mAb (20 μg/mL). At 0 h, 12 h and 18 h (Tfinal), the scratched cultures were photographed and visually compared for differences in cell migration, utilizing an inverted m Zeiss Axiovert 200 M microscope (Carl Zeiss, Jena, Germany). The experiment was conducted in duplicate and cell motility was expressed as (T0-Tfinal) which represents the change in migration over time.
2.11. Densitometric analysis
Immunoblots were scanned using a high-resolution scanner (Hewlett-Packard Co., Palo Alto, CA). Densitometric quantification of images was performed using ImageJ. Densitometry results from several experiments are displayed as mean ± se. The significance (P value) of the differences of pooled results was estimated using t tests.
3. Results
3.1. Effect of GH on GHR signaling pathway in melanoma cell lines
We first examined melanoma cell GH signaling in the human WM35 cell line, which was established from a primary superficial spreading melanoma in radial/vertical growth phase [22]. Cells were treated with GH (500 ng/mL) for 0, 2, 5, 10, 15, 30 or 60min. Detergent cell extracts were either immunoprecipitated with anti-GHR (Fig. 1A and B) or anti-JAK2 (Fig. 1C and D) sera (vs. nonimmune (NI) control serum) or processed without immunoprecipitation (Fig. 1E and F). After resolution by SDS-PAGE, transferred proteins were immunoblotted with anti-phosphotyrosine (anti-pY; Fig. 1A–D) or anti-phosphoSTAT5 (anti-pSTAT5; Fig. 1E and F) and reprobed for GHR, JAK2, and STAT5, as indicated. Consistent with our prior studies of human cancer cells [23], GH induced in WM35 cells rapid and transient tyrosine phosphorylation of both GHR and JAK2 and downregulation of GHR abundance. Notably, there was detectable JAK2 phosphorylation even absent added GH (Fig. 1C and D, 0 min). GH also caused rapid STAT5 phosphorylation that, as anticipated, was more sustained than was GHR or JAK2 phosphorylation.
Fig. 1.
GH Induces Phosphorylation of GHR/JAK2/STAT5 in Melanoma
A-F - Time course of GH-induced phosphorylation of GHR, JAK2, and STAT5 in WM35 cells. Serum-starved cells were treated with vehicle or GH (500 ng/mL) for the indicated periods. Detergent cell extracts were immunoprecipitated with anti-GHR (A) or anti-JAK2 (C) vs NI control serum. Eluates were resolved by SDS-PAGE and serially immunoblotted with anti-pY and anti-GHR (A) or anti-pY and anti-JAK2 (C). Detergent cell extracts were electrophoresed and serially immunoblotted with anti-pSTAT5 and anti-STAT5 (E). Data from three separate experiments, performed as in A,C, and E, were used to densitometrically estimate indicated relative phosphorylation levels. For each experiment, maximum phosphorylation achieved in response to GH was considered 100%. Data are expressed as mean ± SE for each GH treatment duration. IP, immunoprecipitation; NI, nonimmune; WB, Western blot.
G-J, GH concentration dependence of STAT5 and GHR phosphorylation. Serum-starved WM35 and SK-MEL 5 cells were treated with vehicle or the indicated concentrations of GH for 10 min. Detergent cell extracts were either resolved by SDS-PAGE without immunoprecipitation and serially immunoblotted with anti-pSTAT5 and anti-STAT5 (G,I) or immunoprecipitated with anti-GHR (H, J) vs NI control serum. Eluates were resolved by SDS-PAGE and serially immunoblotted with anti-pY and anti-GHR (H, J).
We next compared WM35 with SK-MEL5, another human melanoma cell line previously shown to harbor GHR [14,16], in GH concentration-dependence experiments (Fig. 1G–J). Cells were treated for 10min with varying concentrations (10–500 ng/mL) of GH or binding buffer (BB) only as a diluent control. Anti-pSTAT5 immunoblotting of cell extracts (Fig. 1G and I) revealed GH concentration-dependent acute STAT5 phosphorylation in both cell lines, with STAT5 in SK-MEL 5 being activated at lower GH concentrations than WM35. In addition, STAT5 phosphorylation was detected absent GH treatment in SK-MEL5 cells (Fig. 1I). Anti-GHR immunoprecipitation and sequential anti-pY and anti-GHR immunoblotting of the same extracts revealed similar patterns of GH-induced GHR tyrosine phosphorylation in each cell line as those observed for STAT5 phosphorylation and GHR downregulation (Fig. 1H and J). GHR phosphorylation was also detected absent GH in SK-MEL 5 cells (Fig. 1J). These data indicate that the WM35 and SK-MEL5 human melanoma cell lines bear GHR and respond to GH with GHR, JAK2, and STAT5 phosphorylation. Further, as more readily detected for SK-MEL5, these data suggest that GHR signaling is active even absent added GH.
