Abstract
Adenovirus is a highly evolutionary successful pathogen, as it is widely prevalent across the animal kingdom, infecting hosts ranging from lizards and frogs to dolphins, birds, and humans. Although natural adenovirus infections in humans rarely cause severe pathology, intravenous injection of high doses of adenovirus-based vectors triggers rapid activation of the innate immune system, leading to cytokine storm syndrome, disseminated intravascular coagulation, thrombocytopenia, and hepatotoxicity, which individually or in combination may cause morbidity and mortality. Much of the information on exactly how adenovirus activates the innate immune system has been gathered from mouse experimental systems. Intravenous administration of adenovirus to mice revealed mechanistic insights into cellular and molecular components of the innate immunity that detect adenovirus particles, activate pro-inflammatory signaling pathways and cytokine production, sequester adenovirus particles from the bloodstream, and eliminate adenovirus-infected cells. Collectively, this information greatly improved our understanding of mechanisms of activation of innate immunity to adenovirus and may pave the way for designing safer adenovirus-based vectors for therapy of genetic and acquired human diseases.
Keywords: Innate immunity, inflammation, adenovirus, systemic delivery, disseminated infection
Introduction
Human adenovirus (HAdv) is remarkably efficient at infecting and replicating in human cells. These properties made HAdv-based vectors an attractive platform for developing novel therapeutics to combat genetic diseases and cancer. Unfortunately, early studies revealed that HAdv is a potent activator of the innate and adaptive arms of the immune system, and administration to animals or patients of high doses of HAdv-based vectors, especially via an intravascular route, leads to severe immunopathology, manifested by cytokine storm syndrome, disseminated intravascular coagulation, thrombocytopenia, and hepatotoxicity, which may lead to morbidity and even mortality. To harness the full potential of HAdv as a gene delivery or oncolytic virus platform, a complete understanding of the molecular and cellular components of innate immunity is needed to assist in rational design of safe and effective HAdv-based therapeutics.
There is no animal model system where HAdv would productively replicate to a level observed in susceptible human cells [1]. Nevertheless, numerous animal models faithfully recapitulate certain aspects of HAdv-host interactions, including HAdv interactions with cells of innate and adaptive immunity. In this regard, studies conducted in mouse experimental system with its unparalleled variety of available genetically trackable models and molecular tools, greatly improved our understanding of fundamental mechanisms of adenovirus (Ad) recognition by humoral and cellular components of innate immunity, which mechanistically underlie immune-pathologies observed in clinical trials using HAdv vectors. Importantly, many, but certainly not all, insights learned from mouse models on how innate immunity recognizes HAdv, have been further confirmed to operate in the human system. In this article, we systematically review data obtained upon administering HAdv and HAdv-based vectors to mice. We summarize our current understanding of humoral and cellular factors of the innate immunity that mediate HAdv recognition and how they orchestrate the multifaceted systemic inflammatory responses observed upon delivery of high dose HAdv in gene-transfer applications. We close by discussing the complexity of designing HAdvs that would avoid innate immune recognition through the introduction of structural modifications to the virus capsid.
1. Innate immune mechanisms limiting circulation of HAdv in the blood
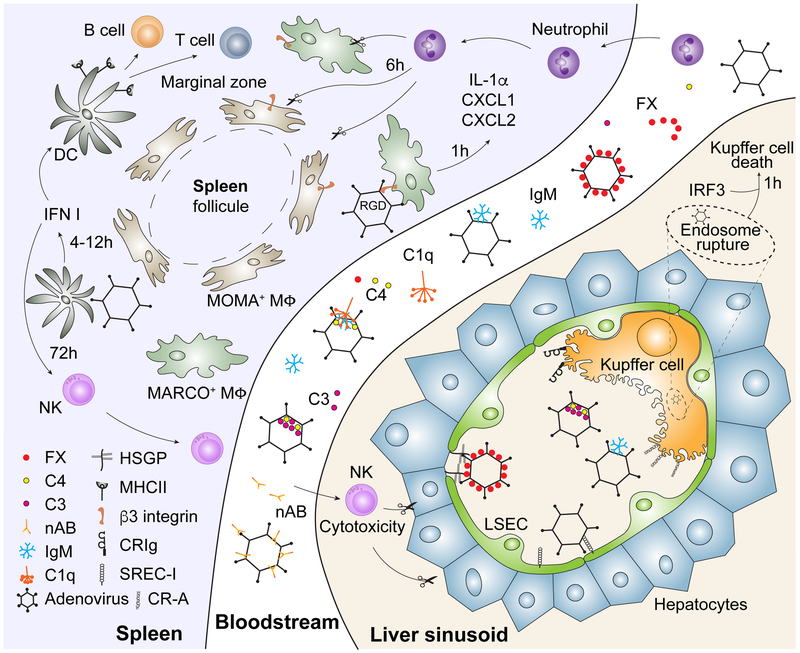
The large number of studies on HAdv-host interactions in a mouse system were conducted by administering high amounts of purified HAdv vectors into the blood stream. Using human species C adenovirus type 5 based HAdv-C5 vectors, Alemany et al showed that the half-life of virus particles in the blood is less than 2 minutes, with near complete elimination of HAdv-C5 from the blood within 30 minutes of virus administration [2]. Such rapid virus clearance suggested the existence of highly efficient mechanism(s) of virus removal from the blood, and later studies indeed confirmed that multiple mechanisms operate in a redundant and synergistic manner to ensure quantitative and rapid removal of HAdv-C5 from the bloodstream [3, 4]. Following intravascular administration, humoral factors bind to HAdv-C5 particles and can mediate virus trapping in tissue-resident macrophages [5, 6] and hepatocytes [7] via a “bridge” mechanism [8-10]. Furthermore, virus particles can be sequestered in platelets, tissue macrophages, and sinusoidal endothelial cells in liver and spleen [3, 11-14] via direct binding to cell surface receptors (Figure 1).
Figure 1. Humoral and cellular components of the innate immune system orchestrating acute response to adenovirus after intravenous virus delivery.
Depicted are the individual roles of blood factors and cell types in mediating the removal of HAdv from the bloodstream and their corresponding receptors and effector molecules, discussed in detail in the body of this review.
1.1. Humoral factors affecting HAdv bio-distribution after intravenous virus injection
Upon intravascular administration, HAdv particles are immediately exposed to humoral components of the blood. An extensive search for the mechanisms mediating the hepatic tropism of HAdv-C5 revealed that GLA-domain-containing blood coagulation factors bind to the major virus capsid protein hexon and that coagulation factors can mediate virus entry into hepatocytes independent of the canonical virus cell attachment receptors, which interact with the fiber knob domain [7-9, 15]. It was established that although several blood coagulation factors (F), including FVII [16], FIX [7], and FX [8, 9], can bind to HAdv-C5 hexon, coagulation FX binds HAdv-C5 hexon with the highest, near picomolar, affinity [8, 9]. Analysis of stoichiometry of FX binding to HAdv-C5 showed that a single virus particle can bind 240 FX molecules, indicating that the entire HAdv-C5 virus capsid, which is composed of 240 hexon trimer capsomers [17], is covered by FX after intravenous virus administration [8, 18]. Additionally, FX can bind to species C HAdv-C2 and human adenoviruses of other species, including species B HAdv-B16, HAdv-B35, HAdv-B21, HAdv-B50, and species F HAdv-F41, albeit with a much lower affinity [8, 9]. Recently, Tian et al. demonstrated that the most abundant Gla domain-containing plasma protein, prothrombin (FII), is able to bind to species C HAdv-C1, HAdv-C2, HAdv-C6, and HAdv-C57 with higher affinity than FX [19], thus potentially pointing to a high significance of HAdv interactions with blood coagulation factors for certain step(s) in the virus life cycle. Indeed, HAdv-C5 mutants unable to bind coagulation FX were shown to be inactivated in fresh mouse and human serum via a natural IgM- and complement-dependent mechanism (reviewed in detail in this issue by Byrnes et al). Because natural IgMs are one of the most abundant plasma proteins, the binding of IgM and coagulation FX to HAdv-C5 appear to be competing processes, since low amounts of IgM were still found to associate with HAdv-C5 virions (as demonstrated by mass spectrometry of HAdv-C5 virions incubated with mouse serum) even in the presence of coagulation FX [20]. Although coagulation FX and natural IgMs are the principal blood factors that bind to HAdv in the blood after intravenous virus administration to non-immune hosts, these factors do not protect the virus from neutralization by HAdv-specific neutralizing antibodies in HAdv-C5 immune hosts. Indeed, liver-directed gene transfer was abrogated upon administering HAdv vector, based on the same serotype that was used for immunization of animals prior to analyzing liver-directed gene delivery [21-23]. Intravenous administration of HAdv to hosts with virus-specific immunity leads to the formation of HAdv-antibody immune complexes that are sequestered by FcR-positive cells including dendritic cells (DCs), neutrophils [24], tissue-resident macrophages. Therefore, although HAdv binding to Gla-domain-containing factors appears to benefit the virus by protecting it from attack by complement and facilitating productive infection of virus-susceptible cells (e.g. hepatocytes), the binding of natural IgMs and virus-specific IgGs to the virus surface triggers its neutralization by complement [25, 26] and targets HAdv-antibody (Ab) complexes for intracellular degradation in circulating and tissue-resident immune cells. It has been found that in mouse and human systems HAdv-Ab complexes are recognized in the cytosol by cytosolic protein tripartite motif containing-21 (TRIM21), which binds to the Fc portion of the Ab and targets HAdv-Ab complexes for proteosomal degradation [27, 28]. Apart from inducing the degradation of the HAdv capsids in the cytosol, TRIM21 activates TBK1, TAB/TAK, and NEMO protein kinases, which initiate innate immune activation through NF-kB, AP-1, and IRF3, IRF5, and IRF7 transcription factors [29, 30]. It should be noted, however, that the TRIM21-dependent mechanism of intracellular degradation of HAdv-Ab complexes does not operate in all cell types. Indeed, HAdv mutants unable to bind to coagulation FX could still transduce hepatocytes with very high efficiency after intravenous administration into complement C1q- or C4-deficient mice [25], which have normal levels of circulating natural IgMs that form HAdv-Ab immune complexes after intravenous virus administration. An in-depth review of TRIM21-dependent neutralization of HAdv-Ab complexes in the cytosol was recently published elsewhere [31].
1.2. Mechanisms of HAdv sequestration in tissue macrophages
Tissue-resident macrophages, most notably Kupffer cells in the liver and CD169-positive and macrophage receptor with collagenous structure (MARCO)-positive macrophages in the marginal zone of the spleen sequester large quantities of HAdv particles after intravenous virus administration [32-37]. HAdv interactions with macrophage receptors that mediate this process remain insufficiently understood. It was clear very early on that the canonical receptors mediating virus entry via a fiber-dependent virus attachment to the coxsackievirus and adenovirus receptor (CAR) [38, 39] or to CD46 [40], both of which are on the cell surface, do not mediate sequestration of HAdv in tissue macrophages after intravenous virus administration, as mouse macrophages do not express CD46 and are yet still able to trap CD46-binding vectors and HAdv-C5-based vectors that do not bind to CAR [3, 34].
Because HAdv particles of most serotypes have a net negative charge, it was proposed that scavenger receptors expressed on tissue-resident microphages may play a role in mediating sequestration of HAdv from the blood by directly binding to the negatively-charged hexon hypervariable loops, located at the surface of the virion. Indeed, administration to mice of poly-inosinic acid, poly-(I), prior to the intravenous injection of HAdv-C5 significantly reduced virus accumulation in Kupffer cells and simultaneously greatly increased virus-mediated gene transfer into hepatocytes [41, 42]. Because poly-(I) is a ligand for a major macrophage scavenger receptor-A (SR-A), it was proposed that HAdv-C5 can bind directly to SR-A, leading to virus clearance from the blood [5, 41, 43, 44]. Using the increase in liver-directed gene transfer as an indirect readout of disruption of HAdv-Kupffer cell interactions, it was also demonstrated that targeted shielding of HAdv-C5 virion surface by attaching small molecular weight polyethylene glycol to hexon hypervariable loops (HVRs) (HVR1, HVR2, HVR5, or HVR7) is sufficient to increase virus-mediated hepatocyte transduction after intravenous virus administration [45]. Based on this data, the authors proposed that HVRs in the HAdv-C5 hexon may be responsible for virus interaction with Kupffer cells. The same group reported that intravenous administration of HAdv-C6 or HAdv-C5-based vector with all hexon HVRs mutated for those of HAdv-C6 (Ad5/6GL) resulted in a highly efficient hepatocyte transduction. Transduction was not further improved by pre-dosing of mice with HAdv-C5 administration (which effectively depletes Kupffer cells), suggesting that HAdv-C6 and Ad5/6GL viruses completely escaped being sequestered in Kupffer cells after intravenous administration[46]. Using the approach of targeted shielding of hexon HVR loops with polyethylene glycol, Krutzke et al. demonstrated that modification of hexon HVR1 loop in HAdv-C5 is sufficient to prevent virus interaction with natural antibodies and complement, leading to an increase in hepatocyte transduction [47].
Although the idea of direct hexon binding to macrophage receptors seems compelling based on the canonical understanding of HAdv cell entry via receptor-mediated attachment and endocytosis [48], several lines of evidence suggest that the interaction of HAdv with Kupffer cells in vivo is unlikely to be direct. First, mouse macrophages are notoriously resistant to direct HAdv-C5 infection in vitro and asanguineous liver perfusion with HAdv-C5 does not lead to virus accumulation in Kupffer cells [7]. In contrast, when liver was perfused with the virus in the presence of coagulation FX, Kupffer cells readily sequestered HAdv-C5 particles from the buffer [7]. Second, although SR-A was suggested to be the principal receptor on Kupffer cells that mediates HAdv elimination from the blood, Kupffer cells in SR-A-deficient mice sequester HAdv at a level comparable to that of wild-type mice [3]. Furthermore, pre-treatment of mice with poly-(I) increases the efficacy of liver-directed gene transfer in both SR-A−/− and SR-A+/+ mice [43]. Third, it has been shown that Kupffer cells express complement receptor CRIg, and HAdv-C5 sequestration in these liver macrophages can be mediated by CRIg in a complement-dependent manner [49]. Finally, it was shown that Kupffer cells in Rag−/− mice, lacking natural IgMs and other classes of antibodies, have a reduced capacity to sequester HAdv from the blood, compared to wild-type mice [5, 6]. Taken together, these data suggest that at least with regard to HAdv-C5, the virus surface is rapidly covered by FX and/or natural IgM and complement after intravenous administration, and therefore, the interaction of the HAdv-C5-blood factor complexes with Kupffer cell receptors occurs for the most part indirectly. Although it is clear that HAdv hexon HVRs are the principal sites on the virus surface responsible for interactions with blood factors, forming a “bridge” between the virus and the receptors on Kupffer cells, the identity of the receptors mediating these interactions remains to be determined.
