Abstract
The relationship between haematological malignancy and chemotherapy on the prevalence of antibiotic allergy label (AAL) is ill-defined. We performed a multicentre retrospective case-control study comparing AAL rates amongst cladribine-treated hairy cell leukaemia (C-HCL) cases, non-HCL cladribine-treated controls (control-1) and fludarabine-treated controls (control-2). The prevalence of AALs in C-HCL patients was 60%, compared with control-1 (14%, p<0.01) and control-2 patients (25%, p<0.01). The predominant phenotype was maculopapular exanthem (92%). The drugs implicated in AAL causality in C-HCL patients included beta-lactams (81%), trimethoprim-sulfamethoxazole (58%) and allopurinol (69%). C-HCL patients demonstrate high rates of AAL, potentially due to immune dysregulation, impacting beta-lactam utilization.
Keywords: Hairy cell leukaemia, Cladribine, Antibiotics, Allergy, Maculopapular exanthem
Introduction:
Antibiotic allergy labels (AAL) are reported in up to 35% of patients with a haematological malignancy and are associated with an increased rate of inappropriate prescribing and inferior patient outcomes(1, 2). Although these patients are exposed to more antibiotics than population controls, the impact of host immune dysregulation and chemotherapy on the prevalence and immunopathogenesis of drug allergic responses has not be elucidated.
Hairy cell leukaemia (HCL) is a mature B-cell leukaemia characterised by progressive splenomegaly and bone marrow failure(3). The first-line treatment of HCL is the purine analogue cladribine, which induces high rates of durable remission(4). Cladribine causes transient neutropenia with prolonged CD4+ T-cell lymphopenia with a median duration of 40 months(5) and has been previously associated with high rates of skin rash (6).
The primary objective of this study is to determine the prevalence of AAL in cladribine-treated hairy cell leukaemia compared with non-HCL cladribine-treated controls and controls with similar degrees of T-cell depletion from treatment with fludarabine.
Methods
Study design and setting
We performed a multicentre retrospective case-control study of adult patients treated with cladribine at two tertiary referral centres (Austin Health and Peter MacCallum Cancer Centre) from 1st January 1998 to 31st July 2018. Cases were extracted from hospital chemotherapy databases by searching for “cladribine” and “fludarabine”. Cladribine-treated patients were divided into those with HCL (C-HCL) and other haematological malignancies (control-1). An additional control group included equivalent T-cell depleted patients treated with fludarabine based-regimens for chronic lymphocytic leukaemia (CLL) or follicular lymphoma (FL)(control-2). C-HCL patients were matched 1:2 for sex and year of diagnosis to control-2 patients.
Data collection
Data collected included baseline demographics, details of malignancy, chemotherapy treatment history (date, dose, number of doses), febrile neutropenia (FN) episodes, drug allergy history (previous history, date of onset, allergy phenotype, phenotypic scores(7)), inpatient antibiotic usage (drug, date of onset, duration, subsequent antibiotic, microbiological diagnosis obtained), and results of allergy testing (patch testing [PT], skin prick testing [SPT] and intradermal testing [IDT]).
Definitions
The diagnosis of HCL, CLL and FL were based on previously published definitions(3, 8). Cladribine was given as a daily intravenous infusion or subcutaneous bolus of 0.14mg/kg. Fludarabine was administered intravenously at 25mg/m2 for 3–5 days. FN was defined according to Australian consensus guidelines(9), and antibiotic utilization examined the proportion of patients receiving second-line non-preferred antibiotic therapy(9). Drug latency was the period of time from drug commencement to onset of rash.
Statistical analysis
For descriptive analysis, categorical variables were summarized using frequency and percentage and compared using a Fisher’s exact test. Continuous variables were summarized using mean and standard deviation or median and interquartile range as appropriate and compared using a paired t-test.
Results:
Forty-three patients with C-HCL were identified. Controls included 14 patients with other haematological malignancies treated with cladribine (4 T-cell lymphoma, 4 systemic mastocytosis, 4 malignant histiocytoses, 1 T-cell prolymphocytic leukaemia and 1 Waldenstrom’s macroglobulinemia) [control-1]. C-HCL patients were matched 1:2 with CLL and FL treated with fludarabine containing regimens (n = 80) [control-2].
