Abstract
Background and Purpose:
American Heart Association guidelines recommend obtaining baseline troponin in all acute ischemic stroke (AIS) patients. Yet, there is a paucity of data on the prevalence of baseline troponin elevation and specifically its diagnostic yield for acute myocardial infarction (AMI) in patients presenting within the time window for thrombolysis.
Methods:
We retrospectively analyzed 1072 consecutive patients admitted for AIS or transient ischemic attack, who presented within 4.5 hours of last known well (LKW). Patients who had baseline cardiac troponin I (bcTnI) obtained within 72 hours from LKW (n=525) were included in the study. Multivariable logistic regression was conducted to determine factors independently related to an elevated bcTnI (>0.04 ng/mL). We calculated the area under receiver-operator curves (AUC) sensitivity, and specificity, to determine the diagnostic accuracy of (i) the bcTnI for AMI stratified by the time to assessment and (ii) the best time cut-off for obtaining bcTnI.
Results:
Among included subjects, the median time from LKW to the bcTnI was 3.8 hours and 113 (21.5%) subjects had an elevated bcTnI. Assessment of bcTnI within 4.5 hours from LKW was significantly more often associated with normal values as compared to assessment between 4.5 and 72 hours (61.7% vs. 38.3%; p=0.001). 15 (2.9%) patients were diagnosed with AMI. After adjustment for pertinent confounders, time to bcTnI assessment was independently associated with AMI (OR 1.04, 95%-CI 1.02 to 1.07, p=0.001). When stratified by time, bcTnI assessed within 4.5 hours had a sensitivity of 25% and specificity of 83.7% for AMI, whereas bcTnI assessment between 4.5-72 hours was associated with a sensitivity of 90.9% and specificity of 74.8%.
Conclusions:
Baseline assessment of cTnI after 4.5 hours from LKW was associated with greater diagnostic accuracy than testing within 4.5 hours. This information may inform routine clinical practice.
Keywords: myocardial infarction, electrocardiogram, thrombolysis, troponin, stroke
Introduction
Severe adverse cardiac events, including acute myocardial infarction (AMI), occur in approximately 20% of patients with ischemic stroke, predominantly within the first 2-3 days.1 The purported pathophysiological mechanisms underlying stroke-associated cardiac dysfunction have been partially related to functional and structural alterations in the central autonomic network that cause dysregulation of normal neural cardiac control.2 Because stroke-associated cardiac dysfunction, most concerning AMI, is associated with unfavorable short- and long-term outcomes after ischemic stroke, early recognition is important to improve patient care;3-5 the American Heart Association guidelines recommend obtaining baseline troponin in all patients presenting with acute ischemic stroke (AIS) if it does not delay thrombolysis (Class I recommendation).6
Elevated cardiac troponin I (cTnI) is a sensitive and specific marker of myocardial injury. cTnI increases approximately 2-3 hours after the onset of symptoms7 and it represents one of the best-studied biomarkers. High sensitivity assays have shown elevations of cardiac troponin T above reference levels in more than 90% of ischemic stroke patients; and up to 60% of these patients were found to have troponin elevations above the reference limit (14 ng/L).3 However, prevalence of AMI is overall low in AIS and there is a distinct paucity of data regarding the diagnostic yield of screening cardiac troponin for AMI in patients presenting within the time window for thrombolysis.
Therefore, we sought to determine the frequency and timing of baseline cTnI (bcTnI) assessments as well as clinical factors associated with an elevated screening cTnI in patients with AIS presenting within 4.5 hours from stroke onset or last known well (LKW). In addition, we explored the association of bcTnI with AMI when obtained before or after 4.5 hours from stroke onset or LKW.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study cohort
We retrospectively analyzed adult patients with AIS or transient ischemic attack (TIA) that were admitted to the University of Massachusetts Memorial Medical Center (UMMMC) between January 2013 and August 2018. Patients presenting more than 4.5 hours from stroke onset or LKW were excluded from the study. In addition, we excluded subjects that had no cTnI obtained within 72 hours from LKW. Subjects ultimately diagnosed with a stroke mimic were included in the main analyses.
Our investigation was approved by our Institutional Review Board and Health Insurance Portability and Accountability Act waiver of informed consent was granted. We adhere to the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines (www.strobe-statement.org).
Clinical characteristics, stroke severity, and stroke etiology
At baseline, all study participants underwent a standardized clinical history, physical examination, head computed tomography (CT) or brain magnetic resonance imaging (MRI) (typically obtained within 24-48 hours after the symptoms onset) per institutional protocol. Patient demographics, laboratory data, co-morbidities, and pre-admission medications were collected from the medical record. Members of the stroke team certified in National Institutes of Health Stroke Scale (NIHSS) scoring graded the severity of stroke at presentation. The stroke etiology was determined based on the Trial of ORG-10172 in Acute Stroke Treatment (TOAST) classification after completion of the stroke workup.8 In addition, we defined embolic stroke of undetermined source (ESUS) according to the criteria proposed by the Cryptogenic Stroke/ESUS International Working Group.9 Patients with transient neurologic dysfunction of less than 24 hours and without evidence for acute ischemia on brain imaging (MRI or CT) were categorized as having a TIA or stroke mimic as previously described.10-12
Cardiac-specific TnI (cTnI)
cTnI was identified using the Access AccuTnI+3 reagent pack. All samples were quantitively analyzed using the UniCel DxI Access Immunoassay Systems. A cTnI ≤0.04 ng/mL was considered normal and cTnI >0.04 ng/mL was considered elevated. cTnI was ordered at the discretion of the treating physician (emergency room physician, neurologist, neurointensivist).