The spleen is the second major organ where tissue-resident macrophages sequester large quantities of HAdv from the blood. However, the mechanisms that mediate HAdv sequestration in the spleen have not been investigated in detail. This is somewhat surprising, as several unique populations of macrophages and dendritic cells reside in the splenic marginal zone, and virus interaction with these cells plays a key role in activating deleterious systemic inflammatory responses that limit the clinical utility of HAdv-based vectors (discussed below). Analysis of mouse spleen sections at 30 minutes after intravenous administration of HAdv-C5 and staining with antibodies specific for the virus and defined macrophage markers revealed that, shortly after intravenous delivery, HAdv particles predominantly accumulate in two distinct populations of macrophages in the splenic marginal zone, with one population expressing CD169 (MOMA-2) and the other macrophage scavenger receptor MARCO (SR-A6; [37]). It is unclear whether HAdv-C5 is sequestered in these cells through the mechanism of a direct virus binding to macrophage cell-surface receptors or via an indirect “bridging” by the blood factors. However, it appears that when HAdv interaction with hepatic cells is reduced via pharmacological conditioning or through ablation of interactions with blood factors by introducing mutations to the virus capsid [12, 50], the accumulation of HAdv in the spleen is significantly increased [50]. One explanation might be that, in the absence of FX binding, the virus surface is rapidly covered by C3b and C4b complement components as a consequence of IgM-mediated complement activation [25]. Accumulation of HAdv in splenic marginal zone macrophages would then occur through interaction of C3b complement-opsonized virus with complement receptors on CD169 and MARCO-positive cells. Another mechanism that could mediate HAdv sequestration in splenic marginal zone macrophages may rely on direct binding of HAdv to scavenger receptor MARCO. Recent studies by Maler et al. demonstrated that mouse alveolar macrophages are readily transduced in vitro by HAdv-C5 via interaction with MARCO [51]. It has also been shown that deletion of the hexon HVR1 loop in HAdv-C5 was sufficient to abrogate virus binding and transduction of alveolar macrophages, and that MARCO can mediate entry into macrophages for adenovirus of species B and D (HAdv-B35 and HAdv-D26, respectively) [52]. Therefore, in the context of intravenous administration of high doses of coagulation FX-binding ablated viruses, upon passing through the splenic marginal zone, the virus particles can directly bind to scavenger receptor MARCO on the surface of macrophages. As is the case with HAdv sequestration in Kupffer cells, it is highly likely that several redundant mechanisms operate in splenic marginal zone macrophages to ensure rapid removal of HAdv from the blood. These mechanisms are also likely to play an essential role in limiting disseminated HAdv infection in susceptible hosts.
1.3. Mechanisms of HAdv sequestration in sinusoidal endothelial cells
Although Kupffer cells and hepatocytes are the principal cell types sequestering adenovirus particles in the liver, several lines of evidence suggest that the liver sinusoidal endothelial cells (LSEC) can also sequester HAdv and thus contribute to its clearance from the blood. The scavenger receptor expressed on endothelial cells, SREC-1, can mediate uptake of the HAdv-C5-based vector into endothelial cells in vivo [43]. Importantly, after intravenous administration, virus particles co-localized with SREC-1 on LSEC and Kupffer cells and pre-treatment of mice with anti-SREC-1 antibody prior to intravenous virus administration significantly increased liver-directed gene transfer, indicating that virus sequestration via SREC-1 limits disseminated virus infection [43]. The interaction of HAdv with LSEC in vivo becomes particularly apparent in mice, when Kupffer cells were inactivated or depleted by pharmacologic conditioning. Using fluorescent microscopy, it was shown that 1 hour after intravenous administration HAdv-C5 virus particles readily co-localized with LSEC in Kupffer cell-depleted mice [11]. However, co-localization of the virus with endothelial cells was significantly reduced for AdL.PB*, a vector with a deletion of the RGD amino acids in the penton base protein of the virus [11]. Upon analysis of factors that lead to HAdv-C5 sequestration in the liver after intravenous virus administration, Di Paolo et al. found that when Kupffer cell-depleted mice were further pre-treated with warfarin, which inactivates blood coagulation factors, large quantities of HAdv-C5 virus particles were trapped in liver sinusoids [3]. However, when mice without Kupffer cells and functional blood coagulation factors were administered HAdv-C5-based vector lacking the RGD amino acid motif in the penton base (Ad5ΔRGD), virus co-localization with liver sinusoids was no longer observed. Importantly, in this setting the total amount of virus trapped in the liver after intravenous administration was significantly lower for Ad5ΔRGD virus compared to the unmodified HAdv-C5 [3]. Further, HAdv5 interaction with endothelial cells is mediated by β3 integrins, as virus sequestration in the liver tissue of the warfarin-pre-treated and Kupffer cell-depleted mice lacking β3 integrins, Itgb3−/−, was significantly lower than in wild-type mice after the same conditioning [3]. Taken together, these data indicate that LSEC become the principal compartment sequestering HAdv from the blood under conditions when virus avoids trapping in Kupffer cells and hepatocytes, and that the HAdv penton RGD amino acid binding to β3 integrins plays a major role in mediating virus interaction with LSECs.
1.4. Mechanisms of HAdv infection of hepatocytes
The highly efficient infection of hepatocytes observed after the intravenous administration of HAdv-C5 was the main rationale for the early development of HAdv-C5-based vectors for numerous gene therapy applications, as liver-directed gene transfer was expected to provide clinical benefits through the expression of a “correct” version of a protein in individuals with monogenic genetic diseases. However, the extreme propensity of HAdv-C5 for transduction of hepatocytes represents a major problem if the expression of a therapeutic gene is required in extrahepatic cells. Upon discovery of CAR as the principal cell surface attachment receptor for HAdv-C5 [38, 39], attempts have been made to reduce HAdv-C5 hepatocyte infection via introduction of mutations into the knob domain of the fiber to ablate virus interaction with CAR. These attempts were unsuccessful as after intravenous administration, the CAR-binding-ablated viruses transduced hepatocytes with efficacy similar to that of the unmodified WT viruses [7, 53]. Furthermore, HAdv-C5-based vectors ablated for interactions with both the CAR and cellular integrins retained their capacity to efficiently transduce hepatocytes after intravenous administration [54, 55]. Using a set of HAdv-C5-based vectors ablated for fiber-mediated receptor interactions in mice, it was found that blood coagulation factors, namely FVII, FIX, and FX can support efficient infection of virus-resistant cells and hepatocytes in vitro or upon isolated liver perfusion ex vivo [7, 15, 16]. Further, pharmacological inactivation of all coagulation factors with warfarin or inactivation of coagulation FX alone is sufficient to greatly reduce liver-directed gene transfer after intravenous administration of HAdv-C5 in mice [15]. The analysis of coagulation factor-mediated virus infection showed that the heparan sulfate proteoglycans (HSPGs) and LDL receptor related protein (LRP) can both serve as functional receptors to mediate cell entry of coagulation factor-HAdv-C5 complexes in vitro [7]. It was also demonstrated that FX and other coagulation factors bind hexon protein with high affinity, corroborating observation that the virus infection of hepatocytes in vivo is a highly efficient process [8, 9]. Although coagulation factors can form a “bridge” with cellular receptors and support CAR-independent HAdv-C5 cell entry in vitro, the role of FX-dependent entry into hepatocytes in vivo had been re-evaluated based on the discovery that in mice lacking natural IgM antibodies or complement components C1q or C4, the FX-binding-ablated HAdv-C5-based vectors were still able to transduce hepatocytes with high efficacy after intravenous administration [25]. Based on this finding, it was suggested that coagulation FX binding to HAdv-C5 is important for protection of the virus from a complement-mediated inactivation and is not important for hepatocyte transduction in vivo [25]. It was also shown that in mice genetically deficient for the expression of HSPGs on hepatocytes, the liver-directed gene transfer with HAdv-C5-based vector was similar to that observed in the WT mice [56]. Although coagulation FX does certainly protect HAdv-C5 from inactivation by the complement [25], a role of coagulation FX in mediating adenovirus entry in hepatocytes cannot be completely ruled out. Specifically, the efficacy of liver-directed gene transfer after intravenous injection is consistently higher for FX-binding WT HAdv-C5-based vectors, compared to virus mutants unable to bind FX, when it tested in Rag2−/− [20], Rag1−/−, JHD−/−, C1qa−/−, and C4b−/− mice [25]. These data indicate that when complement-mediated virus inactivation mechanisms are dysfunctional, the HAdv-C5 that is able to bind FX transduces hepatocytes in vivo with higher efficacy than viruses ablated for FX binding. Another finding that highlights the complexity of mechanisms of HAdv-C5 entry into hepatocytes in vivo relates to a discrepancy in the efficacy of in vivo hepatocyte transduction by HAdv-C5-based vectors with long and short fibers, Ad5/35L and Ad5/35S [34]. Although both of these vectors bind coagulation FX and lack the capacity for a fiber-mediated cell entry due to the lack of CD46 expression in mice [40], Ad5/35L transduces hepatocytes with very high efficacy, while Ad5/35S is poor at transducing hepatocytes after intravenous administration [34]. Moreover, whereas hepatocytes purified from mouse livers 30 minutes after administration of Ad5/35L contained high amounts of virus DNA, hepatocytes purified after intravenous injection of Ad5/35S contained low to no amounts of viral DNA, despite equal amounts of virus genomes for both viruses present in total liver tissue [34]. These data suggest that even under the condition of equal cell entry via FX or other mechanisms, transduction of hepatocytes occurs more efficiently for a virus that can rapidly escape the endosomal compartment (Ad5/35L), compared to a virus which is retained in the endosomes due to a less efficient release of the fiber (Ad5/35S), and thus is promptly returned into the extracellular space through a recycling endosome transport pathway [57]. Indeed, Ad5/35S was found to accumulate in the perisinusoidal Disse space, the anatomical compartment between sinusoid endothelial cells and hepatocytes, and the liver-associated Ad5/35S virus was largely degraded within 24 hours after virus administration, while Ad5/35L persisted in hepatocytes for a prolonged period of time [34].
As is the case with mechanisms of virus sequestration in tissue macrophages and endothelial cells, the mechanisms of HAdv sequestration in hepatocytes may operate in a functionally redundant manner, therefore, identification of “the mechanism” of virus entry into hepatic cells in vivo may prove to be challenging. It is plausible that for the naturally occurring HAdvs from other species, which do not bind coagulation FX and are resistant to complement-mediated inactivation, entry into hepatocytes can still be mediated by a cognate fiber knob receptor and/or cellular integrins expressed on hepatocytes. It is also plausible that due to its anatomical features of fenestrated vascular endothelium and the existence of a subsinusoidal Disse space, some quantities of HAdv will always be trapped and degraded in the liver tissue or even within hepatocytes without measurable physiological sequelae, thus representing default pathways of pathogen elimination from the blood.
2. HAdv infection triggers a multifaceted acute inflammatory response.
Natural respiratory infections caused by several species B HAdv serotypes in immunocompetent hosts and disseminated infections caused by species C adenoviruses in immunocompromised hosts are known to trigger severe acute inflammatory responses, which are manifest in part by high blood levels of inflammatory cytokines and chemokines, namely IL-1, IL-6, TNF-α, IFN-α/β, IFN-γ, and IL-8 (ref). Mouse models have been instrumental in deconvoluting the complexity of HAdv recognition by the innate immune system, and based on the accumulated data, it appears that cells of both the innate and adaptive immune systems, as well as the epithelial and endothelial cells in tissues contribute to activating systemic inflammation in response to HAdv infection in vivo. Although this response limits virus dissemination and prompts elimination of the virus-infected cells from the body, exacerbated virus-induced inflammation is associated with severe immunopathology and collateral damage to the healthy tissues that may lead to multi-organ dysfunction and even death [58, 59]. The acute systemic inflammation that ensues upon delivery of high amounts of HAdv-based vectors in vivo is the key factor limiting their clinical utility in human gene therapy applications and as cancer therapeutics.