Baseline demographics of patients are shown in Table 1. C-HCL patients received a median of 5 doses of cladribine, fewer than patients with other haematological malignancies (median 9, interquartile range [IQR] 5–15). C-HCL patients were more likely to develop FN (31/43; 72%) than control-1 (4/14; 29% [p<0.01]) and control-2 patients (22/80; 28% [p<0.01]). C-HCL patients were also more likely to receive non-prophylactic antimicrobials (32/43; 74%) than control-2 (37/80; 46% [p<0.01]), but not control-1 patients (7/14; 50% [p=0.11]).
Table 1: Baseline characteristics and primary antibiotic allergy comparison:
Maculopapular exanthem (MPE) is defined as a delayed (non-immediate) widespread morbilliform rash. Phenotype severity and drug reaction with eosinophilia and systemic symptoms (DRESS) was classified as per the RegiSCAR scoring algorithm(7).
| Demographics/Clinical characteristics | HCL – Cladribine treated C-HCL, n (%) | Non-HCL – Cladribine treated Control (1), n (%) | P-value | Fludarabine treated controls Control (2), n (%) | P-value vs C-HCL |
|---|---|---|---|---|---|
| Patients (n) | 43 | 14 | 80 | ||
| Age, years (median [IQR]) | 53.7 (49.1–59.5) | 53.85 (48.83–62.53) | 0.90 | 64.7 (57.83–70.45) | <0.01 |
| Sex, male (%) | 76.74 | 71.43 | 0.73 | 68.29 | 0.41 |
| Median cladribine doses (IQR) | 5.0 (5.0–5.0) | 9.0 (5.0–15.0) | <0.01 | * | * |
| Febrile neutropenia | 31 (72.09%) | 4 (28.57%) | <0.01 | 22 (27.5%) | <0.01 |
| Antibiotic exposure post chemotherapy | 32 (74.42%) | 7 (50%) | 0.11 | 37 (46.25%) | <0.01 |
| Allergy in those receiving non-prophylactic antibiotics subsequent to chemotherapy | 22/32 (68.75%) | 0/7 (0%) | <0.01 | 10/37 (27.03%) | <0.01 |
| Antibiotic allergy label | 26 (60.47%) | 2 (14.29%) | <0.01 | 20 (25%) | <0.01 |
| Allergy Type: - Maculopapular exanthem - Urticaria - Other/unknown - DRESS (RegiSCAR score ≥2) |
24/26 (92.31%) 1/26 (3.85%) 1/26 (3.85%) 4/26 (15.38%) |
0/2 (0%) 0/2 (0%) 2/2 (100%) 0/2 (0%) |
<0.01 1.00 0.01 1.00 |
10/20 (50%) 1/20 (5%) 9/20 (45%) 1/20 (5%) |
<0.01 1.00 <0.01 0.37 |
Twenty-six patients (60%) with C-HCL had a current documented antibiotic allergy, compared with 2/14 (14%) control-1 (p<0.01)) and 20/80 (25%) control-2 patients (p<0.01). 25 of 26 (96%) AAL’s in C-HCL patients occurred subsequent to cladribine, as opposed to control-1 (0/2, p<0.01) and control-2 patients (12/20; p<0.01). In patients receiving non-prophylactic antibiotics post chemotherapy, C-HCL patients were more likely to have an antibiotic allergy (22/32; 69%) than control-1 (0/7; p<0.01)) and control-2 patients (10/37; p<0.01). Allergy phenotype was consistent with a delayed maculopapular exanthem (MPE) in 24/26 (92%) of C-HCL patients, of which there were 4/26 (15%) with possible or probable drug reaction with eosinophilia and systemic symptoms (DRESS) defined as a RegiSCAR score >2(7).