Acute myocardial infarction (AMI) and stroke associated cardiac dysfunction (SACD)
To determine the presence of AMI, we retrospectively reviewed all medical records relating to the index hospitalization for clinical and laboratory signs of AMI, including patients’ clinical symptoms during hospitalization, cTnI, and electrocardiography using the European Society of Cardiology/American College of Cardiology criteria. Specifically, AMI was defined based on history (such as presence of chest pain, dyspnea, new onset heart failure), electrocardiographic criteria (including both ST and non-ST elevation myocardial infarcts) as well as elevated serum cTnI with at least one value >0.04 ng/mL during the index admission.13,14 Given the challenge of differentiating type 1 myocardial infarction (infarction due to coronary plaque rupture with the appropriate clinical scenario) from type 2 myocardial infarction (AMI not related to coronary plaque rupture), we combined both types to AMI for the purpose of this study. Lastly, we abstracted potential reasons for any cTnI elevation not related to AMI or systemic disease (e.g., sepsis, chronic kidney disease) and categorized them to stroke associated cardiac dysfunction (SACD)15 including the presence of stress cardiomyopathy (Takotsubo), cardiac arrhythmias, and acute heart failure. Patients with cTnI elevation and without overt cardiac or systemic disease were categorized to undetermined SACD.
Definition of comorbidities
The presence of hypertension was based on the use of antihypertensive medications, or systolic blood pressure of ≥140 mm Hg or diastolic blood pressure of ≥90 mm Hg on two separate occasions.16 Diabetes mellitus was noted in cases with history of fasting glucose ≥126 mg/dL or current use of hypoglycemic drugs as defined according to the National Diabetes Data Group.17 Coronary artery disease (CAD) was defined as history of myocardial infarction within the past 20 years, multivessel coronary disease with symptoms or with history of stable or unstable angina, history of percutaneous coronary intervention, or multivessel coronary artery bypass graft surgery. The diagnosis of congestive heart failure (CHF) was made using modified Framingham clinical criteria.18 Atrial fibrillation (AF) was defined according to the American Heart Association guidelines criteria.19 We considered AF present if AF was documented in the admission history, if AF was present on a per-protocol, admission 12-lead ECG, if detected on telemetry during hospitalization, or if they had a history of oral anticoagulation for AF. Stress cardiomyopathy was characterized by transient wall-motion abnormalities involving the left ventricular apex and mid-ventricle as indicated in echocardiogram or angiography in the absence of obstructive epicardial coronary disease as according to Mayo Clinic criteria.20 Peripheral vascular disease (PVD) was defined as previously described.21 The presence of chronic obstructive pulmonary disease (COPD) was noted if documented in the admission history and/or based on spirometry results as defined by global initiative for chronic obstructive lung disease.22 The CHA2DS2-VASc score (range 0 to 9) was calculated based on 8 factors: CHF (1 point), hypertension (1 point), age 65-74 years (1 point), age ≥75 years (2 points), diabetes mellitus (1 point), prior stroke/TIA (2 points), PVD (1 point), female sex (1 point). The index event was counted towards the total score, thus the minimal possible score in our study was 2.
Statistics
Unless otherwise stated, continuous variables are reported as mean ± S.D. or median (25th-75th percentile). Categorical variables are reported as proportions. Between-group comparisons for continuous and ordinal variables were made with t-Test, Mann-Whitney U test, one-way analysis of variance (ANOVA), and Kruskal-Wallis one-way ANOVA on ranks as appropriate. Categorical variables were compared using the χ2-test or Fisher exact test as appropriate.
We created separate multivariable logistic regression models to determine factors relating to an elevated bcTnI as well as AMI (dependent variables in the respective models). Models were adjusted for the stroke severity (as assessed by the admission NIHSS), age, sex, time to bcTnI assessment from symptom onset/LKW, presence of a TIA (versus stroke), glomerular filtration rate (GFR), as well as history of stroke/TIA, CHF, CAD, and PVD. To avoid model overfitting, variables were sequentially removed (likelihood ratio) from the models at a significance level of 0.1. Collinearity diagnostics were performed (and its presence rejected) for all multivariable regression models. Model calibration was assessed by Hosmer-Lemeshow test and model fit determined by examining the −2 log-likelihood statistic and its associated chi-square statistics.
To determine the diagnostic accuracy of bcTnI for AMI, we calculated the area under receiver-operator curves (AUC; c-statistics), sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and overall accuracy with corresponding 95% confidence intervals (CI). Optimal thresholds were determined by maximizing Youden index (sensitivity+specificity−1). We considered an AUC of 0.6-0.7 poor, 0.7-0.8 acceptable, 0.8-0.9 excellent, and 0.9-1.0 outstanding.23
Two-sided significance tests were used throughout and unless stated otherwise a two-sided P<0.05 was considered statistically significant. All statistical analyses were performed using IBM® SPSS® Statistics Version 24 (IBM®-Armonk, NY).
Results
Study cohort
Over the study period, a total of 3,241 subjects were screened. 1,072 patients presented within 4.5 hours from symptom onset/LKW. Of these, 529 (49.3%) had a bcTnI obtained within 72 hours from symptom onset/LKW. Three subjects were excluded because they had an AMI before the index stroke and one subject because of procedure related cardiac arrest, leaving 525 subjects for analysis. See Supplemental Figure I for the flowchart of patient exclusion.