2.1. Innate immune signaling pathways activated upon HAdv entry into host cells
Tissue-resident macrophages are the first line of defense against invading pathogens and, as discussed above, they are highly potent at sequestering HAdv upon both intra-tracheal and intravenous virus administration [32, 33, 60, 61]. Kupffer cells in the liver and CD169+ and MARCO+ macrophages in the marginal zone of the spleen activate transcription of pro-inflammatory cytokine IL-1α within 10 minutes post intravenous administration of HAdv-C5 [37]. Transcriptional IL-1α activation is followed by its synthesis and functional activation, which triggered IL-1RI-dependent production of pro-inflammatory cytokines, including IL-6 and TNF-α, and chemokines CCL2, CXCL1, and CXCL2 within 30 minutes after intravenous virus injection [37]. Such a rapid response to HAdv in these cells in vivo indicates that the very early steps of virus infection, e.g. virus attachment to the cell surface receptor(s) and/or its internalization into the cell, trigger activation of sensors of the innate immune system, which recognize the virus and alert surrounding cells of the ongoing viral infection. Indeed, when a mutant HAdv-C5-based vector, unable to interact with cellular integrins, was injected intravenously into mice, activation of IL-1α-IL-1RI-dependent signaling cascade did not occur [37]. Further, the virus penton RGD amino acid-dependent interaction with macrophage β3 integrins is required for the induction of IL-1α-dependent inflammatory signaling [37] and pharmacological inhibition of HAdv-C5 interaction with β3 integrins was sufficient to reduce HAdv-mediated systemic toxicity [62]. The virus penton RGD-dependent interaction with cellular integrins is critical for activation of endothelial cells as the expression of E-selectin and VCAM-1 in the liver was significantly lower after intravenous administration of RGD-deleted vector AdL.PB*, compared to unmodified control virus [11]. Another insight into the mechanisms of HAdv sensing by the innate immune system was obtained upon analyzing the innate immune signaling activation in response to adenovirus mutant, ts1, which is unable to escape endosomal compartments due to a single point mutation in viral protease pVI [63]. Compared to its proper control virus of serotype HAdv-C2, intravenous administration of ts1 resulted in a significantly lower systemic inflammatory response, and although transcriptional IL-1α activation was still detectable in the spleen 30 minutes after intravenous virus administration, IL-1α was not functionally matured to initiate IL-1RI signaling and subsequent activation of the entire IL-1RI-dependent pro-inflammatory cytokine and chemokine cascade was muted [37]. These data provide clear evidence that recognition of HAdv by the innate immunity can also occur either at the endosome escape stage of virus infection (via detection of endosome rupture), or upon virus DNA exposure to the cytosolic sensors, which trigger functional maturation of IL-1α and the subsequent activation of IL-1RI-dependent inflammatory cytokines and chemokines. Although the identity of endosome rupture sensor(s) in macrophages remains elusive, several candidates were proposed to serve as HAdv sensors in the cytosol. One of the best characterized signaling pathways implicated in the cytosolic sensing of HAdv is initiated by the cyclic guanine adenine synthase (cGAS) which is activated upon binding to double-stranded DNA in the cytosol [64]. It is proposed that when HAdv escapes from the endosomes and reaches the cytosol, virus genomic DNA becomes exposed to cytosolic cGAS, which catalyzes synthesis of 2'3'-cGAMP second messenger molecules. In its turn, 2'3'-cGAMP activates STING - leading to IRF3-dependent production of type I IFN [64, 65]. Upon intravenous administration of HAdv-C5 to mice deficient in either STING (Tmem173−/−) or cGAS (Cgas−/−), the amounts of IFN-β and several inflammatory cytokines and chemokines in the liver were significantly lower compared to the wild-type mice, when analyzed 5 hours after the intravenous virus administration [66]. Using mouse bone marrow-derived macrophages and an in vitro HAdv infection system, it was further shown that the cytosolic phosphokinase S6K1 was critical for activating IFN-I production in response to HAdv infection by facilitating formation of a complex between STING and TBK1, the principal kinase activating IRF3 through the phosphorylation of serine residues in its C-terminal activation domain [67]. The critical role of TBK-1 in mediating IFN-I production was confirmed by administering HAdv5 into Tbk1−/−Tnf−/− mice [68]. Using this model, it was determined that the transcriptional activation of IFN-α and IFN-β in the spleen 3 hours after intravenous virus administration was completely eliminated in the absence of TBK1, while IFN-I production in the liver was found to be TBK-1-independent [68]. Although HAdv can activate cGAS-dependent IFN-I production, it remains unclear which cells in vivo respond to HAdv infection via a cGAS-dependent mechanism. It was demonstrated that the earliest time points, when the elevation in serum concentrations of IFN-I is detected, fall between 4 hours and 6 hours after intravenous HAdv administration [18, 69]. A very recent report by Wang et al demonstrated that HAdv genomic DNA can be sensed in the nuclei of infected cells by heterogeneous nuclear riboprotein A2B1, hnRNPA2B1. The authors provided evidence that upon binding to viral genomic DNA in the nucleus, hnRNPA2B1 forms a homodimer, which is de-methylated by JMJD6 methyl-transferase, leading to translocation of hnRNPA2B1 into the cytosole, where it activates TBK1. Activation of TBK1 leads to IRF3 phosphorylation, translocation into the nucleus and activation of IFN-I transcription [70]. Thus, transcriptional IFN-I activation occurs upon HAdv DNA recognition by the innate immune sensors both in the cytosol and in the nucleus.
It was also suggested that the cytosolic sensors of the nucleotide-binding and oligomerization domain (NOD) family receptor, NOD2, and a NOD-like (NLR) family receptor NLRP3 contribute to the detection of HAdv in the cytosol [71, 72]. Suzuki at al. has shown that the intravenous administration of HAdv-C5-based vector triggered reduced levels of activation of the inflammatory cytokines IL-6, TNF-a, IL-12p40 and chemokines CCL2 and CCL5 in the livers of Nod2−/− mice, compared to control wild-type mice, when analyzed 6 hours after the intravenous virus administration [71]. Muruve et al has demonstrated that 6 hours after intravenous administration of HAdv5-based vector, the amounts of inflammatory cytokines IL-1β and IL-6 and chemokines CCL2, CCL4, and CXCL10 were significantly lower in the spleens of Nlrp3−/− mice compared to WT mice [72]. In both of these studies, the cell types in vivo in which the NOD2- and NLRP3-dependent sensing of the cytosolic HAdv may have occurred was not established. Importantly, the mechanistic implications of specific receptors of innate immunity in HAdv recognition based on the data obtained at later time points (likely beyond 1 hour after virus administration) is likely to be confounded by the feed-forward inflammatory signaling amplification loops, as these same receptors recognize a wide variety of host “self” ligands [73], which may be released by the virus-infected cells very shortly after the intravenous virus administration. Collectively, these data indicate that the initial phase of HAdv entry recognition and activation of IL-1α -IL-1RI-dependent pro-inflammatory signaling cascade is followed by a phase of cytosolic and nuclear detection of HAdv, activating IFN-I production and continued release of inflammatory cytokines and chemokines. Together these pathways initiate the clearance of the virus-infected cells, establish global antiviral state, and contribute to activation of cells of adaptive immunity to mount protective humoral and cellular antiviral responses.
2.2. The role of Toll-like receptors in activating innate immune responses to HAdv infection.
The innate immune system is endowed with a large array of extracellular and intracellular receptors. Among these, the Toll-like receptors (TLRs) and NOD-like receptors (NLRs) form the sensory arm of the innate immunity enabling host recognition of and discrimination between “self” moieties and the invading pathogens [73, 74]. The role of TLRs in sensing HAdv infection has been extensively studied in mice deficient in various TLRs and their downstream signaling adaptor MyD88 (which transduces the activating signals downstream of all TLRs except for TLR3), and TRIF (which transduces activating signals downstream of TLR3 and TLR4) [75, 76]. Upon engagement with cognate ligands within TLR signaling complexes, the TLR-dependent signaling ultimately leads to activation of pro-inflammatory cytokine and chemokine production via NF-κB and MAPK signaling pathways, and the role of these pathways in activation of early innate immune and inflammatory responses to HAdv in vivo is well established. HAdv-C5-based vector was found to trigger a reduced production of IL-6, IL-12p40, and CCL2 in Tlr2−/− and Myd88−/− mice compared to the wild-type mice, when the concentration of cytokines in the blood was measured 6 hours after intravenous virus administration [77]. In another study, intravenous administration of HAdv-C5-based vector into Tlr2−/− and Myd88−/− mice activated NF-κB and ERK1/2 in the liver in a significantly delayed manner with Myd88−/− mice exhibiting greater phenotype than TLR2-defieint mice [78]. In this study, the elevated levels of IL-6 in plasma at 1 hour and 6 hours after intravenous virus administration was dependent on MyD88 but not on TLR2. It is noteworthy, that MyD88 is also the principal signaling adaptor for IL-1RI [79], and thus the more attenuated induction of inflammatory response to HAdv observed in Myd88−/− is likely due to a defect in both TLR2 and IL-1RI signaling [78]. The mechanistic role of MAPK signaling cascade in activation of inflammatory cytokine production was confirmed by treating mice with chemical inhibitors of ERK and p38 MAPK kinases prior to administration of HAdv-C2 or ts1 mutant viruses [80]. As early as 30 minutes after intravenous injection of HAdv2, ERK and p38 MAPK were phosphorylated in the liver [80]. Pre-treatment of mice with the MEK inhibitor PD0325901 (ERK pathway) reduced levels of TNF-α and CXCL2, but significantly increased levels of IL-6, CXCL10, IL-12p70, and IFN-γ in the blood 6 hours after the intravenous virus administration. Pre-treatment of mice with p38 MAPK inhibitor SB239063 reduced blood levels of IL-1β, IL-10, IFN-γ and CXCL10 after intravenous virus administration, providing direct evidence of the mechanistic link between activation of MAPK signaling cascade and the production of key pro-inflammatory cytokines after intravenous virus administration. Intravenous administration of ts1 mutant virus did not lead to activation of ERK and p38 in the liver, providing support for the notion that the endosome rupture step of HAdv infection is sensed by the innate immune system and is required for activation of a full-scale systemic inflammatory response [80]. The critical role of TLRs and MyD88 in triggering local inflammatory response to HAdv was demonstrated in the mouse model of keratitis upon infection of mice with HAdv37 [81]. In this study, mice deficient in MyD88 had significantly lower corneal inflammation after HAdv37 infection, which was also significantly lower in Tlr2−/−Tlr9−/− double-deficient mice, but not in mice individually deficient in TLR2, TLR9, or IL-1RI expression [81].
As we discussed above, intravenous administration of HAdv-C5 leads to its rapid interaction with blood coagulation FX and natural IgMs, and it is likely that tissue-resident macrophages in the liver and spleen (which sequester virus from the blood), are able to “sense” incoming adenovirus particles in the complex with blood factors. A genome-wide transcriptome analysis of the spleen in mice 30 minutes after intravenous administration of the wild-type HAdv-C5 and a FX-binding ablated HAdv-C5-based vector, Ad-TEA, showed that prototypic NF-κB-signaling targets genes, including IL-1β, IL6, and CCL3, were poorly activated after Ad-TEA virus, compared to HAdv-C5 [18]. The analysis of the upstream signaling components that mediated this differential NF-κB activation between the wild-type and FX-binding-ablated viruses showed that after intravenous virus administration, the transcription of a prototypical NF-κB target gene IL-1β was significantly lower in mice deficient in MyD88, TRIF (Ticam1−/−), and TRAF6, all of which are principal components of the TLR and IL-1RI signaling pathways [18]. It was further established that NF-κB-dependent inflammatory cytokine activation in the spleen after intravenous administration of HAdv5 was significantly reduced in TLR4-deficient, but not in TLR2-, TLR9-, or TLR7/TLR8-double-deficient mice. It is of note that several adenovirus serotypes, including HAdv-C2, HAdv-C5 of species C and HAdv-B16 and HAd-B21 of species B, which bind coagulation FX with high affinity, trigger higher levels of inflammatory cytokine activation in the spleen 30 minutes after intravenous administration, compared to adenovirus serotypes that do not bind FX (HAdv-E4, HAdv-D51) [18]. Based on these data, it was proposed that splenic marginal zone macrophages activate NF-κB-dependent inflammatory cytokine production upon TLR4-dependent detection of HAdv-coagulation FX complexes in the endosomes. As we now know, without coagulation FX binding to HAdv-C5 in the blood, the virus is rapidly inactivated in an IgM- and complement-dependent manner [25]. It was recently shown that the complement C4 binding to a virus prevents its disassembly [82], thus rendering it phenotypically similar to ts1 virus, which cannot escape the endosomal compartments and which induces a greatly attenuated inflammatory response after intravenous administration [37, 80]. Therefore, a lower efficacy of endosome escape of FX-binding ablated mutants may potentially contribute to their poor induction of TLR4-dependent pro-inflammatory signaling in splenic macrophages in vivo. Although plausible, the implication of TLR4 as a sensor of HAdv-induced endosome rupture requires further investigation.
The HAdv-dependent activation of TLR signaling leads to qualitatively different transcriptional responses in different cell types. Although TLR-dependent NF-κB and MAPK signaling leads to activation of broadly pro-inflammatory cytokines and chemokines in macrophages, in plasmocytoid dendritic cells (pDCs) TLR-dependent recognition of HAdv leads to a very high level of IFN-I production. Basner-Tschakarjan et al. found that in vitro differentiated pDCs and cDCs activate IFN-I production in response to HAdv infection and pDCs, but not cDCs, secrete IFN-I in a TLR9-dependent manner [83]. Zhu et al. have analyzed IFN-I production after exposure of mouse splenic pDCs, conventional DCs (cDCs), Kupffer cells, and peritoneal macrophages to HAdv-C5 in vitro. It was found that upon exposure to HAdv, pDCs produced very high amounts of both IFN-α and IFN-β [84]. The production of IFN-I by pDCs in response to HAdv was dependent on MyD88 and TLR9 but not on TRIF, indicating that the endosomal sensing of viral DNA by TLR9 was critical for activating of IFN-I production, which in this cell type is coupled to IRF7 activation downstream of MyD88 signalosome [85]. It was further determined that although cDCs, Kupffer cells, and peritoneal macrophages produced low levels of IFN-I upon exposure to HAdv in vitro, HAdv activation of IFN-I signaling in these cells occurred independently of TLR9, MyD88, and TRIF [84]. The cellular source of IFN-I production in vivo has been characterized in detail by Fejer et al [69]. Four to twelve hours after intravenous administration of HAdv-B3, both IFN-α and IFN-β expression were detectable in the spleen. Purification of pDCs and myeloid cDCs from spleen and their exposure to HAdv-B3 in vitro showed that while IFN-β was activated by virus in both cell types, only pDCs activated IFN-α in response to virus infection. Importantly, it was established that late phase IL-6 production, determined 6-8 hours after intravenous virus administration, required functional IFNAR signaling as serum levels of IL-6 were significantly lower in IFNAR-deficient mice, compared to wild-type mice after intravenous administration of HAdv-C5 [84] or HAdv-B3 [69].
2.3. Functional consequences of innate immune activation by HAdv at a cellular level.
The interaction of HAdv with cell surface receptors leads to activation of signaling pathways that trigger the secretion of pro-inflammatory cytokines and chemokines and initiates other cell-intrinsic anti-viral response programs. One of the most dramatic phenomena observed in mice after intravenous HAdv administration is the induction of necrotic death of Kupffer cells [36]. As we discussed above, Kupffer cells sequester large quantities of HAdv from the blood and within minutes activate transcription of pro-inflammatory cytokine IL-1α [37]. IL-1α functions as a membrane-bound cytokine and typically orchestrates local inflammatory responses by infected cells or under cell stress conditions [86]. Nevertheless, IL-1α can initiate both local and systemic IL-1RI signaling upon release from necrotic cells [86]. Upon sequestering HAdv from the blood, Kupffer cells undergo necrotic death within 1 hour and the loss of Kupffer cell population in the liver can be quantified on liver sections as early as 6 hours after intravenous HAdv administration [35, 36]. Although the exact molecular mechanism and key mediators executing necrotic cell death in Kupffer cells after HAdv exposure in vivo remain unknown, it was determined that Kupffer cells in mice genetically deficient in principal components of all currently known pathways of regulated cell death, including apoptosis, necroptosis, and pyroptosis, are still able to execute necrosis upon sequestering HAdv from the blood [87]. It was found that mice deficient in transcription factor IRF3 are resistant to HAdv-induced cell death [87]. However, the exact mechanism of how IRF3 regulates the execution of necrotic Kupffer cell death requires further investigation. It has been established that virus-induced endosome rupture is required for Kupffer cell death to occur, as viruses unable to escape the endosomal compartment efficiently (e.g. ts1 and a mutant HAvd-C5-based virus with HAdv-B35 fiber, Ad5/35) fail to trigger rapid death of Kupffer cells after intravenous virus administration [36]. One critical functional consequence of necrosis execution by Kupffer cells after sequestering HAdv from the blood is curtailing systemic virus dissemination and thus protecting the susceptible cell types, such as hepatocytes, from infection [87]. The second outcome of necrotic Kupffer cell death is activation of the systemic inflammatory response, whereby a pre-synthesized cytosolic IL-1α and other pro-inflammatory mediators, including HMGB1 [88], are released in the circulation.