Table 2 describes the drugs implicated in allergy causality in C-HCL patients. The majority of patients had a number of possible implicated drugs including beta-lactams (21/26; 81%), trimethoprim-sulfamethoxazole (15/26; 58%) and allopurinol (18/26; 69%). Duration between rash onset and drug commencement varied between drug classes with beta lactams having a shorter latency period (median 2 days, IQR 1.25–7) than trimethoprim-sulfamethoxazole (median 12 days; IQR 10–14.5), allopurinol (median 13 days; IQR 10–14), and cladribine itself(median 12 days; IQR 10–13). The median onset between onset of rash from cladribine cessation was 8 days. Beta-lactams associated with skin rash included piperacillin-tazobactam (14/21; 67%), cefepime (4/21; 19%), meropenem (2/21; 10%) and ceftazidime (1/21; 5%). Alternative antimicrobials for FN were prescribed in 12/21 (57%) patients with C-HCL who had a beta-lactam implicated antibiotic allergy. Alternative antimicrobials used included cefepime (7/21; 33%), ciprofloxacin (4/21; 19%), meropenem (3/21; 14%) and moxifloxacin (1/21; 5%).
Table 2: Characteristics of C-HCL patients with allergy.
(abbreviations – M, male; F, female; PIP-TAZ, piperacillin-tazobactam; TMP-SMX, trimethoprim-sulfamethoxazole; MPE, maculopapular exanthem; DRESS, drug reaction with eosinophilia and systemic symptoms; NA, not applicable).
| Patient | Age (years) | Gender | Febrile neutropenia | Allergy phenotype | Regi-SCAR score | Implicated drugs | Beta lactam latency period (days) | Alloprinol latency period (days) | TMP-SMX latency period (days) | Cladribine latency period (days) | Replacement antimicrobial |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 37.4 | F | Y | MPE+DRESS | 4 | PIP-TAZ, allopurinol | 5.00 | 14.00 | NA | 13.00 | nil |
| 2 | 53.3 | M | Y | MPE | 0 | PIP-TAZ, allopurinol | 7.00 | 10.00 | NA | 10.00 | nil |
| 3 | 36.7 | M | Y | urticarial rash | 0 | PIP-TAZ, allopurinol, TMP-SMX | 1.00 | 11.00 | 11.00 | 11.00 | Cefepime |
| 4 | 36.7 | M | Y | MPE | 0 | PIP-TAZ, allopurinol, TMP-SMX | 10.00 | 12.00 | 12.00 | 13.00 | Cefepime |
| 5 | 47.9 | M | Y | MPE | 0 | PIP-TAZ, allopurinol | 7.00 | 12.00 | NA | 12.00 | Meropenem |
| 6 | 77.9 | F | Y | MPE | 1 | PIP-TAZ, allopurinol | 3.00 | 9.00 | NA | 9.00 | Cefepime+moxifloxacin |
| 7 | 29.3 | F | Y | MPE | 0 | PIP-TAZ, TMP-SMX | 2.00 | NA | 11.00 | 11.00 | nil |
| 8 | 51.7 | M | Y | MPE | 0 | PIP-TAZ, allopurinol, TMP-SMX | 4.00 | 16.00 | 16.00 | 12.00 | nil |
| 9 | 50.6 | F | Y | MPE | 1 | PIP-TAZ, TMP-SMX | 2.00 | NA | 14.00 | 11.00 | Cefepime+ciprofloxacin |
| 10 | 73.1 | M | Y | MPE | 0 | Allopurinol | NA | 6.00 | NA | 8.00 | nil |
| 11 | 49.1 | M | Y | MPE | 0 | PIP-TAZ, allopurinol | 0.00 | 14.00 | NA | 11.00 | Cefepime |
| 12 | 73.7 | F | Y | MPE+DRESS | 4 | Cefepime, allopurinol, TMP-SMX | 2.00 | 10.00 | 10.00 | 11.00 | Meropenem/Pentamidine |
| 13 | 76.2 | M | Y | MPE | 0 | TMP-SMX | NA | NA | 22.00 | 11.00 | Pentamidine |