Overall, baseline characteristics of included patients were similar to excluded patients who presented within 4.5 hours, except for a more frequent history of CHF (p=0.021), CAD (p=0.003), PVD (p=0.001), and COPD (p=0.014) among included patients (Supplemental Table I).
Factors relating to an elevated bcTnI
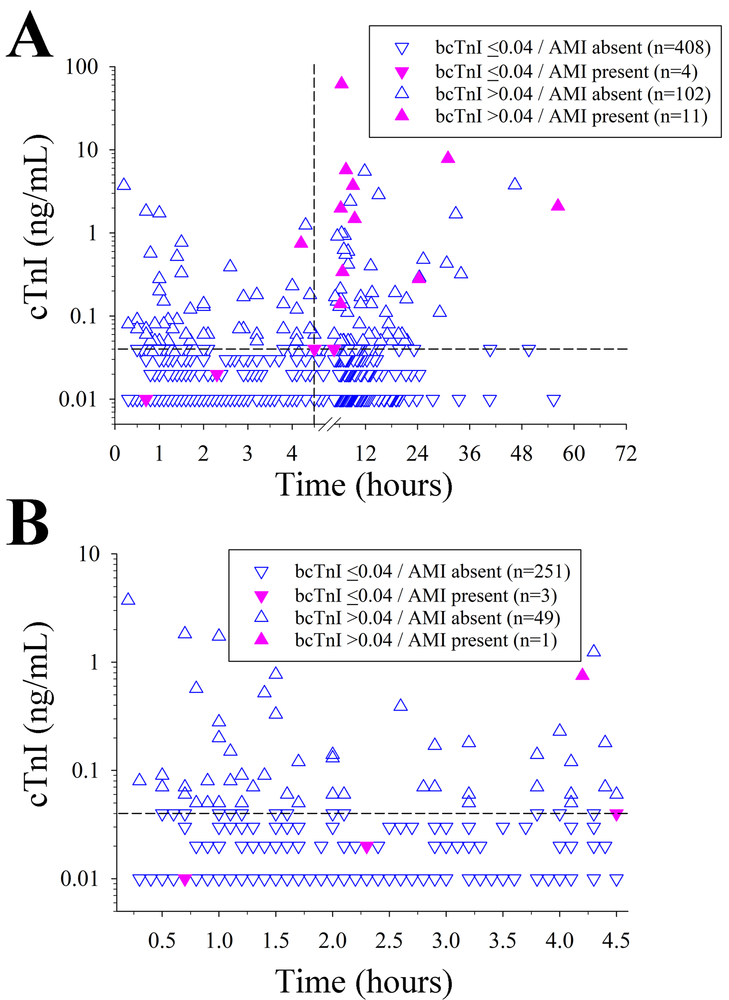
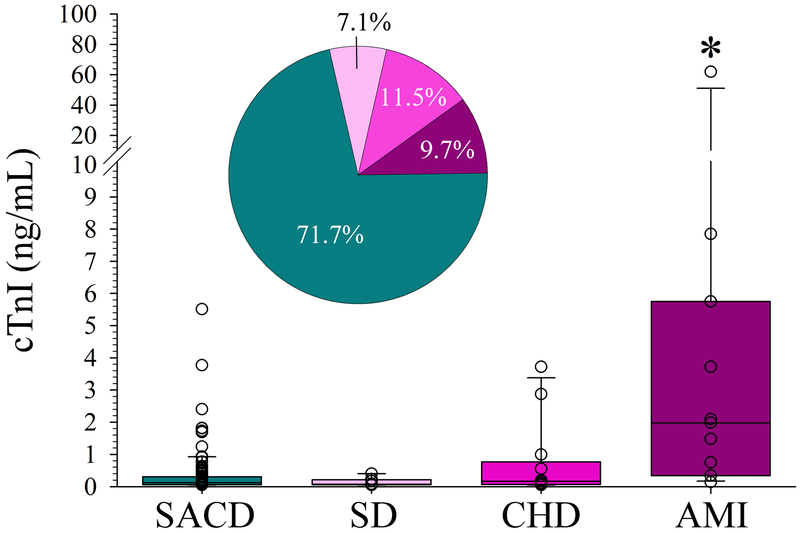
Among all included patients, 412 (78.5%) had a normal bcTnI, and 113 (21.5%) had an elevated bcTnI >0.04 ng/mL. Supplemental Figure II depicts the distribution of time to bcTnI assessment as stratified by a normal versus elevated bcTnI level. Serial cTnI were obtained in 98 (86.7%) subjects with increased bcTnI and 73 (17.7%) patients with normal bcTnI. The median time from symptom onset/LKW time to the bcTnI was 3.8 hours (IQR 1.5 hours to 8.0 hours), with 58% (n=304) of patients having their bcTnI obtained ≤ 4.5 hours from symptom onset/LKW (Figure 1A). Patients with bcTnI >0.04 ng/mL were tested 2.9 hours later than subjects with normal bcTnI (p=0.001; Table 1). When bcTnI was assessed within 4.5 hours from symptoms onset, it was significantly more often normal as compared to bcTnI obtained between 4.5 and 72 hours (83.6% vs. 71.5%; p=0.001, Figure 1B). These results were similar when restricted to patients treated with recombinant tissue-type plasminogen activator (n=287; 85.4% vs. 71.3%; p=0.005, data not shown). Additional factors relating to a bcTnI >0.04 ng/mL on unadjusted analyses are shown in Table 1. When we restricted these analyses to patients with bcTnI within 4.5 hours from symptom onset (n=304) there was no association between time to bcTnI with bcTnI >0.04ng/mL (p=0.808); and only the composite CHA2DS2-VAsc score (p=0.025), and history of CHF (p=0.028) related to an increased bcTnI above the cut-off value. Notably, among the 29 included stroke mimics only one patient (who had a seizure as the underlying cause) had an elevated bcTnI (0.05 ng/mL). After adjustment, time to bcTnI (p=0.001) and CHF (p<0.001) were independently associated with an elevated bcTnI (Table 2). Figure 2 depicts the mechanism underlying the troponin elevation. In most cases (71.3%), bcTnI elevation was attributed to SACD including 10 (8.8%) subjects with stress cardiomyopathy (Takotsubo), 20 (17.7%) subjects with cardiac arrhythmia, 4 (3.5%) subjects with acute heart failure, and 47 (41.6%) of subjects with undetermined SACD.