Unlike Kupffer cells in the liver, after sequestering HAdv from the blood, marginal zone macrophages do not execute necrotic death program. Instead, MARCO-positive macrophages are cleared from the spleen in a cell-extrinsic manner. Although IL-1α is promptly activated in MARCO+ and CD169+ macrophages within minutes of intravenous virus administration, the early acute local inflammatory response in the spleen involves IL-1RI-dependent production of chemokines CXCL1, CXCL2, CXCL10, and CCL2 [37]. This chemokine production leads to the influx and retention of polymorphonuclear leukocytes (PMNs) in the splenic marginal zone, which further receive local activating signals and trigger the induction of cell death in MARCO+ macrophages without the loss of plasma membrane integrity [89]. The anatomical disintegration of the splenic marginal zone is rather rapid and the MARCO+ macrophage population is completely lost 6 hours after intravenous administration of HAdv-C5 [89]. Interfering with both the IL-1α signaling and complement activation is needed to prevent PMN-dependent elimination of marginal zone macrophages after intravenous virus administration, suggesting that although chemokine production allows for the recruitment of PMNs into the spleen, local complement-dependent responses may ultimately be needed to trigger cytotoxic PMN activity [89].
IFN-I production by splenic dendritic cells plays a critical role in orchestrating the subsequent innate and adaptive immune responses targeted at both the virus and the virus-encoded transgenes. In the mouse model of a liver-directed gene transfer, it was found that natural killer (NK) cells play the principal role in eliminating liver cells infected with HAdv-C5-based vector[90]. Within 72 hours of intravenous vector administration, NK cells in the spleen acquired a highly activated state manifested by the expression of perforin, granzyme B, and IFN-γ and the expression of these cytotoxic effectors in NK cells was completely dependent on functional IFN-I signaling. Importantly, clearance of virus-infected cells from the liver at this time point was principally mediated by activated NK cells and the lack of IFN-I signaling resulted in both a dysfunctional NK cell population and a prolong persistence of virus-infected transgene-expressing liver cells [90]. The same group demonstrated that the principal NK cell activating receptor NKG2D is required for the NK cell-mediated cytolysis of virus-infected cells [91]. It is apparent that certain early viral genes encoded in the HAdv genome and expressed in hepatocytes after systemic delivery of replication-deficient first-generation vectors, are direct activators of NK cell-mediated cytotoxicity. Intravenous delivery of HAdv-based vectors devoid of all viral genes, so called “gutless” vectors [92, 93], results in a greatly prolong transgene expression in the liver of mice [94, 95], dogs [96, 97], and non-human primates [21, 98, 99]. Although NK cell-mediated toxicity is avoided by deleting all viral genes, systemic delivery of “gutless” viruses still potently activates acute systemic inflammatory responses that are driven entirely by interaction of virus capsid with innate immune cell compartments.
The induction of IFN-I production by DCs after their exposure to HAdv also leads to their functional maturation. Upon analysis of the development of protective antibody responses to HAdv5 it is was found that mice deficient in IFN-I signaling failed to develop virus-neutralizing antibodies [100]. Specifically, it was found that IFNAR-signaling on DCs, CD4+ T cells and B cells was required for the production of virus-specific IgM and IgG antibodies and without IFN-I signaling, multiple steps of functional B cell maturation failed to occur [100]. The role of IFN-I in promoting maturation of DCs and the development of CD8+ T cell responses to vaccine vectors based on HAdv-C5 and chimpanzee adenovirus serotype CAdv-68 was evaluated in mice after intramuscular virus administration. Although IFN-I signaling was required for efficient maturation of DCs after their exposure to both vaccine vectors, the development of strong transgene-specific CD8+ T cell responses was independent of IFN-I signaling, as the numbers of transgene-specific CD8+ T cells were not different between wild-type mice and mice deficient in IFNAR [100, 101]. Taken together, the functional consequences of early innate antiviral response are i) the elimination of virus-containing phagocytes through cell-intrinsic and cell-extrinsic mechanisms, within 24 hours of virus administration; ii) DC cell-mediated IFN-I production that activates NK cells resulting in elimination of virus-infected parenchymal cells; and iii) virus-induced infection and maturation of DCs and other antigen-presenting cells, which promote strong anti-viral humoral and cellular adaptive immune responses.
2.4. Systemic pathological sequelae of adenovirus-induced activation of innate immunity.
Although individual aspects of innate immune activation by HAdv do not appear to be detrimental to the host and aim at limiting virus dissemination and the induction of adaptive immunity, the concerted activation of a multitude of pro-inflammatory and cell death-inducing signaling pathways by many cell types simultaneously creates a major burden on host homeostatic processes and can drive severe systemic immunopathology. Whereas the induction of certain components of systemic HAdv-induced host responses, such as the production of pro-inflammatory cytokines, is fairly well understood, the molecular mechanisms underlying the development of systemic complement activation, disseminated intravascular coagulation, thrombocytopenia, and shock, which are observed after high dose intravenous virus administration remain poorly defined. Nonetheless, these symptoms are the principal signs of acute systemic toxicity which limit clinical utility of HAdv-based vectors for application in humans. After intravenous administration of HAdv-C5 or HAdv-C2, the plasma concentrations of cleaved complement component C3 fragment C3a increases rapidly to a peak 1 hour post virus injection [102]. This rapid systemic complement activation did not require circulating antibodies, and could be initiated via both the classical and alternative complement activation pathways [102]. The depletion of phagocytic cells in liver and spleen with clodronate liposomes significantly reduced systemic complement activation, implicating these cells in the induction of complement activation cascade after their interaction with HAdv. It is noteworthy, that intravenous administration of the ts1 mutant virus failed to trigger in vivo complement activation, indicating that virus escape from cellular endosomal compartments is required to initiate the signaling triggering complement activation cascade [102]. Complement components C3a and C5a are potent activators of the innate immune signaling and administration of HAdv-C5 into complement component C3-deficient mice leads to significantly lower circulating levels of CXCL1, IL-5, G-CSF, GM-CSF, and IL-6 in serum [103]. Global liver-specific transcriptome analysis in C3-deficient and wild-type mice showed that 6 hours after intravenous virus administration, a large number of genes were not transcriptionally activated in C3−/− mice. Annotation of those complement C3-dependent virus-induced genes demonstrated that they are involved in NIK-I-κB/NF-κB signaling cascade, T helper 1-type immune response and cell-mediated immune response, pyrimidine metabolism pathways, apoptosis pathways, IL-6 signaling, and N-glycan biosynthesis pathways [104]. Additionally, intravenous administration of HAdv-C5 into mice deficient in complement components C3 and Factor B (FB) failed to trigger thrombocytopenia. As thrombocytopenia was still induced after virus injection in C1q- and C4-deficient mice, these data suggest that Factor B- and C3-dependent alternative complement activation pathway is responsible for triggering systemic thrombocytopenia upon intravenous virus administration [104]. The broad analysis of the induction of thrombocytopenia and inflammatory cytokine production in wild-type and complement C3- and FB-deficient mice was performed after intravenous administration of several serotypes from all human virus species, including HAdv-A31, HAdv-B3, HAdv-C5, HAdv-D37, HAdv-F41, and SAdv-23 [105]. Although this analysis confirmed that inflammatory cytokine and chemokine activation was muted after administration of virus in complement-deficient mice, compared to the wild-type animals, the thrombocytopenia was only observed after administration of HAdv-B3, HAdv-D37, and SAdv-23 in the wild-type mice and did not depend on complement [105]. The authors suggested that higher virus doses are needed to trigger complement-dependent thrombocytopenia as compared to complement-dependent inflammatory cytokine activation, which occurs after intravenous injection of even relatively low virus doses [105]. Othman et al. explored the mechanisms of platelet clearance from the blood after intravenous administration of HAdv-C5 [106]. It was determined that platelet activation occurs rapidly after their incubation with HAdv-C5 and that platelets express HAdv-C5 attachment receptor, CAR, suggesting that direct HAdv-C5 binding to CAR on platelets may be responsible for virus-mediated platelet activation. Further, administration of high dose HAdv-C5 into mice deficient in von-Willebrand factor does not trigger systemic thrombocytopenia, implicating this factor in mediating this virus-activated thrombocyte-consuming systemic response [106]. With regards to CAR-dependent mechanisms of thrombocytopenia induction, it is worth noting that HAdv serotypes that does not use CAR for cell entry, i.e. HAdv-B3, still trigger thrombocytopenia after intravenous administration even at relatively low doses [105]. This suggests that although virus binding to CAR on platelets can contribute to systemic thrombocytopenia after intravenous administration of CAR-interacting virus serotypes, other aspects of virus-host interactions, including systemic complement activation, activation of tissue macrophages and vascular endothelium in liver and spleen [107], and disseminated intravascular coagulation [13] are likely to contribute significantly to the etiology of acute consumptive thrombocytopenia. The etiology of disseminated intravascular coagulation that ensues after intravenous administration of HAdv remain unexplored. Stone et al. found that disseminated intravascular coagulation was uniformly observed after intravenous administration of various HAdv serotypes, including HAdv-B3, HAdv-E4, HAdv-C5, HAdv-B11, HAdv-B35, and HAdv-F41 in hCD46-Tg mice [108]. Upon analysis of plasma levels of sCD62P and D-dimer it was found that 6 hours after intravenous virus administration their concentrations were significantly higher compared to mock-treated mice, with the highest concentrations of D-dimer observed after intravenous administration of HAdv-C5, HAdv-B11, HAdv-B35, and HAdv-F41 [108]. It was recently discovered that upon executing necrotic type death, macrophages release Tissue Factor (TF), one of the principal factors activating blood coagulation cascade [109]. Because large numbers of Kupffer cells die via a necrotic type death after interaction with HAdv, it is plausible that Kupffer cells release TF, which activates systemic intravascular coagulation. Further studies are needed to address this possibility.
Another pathological sequelae observed upon intravenous HAdv administration is the induction of shock, which involves hypotension, hemoconcentration, tissue edema, and vasocongestion. Using rats as a model, Xu et al. have found that multiple shock-associated symptoms depend on virus-induced activation of lipid mediator platelet-activating factor, PAF [110]. Blood levels of PAF increase by more than 10-fold within 5 minutes of intravenous virus administration. Pharmacologic interference with PAF receptor signaling using the inhibitor ABT-491 prevented increase in hematocrit and drop in systolic blood pressure shortly after intravenous virus administration. It was also established that macrophages in spleen were the likely source of PAF, because splenectomy or depletion of phagocytes with clodronate liposomes prior to the intravenous virus administration prevented PAF release and shock. The pre-treatment of rats with dexamethasone alleviated the shock symptoms. The authors found that PAF also plays a role in the induction of shock in mice, albeit a much higher doses of the virus were needed to trigger PAF release in the mouse model [110].
The cytokine storm, triggered by intravenous administration of HAdv, is likely to dramatically affect host responses to secondary infections. Indeed, Fejer et al. have shown that intravenous or intra-peritoneal administration of HAdv-C2 or HAdv-C5 leads to the development of hypersensitivity to secondary challenge of mice with LPS [111]. This hypersensitivity was manifest by a dramatic increase in blood levels of TNF-α and the expression of pro-inflammatory cytokines and chemokines in liver, spleen, kidney and lungs after administration of LPS to HAdv-pre-treated mice. Furthermore, pretreatment of mice with either HAdv-B3 or HAdv-C5 significantly increased lethality after their challenge with sub-lethal doses of LPS [111]. Mechanistically, LPS lethality depends on intracellular sensing of LPS by caspase-11, which triggers pyroptotic cell death [112, 113], and on collaboration of caspase-11 with caspase-8, which triggers TNF-dependent death of intestinal epithelial cells, leading to intestinal epithelial permeability [114]. Because caspase-11 expression is upregulated by IFN-I [115] and because IFN-I is produced in high amounts after intravenous HAdv administration [69], the intriguing possibility arises that HAdv administration triggers LPS hypersensitivity via an IFN-I-dependent upregulation of caspase-11 expression. If this is the case, then HAdv administration is likely to lead to a global modulation of numerous capsase-11- and caspase-8-dependent host defense programs, which may enable host hypersensitivity to secondary infection with bacterial pathogens. Taken together, these data suggest that the induction of acute systemic pathological responses predominantly stems from the virus interaction with phagocytes in liver and spleen after intravenous virus delivery, as the depletion of these cells alleviates the symptoms of shock and prevents systemic complement activation. The activation of complement, most notably the complement component C3, initiates the complement-dependent feed-forward pro-inflammatory signaling cascade and triggers thrombocytopenia. The cytokine storm, observed after high dose intravenous virus administration, is likely to mechanistically underlie the hypersensitivity to LPS and may potentially lead to exacerbated host responses to secondary challenge with bacterial pathogens.
3. Complexity of designing HAdv vectors with reduced innate immune recognition
Since the discovery of the detrimental impact of innate immune activation on the safety of HAdv-based vectors in gene therapy settings over two decades ago, a large number of studies have been conducted with the goal of deriving a structural configuration of the virus that escapes recognition by the innate immune system and thus, is safe for application in people with genetic diseases and cancer patients. The rationale for this approach is rooted in observations that the systemic toxicity of different wild-type HAdv serotypes after intravenous administration can vary greatly, and although some serotypes, including HAdv-B3, HAdv-E4 and HAdv-B11, exhibit strong toxicity after intravenous administration [108], others exhibit a more muted activation of acute innate inflammatory response. As recognition of HAdv by the innate immune system occurs within minutes after intravenous virus administration, and the amino acid sequences of the solvent-exposed receptor-interacting surfaces on the virus capsid differ between different serotypes, the introduction of mutations into the capsid should reduce its interactions with innate immune receptors. Although this approach yielded notable successes in a hit-and-miss manner, a comprehensive mechanistic understanding of steps necessary for the construction of HAdv variants that would avoid innate immune recognition is still lacking. Furthermore, with the intrinsic redundancy of innate immune signaling pathways, the multitude of receptors and cell types that respond to HAdv after its intravenous delivery, and a functional redundancy in the sequestration mechanisms that ensure prompt removal of pathogens from the blood [3, 4], design of HAdv variants that would completely avoid innate immune recognition may not be feasible. Nevertheless, based on our understanding of the role of individual virus-host receptor interactions, a reduction in the magnitude of harmful systemic responses to HAdv administration is possible through modifications to the virus capsid.