3.2. GH treatment enhances WM35 cell invasion and migration
Cell invasion is a key step involved in tumor metastasis [24]. Using a Boyden chamber cell invasion assay, we found that treatment of WM35 cells with GH (500 ng/mL; 16 h) yielded ~1.8 fold increase in invasion (Fig. 2A and B). To further define this effect, we employed our GHR-specific antagonist monoclonal antibody, anti-GHRext-mAb. This antibody recognizes human GHR; its conformation-sensitive epitope resides in extracellular subdomain 2, and pre-treatment of cells that bear GHR with anti-GHRext-mAb inhibits GH-induced GHR-mediated signaling without significantly impairing GH binding [20,25]. The GH-induced increase in WM35 cell invasion was inhibited by anti-GHRext-mAb, but not by anti-GHRcyt-mAb (a control mAb which is directed at the intracellular domain of GHR) (Fig. 2C and D), suggesting GH exerted this effect specifically via the GHR.
Fig. 2.
GH induces invasion and migration in WM35 cells
A, B - Melanoma cells (2 × 104 cells/500 μl + 10% FBS-containing medium) were placed in the upper chamber of Boyden chamber containing buffer (BB) or 500 ng/mL of GH. The lower chamber contained 750 μl of medium supplemented with 10% FBS and 10 μg/mL of Fibronectin. After 16 h, invaded cells on the lower surface of the membranes were fixed with chilled paraformaldehyde and stained with crystal violet. A representative image from eight independent experiments is shown (A). The invaded cells were counted in eight randomly selected microscopic fields on the membrane and the results are summarized and expressed as the mean number of invaded cells ± SEM per microscopic field (B). Significant difference versus control group, P = .0004. C, D - WM35 cells were treated with BB or GH (25 ng/mL) or co-treated with anti-GHRcyt-mAb and GH or anti-GHRext-mAb and GH for 16 h. Control compared to GH, P = .002. GH compared to GHRext-mAb, P = .02. Anti-GHRcyt-mAb compared to anti-GHRext-mAb, P = .05. E,F- WM35 cells were subjected to a scratch wound and treated with GH (500 ng/mL) or BB for 6 h. A representative image is shown in (E). Migration was estimated as the relative fold change of wound closure, P = .015 (F).
We next performed wound healing (scratch) assays to examine the GH's effect on cell migration (Fig. 2E and F). WM35 cell migration, as assayed by wound closure, was significantly increased by 57% after treatment with GH (Fig. 2F). The data in Fig. 2 suggest that GH, via engagement of GHR, promotes WM35 cell invasion and migration.