Figure 1.
(A) Relationship between time to baseline cardiac troponin I (bcTnI) and diagnosis of acute myocardial infarction (AMI) in all included patients indicates that significantly fewer patients (p=0.013) were diagnosed with AMI within the first 4.5 hours from symptom onset (n=4 [1.3%]) than afterwards (n=11 [5.0%]). (B) When focusing on the subset of patients with bcTnI assessment within 4.5 hours of symptom onset, only a single patient diagnosed with AMI had an elevated bcTnI (assessed at 4.2 hours, bcTnI = 0.75 ng/mL). Vertical dashed line indicates the 4.5 hour time point. Horizontal dashed line indicates the 0.04 ng/mL cTnI cut off.
Table 1.
Baseline characteristics of included patients as stratified according to the bcTnI cut off.
| Characteristics | All patients (n=525) |
bcTnI ≤0.04 (n=412) |
bcTnI >0.04 (n=113) |
P-value |
|---|---|---|---|---|
| Time to bcTnI, hours | 3.8 (1.5-8.0) | 3.3 (1.4-7.5) | 6.2 (1.9-11.6) | 0.001 |
| AMI present | 15 (3.4) | 5 (1.2) | 13 (11.5) | <0.001 |
| Age, years | 73 (63-84) | 72 (62-83) | 77 (65-86) | 0.005 |
| Female sex | 250 (47.6) | 194 (47.1) | 56 (49.6) | 0.671 |
| Admission NIHSS | 8 (3-16) | 7 (3-15) | 11 (5-17) | 0.003 |
| TIA (versus stroke) | 41 (7.8) | 36 (8.7) | 5 (4.4) | 0.126 |
| Stroke mechanism (n=496)* | 0.108 | |||
| Large artery atherosclerosis | 82 (16.5) | 68 (17.7) | 14 (12.5) | |
| Cerebral small vessel disease | 57 (11.5) | 50 (13.0) | 7 (6.3) | |
| Cardioembolic | 215 (43.3) | 157 (40.9) | 58 (51.8) | |
| Embolic stroke of undetermined source | 130 (26.2) | 100 (26.0) | 30 (26.8) | |
| Other determined | 12 (2.4) | 9 (2.3) | 3 (2.7) | |
| Mimic* | 29 (5.5) | 28 (6.8) | 1 (0.9) | 0.010 |
| Comorbidities | ||||
| Congestive heart failure | 79 (15.0) | 43 (10.4) | 36 (31.9) | <0.001 |
| Hypertension | 400 (76.2) | 312 (75.7) | 88 (77.9) | 0.709 |
| Diabetes | 143 (27.2) | 112 (27.2) | 31 (27.4) | 1.000 |
| Prior stroke or TIA | 98 (18.7) | 70 (17.0) | 28 (24.8) | 0.076 |
| Peripheral vascular disease | 169 (32.2) | 121 (29.4) | 48 (42.5) | 0.012 |
| Coronary artery disease | 138 (26.3) | 99 (24.0) | 39 (34.5) | 0.030 |
| Atrial fibrillation | 205 (39.0) | 154 (37.4) | 51 (45.1) | 0.157 |
| Chronic obstructive pulmonary disease | 131 (24.9) | 104 (25.3) | 27 (23.5) | 0.716 |
| CHA2DS2-VASc score | 4 (2-5) | 3 (2-5) | 4 (3-5) | <0.001 |
| Creatinine (mg/dl) | 0.95 (0.81-1.22) | 0.93 (0.79-1.19) | 1.05 (0.87-1.32 | 0.009 |
| GFR (ml/min/1.73m2) | 60 (51-60) | 60 (53-60) | 60 (44-60) | 0.009 |
| Systemic thrombolysis | 287 (54.7) | 227 (55.1) | 60 (53.1) | 0.749 |
Data are n (%) or median (25th-75th quartile). All five subjects with acute myocardial infarction (AMI) and baseline cardiac troponin I (bcTnI) ≤0.04ng/mL had subsequent cTnI >0.04. GFR = glomerular filtration rate; TIA=transient ischemic attack, NIHSS=National Institutes of Health Stroke Scale.
Analysis excludes stroke mimics (encephalopathy [n=8], complex migraine [n=8], functional deficits [n=5], seizure [n=4], miscellaneous [n=4]).
Table 2.