Upon analysis of transcriptional activation of inflammatory cytokines and chemokines in the liver 30 minutes and plasma cytokine levels 6 hours after intravenous virus administration, it was found that the HAdv5-based vectors with short fibers, derived from either HAdv9 or HAdv35, triggered a significantly lower inflammatory response compared to the virus variants with long fiber shaft domains [34]. Further, a HAdv-C5-based vector with short HAdv-B35-derived fibers (Ad5/35S) triggers Kupffer cell death with a much lower efficacy compared to parental HAdv-C5 virus [36], despite its highly efficient accumulation in Kupffer cells after intravenous administration [3]. The fiber-mutated Ad5/35S vector triggered muted inflammatory response in the spleen and recruitment and retention of PMNs into the splenic marginal zone after intravenous administration of Ad5/35S was similar to that of the ts1 mutant virus, which is unable to escape cellular endosomal compartments [89]. Because both Ad5/35S and ts1 produce poor induction of the pro-inflammatory responses after intravenous administration and because Ad5/35S triggers inflammatory responses at significantly lower levels compared to both of its parental viruses HAdv-C5 and HAdv-B35 [116], it is likely that the substitution of the long fiber in HAdv-C5 capsid for a short fiber from HAdv-B35 reduces the efficacy of Ad5/35S escape from endosomal compartments in phagocytic cells. Importantly, the total amounts of Ad5/35S virus accumulated in liver and spleen after intravenous administration and its sequestration in Kupffer cells and splenic macrophages were not reduced compared to parental HAdv-C5 [3, 89].
As we discussed above, the critical role of virus penton RGD motif-dependent interactions with cellular integrins in triggering activation of pro-inflammatory cytokine and chemokine expression is well established. The RGD amino acid motif is present in penton base proteins of all known human adenovirus serotypes, except for species F viruses HAdv-F40 and HAdv-F41 and species D HAdv-D60 [117, 118]. Such a strong evolutionary conservation of this moiety is easily understood given that efficient virus internalization into the cell critically rely on penton RGD- integrins interactions [48, 119]. Indeed, HAdv-C5-based vectors lacking the RGD amino acid motif in their pentons internalize into cells and escape from the endosomal compartment with reduced efficacy compared to the wild-type virus [120]. Although the intravenous administration of the RGD motif-deleted vectors triggers a profoundly lower inflammatory response compared to parental viruses with wild-type pentons [37], the utility of these vectors for gene transfer applications may be limited due to their reduced infectivity of susceptible target cells. Similar to vectors with mutated fibers, the deletion of the RGD amino acids from the penton or even a combination of both the fiber substitution and penton RGD deleting mutations do not reduce the sequestration of the mutant viruses in the reticulo-endothelial system of the liver and spleen after intravenous virus administration [3, 121].
Since the discovery of the role of hexon HVRs in mediating coagulation FX binding to HAdv-C5 and specific mutations that abrogate hexon-FX interactions, several studies have compared the innate immune activation observed after intravenous injection of FX-binding ablated vectors versus unmodified wild-type HAdv-C5. Upon analysis of transcriptional activation of pro-inflammmatory cytokines and chemokines in the spleen 30 minutes after intravenous administration of a FX-binding ablated virus with a T425A single point mutation in the hexon HVR7, it was determined that a set of NK-kB dependent genes were activated at significantly lower levels compared to their level of activation after administration of HAdv-C5 [18]. It is important to point out that the IL-1α -dependent pro-inflammatory signaling cascade was still activated in the spleen and virus sequestration in macrophages in the liver and spleen after intravenous administration was not reduced upon ablation of FX binding [18]. In an attempt to avoid virus sequestration in the liver and its neutralization by an HAdv-C5-specific serum, Coughlan et al. constructed vector Ad5HVR48(1-7), in which all HAdv-C5 hexon HVRs were substituted for hexon HVRs from a rare species D HAdv-D48 [122]. Although Ad5HVR48(1-7) exhibited lower efficacy of liver-directed gene transfer and reduced virus accumulation in Kupffer cells after intravenous administration, compared to parental viruses HAdv-C5 or HAdv-D48, this hexon-mutated virus was very potent at activating pro-inflammatory cytokine production [122]. Using a similar approach, Khare et al. replaced HAdv-C5 hexon HVRs for those of HAdv-C6 (designated Ad5/6) and analyzed in vivo liver-directed gene transfer, sequestration in tissues, and production of inflammatory cytokine IL-6 after intravenous administration of this mutant virus [123]. It was determined that Ad5/6 vector was able to bind coagulation FX, transduced liver with a 10-fold higher efficacy compared to parental HAdv-C5 virus, and avoided sequestration in Kupffer cells after intravenous administration. However, the levels of IL-6 in the blood after administration of Ad5/6 to mice were still elevated and not different compared to parental HAdv-C5 virus [123]. Although introduction of mutations that ablate FX binding to HAdv-C5 efficiently reduces liver directed gene transfer, the efficacy of virus sequestration in the spleen is significantly increased. Alba et al. found that 1 hour after intravenous virus administration to mice, significantly higher amounts of HAdv-C5-based FX-binding ablated vector accumulated in the spleen compared to the unmodified HAdv-C5 [50]. It was further determined that FX binding-ablated vector efficiently transduced cells in splenic marginal zone, and splenic marginal zone CD11c+, ER-TR7+, and MAdCAM-1+ cells expressed vector-encoded b-galactosidase transgene 48 hours after intravenous virus administration [50]. Bradshaw et al. combined an FX binding ablated mutation in the hexon and the RGD motif to RGE mutation in penton into a single virus, AdT*RGE [12]. Intravenous administration of AdT*RGE resulted in activation of inflammatory cytokines and chemokines similar to that observed after administration of Ad5RGE vector, comprising penton RGE motif mutation only. However, the amount of AdT*RGE virus in the spleen 48 hours after virus administration was 5-fold lower compared to unmodified HAdv-C5-based vector [12]. Collectively, these data suggest that although the interactions of virus capsid proteins with host factors and cellular receptors undoubtedly govern virus recognition by the innate immune system, more targeted and complexed modifications of the virus capsid are likely necessary to avoid virus sequestration in tissue phagocytes and virus triggering of the receptors of innate immunity.
The most radical approach to avoiding HAdv recognition by the immune system has been through shielding the entire HAdv virion with polyethylene glycol (PEG) [124-128], or coating the virus with engineered protein shields [129-131]. These approaches have demonstrated both a significantly improved persistence of HAdv in the circulation after intravenous virus administration and a reduction in the amount of inflammatory cytokines in the blood [47, 132]. Although shielding of HAdv with PEG effectively prevents virus neutralization by pre-existing neutralizing antibodies, allowing for repeated gene delivery in a gene transfer or vaccination setting [133, 134], the utility of this approach in other applications has limitations. Specifically, although shielding oncolytic HAdv with PEG or protein conjugates significantly increases the efficacy of oncolytic HAdv-based vectors in mouse models [131, 133], after the initial round of replication, the progeny of oncolytic virus produced in tumor sites would not be covered with PEG or protein shields, thus exposing the virus to receptors and cells of the innate immune system. Moreover, certain cytokines, most notably IL-6 and IL-12, still remain elevated after systemic delivery [135] or direct blood cell exposure to PEGylated HAdv vectors [47], demonstrating that any single-pronged approach to avoiding virus recognition by the innate immune system is unlikely to prevent or alleviate all factors and mechanisms responsible for triggering multi-faceted host innate immune and inflammatory responses to HAdv.
4. Relevance of mice and other animals as models of human immune response to HAdv.
The major limitation of analyzing immune responses to HAdv in mouse experimental systems is that mice are not permissive to HAdv [1, 136], thus little, if any, information on specific tissues, cell types, and cellular receptors pertinent to explaining the pathogenesis of natural HAdv infection in humans can be derived from analyzing low-dose HAdv infections in mice. Nevertheless, the vast majority of findings discussed and conceptualized in this review were obtained upon administering mice intravenously with extremely high doses of viruses, a clinical scenario only observed in immunocompromised hosts with disseminated and often lethal natural HAdv infections [137-141]. The complexity of evaluating the relevance of findings in mouse system to humans stems from the fact that exquisite and definitive data on tissue-resident immune cells that sequester HAdv from the blood, their immediate and delayed responses, the innate immune receptors utilized by these cells to activate inflammatory signaling at time points relevant to the activation state of the immune response, are not available for the human system, due to obvious practical and ethical limitations. Although much information on human immune system activation by HAdv was obtained by infecting cells of epithelial origin, like Hela and human embryonic kidney HEK293 cells, and human peripheral blood mononuclear cells, differentiated in vitro in the presence of supra-physiological amounts of lineage-committing cytokines, the differentiating state and transcriptional and epigenetic programs operating in these in vitro differentiated cells are certainly unlike true tissue-resident macrophages and dendritic cells that ontogenetically arise from bone marrow precursors and home to and reside in human liver, spleen, and other organs. Despite these objective limitations, many aspects of HAdv-host interactions initially discovered in mice were shown to function in human experimental systems and vice versa. First, HAdv serotypes of species A, C, D, E, and F can utilize CAR of both human and mouse origin as a primary high-affinity attachment receptor to gain entry into human and mouse cells [38, 39]. Second, the highly efficient binding of coagulation FX of human origin to HAdv-C5 was also shown for mouse coagulation FX [8]. Third, the natural IgM- and complement-mediated inactivation of FX-binding ablated HAdv-C5-based vectors occurs in both mouse and human sera [25, 26]. Fourth, the cytosolic inactivation of HAdv-Ab complexes by TRIM21 appears to function in both mouse and human systems [142, 143]. Fifth, high-dose intravenous delivery to mice [144] and non-human primates [58] of HAdv triggers an acute release of IL-6 and TNF-α, a response stereotypically observed in patients with severe natural HAdv infection [145-147] and during clinical gene therapy trials with systemic delivery of high doses of HAdv-based vectors [59, 148, 149]. Sixth and last, leukocytopenia and thrombocytopenia observed after intravenous administration of HAdv to mice [14, 103, 150] is also observed in rabbits [151], non-human primates [152] and humans [149] but not in pigs [153]. The vast majority of humans are exposed to various HAdv serotypes through natural infections [154]. It is therefore plausible that pre-existing neutralizing and non-neutralizing Ads may modulate HAdv bio-distribution after intravenous delivery by binding to the virus capsid and directing HAdv-Ab complexes to FcR-positive immune cells, further potentiating the magnitude and scope of innate immune activation. Findings from the mouse system indicate that mice with pre-existing HAdv-C5-specific immunity release significantly higher amounts of TNF-α, IL-2, and IFN-γ after systemic delivery of HAdv-C5-based virus, compared to virus-naïve animals [150]. Likewise, the amounts IL-6 in the blood of pre-immunized rhesus monkeys after intravenous administration of HAdv-C5-based vector was even higher than in naïve animals [155]. Ongoing gene and cancer therapy trials with repeated HAdv administration regimens will provide clarity on whether the same phenomena previously observed in mice will also be seen in humans. With paucity of human data obtained in the same experimental settings that can be directly compared to findings in mice and other animal experimental systems, consideration of limitations objectively existing in each system is warranted. Caution should always be exercised upon directly extrapolating findings to different species, since known differences in host factors, receptors, and regulatory mechanisms of the innate immune systems between mice, other animal models, and humans are abundant and incompletely understood.
Conclusions and Perspectives
Despite the fact that mouse systems are not permissive to infection with human adenoviruses, virus administration to mice has provided a wealth of information on how innate immunity recognizes and responds to infection with this human pathogen. Over the past decade, based largely on studies in mice, there has been a dramatic advance in our understanding of specific factors and mechanisms of virus recognition by the innate immune system. Notably, many of the lessons learned from these experiments do certainly improve our understanding of the virus-host interactions in humans. The main message that we have learned thus far is that the innate immunity deploys each and every remedy at its disposal to limit the dissemination of adenovirus through the blood, to suppress its persistence in transduced tissues, and to activate numerous functionally redundant signaling cascades to ensure that the pathogen is cleared from the body and a strong adaptive immunity arise to prevent future reinfection with the same virus serotype.
Based on the accumulated data, it appears that virus interactions with tissue-resident phagocytes (most notably Kupffer cells in the liver and marginal-zone macrophages in the spleen) and virus escape from the endosomal compartments are two principal factors that are mechanistically responsible for and drive the development of the majority of the pathological sequelae observed after host exposure to a high dose of HAdv or HAdv-based vectors. Future efforts should be focused on improving our understanding of structural aspects of HAdv interactions with IgM and complement components, on uncovering the identity of receptors on phagocytic cells that sequester HAdv from the blood, and on elucidating the mechanisms of their interaction with the virus. We still have much to learn about mechanisms of HAdv recognition by the innate immunity and studies in mouse systems will surely continue to provide new insights.
Acknowledgements
We are thankful to Becky Kinkead (Emory University) for manuscript editing. This work was supported by the US NIH grants AI065429 and AI107960, David C. Lowance Endowment Fund, and Children’s Healthcare of Atlanta Research Trust to D.M.S.
Footnotes
Conflict of interest
Dmitry Shayakhmetov is a founder, shareholder, and chief scientific officer of AdCure Bio, LLC, which develops adenovirus-based therapies for clinical use.