3.3. GH is detected in human melanoma cell conditioned media
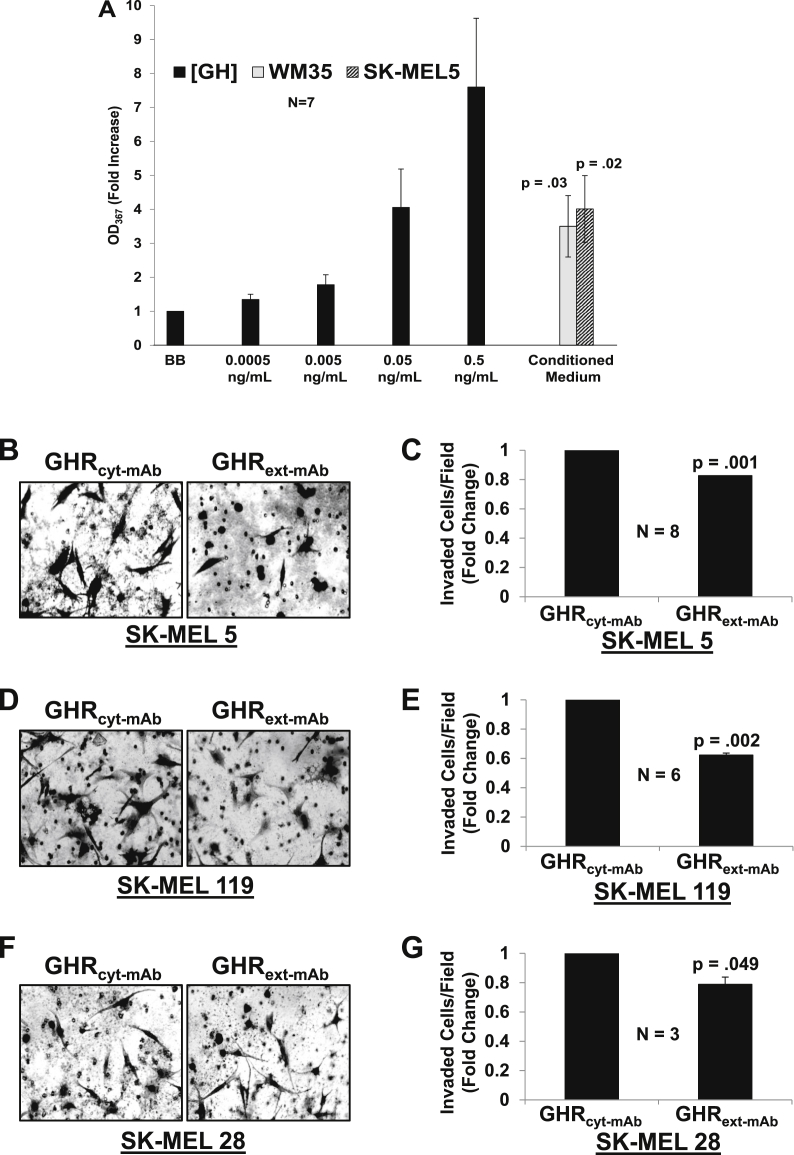
The basal GHR and STAT5 phosphorylation observed in SK-MEL5 and basal JAK2 phosphorylation observed in WM35 melanoma cells (Fig. 1) suggests GH production and autocrine GH action in these cells. To investigate this, we sampled WM35 and SK-MEL5 melanoma cell conditioned media for the presence of GH, using our previously described assay bioassay [17] in which survival of GH-dependent 32D-GHR promonocytes is monitored by MTT assay (Fig. 3A). As anticipated, treatment of 32D-GHR cells with recombinant GH dose-dependently promoted 32D-GHR survival. Serum-free conditioned media (CM) from confluent WM35 or SK-MEL5 cells were collected and incubated at 1:2 dilution with 32D-GHR cells and yielded markedly enhanced survival compared to control. These data suggest both melanoma cell lines secrete bioactive GH into their media. (This result was confirmed in other experiments (not shown), in which hGH was specifically detected in both WM35 and SK-MEL-5 CM by ELISA, albeit at ~5-10-fold lower concentrations than by bioassay; reasons for this quantitative difference are currently unknown.) Thus, these melanoma cells produce bioactive and immunoreactive GH under cell culture conditions, lending credence to the idea that GH plays a role in tumor cell migration.
Fig. 3.
Autocrine GH production and action in melanoma cells.
A - Melanoma Conditioned Medium (CM) promotes survival of 32D-GHR cells Serum-starved 32D-GHR cells were exposed to the indicated concentrations of hGH or CM from WM35 or SK-MEL 5 cells (1:2 dilution) for 48 h and cell viability was measured by MTT assay, as detailed in Materials and Methods. Data (mean ± SEM of quadruplicate determinations) for this experiment are plotted as the fold increase in OD567 relative to the value determined when no GH was added (BB). The experiment shown is representative of three such experiments. Control compared to WM35, P = .03. Control compared to SK-MEL 5, P = .02.B-G - Anti-GHRext-mAbinhibits melanoma invasion in absent added GH in SK-MEL 5, SK-MEL 119, and SK-MEL 28 cells. Melanoma cells (2 × 104 cells/500 μl + 10%FBS containing medium) were placed in the upper chamber of Boyden chamber containing anti-GHRext-mAb or anti-GHRcyt-mAb (20 μg/mL each). The lower chamber contained 750 μl of medium supplemented with 10% FBS and 10 μg/mL of Fibronectin. Invasion was measured as in Fig. 2. Representative images and quantitation of multiple experiments using SK-MEL5 (B,C; n = 8), SK-MEL 119 (D,E; n = 6), and SK-MEL 28 (F,G; n = 3) are shown. P-values compared to control mAb are as indicated.