Multivariable analysis of factors relating to an elevated bcTnI
| Independent variable | Crude OR (95% CI) | p-value | Adjusted OR (95% CI)* | p-value |
|---|---|---|---|---|
| Time to bcTnI assessment, hours | 1.051 (1.025 to 1.078) | <0.001 | 1.046 (1.019 to 1.074) | 0.001 |
| Congestive heart failure | 4.012 (2.418 to 6.657) | <0.001 | 3.087 (1.814 to 5.254) | <0.001 |
| Admission NIHSS, per point | 1.034 (1.011 to 1.058) | 0.004 | 1.025 (1.000 to 1.051) | 0.050 |
| Age, years | 1.024 (1.008 to 1.041) | 0.003 | 1.016 (0.999 to 1.033) | 0.067 |
| Peripheral vascular disease | 1.776 (1.156 to 2.728) | 0.009 | 1.498 (0.949 to 2.363) | 0.083 |
| Stroke (versus TIA and mimic*) | 2.068 (0.792 to 5.399) | 0.138 | -- | -- |
| Female sex | 1.104 (0.728 to 1.674) | 0.641 | -- | -- |
| Coronary artery disease | 1.666 (1.064 to 2.610) | 0.026 | -- | -- |
| Creatinine, mg/dL | 1.162 (0.895 to 1.508) | 0.259 | -- | -- |
| GFR, ml/min/1.73m2 | 0.980 (0.964 to 0.995) | 0.011 | -- | -- |
Odds ratios indicate the increased or decreased odds of the clinical factor being present, for a 1 hour increase in time to bcTnI assessment, 1 year increase in patient age, 1 point increase in the admission National Institutes of Health Stroke Scale (NIHSS) score, 1 mg/dL increase in creatinine, and 1 ml/min/1.73m2 increase in the glomerular filtration rate (GFR). Blank cells represent variables that were dropped as non-significant during stepwise selection (p≤0.1 for removal). The units for NIHSS score are points on a scale from 0 to 40 (maximum observed in this study). Hosmer-Lemeshow goodness of fit χ2 6.248, p=0.619. TIA indicates transient ischemic attack. Presence of acute myocardial infarction was not included into the model because it was not established at admission.
Excluding stroke mimics did not meaningfully change the results (not shown).
Figure 2.
Association of baseline cardiac troponin I (bcTnI) elevation with the underlying mechanism. Among patients with bcTnI >0.04 ng/mL (n=113), subjects with an acute myocardial infarction (AMI) had a significantly greater bcTnI than all other defined causes (*p<0.05, each; ANOVA on Ranks with post hoc Dunn’s). Box plots are median ± interquartile range with open circles depicting all individual data points. Pie-chart indicates the relative distribution of underlying mechanism (color coding consistent with the box plot). SACD indicates stroke associated cardiac dysfunction (see text for details), SD=systemic disease, CHD=chronic heart disease.
Association of baseline characteristics and bcTnI with AMI
Overall, 15 (2.9%) patients were diagnosed with an AMI. Among AMI patients, 11 (73.3%) had an elevated bcTnI above 0.04 ng/mL. Figure 1 summarizes the association between level and time to bcTnI assessment as stratified by AMI diagnosis. Among the 304 patients who had bcTnI assessed within 4.5 hours from symptom onset, 4 (1.3%) were diagnosed with an AMI. In the single patient with elevated bcTnI, troponin was assessed at 4.2 hours; whereas bcTnI in the patients with normal values was assessed at 0.7, 2.3, and 4.5 hours. In all three subjects with normal bcTnI further serial cTnI was obtained for complaints of chest pain (n=2) and acute heart failure (n=1). Except for the admission NIHSS, none of the other assessed baseline characteristics were associated with the diagnosis of AMI (p>0.05, each; not shown). Specifically, AMI patients had a greater median NIHSS (median 14, IQR 9-20) than patients without AMI (median 7, IQR 3-15) (p=0.005). After adjustment for the NIHSS, the time to bcTnI remained independently associated with the diagnosis of AMI (per hour: OR 1.06, 95%-CI 1.02-1.1, p=0.004).
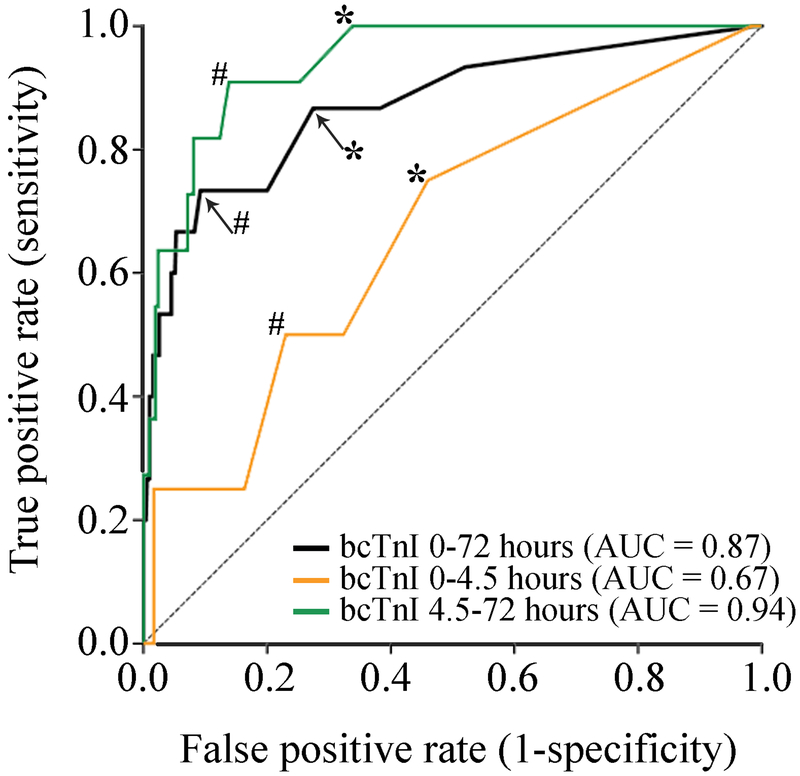
The sensitivity, specificity, and overall accuracy of a bcTnI >0.04 ng/mL assessed within 72 hours form symptom onset/LKW for AMI was 73.3%, 80.0%, and 79.8%, respectively (Table 3, Figure 3). When we stratified this analysis by the time to bcTnI assessment within 4.5 hours (n=304 [57.9%]) versus assessment beyond 4.5 hours (n=221 [42.1%]), the sensitivity of a bcTnI >0.04 ng/mL for AMI was markedly reduced to 25.0% whereas the specificity remained high at 83.7% (Table 3; Figure 3). In contrast, bcTnI >0.04 ng/mL obtained after 4.5 hours had 91% sensitivity and 75% specificity for AMI (Table 3).