References:
- 1.Jogler C, Hoffmann D, Theegarten D, Grunwald T, Uberla K & Wildner O (2006) Replication properties of human adenovirus in vivo and in cultures of primary cells from different animal species, J Virol. 80, 3549–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alemany R, Suzuki K & Curiel DT (2000) Blood clearance rates of adenovirus type 5 in mice, The Journal of general virology. 81, 2605–9. [DOI] [PubMed] [Google Scholar]
- 3.Di Paolo NC, van Rooijen N, Shayakhmetov DM (2009) Redundant and synergistic mechanisms control the sequestration of blood-born adenovirus in the liver., Mol Ther. 17, 675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Paolo NC & Shayakhmetov DM (2009) Adenovirus de-targeting from the liver, Curr Opin Mol Ther. 11, 523–31. [PubMed] [Google Scholar]
- 5.Xu Z, T. J., Smith JS, Byrnes AP (2008) Clearance of adenovirus by Kupffer cells is mediated by scavenger receptors, natural antibodies, and complement. J Virol. 82, 11705–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khare R, Hillestad ML, Xu Z, Byrnes AP & Barry MA (2013) Circulating antibodies and macrophages as modulators of adenovirus pharmacology, J Virol. 87, 3678–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shayakhmetov DM, Gaggar A, Ni SH, Li ZY & Lieber A (2005) Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity, Journal of Virology. 79, 7478–7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalyuzhniy O , D. P. N., Silvestry M , Hofherr SE , Barry MA , Stewart PL , Shayakhmetov DM (2008) Adenovirus serotype 5 hexon is critical for virus infection of hepatocytes in vivo., Proc Natl Acad Sci U S A. 105, 5483–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waddington SN, Mcvey JH, Bhella D, Parker AL, Barker K, Atoda H, Pink R, Buckley SMK, Greig JA, Denby L, Custers J, Morita T, Francischetti IMB, Monteiro RQ, Barouch DH, van Rooijen N, Napoli C, Hlavenga MJE, Nicklin SA & Baker AH (2008) Adenovirus serotype 5 hexon mediates liver gene transfer, Cell. 132, 397–409. [DOI] [PubMed] [Google Scholar]
- 10.Baker A, McVey JH, Waddington SN, Di Paolo NC, Shayakhmetov DM. (2007) The influence of blood on in vivo adenovirus bio-distribution and transduction., Mol Ther, 15, 1410–6. [DOI] [PubMed] [Google Scholar]
- 11.Liu Q, Zaiss AK, Colarusso P, Patel K, Haljan G, Wickham TJ & Muruve DA (2003) The role of capsid-endothelial interactions in the innate immune response to adenovirus vectors, Hum Gene Ther. 14, 627–43. [DOI] [PubMed] [Google Scholar]
- 12.Bradshaw AC, Coughlan L, Miller AM, Alba R, van Rooijen N, Nicklin SA & Baker AH (2012) Biodistribution and inflammatory profiles of novel penton and hexon double-mutant serotype 5 adenoviruses, Journal of Controlled Release. 164, 394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stone D, Liu Y, Shayakhmetov D, Li ZY, Ni SH & Lieber A (2007) Adenovirus-platelet interaction in blood causes virus sequestration to the reticuloendothelial system of the liver, Journal of Virology. 81, 4866–4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Othman M, Labelle A, Mazzetti I, Elbatarny HS & Lillicrap D (2007) Adenovirus-induced thrombocytopenia: the role of von Willebrand factor and P-selectin in mediating accelerated platelet clearance, Blood. 109, 2832–2839. [DOI] [PubMed] [Google Scholar]
- 15.Parker AL, Waddington SN, Nicol CG, Shayakhmetov DM, Buckley SM, Denby L, Kemball-Cook G, Ni SH, Lieber A, McVey JH, Nicklin SA & Baker AH (2006) Multiple vitamin K-dependent coagulation zymogens promote adenovirus-mediated gene delivery to hepatocytes, Blood. 108, 2554–2561. [DOI] [PubMed] [Google Scholar]
- 16.Irons EE, Flatt JW, Doronin K, Fox TL, Acchione M, Stewart PL & Shayakhmetov DM (2013) Coagulation factor binding orientation and dimerization may influence infectivity of adenovirus-coagulation factor complexes, J Virol. 87, 9610–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reddy VS, Natchiar SK, Stewart PL & Nemerow GR (2010) Crystal Structure of Human Adenovirus at 3.5 angstrom Resolution, Science. 329, 1071–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doronin K, Flatt JW, Di Paolo NC, Khare R, Kalyuzhniy O, Acchione M, Sumida JP, Ohto U, Shimizu T, Akashi-Takamura S, Miyake K, MacDonald JW, Bammler TK, Beyer RP, Farin FM, Stewart PL & Shayakhmetov DM (2012) Coagulation Factor X Activates Innate Immunity to Human Species C Adenovirus, Science. 338, 795–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tian J, X. Z., Byrnes AP (2019) Serotype-specific binding of prothrombin to adenovirus vectors, Molecular therapy. 27, 195–196. [Google Scholar]
- 20.Doszpoly A, de la Cuesta F, Lopez-Gordo E, Benezech C, Nicklin SA & Baker AH (2019) Human Adenovirus Serotype 5 Is Sensitive to IgM-Independent Neutralization In Vitro and In Vivo, Viruses. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morral N, O'Neal W, Rice K, Leland M, Kaplan J, Piedra PA, Zhou H, Parks RJ, Velji R, Aguilar-Cordova E, Wadsworth S, Graham FL, Kochanek S, Carey KD & Beaudet AL (1999) Administration of helper-dependent adenoviral vectors and sequential delivery of different vector serotype for long-term liver-directed gene transfer in baboons, Proc Natl Acad Sci U S A. 96, 12816–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benihoud K, Saggio I, Opolon P, Salone B, Amiot F, Connault E, Chianale C, Dautry F, Yeh P & Perricaudet M (1998) Efficient, repeated adenovirus-mediated gene transfer in mice lacking both tumor necrosis factor alpha and lymphotoxin alpha, Journal of Virology. 72, 9514–9525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Croyle MA, Chirmule N, Zhang Y & Wilson JM (2002) PEGylation of E1-deleted adenovirus vectors allows significant gene expression on readministration to liver, Human Gene Therapy. 13, 1887–1900. [DOI] [PubMed] [Google Scholar]
- 24.Cotter MJ, Zaiss AK & Muruve DA (2005) Neutrophils interact with adenovirus vectors via Fc receptors and complement receptor 1, Journal of Virology. 79, 14622–14631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu Z, Q. Q., Tian J, Smith JS, Conenello GM, Morita T, Byrnes AP. (2013) Coagulation factor X shields adenovirus type 5 from attack by natural antibodies and complement, Nature Medicine, advanced online publication; published March 24th 2013; doi: 10.1038/nm.3107. [DOI] [PubMed] [Google Scholar]
- 26.Harmon AW, Moitra R, Xu Z & Byrnes AP (2018) Hexons from adenovirus serotypes 5 and 48 differentially protect adenovirus vectors from neutralization by mouse and human serum, PLoS One. 13, e0192353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fletcher AJ & James LC (2016) Coordinated Neutralization and Immune Activation by the Cytosolic Antibody Receptor TRIM21, J Virol. 90, 4856–4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mallery DL, McEwan WA, Bidgood SR, Towers GJ, Johnson CM & James LC (2010) Antibodies mediate intracellular immunity through tripartite motif-containing 21 (TRIM21), Proc Natl Acad Sci U S A. 107, 19985–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McEwan WA, Tam JC, Watkinson RE, Bidgood SR, Mallery DL & James LC (2013) Intracellular antibody-bound pathogens stimulate immune signaling via the Fc receptor TRIM21, Nat Immunol. 14, 327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fletcher AJ, Mallery DL, Watkinson RE, Dickson CF & James LC (2015) Sequential ubiquitination and deubiquitination enzymes synchronize the dual sensor and effector functions of TRIM21, Proc Natl Acad Sci U S A. 112, 10014–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foss S, Bottermann M, Jonsson A, Sandlie I, James LC & Andersen JT (2019) TRIM21-From Intracellular Immunity to Therapy, Front Immunol. 10, 2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolff G, Worgall S, van Rooijen N, Song WR, Harvey BG & Crystal RG (1997) Enhancement of in vivo adenovirus-mediated gene transfer and expression by prior depletion of tissue macrophages in the target organ, J Virol. 71, 624–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lieber A, He CY, Meuse L, Schowalter D, Kirillova I, Winther B & Kay MA (1997) The role of Kupffer cell activation and viral gene expression in early liver toxicity after infusion of recombinant adenovirus vectors, J Virol. 71, 8798–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shayakhmetov DM, Li ZY, Ni S & Lieber A (2004) Analysis of adenovirus sequestration in the liver, transduction of hepatic cells, and innate toxicity after injection of fiber-modified vectors, J Virol. 78, 5368–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith JS, Xu ZL & Byrnes AP (2008) A quantitative assay for measuring clearance of adenovirus vectors by Kupffer cells, Journal of Virological Methods. 147, 54–60. [DOI] [PubMed] [Google Scholar]
- 36.Smith JS, Xu ZL, Tian J, Stevenson SC & Byrnes AP (2008) Interaction of systemically delivered adenovirus vectors with Kupffer cells in mouse liver, Human Gene Therapy. 19, 547–554. [DOI] [PubMed] [Google Scholar]
- 37.Di Paolo NC, Miao EA, Iwakura Y, Murali-Krishna K, Aderem A, Flavell RA, Papayannopoulou T & Shayakhmetov DM (2009) Virus Binding to a Plasma Membrane Receptor Triggers Interleukin-1 alpha-Mediated Proinflammatory Macrophage Response In Vivo, Immunity. 31, 110–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL & Finberg RW (1997) Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5, Science. 275, 1320–3. [DOI] [PubMed] [Google Scholar]
- 39.Tomko RP, Xu R & Philipson L (1997) HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses, Proc Natl Acad Sci U S A. 94, 3352–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gaggar A, Shayakhmetov D & Lieber A (2003) CD46 is a cellular receptor for group B adenoviruses, Nature Medicine. 9, 1408–1412. [DOI] [PubMed] [Google Scholar]
- 41.Haisma HJ, Kamps JA, Kamps GK & Bellu AR (2007) Poly I prevents adenoviral vector clearance by Kupffer cells and increases efficiency in gene therapy applications, Human Gene Therapy. 18, 981–982. [Google Scholar]
- 42.Haisma HJ, Kamps JAAM, Kamps GK, Plantinga JA, Rots MG & Bellu AR (2008) Polyinosinic acid enhances delivery of adenovirus vectors in vivo by preventing sequestration in liver macrophages, Journal of General Virology. 89, 1097–1105. [DOI] [PubMed] [Google Scholar]
- 43.Piccolo P, Vetrini F, Mithbaokar P, Grove NC, Bertin T, Palmer D, Ng P & Brunetti-Pierri N (2013) SR-A and SREC-I Are Kupffer and Endothelial Cell Receptors for Helper-dependent Adenoviral Vectors, Mol Ther. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haisma HJ, Boesjes M, Beerens AM, van der Strate BW, Curiel DT, Pluddemann A, Gordon S & Bellu AR (2009) Scavenger receptor A: a new route for adenovirus 5, Mol Pharm. 6, 366–74. [DOI] [PubMed] [Google Scholar]
- 45.Khare R, Reddy VS, Nemerow GR & Barry MA (2012) Identification of adenovirus serotype 5 hexon regions that interact with scavenger receptors, J Virol. 86, 2293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khare R, May SM, Vetrini F, Weaver EA, Palmer D, Rosewell A, Grove N, Ng P & Barry MA (2011) Generation of a Kupffer Cell-evading Adenovirus for Systemic and Liver-directed Gene Transfer, Molecular Therapy. 19, 1254–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krutzke L, Prill JM, Engler T, Schmidt CQ, Xu Z, Byrnes AP, Simmet T & Kreppel F (2016) Substitution of blood coagulation factor X-binding to Ad5 by position-specific PEGylation: Preventing vector clearance and preserving infectivity, J Control Release. 235, 379–392. [DOI] [PubMed] [Google Scholar]
- 48.Nemerow GR (2000) Cell receptors involved in adenovirus entry, Virology. 274, 1–4. [DOI] [PubMed] [Google Scholar]
- 49.He JQ, Katschke KJ Jr., Gribling P, Suto E, Lee WP, Diehl L, Eastham-Anderson J, Ponakala A, Komuves L, Egen JG & van Lookeren Campagne M (2013) CRIg mediates early Kupffer cell responses to adenovirus, J Leukoc Biol. 93, 301–6. [DOI] [PubMed] [Google Scholar]
- 50.Alba R, Bradshaw AC, Coughlan L, Denby L, McDonald RA, Waddington SN, Buckley SMK, Greig JA, Parker AL, Miller AM, Wang HJ, Lieber A, van Rooijen N, McVey JH, Nicklin SA & Baker AH (2010) Biodistribution and retargeting of FX-binding ablated adenovirus serotype 5 vectors, Blood. 116, 2656–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maler MD, Nielsen PJ, Stichling N, Cohen I, Ruzsics Z, Wood C, Engelhard P, Suomalainen M, Gyory I, Huber M, Muller-Quernheim J, Schamel WWA, Gordon S, Jakob T, Martin SF, Jahnen-Dechent W, Greber UF, Freudenberg MA & Fejer G (2017) Key Role of the Scavenger Receptor MARCO in Mediating Adenovirus Infection and Subsequent Innate Responses of Macrophages, MBio. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stichling N, Suomalainen M, Flatt JW, Schmid M, Pacesa M, Hemmi S, Jungraithmayr W, Maler MD, Freudenberg MA, Pluckthun A, May T, Koster M, Fejer G & Greber UF (2018) Lung macrophage scavenger receptor SR-A6 (MARCO) is an adenovirus type-specific virus entry receptor, PLoS Pathog. 14, e1006914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith T, Idamakanti N, Kylefjord H, Rollence M, King L, Kaloss M, Kaleko M & Stevenson SC (2002) In vivo hepatic adenoviral gene delivery occurs independently of the coxsackievirus-adenovirus receptor, Mol Ther. 5, 770–9. [DOI] [PubMed] [Google Scholar]
