Abstract
Using data from the National Cancer Data Base, 2010–2015, we examined characteristics and outcomes of T-cell/histiocyte-rich large B-cell lymphoma (THRLBCL, N=622) relative to unspecified diffuse large B-cell lymphoma (DLBCL-NOS, N=91,588) and nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL, N=2,240). Socio-demographic characteristics of patients with THRLBCL resembled more NLPHL than DLBCL-NOS. Five-year overall survival in THRLBCL was 66% (95%CI, 60–71%). Adjusting for clinical and socio-economic covariates, THRLBCL was associated with better survival than DLBCL-NOS (adjusted hazard ratio, 0.80; 95%CI, 0.67–0.94). This association was similar in academic and community hospitals and consistent in a model stratified by the revised International Prognostic Index. Prognostic factors in THRLBCL included age, comorbidity index, and extranodal primary site, but not stage. Adjusted odds of prior NLPHL were 18.2 higher for THRLBCL (95% confidence interval [CI], 7.2–45.7) than DLBCL-NOS. These large-scale epidemiologic data support the relationship between THRLBCL and NLPHL, and suggest improved prognosis with modern rituximab-based immunochemotherapy.
Keywords: diffuse large B-cell lymphoma, T-cell/histiocyte-rich large B-cell lymphoma, nodular lymphocyte predominant Hodgkin lymphoma, epidemiology, survival analysis
Introduction
T-cell/histiocyte-rich large B-cell lymphoma (THRLBCL) is an uncommon histologic variant of diffuse large B-cell lymphoma (DLBCL), characterized by low (1–10%) content of lymphoma cells in the tumor, with an abundant surrounding infiltrate composed of predominantly CD8+TIA-1+granzyme B− T-cells and histiocytes.[1, 2] The disease was initially described in small case series as a poor-prognosis subtype frequently involving the spleen and bone marrow, with 3-year event-free survival (EFS) of only about 40%.[3, 4] However, subsequent case-control analyses suggested no difference in outcomes compared with other forms of DLBCL.[5] Many studies remarked on morphologic and genomic similarities between THRLBCL and nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL), pointing to the fact that in some NLPHL patients THRLBCL may develop as a synchronous or metachronous pathology.[6–11] The knowledge about clinical outcomes in THRLBCL has remained limited to small clinicopathologic series, as it was only a provisional subtype in the World Health Organization (WHO) classification of lymphoid neoplasms until 2008, and thus was not identifiable in population-based registries.
In 2010, cancer registries in the United States (US) started to distinguish THRLBCL as a separate entity from DLBCL not otherwise specified (DLBCL-NOS). With a consistent description of histologic criteria, these newly available epidemiologic data allow for a more comprehensive evaluation of clinical features and outcomes among patients diagnosed with THRLBCL in the community. One study used the Surveillance, Epidemiology, and End Results (SEER) program data to describe overall survival of 270 THRLBCL cases, which was higher (72% at 3 years) than in the historical reports, raising a question about adequacy of diagnosis in this rare lymphoma.[12] The study had also remarked on a 68% higher incidence of THRLBCL among black Americans, similar to observations in NLPHL.[13, 14] Our objectives were to use a richer dataset to compare clinical characteristics of THRLBCL with DLBCL-NOS and NLPHL in the context of diagnostic patterns and outcomes in academic and community-based institutions. We also aimed to more comprehensively describe the relationship between THRLBCL and pre-existing NLPHL from the epidemiologic perspective, as well as overall and disease-specific survival relative to DLBCL-NOS, accounting for receipt of chemotherapy and the Revised International Prognostic Index (R-IPI).[15]
Methods
Patients and data source
We analyzed data on all adult (18 years or older) patients with THRLBCL reported to the National Cancer Data Base (NCDB) between 2010 and 2015, and compared them with contemporary cases of DLBCL and NLPHL. Cases were selected according to WHO histology codes 9688/3 (THRLBCL), 9680/3 and 9684/3 (DLBCL-NOS), and 9659/3 (NLPHL). In the NCDB, THRLBCL were designated as such regardless of presentation as the first lymphoma, or as a transformation from prior NLPHL. The NCDB is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society, and contains over 34 million records from over 1,500 hospital registries accredited by the Commission on Cancer.[16] The NCDB captures about 84% of all incident lymphomas in the US.[17] It has been extensively used for analysis of epidemiology and outcomes in lymphomas, including rare subtypes.[18–20] Participating hospitals are subject to periodic audits and must report survival follow up for >90% of cases within 5 years from diagnosis.