| 14 | 53.7 | M | Y | MPE | 0 | Ceftazidime | 6.00 | NA | NA | 11.00 | nil |
| 15 | 76.8 | M | Y | MPE | 0 | Cefepime, TMP-SMX | 1.00 | NA | 10.00 | 10.00 | nil |
| 16 | 49.1 | M | Y | MPE | 0 | Meropenem, TMP-SMX | 13.00 | NA | 19.00 | 30.00 | nil |
| 17 | 54.8 | F | Y | MPE+DRESS | 2 | PIP-TAZ, allopurinol, TMP-SMX | 9.00 | 14.00 | 14.00 | 14.00 | nil |
| 18 | 49.2 | M | N | MPE | 0 | Allopurinol, TMP-SMX | NA | 10.00 | 10.00 | 10.00 | nil |
| 19 | 30.7 | M | Y | MPE | 0 | Meropenem | 31.00 | NA | NA | 26.00 | Cefepime |
| 20 | 50.6 | M | Y | MPE+ DRESS | 4 | PIP-TAZ, allopurinol, TMP-SMX | 0.00 | 12.00 | 12.00 | 9.00 | Meropenem, Ciprofloxacin |
| 21 | 59.5 | M | N | MPE | 0 | Penicillin, allopurinol | No data | No data | No data | No data | nil |
| 22 | 56.8 | F | Y | MPE | 0 | PIP-TAZ, allopurinol, TMP-SMX | 2.00 | 10.00 | 10.00 | 10.00 | Cefepime |
| 23 | 76.2 | M | N | MPE | 0 | Allopurinol, TMP-SMX | NA | 6.00 | 6.00 | 8.00 | nil |
| 24 | 32.8 | F | Y | MPE | 0 | PIP-TAZ, allopurinol, TMP-SMX | No data | No data | No data | No data | nil |
| 25 | 59.3 | F | Y | MPE | 0 | Cefepime | 2.00 | NA | NA | 13.00 | Ciprofloxacin |
| 26 | 52.5 | M | Y | MPE | 0 | Cefepime, allopurinol | 1.00 | 43.00 | NA | 40.00 | Ciprofloxacin |
A microbiological diagnosis was found in only 3/32 C-HCL patients prescribed antibiotics, all during FN. Patient 1 developed candidaemia and Enterococcus faecium bacteraemia, patient 2 developed Pseudomonas aeruginosa and Enterococcus faecium bacteraemia and patient 3 developed Streptococcal pyogenes bacteraemia.
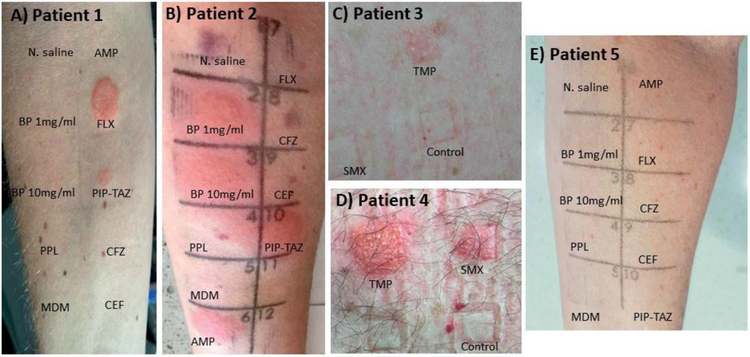
Follow up skin testing, including intradermal and patch testing was performed on five HCL patients with a reported AAL post cladribine. One of the patients was referred from a separate tertiary referral centre. Four of the five patients had positive allergy tests (Fig 1). The clinical characteristics and demographics of these five patients are provided in Supplementary Table 1.
Figure 1. Skin testing in Cladribine-treated HCL patients:
Skin testing either via intradermal testing (IDT) [0.02mls of highest non-irritating concentration] or patch testing (PT) utilizing % concentration of drug in petrolatum. For IDT Normal Saline utilized as negative control and for PT petrolatum alone. All photos provided are from 48-hour reads following testing. A positive IDT was considered to be a greater than 5mm erythematous, raised and indurated lesion present at 6 to 48 hours after IDT (at the site of IDT).(15) Patch tests were graded according to previously published International Contact Dermatitis Research Group Definitions.(16)
(a) Positive delayed intradermal test to flucloxacillin and piperacillin-tazobactam, negative to other tested beta-lactams
(b) Positive delayed intradermal test to benzylpenicillin, ampicillin, piperacillin-tazobactam and flucloxacillin and negative to cephalosporins. Note non-specific brusing in Normal Saline (1) and CFZ (9) panels.