- 54.Mizuguchi H, Koizumi N, Hosono T, Ishii-Watabe A, Uchida E, Utoguchi N, Watanabe Y & Hayakawa T (2002) CAR- or alphav integrin-binding ablated adenovirus vectors, but not fiber-modified vectors containing RGD peptide, do not change the systemic gene transfer properties in mice, Gene Ther. 9, 769–76. [DOI] [PubMed] [Google Scholar]
- 55.Martin K, Brie A, Saulnier P, Perricaudet M, Yeh P & Vigne E (2003) Simultaneous CAR- and alpha V integrin-binding ablation fails to reduce Ad5 liver tropism, Mol Ther. 8, 485–94. [DOI] [PubMed] [Google Scholar]
- 56.Zaiss AK, Foley EM, Lawrence R, Schneider LS, Hoveida H, Secrest P, Catapang AB, Yamaguchi Y, Alemany R, Shayakhmetov DM, Esko JD & Herschman HR (2016) Hepatocyte Heparan Sulfate Is Required for Adeno-Associated Virus 2 but Dispensable for Adenovirus 5 Liver Transduction In Vivo, J Virol. 90, 412–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Enrich C, Pol A, Calvo M, Pons M & Jackle S (1999) Dissection of the multifunctional “Receptor-Recycling” endocytic compartment of hepatocytes, Hepatology. 30, 1115–20. [DOI] [PubMed] [Google Scholar]
- 58.Brunetti-Pierri N, Palmer DJ, Beaudet AL, Carey KD, Finegold M, Ng P (2004) Acute toxicity after high-dose systemic injection of helper-dependent adenoviral vectors into non-human primates, Human Gene Therapy. 15, 35–46. [DOI] [PubMed] [Google Scholar]
- 59.Raper SE, Chirmule N, Lee FS, Wivel NA, Bagg A, Gao GP, Wilson JM & Batshaw ML (2003) Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer, Mol Genet Metab. 80, 148–58. [DOI] [PubMed] [Google Scholar]
- 60.Worgall S, Leopold PL, Wolff G, Ferris B, VanRoijen N & Crystal RG (1997) Role of alveolar macrophages in rapid elimination of adenovirus vectors administered to the epithelial surface of the respiratory tract, Human Gene Therapy. 8, 1675–1684. [DOI] [PubMed] [Google Scholar]
- 61.Worgall S, Wolff G, Falck-Pedersen E & Crystal RG (1997) Innate immune mechanisms dominate elimination of adenoviral vectors following in vivo administration, Hum Gene Ther. 8, 37–44. [DOI] [PubMed] [Google Scholar]
- 62.Browne A, Tookman LA, Ingemarsdotter CK, Bouwman RD, Pirlo K, Wang Y, McNeish IA & Lockley M (2015) Pharmacological Inhibition of beta3 Integrin Reduces the Inflammatory Toxicities Caused by Oncolytic Adenovirus without Compromising Anticancer Activity, Cancer Res. 75, 2811–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weber J (1976) Genetic analysis of adenovirus type 2 III. Temperature sensitivity of processing viral proteins, J Virol. 17, 462–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Motwani M, Pesiridis S & Fitzgerald KA (2019) DNA sensing by the cGAS-STING pathway in health and disease, Nat Rev Genet. [DOI] [PubMed] [Google Scholar]
- 65.Barber GN (2011) Innate immune DNA sensing pathways: STING, AIMII and the regulation of interferon production and inflammatory responses, Curr Opin Immunol. 23, 10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Anghelina D, Lam E & Falck-Pedersen E (2016) Diminished Innate Antiviral Response to Adenovirus Vectors in cGAS/STING-Deficient Mice Minimally Impacts Adaptive Immunity, J Virol. 90, 5915–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang F, Alain T, Szretter KJ, Stephenson K, Pol JG, Atherton MJ, Hoang HD, Fonseca BD, Zakaria C, Chen L, Rangwala Z, Hesch A, Chan ESY, Tuinman C, Suthar MS, Jiang Z, Ashkar AA, Thomas G, Kozma SC, Gale M Jr., Fitzgerald KA, Diamond MS, Mossman K, Sonenberg N, Wan Y & Lichty BD (2016) S6K-STING interaction regulates cytosolic DNA-mediated activation of the transcription factor IRF3, Nat Immunol. 17, 514–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tsuzuki S, Tachibana M, Hemmi M, Yamaguchi T, Shoji M, Sakurai F, Kobiyama K, Kawabata K, Ishii KJ, Akira S & Mizuguchi H (2016) TANK-binding kinase 1-dependent or -independent signaling elicits the cell-type-specific innate immune responses induced by the adenovirus vector, Int Immunol. 28, 105–15. [DOI] [PubMed] [Google Scholar]
- 69.Fejer G, Drechsel L, Liese J, Schleicher U, Ruzsics Z, Imelli N, Greber UF, Keck S, Hildenbrand B, Krug A, Bogdan C & Freudenberg MA (2008) Key role of splenic myeloid DCs in the IFN-alphabeta response to adenoviruses in vivo, PLoS Pathog. 4, e1000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang L, Wen M & Cao X (2019) Nuclear hnRNPA2B1 initiates and amplifies the innate immune response to DNA viruses, Science. 365. [DOI] [PubMed] [Google Scholar]
- 71.Suzuki M, Cela R, Bertin TK, Sule G, Cerullo V, Rodgers JR & Lee B (2011) NOD2 signaling contributes to the innate immune response against helper-dependent adenovirus vectors independently of MyD88 in vivo, Hum Gene Ther. 22, 1071–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Muruve DA, Petrilli V, Zaiss AK, White LR, Clark SA, Ross PJ, Parks RJ & Tschopp J (2008) The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response, Nature. 452, 103–U11. [DOI] [PubMed] [Google Scholar]
- 73.Ting JP, Willingham SB & Bergstralh DT (2008) NLRs at the intersection of cell death and immunity, Nat Rev Immunol. 8, 372–9. [DOI] [PubMed] [Google Scholar]
- 74.Beutler B (2004) Inferences, questions and possibilities in Toll-like receptor signalling, Nature. 430, 257–63. [DOI] [PubMed] [Google Scholar]
- 75.Doyle SL & O'Neill LA (2006) Toll-like receptors: from the discovery of NFkappaB to new insights into transcriptional regulations in innate immunity, Biochem Pharmacol. 72, 1102–13. [DOI] [PubMed] [Google Scholar]
- 76.O'Neill LA (2006) How Toll-like receptors signal: what we know and what we don't know, Curr Opin Immunol. 18, 3–9. [DOI] [PubMed] [Google Scholar]
- 77.Suzuki M, Cerullo V, Bertin TK, Cela R, Clarke C, Guenther M, Brunetti-Pierri N & Lee B (2010) MyD88-dependent silencing of transgene expression during the innate and adaptive immune response to helper-dependent adenovirus, Hum Gene Ther. 21, 325–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Appledorn DM, Patial S, McBride A, Godbehere S, Van Rooijen N, Parameswaran N & Amalfitano A (2008) Adenovirus vector-induced innate inflammatory mediators, MAPK signaling, as well as adaptive immune responses are dependent upon both TLR2 and TLR9 in vivo, J Immunol. 181, 2134–44. [DOI] [PubMed] [Google Scholar]
- 79.Deguine J & Barton GM (2014) MyD88: a central player in innate immune signaling, F1000Prime Rep. 6, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Smith JS, Xu Z, Tian J, Palmer DJ, Ng P & Byrnes AP (2011) The role of endosomal escape and mitogen-activated protein kinases in adenoviral activation of the innate immune response, PLoS One. 6, e26755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhou X, Ramke M, Chintakuntlawar AV, Lee JY, Rajaiya J & Chodosh J (2017) Role of MyD88 in adenovirus keratitis, Immunol Cell Biol. 95, 108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bottermann M, Foss S, Caddy SL, Clift D, van Tienen LM, Vaysburd M, Cruickshank J, O'Connell K, Clark J, Mayes K, Higginson K, Lode HE, McAdam MB, Sandlie I, Andersen JT & James LC (2019) Complement C4 Prevents Viral Infection through Capsid Inactivation, Cell Host Microbe. 25, 617–629 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Basner-Tschakarjan E, Gaffal E, O'Keeffe M, Tormo D, Limmer A, Wagner H, Hochrein H & Tuting T (2006) Adenovirus efficiently transduces plasmacytoid dendritic cells resulting in TLR9-dependent maturation and IFN-alpha production, Journal of Gene Medicine. 8, 1300–1306. [DOI] [PubMed] [Google Scholar]
- 84.Zhu JG, Huang XP & Yang YP (2007) Innate immune response to adenoviral vectors is mediated by both Toll-like receptor-dependent and -independent pathways, Journal of Virology. 81, 3170–3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gilliet M, Cao W & Liu YJ (2008) Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases, Nat Rev Immunol. 8, 594–606. [DOI] [PubMed] [Google Scholar]
- 86.Di Paolo NC & Shayakhmetov DM (2016) Interleukin 1[alpha] and the inflammatory process, Nat Immunol. 17, 906–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Di Paolo NC, Doronin K, Baldwin LK, Papayannopoulou T & Shayakhmetov DM (2013) The transcription factor IRF3 triggers “defensive suicide” necrosis in response to viral and bacterial pathogens, Cell reports. 3, 1840–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tang Z, Zang N, Fu Y, Ye Z, Chen S, Mo S, Ren L & Liu E (2018) HMGB1 mediates HAdV-7 infection-induced pulmonary inflammation in mice, Biochem Biophys Res Commun. 501, 1–8. [DOI] [PubMed] [Google Scholar]
- 89.Di Paolo NC, Baldwin LK, Irons EE, Papayannopoulou T, Tomlinson S & Shayakhmetov DM (2014) IL-1alpha and complement cooperate in triggering local neutrophilic inflammation in response to adenovirus and eliminating virus-containing cells, PLoS Pathog. 10, e1004035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhu J, Huang X & Yang Y (2008) A critical role for type I IFN-dependent NK cell activation in innate immune elimination of adenoviral vectors in vivo, Mol Ther. 16, 1300–7. [DOI] [PubMed] [Google Scholar]
- 91.Zhu J, Huang X & Yang Y (2010) NKG2D is required for NK cell activation and function in response to E1-deleted adenovirus, J Immunol. 185, 7480–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kochanek S, Schiedner G & Volpers C (2001) High-capacity ‘gutless' adenoviral vectors, Curr Opin Mol Ther. 3, 454–63. [PubMed] [Google Scholar]
- 93.Jager L & Ehrhardt A (2009) Persistence of high-capacity adenoviral vectors as replication-defective monomeric genomes in vitro and in murine liver, Hum Gene Ther. 20, 883–96. [DOI] [PubMed] [Google Scholar]
- 94.Reddy PS, Sakhuja K, Ganesh S, Yang L, Kayda D, Brann T, Pattison S, Golightly D, Idamakanti N, Pinkstaff A, Kaloss M, Barjot C, Chamberlain JS, Kaleko M & Connelly S (2002) Sustained human factor VIII expression in hemophilia A mice following systemic delivery of a gutless adenoviral vector, Mol Ther. 5, 63–73. [DOI] [PubMed] [Google Scholar]
- 95.McCaffrey AP, Fawcett P, Nakai H, McCaffrey RL, Ehrhardt A, Pham TT, Pandey K, Xu H, Feuss S, Storm TA & Kay MA (2008) The host response to adenovirus, helper-dependent adenovirus, and adeno-associated virus in mouse liver, Mol Ther. 16, 931–41. [DOI] [PubMed] [Google Scholar]
- 96.Chuah MK, Schiedner G, Thorrez L, Brown B, Johnston M, Gillijns V, Hertel S, Van Rooijen N, Lillicrap D, Collen D, VandenDriessche T & Kochanek S (2003) Therapeutic factor VIII levels and negligible toxicity in mouse and dog models of hemophilia A following gene therapy with high-capacity adenoviral vectors, Blood. 101, 1734–43. [DOI] [PubMed] [Google Scholar]
- 97.Brown BD, Shi CX, Powell S, Hurlbut D, Graham FL & Lillicrap D (2004) Helper-dependent adenoviral vectors mediate therapeutic factor VIII expression for several months with minimal accompanying toxicity in a canine model of severe hemophilia A, Blood. 103, 804–10. [DOI] [PubMed] [Google Scholar]
- 98.Brunetti-Pierri N, Ng T, Iannitti D, Cioffi W, Stapleton G, Law M, Breinholt J, Palmer D, Grove N, Rice K, Bauer C, Finegold M, Beaudet A, Mullins C & Ng P (2013) Transgene expression up to 7 years in nonhuman primates following hepatic transduction with helper-dependent adenoviral vectors, Hum Gene Ther. 24, 761–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rastall DP, Seregin SS, Aldhamen YA, Kaiser LM, Mullins C, Liou A, Ing F, Pereria-Hicks C, Godbehere-Roosa S, Palmer D, Ng P & Amalfitano A (2016) Long-term, high-level hepatic secretion of acid alpha-glucosidase for Pompe disease achieved in non-human primates using helper-dependent adenovirus, Gene Ther. 23, 743–752. [DOI] [PubMed] [Google Scholar]
- 100.Zhu JG, Huang XP & Yang YP (2007) Type IIFN signaling on both B and CD4 T cells is required for protective antibody response to adenovirus, Journal of Immunology. 178, 3505–3510. [DOI] [PubMed] [Google Scholar]
- 101.Hensley SE, Giles-Davis W, McCoy KC, Weninger W & Ertl HC (2005) Dendritic cell maturation, but not CD8+ T cell induction, is dependent on type I IFN signaling during vaccination with adenovirus vectors, J Immunol. 175, 6032–41. [DOI] [PubMed] [Google Scholar]
- 102.Tian J, Xu ZL, Smith JS, Hofherr SE, Barry MA & Byrnes AP (2009) Adenovirus Activates Complement by Distinctly Different Mechanisms In Vitro and In Vivo: Indirect Complement Activation by Virions In Vivo, Journal of Virology. 83, 5648–5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kiang A, Hartman ZC, Everett RS, Serra D, Jiang HX, Frank MM & Amalfitano A (2006) Multiple innate inflammatory responses induced after systemic adenovirus vector delivery depend on a functional complement system, Molecular Therapy. 14, 588–598. [DOI] [PubMed] [Google Scholar]
- 104.Appledorn DM, McBride A, Seregin S, Scott JM, Schuldt N, Kiang A, Godbehere S & Amalfitano A (2008) Complex interactions with several arms of the complement system dictate innate and humoral immunity to adenoviral vectors, Gene Ther. 15, 1606–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Appledorn DM, Kiang A, McBride A, Jiang H, Seregin S, Scott JM, Stringer R, Kousa Y, Hoban M, Frank MM & Amalfitano A (2008) Wild-type adenoviruses from groups A-F evoke unique innate immune responses, of which HAd3 and SAd23 are partially complement dependent, Gene Ther. 15, 885–901. [DOI] [PubMed] [Google Scholar]
- 106.Othman M, Labelle A, Mazzetti I & Lillicrap D (2005) Adenovirus induced thrombocytopenia: The role of P-selectin and von Willebrand factor in virus-mediated platelet clearance., Blood. 106, 68A–68A. [DOI] [PubMed] [Google Scholar]