Table 3.
Diagnostic accuracy of bcTnI >0.04 ng/mL for AMI stratified by the time to assessment.
| 0-72h (n=525) |
0-4.5h (n=304) |
4.5-72h (n=221) |
|
|---|---|---|---|
| Sensitivity (95% CI) | 73.3 (44.9-92.2) | 25.0 (0.63-80.6) | 90.9 (58.7-99.8) |
| Specificity (95% CI) | 80.0 (76.3-83.4) | 83.7 (79.0-87.7) | 74.8 (68.3-80.4) |
| Positive predictive value (95% CI) | 9.7 (7.0-13.2) | 2.0 (0.37-10.2) | 15.9 (12.3-20.3) |
| Negative predictive value (95% CI) | 99.0 (97.8-99.6) | 98.8 (97.9-99.3) | 99.4 (96.0-99.9) |
| Accuracy (95% CI) | 79.8 (76.1-83.2) | 82.9 (78.2-87.0) | 75.6 (69.3-81.1) |
Figure 3.
Area under the receiver-operating-characteristics curve analysis for baseline cardiac troponin I (bcTnI) predicting the diagnosis of acute myocardial infarction (AMI) stratified by the time to assessment. There was overall good diagnostic accuracy of the bcTnI (ng/mL) obtained within 72 hours to predict AMI. However, when stratified by time to assessment, we found poor diagnostic accuracy of bcTnI to depict an AMI if measured within 4.5 hours from stroke onset as compared to excellent diagnostic accuracy when assessed after 4.5 hours. *Indicates the sensitivity and specificity for bcTnI >0.04 ng/mL for AMI. #Indicates the optimal screening bcTnI cut-off for AMI (0-72 hours: 0.14 ng/mL, sensitivity=0.73, specificity=0.91, Youden’s J=0.64; 0-4.5 hours: 0.02 ng/mL, sensitivity=0.75, specificity=0.54, Youden’s J=0.29; 4.5-72 hours: 0.14 ng/mL, sensitivity=0.91, specificity=0.86, Youden’s J=0.77).
Finally, among all included patients, the optimal time-threshold to detect AMI was 252 minutes (4.2 hours; sensitivity=0.87, specificity=0.55, Youden’s J=0.42) and the optimal time-threshold to detect an elevated bcTnI >0.04 ng/mL was 324 minutes (5.4 hours; sensitivity=0.54, specificity=0.66, Youden’s J=0.20).
Discussion
Current guidelines for the management of AIS recommend routine baseline assessment of cardiac troponin. Yet, little is known regarding the relationship between cTnI elevation in AIS patients in general and specifically for patients presenting within the standard time window for thrombolysis. Understanding this association is important because cTnI elevation above the critical threshold typically occurs only with several hours after onset of cardiac injury. Arguably, bcTnI assessment in AIS patients may thus related to low diagnostic yield.
Indeed, our data provides novel insight into this issue by showing that cTnI screening within 4.5 hours from symptoms onset was significantly more often associated with a normal level as compared to cTnI screening after 4.5 hours. Perhaps more important, bcTnI within 4.5 hours from symptom onset was associated with a high false negative rate for AMI resulting in poor sensitivity, correctly identifying only 1 in 4 subjects with AMI (sensitivity = 25%). In contrast, the sensitivity substantially increased to >90% when the bcTnI was assessed more than 4.5 hours after symptom onset. The importance of this finding is further highlighted by the fact that only 1 in 6 patients with normal screening cTnI underwent further serial troponin testing in our cohort. Although the possible reasons for lack of serial cTnI were not documented, it appears likely that in the absence of overt cardiac symptoms physicians relied on the negative bcTnI to exclude an AMI. If true, this approach may be problematic among AIS patients who are aphasic, are sedated and intubated after mechanical thrombectomy/other procedure, or have pre-existing cognitive impairment, which may impair their ability to communicate AMI-related symptoms. Further study is required to elucidate this issue. Regardless, our observations indicate that routine cTnI screening in AIS patients without overt cardiac symptoms is of low diagnostic accuracy in the hyperacute phase. Instead, diagnostic accuracy may be improved by screening after approximately 6 hours of symptom onset. Whether consideration of clinical factors relating to bcTnI (such as older age, greater stroke severity, and cardiac comorbidities24-27) can further improve patient selection for screening remains to be shown.