(c) Positive patch testing to trimethoprim (5% conc.) (grade 3 reaction), negative to control (petrolatum) and sulfamethoxazole (10% conc.)
(d) Positive patch testing to trimethoprim (5% conc.) (grade 4 reaction) negative to control (petrolatum) and sulfamethoxazole (10% conc.) Non specific reaction to SMX, this was repeated in isolation with a negative result.
(e) Negative delayed intradermal testing to all tested beta-lactam reagents.
Abbreviations: TMP, trimethoprim (5%) SMX; sulfamethoxazole (10%); PC, petrolatum control; BP, benzylpenicillin (1mg/ml & 10mg/ml); AMP, ampicillin (25mg/ml); PIP-TAZ, piperacillin-tazobactam (4.5mg/ml [piperacillin component]); FLX, flucloxacillin (2mg/ml); MER, meropenem (2.5mg/ml); NSC, Normal Saline control (0.9%); MDM, minor determinant mixture; PPL, benzyl penicilloyl-polylysine.
Discussion:
We demonstrated high rates of AAL in C-HCL patients (60%) compared with cladribine (14%) and fludarabine (25%) treated controls. C-HCL patients had the highest rate of AAL in patients who developed an antibiotic allergy after chemotherapy. Despite cladribine being T-cell depleting, all allergy phenotypes were consistent with a delayed T-cell mediated hypersensitivity.
These findings are consistent with previous reports of high rates of skin rash in C-HCL patients(6). Although cladribine has been reported to cause rash during therapy(10, 11), we conclude that the exanthems are unlikely to be directly attributable to cladribine because the timing of onset of the rash from cladribine cessation (median 8 days) exceeded 5 half-lives of cladribine and its metabolites. In addition all C-HCL patients with a drug allergy received at least 1 other implicated drug.
The majority patients with AAL post cladribine (81%) received beta-lactams (often for FN [72%]) and developed rash within a median of 2 days after its commencement, consistent with known latency periods for MPE (12). Whilst many of these patients were not deemed as possible/probable/definite DRESS, this diagnosis is problematic in a haematology population where eosinophilia is difficult functionally to achieve subsequent to bone marrow suppression. Therefore it is likely we may have underestimated severity of delayed phenotypes and diagnosis of DRESS in this cohort.
We postulate that the high rates of delayed hypersensitivity are due to immune dysregulation in the setting of profound CD4+ lymphopenia caused by cladribine in HCL patients(5), similar to HIV where high rates of antibiotic hypersensitivity have been observed(13). However, in this study, the AAL rate in HCL patients exceeded those in the control-1 group who were also treated with cladribine. Hence excess AAL may be due to the combination of cladribine and unique features of the underlying HCL, and requires further investigation. We were unable to separate treated and untreated HCL as all available patients were treated with cladribine during this period.
Alternative antimicrobials were used in 57% of patients after beta-lactam implicated allergy onset, with cefepime being the most commonly used agent, in accordance with Australian FN guidelines(9). 43% of patients did not receive alternative antimicrobials, perhaps illustrating clinician awareness of the high rates of drug-related fevers in cladribine-treated patients. In our study only 9% of patients with FN post cladribine had bacteraemia, similar to a historical cohort of over 300 patients(14). Although the empiric treatment of FN with broadspectrum antibiotics has long been standard practice, in C-HCL patients the risk of antibiotic-related severe delayed hypersensitivity appears higher than the rate of microbiological diagnosis, and calls this umbrella-concept into question.
Limitations to this study include key differences in patient characteristics between cases and controls. Patients treated with cladribine were younger than fludarabine-treated controls, reflecting the underlying epidemiology of HCL and CLL/FL respectively. Cases were more likely to have FN than both control-1 and 2 groups, which may have confounded the rates of AAL. However even with this discrepancy the rate of AAL still remains much higher than other cohorts with similar rates of neutropenia(1, 2). In addition, the rates of skin-testing amongst cases were low as this practice has only become standard-of-care in our institutions in 2015. This meant we were unable to confirm the implicated allergen in the majority of cases.