- 107.Li Y, Muruve DA, Collins RG, Lee SS & Kubes P (2002) The role of selectins and integrins in adenovirus vector-induced neutrophil recruitment to the liver, Eur J Immunol. 32, 3443–52. [DOI] [PubMed] [Google Scholar]
- 108.Stone D, Liu Y, Li ZY, Tuve S, Strauss R & Lieber A (2007) Comparison of adenoviruses from species B, C, E, and F after intravenous delivery, Mol Ther. 15, 2146–53. [DOI] [PubMed] [Google Scholar]
- 109.Wu C, Lu W, Zhang Y, Zhang G, Shi X, Hisada Y, Grover SP, Zhang X, Li L, Xiang B, Shi J, Li XA, Daugherty A, Smyth SS, Kirchhofer D, Shiroishi T, Shao F, Mackman N, Wei Y & Li Z (2019) Inflammasome Activation Triggers Blood Clotting and Host Death through Pyroptosis, Immunity. 50, 1401–1411 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xu Z, Smith JS, Tian J & Byrnes AP (2010) Induction of shock after intravenous injection of adenovirus vectors: a critical role for platelet-activating factor, Mol Ther. 18, 609–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fejer G, Szalay K, Gyory I, Fejes M, Kusz E, Nedieanu S, Pali T, Schmidt T, Siklodi B, Lazar G Jr., Lazar G Sr. & Duda E (2005) Adenovirus infection dramatically augments lipopolysaccharide-induced TNF production and sensitizes to lethal shock, J Immunol. 175, 1498–506. [DOI] [PubMed] [Google Scholar]
- 112.Kayagaki N, Wong MT, Stowe IB, Ramani SR, Gonzalez LC, Akashi-Takamura S, Miyake K, Zhang J, Lee WP, Muszynski A, Forsberg LS, Carlson RW & Dixit VM (2013) Noncanonical inflammasome activation by intracellular LPS independent of TLR4, Science. 341, 1246–9. [DOI] [PubMed] [Google Scholar]
- 113.Hagar JA, Powell DA, Aachoui Y, Ernst RK & Miao EA (2013) Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock, Science. 341, 1250–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mandal P, Feng Y, Lyons JD, Berger SB, Otani S, DeLaney A, Tharp GK, Maner-Smith K, Burd EM, Schaeffer M, Hoffman S, Capriotti C, Roback L, Young CB, Liang Z, Ortlund EA, DiPaolo NC, Bosinger S, Bertin J, Gough PJ, Brodsky IE, Coopersmith CM, Shayakhmetov DM & Mocarski ES (2018) Caspase-8 Collaborates with Caspase-11 to Drive Tissue Damage and Execution of Endotoxic Shock, Immunity. 49, 42–55 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rathinam VA, Vanaja SK, Waggoner L, Sokolovska A, Becker C, Stuart LM, Leong JM & Fitzgerald KA (2012) TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by gram-negative bacteria, Cell. 150, 606–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sakurai F, Nakashima K, Yamaguchi T, Ichinose T, Kawabata K, Hayakawa T & Mizuguchi H (2010) Adenovirus serotype 35 vector-induced innate immune responses in dendritic cells derived from wild-type and human CD46-transgenic mice: Comparison with a fiber-substituted Ad vector containing fiber proteins of Ad serotype 35, J Control Release. 148, 212–8. [DOI] [PubMed] [Google Scholar]
- 117.Robinson CM, Zhou X, Rajaiya J, Yousuf MA, Singh G, DeSerres JJ, Walsh MP, Wong S, Seto D, Dyer DW, Chodosh J & Jones MS (2013) Predicting the next eye pathogen: analysis of a novel adenovirus, MBio. 4, e00595–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Madisch I, Hofmayer S, Moritz C, Grintzalis A, Hainmueller J, Pring-Akerblom P & Heim A (2007) Phylogenetic analysis and structural predictions of human adenovirus penton proteins as a basis for tissue-specific adenovirus vector design, Journal of Virology. 81, 8270–8281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nemerow GR & Stewart PL (1999) Role of alpha(v) integrins in adenovirus cell entry and gene delivery, Microbiol Mol Biol Rev. 63, 725–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shayakhmetov DM, Eberly AL, Li ZY & Lieber A (2005) Deletion of penton RGD motifs affects the efficiency of both the internalization and the endosorne escape of viral particles containing adenovirus serotype 5 or 35 fiber knobs (vol 79, pg 1053, 2005), Journal of Virology. 79, 4553–4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Koizumi N, Kawabata K, Sakurai F, Watanabe Y, Hayakawa T & Mizuguchi H (2006) Modified adenoviral vectors ablated for coxsackievirus-adenovirus receptor, alphav integrin, and heparan sulfate binding reduce in vivo tissue transduction and toxicity, Hum Gene Ther. 17, 264–79. [DOI] [PubMed] [Google Scholar]
- 122.Coughlan L, Bradshaw AC, Parker AL, Robinson H, White K, Custers J, Goudsmit J, Van Roijen N, Barouch DH, Nicklin SA & Baker AH (2012) Ad5:Ad48 Hexon Hypervariable Region Substitutions Lead to Toxicity and Increased Inflammatory Responses Following Intravenous Delivery, Molecular Therapy. 20, 2268–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Khare R, May SM, Vetrini F, Weaver EA, Palmer D, Rosewell A, Grove N, Ng P & Barry MA (2012) Generation of a Kupffer cell-evading adenovirus for systemic and liver-directed gene transfer, Mol Ther. 19, 1254–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.O'Riordan CR, Lachapelle A, Delgado C, Parkes V, Wadsworth SC, Smith AE & Francis GE (1999) PEGylation of adenovirus with retention of infectivity and protection from neutralizing antibody in vitro and in vivo, Hum Gene Ther. 10, 1349–58. [DOI] [PubMed] [Google Scholar]
- 125.Croyle MA, Chirmule N, Zhang Y & Wilson JM (2002) PEGylation of E1-deleted adenovirus vectors allows significant gene expression on readministration to liver, Hum Gene Ther. 13, 1887–900. [DOI] [PubMed] [Google Scholar]
- 126.Kreppel F & Kochanek S (2008) Modification of adenovirus gene transfer vectors with synthetic polymers: a scientific review and technical guide, Mol Ther. 16, 16–29. [DOI] [PubMed] [Google Scholar]
- 127.Capasso C, Garofalo M, Hirvinen M & Cerullo V (2014) The evolution of adenoviral vectors through genetic and chemical surface modifications, Viruses. 6, 832–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wonganan P & Croyle MA (2010) PEGylated Adenoviruses: From Mice to Monkeys, Viruses. 2, 468–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Schmid M, Ernst P, Honegger A, Suomalainen M, Zimmermann M, Braun L, Stauffer S, Thom C, Dreier B, Eibauer M, Kipar A, Vogel V, Greber UF, Medalia O & Pluckthun A (2018) Adenoviral vector with shield and adapter increases tumor specificity and escapes liver and immune control, Nature communications. 9, 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rojas LA, Condezo GN, Moreno R, Fajardo CA, Arias-Badia M, San Martin C & Alemany R (2016) Albumin-binding adenoviruses circumvent pre-existing neutralizing antibodies upon systemic delivery, J Control Release. 237, 78–88. [DOI] [PubMed] [Google Scholar]
- 131.Capasso C, Hirvinen M, Garofalo M, Romaniuk D, Kuryk L, Sarvela T, Vitale A, Antopolsky M, Magarkar A, Viitala T, Suutari T, Bunker A, Yliperttula M, Urtti A & Cerullo V (2016) Oncolytic adenoviruses coated with MHC-I tumor epitopes increase the antitumor immunity and efficacy against melanoma, Oncoimmunology. 5, e1105429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Choi JW, Lee YS, Yun CO & Kim SW (2015) Polymeric oncolytic adenovirus for cancer gene therapy, J Control Release. 219, 181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Doronin K, Shashkova EV, May SM, Hofherr SE & Barry MA (2009) Chemical modification with high molecular weight polyethylene glycol reduces transduction of hepatocytes and increases efficacy of intravenously delivered oncolytic adenovirus, Hum Gene Ther. 20, 975–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Choi JH, Schafer SC, Freiberg AN & Croyle MA (2015) Bolstering Components of the Immune Response Compromised by Prior Exposure to Adenovirus: Guided Formulation Development for a Nasal Ebola Vaccine, Mol Pharm. 12, 2697–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wonganan P, Clemens CC, Brasky K, Pastore L & Croyle MA (2011) Species differences in the pharmacology and toxicology of PEGylated helper-dependent adenovirus, Mol Pharm. 8, 78–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Illingworth S, Di Y, Bauzon M, Lei J, Duffy MR, Alvis S, Champion B, Lieber A, Hermiston T, Seymour LW, Beadle J & Fisher K (2017) Preclinical Safety Studies of Enadenotucirev, a Chimeric Group B Human-Specific Oncolytic Adenovirus, Molecular therapy oncolytics. 5, 62–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Takamatsu A, Tagashira Y, Hasegawa S & Honda H (2019) Disseminated adenovirus infection in a patient with a hematologic malignancy: a case report and literature review, Future Sci OA. 5, FSO412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lee B, Park E, Ha J, Ha IS, Cheong H Il & Kang HG (2018) Disseminated adenovirus infection in a 10-year-old renal allograft recipient, Kidney Res Clin Pract. 37, 414–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Radke JR & Cook JL (2018) Human adenovirus infections: update and consideration of mechanisms of viral persistence, Curr Opin Infect Dis. 31, 251–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Seo YM, Hwang-Bo S, Kim SK, Han SB, Chung NG & Kang JH (2016) Fatal systemic adenoviral infection superimposed on pulmonary mucormycosis in a child with acute leukemia: A case report, Medicine (Baltimore). 95, e5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.McKillop SJ, Belletrutti MJ, Lee BE, Yap JY, Noga ML, Desai SJ & Sergi C (2015) Adenovirus necrotizing hepatitis complicating atypical teratoid rhabdoid tumor, Pediatr Int. 57, 974–7. [DOI] [PubMed] [Google Scholar]
- 142.Bottermann M, Foss S, van Tienen LM, Vaysburd M, Cruickshank J, O'Connell K, Clark J, Mayes K, Higginson K, Hirst JC, McAdam MB, Slodkowicz G, Hutchinson E, Kozik P, Andersen JT & James LC (2018) TRIM21 mediates antibody inhibition of adenovirus-based gene delivery and vaccination, Proc Natl Acad Sci U S A. 115, 10440–10445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.McEwan WA, Hauler F, Williams CR, Bidgood SR, Mallery DL, Crowther RA & James LC (2012) Regulation of virus neutralization and the persistent fraction by TRIM21, J Virol. 86, 8482–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang Y, Chirmule N, Gao GP, Qian R, Croyle M, Joshi B, Tazelaar J & Wilson JM (2001) Acute cytokine response to systemic adenoviral vectors in mice is mediated by dendritic cells and macrophages, Molecular Therapy. 3, 697–707. [DOI] [PubMed] [Google Scholar]
- 145.Kawasaki Y, Hosoya M, Katayose M & Suzuki H (2002) Correlation between serum interleukin 6 and C-reactive protein concentrations in patients with adenoviral respiratory infection, Pediatr Infect Dis J. 21, 370–4. [DOI] [PubMed] [Google Scholar]
- 146.Mistchenko AS, Robaldo JF, Rosman FC, Koch ERR & Kajon AE (1998) Fatal adenovirus infection associated with new genome type, Journal of Medical Virology. 54, 233–236. [DOI] [PubMed] [Google Scholar]
- 147.Mistchenko AS, Diez RA, Mariani AL, Robaldo J, Maffey AF, Bayley-Bustamante G & Grinstein S (1994) Cytokines in adenoviral disease in children: association of interleukin-6, interleukin-8, and tumor necrosis factor alpha levels with clinical outcome, J Pediatr. 124, 714–20. [DOI] [PubMed] [Google Scholar]
- 148.Machiels JP, Salazar R, Rottey S, Duran I, Dirix L, Geboes K, Wilkinson-Blanc C, Pover G, Alvis S, Champion B, Fisher K, McElwaine-Johnn H, Beadle J & Calvo E (2019) A phase 1 dose escalation study of the oncolytic adenovirus enadenotucirev, administered intravenously to patients with epithelial solid tumors (EVOLVE), Journal for immunotherapy of cancer. 7, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Garcia M, Moreno R, Gil-Martin M, Cascallo M, de Olza MO, Cuadra C, Piulats JM, Navarro V, Domenech M, Alemany R & Salazar R (2019) A Phase 1 Trial of Oncolytic Adenovirus ICOVIR-5 Administered Intravenously to Cutaneous and Uveal Melanoma Patients, Hum Gene Ther. 30, 352–364. [DOI] [PubMed] [Google Scholar]
- 150.Varnavski AN, Calcedo R, Bove M, Gao G & Wilson JM (2005) Evaluation of toxicity from high-dose systemic administration of recombinant adenovirus vector in vector-naive and pre-immunized mice, Gene Ther. 12, 427–36. [DOI] [PubMed] [Google Scholar]
- 151.Cichon G, Schmidt HH, Benhidjeb T, Loser P, Ziemer S, Haas R, Grewe N, Schnieders F, Heeren J, Manns MP, Schlag PM & Strauss M (1999) Intravenous administration of recombinant adenoviruses causes thrombocytopenia, anemia and erythroblastosis in rabbits, J Gene Med. 1, 360–71. [DOI] [PubMed] [Google Scholar]
- 152.Brunetti-Pierri N, Palmer DJ, Beaudet AL, Carey KD, Finegold M & Ng P (2004) Acute toxicity after high-dose systemic injection of helper-dependent adenoviral vectors into nonhuman primates, Hum Gene Ther. 15, 35–46. [DOI] [PubMed] [Google Scholar]
- 153.Koodie L, Robertson MG, Chandrashekar M, Ruth G, Dunning M, Bianco RW & Davydova J (2019) Rodents Versus Pig Model for Assessing the Performance of Serotype Chimeric Ad5/3 Oncolytic Adenoviruses, Cancers (Basel). 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mennechet FJD, Paris O, Ouoba AR, Salazar Arenas S, Sirima SB, Takoudjou Dzomo GR, Diarra A, Traore IT, Kania D, Eichholz K, Weaver EA, Tuaillon E & Kremer EJ (2019) A review of 65 years of human adenovirus seroprevalence, Expert Rev Vaccines. 18, 597–613. [DOI] [PubMed] [Google Scholar]
- 155.Varnavski AN, Zhang Y, Schnell M, Tazelaar J, Louboutin JP, Yu QC, Bagg A, Gao GP & Wilson JM (2002) Preexisting immunity to adenovirus in rhesus monkeys fails to prevent vector-induced toxicity, Journal of Virology. 76, 5711–5719. [DOI] [PMC free article] [PubMed] [Google Scholar]