A second important observation of our study was that among included subjects 25% had an elevated bcTnI attributed to reasons other than AMI and that this association was similar for patients with bcTnI assessment within 4.5 hours (approximately 20%). These observations are in general agreement with previous investigations, which found serum troponin elevation in 15% to 20% of AIS patients.28-30 This adds to the notion that troponin elevations are common after AIS and in the majority of cases related to neurogenic SACD.15 Future studies may benefit from additional assessment of neuroimaging data to determine the potential association of specific brain regions and stroke extent with early bcTnI elevations. The importance of this finding is highlighted by the more frequent occurrence of SACD-related bcTnI elevation relative to AMI and the fact that SACD may aid identification of the stroke etiology such as AF31 and identify patients at risk for worse in-hospital and long term functional outcomes.3,4,25
Strengths of our study relate to the inclusion of well-characterized patients that presented within 4.5 hours of last known well with careful delineation of the association between bcTnI elevation relative to the time of symptoms onset as well as conducted a detailed chart review to determine causes of bcTnI elevation in patients without AMI (false positives), as well as those with AMI but had normal bcTnI (false negatives). Furthermore, we adjusted our analyses for pertinent confounders to identify factors relating to an elevated bcTnI.
Limitations of our study relate to its single center, retrospective design, for which reason results should be considered hypothesis-generating. Second, 50% of screened patients presenting within 4.5 hours had no bcTnI assessed and were excluded. However, their baseline characteristics were similar to included patients assuaging concerns of bias. Third, our analyses focused on the association between the bcTnI and AMI. For this reason, and because serial cTnI were not routinely collected when the screening cTnI was normal, we may have overestimated the false negatives for AMI and possibly overestimated the presence of SACD. In addition, since bcTnI was not routinely obtained at the time of presentation, early (within 4.5 hours) elevation of bcTnI could have been missed in subjects found to have bcTnI elevation after 4.5 hours leading to an underestimation of the true sensitivity. Nevertheless, it is worth noting that the negative predictive value was high, indicating a high probability that subjects with a negative bcTnI truly didn’t have an AMI. Further study is needed to learn the clinical implication of assessing cTnI in the acute phase. In particular, it would be interesting to determine possible associations between elevations in troponin and other cardiac biomarkers such as ECG (and echocardiographic) indices of cardiopathy, which may provide important insight into the underlying stroke mechanism and risk of future events. Nevertheless, we reviewed medical record regardless of the available bcTnI levels to determine possible causes for any bcTnI elevation as well as clinical signs of AMI, assuaging concerns of miscategorization. Hence, our approach and the study of a real-life cohort provides valuable insight into clinical practice, which may facilitate translation of our findings to common clinical settings if confirmed by prospective studies.
Conclusion
Among patients presenting within the time window for thrombolysis treatment, troponin elevations are common; yet, less than 3% of patients were found to have an AMI. The diagnostic accuracy of an initial screening cTnI for AMI is substantially greater when obtained after 4.5 hours from LKW as compared to testing within 4.5 hours. However, pending confirmation by prospective studies with systematic assessment of serial cTnI, information should not be used to inform routine clinical practice. We hope that our real world cohort findings will be the impetus for further systematic study of this issue to ultimately improve patient care and inform clinical guidelines.
Supplementary Material
Acknowledgments
Sources of Funding
Dr. Henninger is supported by K08NS091499 from the National Institute of Neurological Disorders and Stroke and R44NS076272 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures
Dr. Henninger serves on the advisory board of Omniox, Inc. and as consultant to Astrocyte Pharmaceuticals, Inc. Dr. Silver received compensation for medicolegal malpractice review, adjudication for the Women’s Health Initiative, and authorship for Ebix, MedLink, and Medscape.
References
- 1.Prosser J, MacGregor L, Lees KR, Diener HC, Hacke W, Davis S, et al. Predictors of early cardiac morbidity and mortality after ischemic stroke. Stroke. 2007;38:2295–2302 [DOI] [PubMed] [Google Scholar]
- 2.Yperzeele L, van Hooff RJ, Nagels G, De Smedt A, De Keyser J, Brouns R. Heart rate variability and baroreceptor sensitivity in acute stroke: a systematic review. Int J Stroke. 2015;10:796–800 [DOI] [PubMed] [Google Scholar]
- 3.Scheitz JF, Mochmann HC, Erdur H, Tutuncu S, Haeusler KG, Grittner U, et al. Prognostic relevance of cardiac troponin T levels and their dynamic changes measured with a high-sensitivity assay in acute ischaemic stroke: analyses from the TRELAS cohort. Int J Cardiol. 2014;177:886–893 [DOI] [PubMed] [Google Scholar]