In conclusion, C-HCL patients are at higher risk for developing AAL’s compared with non-HCL treated cladribine patients and non-HCL malignancies those treated with fludarabine, possibly related to the underlying immune dysregulation. This study demonstrates that the risk of developing drug allergy varies according to host genomic factors, immune dysregulation, chemotherapeutics and antibiotic factors.
Supplementary Material
Acknowledgements:
This work was supported by a grant from the Australian National Health and Medical Research Council (1139902 to JAT)
All authors were involved in the critical appraisal of the paper and have approved the final submitted version.
ZM, JS, JAT, NEH, KYLC performed the research
ZM, JFS, CST, JAT designed the research study
ZM, JFS, CST, EJP, MAS, JAT analysed the data
ZM, JAT wrote the paper
References
- 1.Huang K, Cluzet V, Hamilton K, et al. The impact of reported beta-lactam allergy in hospitalized patients with hematologic malignancies requiring antibiotics. Clinical Infectious Diseases 2018;67(1):27–33. [DOI] [PubMed] [Google Scholar]
- 2.Trubiano JA, Leung VK, Chu MY, et al. The impact of antimicrobial allergy labels on antimicrobial usage in cancer patients. Antimicrobial resistance and infection control 2015;4(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grever MR, Abdel-Wahab O, Andritsos LA, et al. Consensus guidelines for the diagnosis and management of patients with classic hairy cell leukemia. Blood 2017;129(5):553–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodman GR, Burian C, Koziol JA, et al. Extended follow-up of patients with hairy cell leukemia after treatment with cladribine. Journal of Clinical Oncology 2003;21(5):891–6. [DOI] [PubMed] [Google Scholar]
- 5.Seymour J, Kurzrock R, Freireich E, et al. 2-chlorodeoxyadenosine induces durable remissions and prolonged suppression of CD4+ lymphocyte counts in patients with hairy cell leukemia. Blood 1994;83(10):2906–11. [PubMed] [Google Scholar]
- 6.Ganzel C, Gatt ME, Maly A, et al. High incidence of skin rash in patients with hairy cell leukemia treated with cladribine. Leukemia & lymphoma 2012;53(6):1169–73. [DOI] [PubMed] [Google Scholar]
- 7.Kardaun SH, Sekula P, Valeyrie-Allanore L, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. The British journal of dermatology 2013;169(5):1071–80. [DOI] [PubMed] [Google Scholar]
- 8.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016;127(20):2375–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tam CS, O’Reilly M, Andresen D, et al. Use of empiric antimicrobial therapy in neutropenic fever. Australian Consensus Guidelines 2011 Steering Committee. Internal medicine journal 2011;41(1b):90–101. [DOI] [PubMed] [Google Scholar]
- 10.Hendrick A Purpuric rash following treatment with 2-chlorodeoxyadenosine. Clinical and laboratory haematology 2001;23(1):67–8. [DOI] [PubMed] [Google Scholar]
- 11.Rossini MS, de Souza EM, Cintra ML, et al. Cutaneous adverse reaction to 2-chlorodeoxyadenosine with histological flame figures in patients with chronic lymphocytic leukaemia. Journal of the European Academy of Dermatology and Venereology 2004;18(5):538–42. [DOI] [PubMed] [Google Scholar]
- 12.Konvinse KC, Phillips EJ, White KD, et al. Old dog begging for new tricks: current practices and future directions in the diagnosis of delayed antimicrobial hypersensitivity. Current opinion in infectious diseases 2016;29(6):561–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carr A, Cooper DA. Pathogenesis and management of HIV-associated drug hypersensitivity. AIDS clinical review 1995:65–97. [PubMed]
- 14.Saven A, Burian C, Koziol JA, et al. Long-term follow-up of patients with hairy cell leukemia after cladribine treatment. Blood 1998;92(6):1918–26. [PubMed] [Google Scholar]
- 15.Trubiano JA, Douglas AP, Goh M, et al. The safety of antibiotic skin testing in severe T-cell-mediated hypersensitivity of immunocompetent and immunocompromised hosts. The journal of allergy and clinical immunology In practice 2018. [DOI] [PMC free article] [PubMed]
- 16.Fregert S Manual of Contact Dermatitis 2nd ed. Copenhagen: Year Book Medical Publishers; 1981. 10 p. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.