3.4. GH receptor monoclonal antibody inhibits melanoma invasion and migration
Reasoning that melanoma cell production of GH might influence cell behavior in an autocrine manner, we tested the effects of GHR antagonism alone on invasion and migration. We used the Boyden chamber cell invasion assay to compare SK-MEL-5 to two other human melanoma cell isolates - SK-MEL-28 cells and SK-MEL119 cells [26,27]. Cells were treated with anti-GHRext-mAb or control monoclonal antibody (anti-GHRcyt-mAb) for 16hr during the invasion assay (Fig. 3B–G). Treatment with control mAb had no effect compared with vehicle alone (not shown). In contrast, anti-GHRext-mAb treatment for 16 h reduced the invasive potential of: SK-MEL 5 cells by 18% (Fig. 3C), SK-MEL 119 by 38% (Fig. 3E) and SK-MEL 28 by 22% (Fig. 3G).
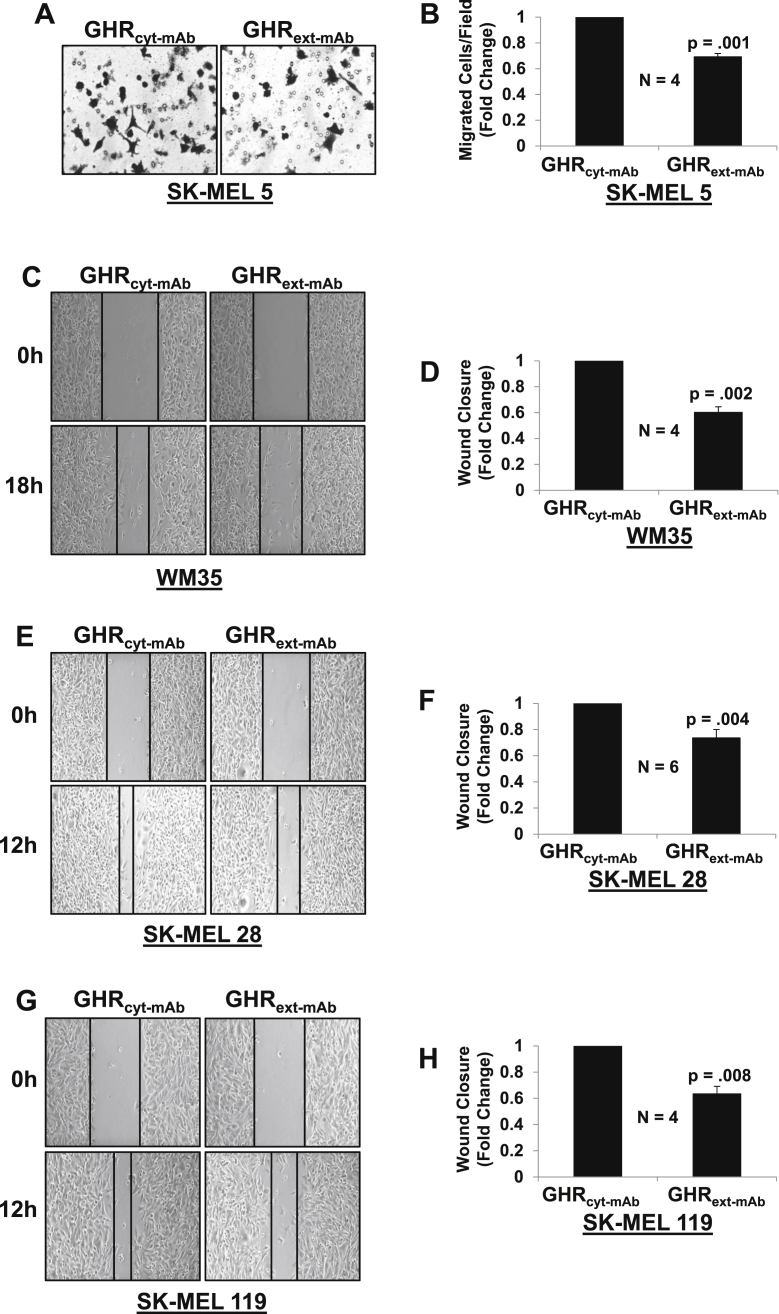
We next investigated the influence of anti-GHRext-mAb on melanoma cell migration (Fig. 4). Utilizing a transwell chamber assay (Fig. 4A and B), for SK-MEL 5, cell migration was significantly reduced by 31% after treatment with anti-GHRext-mAb compared to control antibody. Scratch assays were performed to examine the effect anti-GHRext-mAb on WM35, SK-MEL 28, and SK-MEL 119 migration. For each melanoma cell line, the migration was significantly decreased by GHR antagonism. Anti-GHRext-mAb inhibited migration in WM35 by 34% (Fig. 4C and D), SK-MEL 28 by 38% (Fig. 4E and F), and SK-MEL 119 by 30% (Fig. 4G and H). In concert with the data in Fig. 3, the results in Fig. 4 suggest that melanoma cell-produced GH exerts autocrine/paracrine effects on the propensity of these cell lines to invade and migrate, as determined by in vitro assays. Further, they indicate that anti-GHRext-mAb can inhibit these actions.