- 4.Wrigley P, Khoury J, Eckerle B, Alwell K, Moomaw CJ, Woo D, et al. Prevalence of Positive Troponin and Echocardiogram Findings and Association With Mortality in Acute Ischemic Stroke. Stroke. 2017;48:1226–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azher I, Kaushal A, Chang A, Cutting S, Mac Grory B, Burton T, et al. Serum troponin level in acute ischemic stroke identifies patients with visceral infarcts. J Stroke Cerebrovasc Dis. 2019;28:1173–1177 [DOI] [PubMed] [Google Scholar]
- 6.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2018;49:e46–e110 [DOI] [PubMed] [Google Scholar]
- 7.Macrae AR, Kavsak PA, Lustig V, Bhargava R, Vandersluis R, Palomaki GE, et al. Assessing the requirement for the 6-hour interval between specimens in the American Heart Association Classification of Myocardial Infarction in Epidemiology and Clinical Research Studies. Clin Chem. 2006;52:812–818 [DOI] [PubMed] [Google Scholar]
- 8.Adams HP Jr, Woolson RF, Clarke WR, Davis PH, Bendixen BH, Love BB, et al. Design of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Control Clin Trials. 1997;18:358–377 [DOI] [PubMed] [Google Scholar]
- 9.Hart RG, Diener HC, Coutts SB, Easton JD, Granger CB, O’Donnell MJ, et al. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. 2014;13:429–438 [DOI] [PubMed] [Google Scholar]
- 10.Easton JD, Saver JL, Albers GW, Alberts MJ, Chaturvedi S, Feldmann E, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009;40:2276–2293 [DOI] [PubMed] [Google Scholar]
- 11.Nagy M, Azeem MU, Soliman Y, Nawab SA, Jun-O’Connell AH, Goddeau RP Jr., et al. Pre-existing White Matter Hyperintensity Lesion Burden and Diagnostic Certainty of Transient Ischemic Attack. J Stroke Cerebrovasc Dis. 2019;28:944–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jun-O’connell A H, Henninger N, Moonis M, Silver B, Ionete C, Goddeau RP. Recrudescence of Old Stroke Deficits Among Transient Neurological Attacks. Neurohospitalist. 2019;9:183–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Myocardial infarction redefined--a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. Eur Heart J. 2000;21:1502–1513 [DOI] [PubMed] [Google Scholar]
- 14.Luepker RV, Apple FS, Christenson RH, Crow RS, Fortmann SP, Goff D, et al. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003;108:2543–2549 [DOI] [PubMed] [Google Scholar]
- 15.Scheitz JF, Nolte CH, Doehner W, Hachinski V, Endres M. Stroke-heart syndrome: clinical presentation and underlying mechanisms. Lancet Neurol. 2018;17:1109–1120 [DOI] [PubMed] [Google Scholar]
- 16.Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127–e248 [DOI] [PubMed] [Google Scholar]
- 17.Expert Committee on the D, Classification of Diabetes M. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26 Suppl 1:S5–20 [DOI] [PubMed] [Google Scholar]
- 18.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–1446 [DOI] [PubMed] [Google Scholar]
- 19.Writing Group M, January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2019;16:e66–e93 [DOI] [PubMed] [Google Scholar]
- 20.Bybee KA, Kara T, Prasad A, Lerman A, Barsness GW, Wright RS, et al. Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST-segment elevation myocardial infarction. Ann Intern Med. 2004;141:858–865 [DOI] [PubMed] [Google Scholar]
- 21.Olin JW, Sealove BA. Peripheral artery disease: current insight into the disease and its diagnosis and management. Mayo Clin Proc. 2010;85:678–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burkes RM, Donohue JF. An Update on the Global Initiative for Chronic Obstructive Lung Disease 2017 Guidelines With a Focus on Classification and Management of Stable COPD. Respir Care. 2018;63:749–758 [DOI] [PubMed] [Google Scholar]
- 23.Hosmer DW, Lemeshow W. Area Under the ROC Curve. Applied Logistic Regression 2nd Edition New York: John Wiley & Sons, Inc; 2000:160–164 [Google Scholar]
- 24.Thalin C, Rudberg AS, Johansson F, Jonsson F, Laska AC, Nygren AT, et al. Elevated Troponin Levels in Acute Stroke Patients Predict Long-term Mortality. J Stroke Cerebrovasc Dis. 2015;24:2390–2396 [DOI] [PubMed] [Google Scholar]
- 25.Batal O, Jentzer J, Balaney B, Kolia N, Hickey G, Dardari Z, et al. The prognostic significance of troponin I elevation in acute ischemic stroke. J Crit Care. 2016;31:41–47 [DOI] [PubMed] [Google Scholar]
- 26.Ahn SH, Lee JS, Kim YH, Kim BJ, Kim YJ, Kang DW, et al. Prognostic Significance of Troponin Elevation for Long-Term Mortality after Ischemic Stroke. J Stroke. 2017;19:312–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Su YC, Huang KF, Yang FY, Lin SK. Elevation of troponin I in acute ischemic stroke. PeerJ. 2016;4:e1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ali F, Young J, Rabinstein AA, Flemming KD, Fugate JE. Routine Troponin Measurements Are Unnecessary to Exclude Asymptomatic Coronary Events in Acute Ischemic Stroke Patients. J Stroke Cerebrovasc Dis. 2016;25:1215–1221 [DOI] [PubMed] [Google Scholar]
- 29.VanHouten J, Fricker G, Collins B, Bhatia R, Ellis C, Schrag M. Circulating Troponin I Level in Patients with Acute Ischemic Stroke. Curr Neurol Neurosci Rep. 2018;18:32. [DOI] [PubMed] [Google Scholar]
- 30.Kerr G, Ray G, Wu O, Stott DJ, Langhorne P. Elevated troponin after stroke: a systematic review. Cerebrovasc Dis. 2009;28:220–226 [DOI] [PubMed] [Google Scholar]
- 31.Lasek-Bal A, Gasior Z, Kowalewska-Twardela T, Urbanek T. New-onset atrial fibrillation in patients with elevated troponin I levels in the acute phase of stroke. Int J Cardiol. 2015;195:210–211 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.