Fig. 4.
Anti-GHRext-mAbinhibits melanoma migration in the absence of GH in SK-MEL 5, WM35 and SK-MEL 28 and SK-MEL 119 cells.
A,B – SK-MEL 5. Boyden Chamber migration assay was performed as in Methods. Representative images (A) and quantitation of n = 4 experiments (B) are shown. C–H - Scratch migration assays were performed as in Methods. Representative images (C,E,G) and quantitation of multiple experiments (D,F, H) are shown for WM35 (C, D; n = 4), SK-MEL 28 (E, F; n = 6), and SK-MEL 119 (G,H; n = 4) cells. P-values compared to controls are as indicated.
4. Discussion
Emerging data indicate that GHR is abundant in melanoma cells [13,15,16]. Herein we verify that GHR protein is present and immunoprecipitable in melanoma and we detect GH-inducible phosphorylation of GHR, JAK2 and STAT5 in time-course and concentration-dependence experiments, consistent with previous findings of Basu et al. and Sustaric et al. [[14], [15], [16]]. We also detect phosphorylation of the GHR and JAK2 in some of the cultured melanoma cells in the absence of added GH, suggesting autocrine/paracrine activation of the GHR in melanoma. Accordingly, we detect human GH in the melanoma cell conditioned media, consistent with autocrine production. These conclusions align with observed local GH and GHR expression in a range of different human cancers [9].
Two features of the plasticity observed in melanoma and other cancers related to epithelial-mesenchymal transformation are the abilities to invade and to migrate, thus enabling tumor metastasis [28]. We found that GH treatment potentiated melanoma cell invasion and migration in in vitro assays. Furthermore, in experiments that did not include GH treatment, our GHR antagonist mAb, anti-GHRext-mAb, specifically inhibited melanoma cell migration and invasion, strongly supporting the notion that these behaviors are promoted by autocrine/paracrine signaling by melanoma-produced GH via melanoma-expressed GHR.
It is increasingly appreciated that GH can act in an endocrine as well as an autocrine/paracrine fashion, having an impact on both cancer cells and the tumor microenvironment, and contributes to multiple aspects of cancer progression [9]. We interpret our studies to suggest that even relatively low levels of melanoma-produced GH may have substantial effects on melanoma cell behavior. Further exploration of these effects, the mechanisms of regulation of GH production, and the translation to in vivo model systems are all topics worthy of investigation. Likewise, future studies of the potential of our antagonist GHR mAb as a therapeutic, alone or in combination with other therapies, appear warranted.
CRediT authorship contribution statement
Ashiya Buckels: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing - original draft, Visualization. Yue Zhang: Conceptualization, Validation, Investigation. Jing Jiang: Methodology, Validation, Investigation. Mohammad Athar: Methodology, Resources. Farrukh Afaq: Methodology, Resources. Lalita Shevde-Samant: Methodology, Resources. Stuart J. Frank: Conceptualization, Methodology, Formal analysis, Resources, Writing - review & editing, Visualization, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare no potential conflicts of interest.
Acknowledgements
This work was supported in part by NIDDK R01-DK107441 and a VA Merit Review grant (1I01BX003718), both awarded to SJF
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2019.100716.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Domingues B., Lopes J.M., Soares P., Populo H. Melanoma treatment in review. ImmunoTargets Ther. 2018;7:35–49. doi: 10.2147/ITT.S134842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.An S., Yang Y., Ward R., Liu Y., Guo X.X., Xu T.R., A-Raf A new star of the family of raf kinases. Crit. Rev. Biochem. Mol. Biol. 2015;50:520–531. doi: 10.3109/10409238.2015.1102858. [DOI] [PubMed] [Google Scholar]
- 3.Meador C.B., Pao W. Old habits die hard: addiction of BRAF-mutant cancer cells to MAP kinase signaling. Cancer Discov. 2015;5:348–350. doi: 10.1158/2159-8290.CD-15-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapman P.B. Mechanisms of resistance to RAF inhibition in melanomas harboring a BRAF mutation. Am. Soc. Clin. Oncol. Educ. Book. 2013 doi: 10.14694/EdBook_AM.2013.33.e80. [DOI] [PubMed] [Google Scholar]
- 5.Isaksson O.G., Eden S., Jansson J.O. Mode of action of pituitary growth hormone on target cells. Annu. Rev. Physiol. 1985;47:483–499. doi: 10.1146/annurev.ph.47.030185.002411. [DOI] [PubMed] [Google Scholar]
- 6.Argetsinger L.S., Campbell G.S., Yang X., Witthuhn B.A., Silvennoinen O., Ihle J.N., Carter-Su C. Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase. Cell. 1993;74:237–244. doi: 10.1016/0092-8674(93)90415-m. [DOI] [PubMed] [Google Scholar]
- 7.Frank S.J., Messina J.L. Growth hormone receptor. In: Oppenheim J.J., Feldman M., editors. Cytokine Reference On-Line. Academic Press, Harcourt; London, UK: 2002. pp. 1–21. [Google Scholar]
- 8.Woelfle J., Billiard J., Rotwein P. Acute control of insulin-like growth factor-I gene transcription by growth hormone through Stat5b. J. Biol. Chem. 2003;278:22696–22702. doi: 10.1074/jbc.M301362200. [DOI] [PubMed] [Google Scholar]
- 9.Perry J.K., Wu Z.S., Mertani H.C., Zhu T., Lobie P.E. Tumour-derived human growth hormone as a therapeutic target in oncology. Trends Endocrinol. Metab. 2017;28:587–596. doi: 10.1016/j.tem.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Chesnokova V., Zonis S., Zhou C., Recouvreux M.V., Ben-Shlomo A., Araki T., Barrett R., Workman M., Wawrowsky K., Ljubimov V.A., Uhart M., Melmed S. Growth hormone is permissive for neoplastic colon growth. Proc. Natl. Acad. Sci. U. S. A. 2016;113:E3250–E3259. doi: 10.1073/pnas.1600561113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guevara-Aguirre J., Balasubramanian P., Guevara-Aguirre M., Wei M., Madia F., Cheng C.W., Hwang D., Martin-Montalvo A., Saavedra J., Ingles S., de Cabo R., Cohen P., Longo V.D. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci. Transl. Med. 2011;3 doi: 10.1126/scitranslmed.3001845. 70ra13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swanson S.M., Unterman T.G. The growth hormone-deficient Spontaneous Dwarf rat is resistant to chemically induced mammary carcinogenesis. Carcinogenesis. 2002;23:977–982. doi: 10.1093/carcin/23.6.977. [DOI] [PubMed] [Google Scholar]
- 13.Chabner B.A. NCI-60 cell line screening: a radical departure in its time. J. Natl. Cancer Inst. 2016;108 doi: 10.1093/jnci/djv388. [DOI] [PubMed] [Google Scholar]
- 14.Sustarsic E.G., Junnila R.K., Kopchick J.J. Human metastatic melanoma cell lines express high levels of growth hormone receptor and respond to GH treatment. Biochem. Biophys. Res. Commun. 2013;441:144–150. doi: 10.1016/j.bbrc.2013.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basu R., Baumgaertel N., Wu S., Kopchick J.J. Growth hormone receptor knockdown sensitizes human melanoma cells to chemotherapy by attenuating expression of ABC drug efflux pumps. Horm. Can. 2017;8:143–156. doi: 10.1007/s12672-017-0292-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basu R., Wu S., Kopchick J.J. Targeting growth hormone receptor in human melanoma cells attenuates tumor progression and epithelial mesenchymal transition via suppression of multiple oncogenic pathways. Oncotarget. 2017;8:21579–21598. doi: 10.18632/oncotarget.15375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang L., Zhou T., Jiang J., Pierce J.H., Gustafson T.A., Frank S.J. Insulin receptor substrate-1 enhances growth hormone-induced proliferation. Endocrinology. 1999;140:1972–1983. doi: 10.1210/endo.140.5.6724. [DOI] [PubMed] [Google Scholar]
- 18.Gan Y., Zhang Y., Buckels A., Paterson A.J., Jiang J., Clemens T.L., Zhang Z.Y., Du K., Chang Y., Frank S.J. IGF-1R modulation of acute GH-induced STAT5 signaling: role of protein tyrosine phosphatase activity. Mol. Endocrinol. 2013;27:1969–1979. doi: 10.1210/me.2013-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang J., Liang L., Kim S.O., Zhang Y., Mandler R., Frank S.J. Growth hormone-dependent tyrosine phosphorylation of a GH receptor-associated high molecular weight protein immunologically related to JAK2. Biochem. Biophys. Res. Commun. 1998;253:774–779. doi: 10.1006/bbrc.1998.9793. [DOI] [PubMed] [Google Scholar]
- 20.Jiang J., Wang X., He K., Li X., Chen C., Sayeski P.P., Waters M.J., Frank S.J. A conformationally sensitive GHR [growth hormone (GH) receptor] antibody: impact on GH signaling and GHR proteolysis. Mol. Endocrinol. 2004;18:2981–2996. doi: 10.1210/me.2004-0102. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y., Jiang J., Kopchick J.J., Frank S.J. Disulfide linkage of growth hormone (GH) receptors (GHR) reflects GH-induced GHR dimerization. Association of JAK2 with the GHR is enhanced by receptor dimerization. J. Biol. Chem. 1999;274:33072–33084. doi: 10.1074/jbc.274.46.33072. [DOI] [PubMed] [Google Scholar]
- 22.Wasinger C., Hofer A., Spadiut O., Hohenegger M. Amino acid signature in human melanoma cell lines from different disease stages. Sci. Rep. 2018;8:6245. doi: 10.1038/s41598-018-24709-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gan Y., Buckels A., Liu Y., Zhang Y., Paterson A.J., Jiang J., Zinn K.R., Frank S.J. Human GH receptor-IGF-1 receptor interaction: implications for GH signaling. Mol. Endocrinol. 2014;28:1841–1854. doi: 10.1210/me.2014-1174. me20141174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guarino M. Epithelial-mesenchymal transition and tumour invasion. Int. J. Biochem. Cell Biol. 2007;39:2153–2160. doi: 10.1016/j.biocel.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 25.Jiang J., Wan Y., Wang X., Xu J., Harris J.M., Lobie P.E., Zhang Y., Zinn K.R., Waters M.J., Frank S.J. Inhibitory GH receptor extracellular domain monoclonal antibodies: three-dimensional epitope mapping. Endocrinology. 2011;152:4777–4788. doi: 10.1210/en.2011-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zecena H., Tveit D., Wang Z., Farhat A., Panchal P., Liu J., Singh S.J., Sanghera A., Bainiwal A., Teo S.Y., Meyskens F.L., Jr., Liu-Smith F., Filipp F.V. Systems biology analysis of mitogen activated protein kinase inhibitor resistance in malignant melanoma. BMC Syst. Biol. 2018;12:33. doi: 10.1186/s12918-018-0554-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prasad R., Kappes J.C., Katiyar S.K. Inhibition of NADPH oxidase 1 activity and blocking the binding of cytosolic and membrane-bound proteins by honokiol inhibit migratory potential of melanoma cells. Oncotarget. 2016;7:7899–7912. doi: 10.18632/oncotarget.6860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tulchinsky E., Pringle J.H., Caramel J., Ansieau S. Plasticity of melanoma and EMT-TF reprogramming. Oncotarget. 2014;5:1–2. doi: 10.18632/oncotarget.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.