We excluded cases treated completely outside of the reporting institution, for which the NCDB does not require treatment or follow-up data. Hospitals were classified as academic/research centers (including the National Cancer Institute-designated Comprehensive Cancer Centers) and other types (community or integrated network cancer programs). The NCDB records patients’ socio-demographic status and a comorbidity index derived from hospital records, predictive of mortality risk.[21] Lymphoma-specific data include histology according to the WHO classification, stage according to the Ann Arbor schema, presence of B symptoms, and primary site. The IPI is recorded in a subset of observations.[22, 23] The registry also records receipt of chemotherapy, radiation, or stem cell transplantation as part of the first course of treatment (i.e. before any recurrence or progression of disease), but without specifying regimens, doses, or response to therapy. Overall survival (OS) from diagnosis is the only recorded outcome, and was available for patients diagnosed in 2010–2014.
For the analysis of NLPHL preceding the diagnosis of THRLBCL or DLBCL-NOS, we separately extracted data from the SEER registry on cases of THRLBCL and DLBCL-NOS (2010–2015), as well as all prior malignant tumors that occurred in these patients.[24] The SEER program records up to 10 cancers for each subject, and has been collecting population-based data from 18 geographical areas in the US, currently covering about 34.6% of the US population.
Statistical analysis
Clinical characteristics were tabulated and compared using Wilcoxon rank-sum test or chi-squared test for continuous or categorical variables, respectively. OS was plotted using the Kaplan-Meier method, without any univariate survival comparisons to avoid bias in this observational dataset. All multivariable models included the same set of clinically relevant variables, regardless of statistical significance: age, sex, race, comorbidity index, median income in patient’s county of residence, lymphoma stage, presence of B symptoms, nodal or extranodal primary site, and type of reporting hospital. In DLBCL, we additionally distinguished central nervous system, lung, liver, pancreas, gastrointestinal tract, and bone marrow as “high-risk” extranodal sites based on prior studies evaluating survival in DLBCL.[23, 25] For optimal specification, age was introduced as a continuous variable using a fractional polynomial.[26] In order to compare survival between THRLBCL and DLBCL-NOS, we generated an expected survival curve for DLBCL-NOS patients with a distribution of all available covariates identical to the THRLBCL subgroup. For this purpose, we fitted a multivariable flexible parametric model with time-varying effects for all covariates (with 5 degrees of freedom for baseline hazard, and 3 degrees of freedom for all time-varying variables) in the combined THRLBCL/DLBCL-NOS cohort, and then predicted survival in the THRLBCL subcohort, as described previously.[27, 28] We studied analogous models stratified by the R-IPI in the subset of cases with available IPI (~12%),[15] in the subset reported from academic/research institutions, or with a record of multi-agent chemotherapy. For analysis of prognostic factors in THRLBCL, we modeled relative survival (RS) in addition to OS. RS accounts for a baseline mortality risk for patient’s specific age, sex, race, and calendar year, based on national mortality statistics, and can approximate excess (net) mortality related to lymphoma and its therapy rather than extraneous causes.[29] Missing values for race, stage, and B symptoms in the prognostic models were assumed to be missing at random and were filled in by multiple imputation using chained equations (which included vital status and Nelson-Aalen cumulative hazard of death as covariates) in 10 imputed datasets.[30] All model estimates are presented with 95% confidence intervals (95%CI). Statistical analyses were conducted using Stata/MP 15.1 (College Station, TX) with stpm2 module (v.1.7.1, Lambert P, Andersson T, Royston P, 2018).
Results
Between 2010 and 2015, the NCDB recorded 622 cases of THRLBCL, 91,588 of DLBCL-NOS, and 2,240 of NLPHL (Table 1). Median age of patients with THRLBCL was 11 years higher than in NLPHL, and 10 years lower than in DLBCL-NOS (Fig. 1; age range was 18 to >90 in all groups). The socio-demographic profile significantly differed between THRLBCL and DLBCL-NOS: THRLBCL patients were more often male (66%) or black (23%) than those with DLBCL-NOS (55% and 8%, respectively). In contrast, distribution of sex and race was similar between THRLBCL and NLPHL. THRLBCL was more often (relative to DLBCL-NOS) reported by academic/research hospitals (47% versus 41%, respectively), at an advanced stage (81% versus 56%), with B symptoms (44% versus 28%), and with a primary nodal site (95% versus 66%). The most common primary extranodal sites in THRLBCL included liver, spleen, and bone marrow (each with <10 cases). In the subgroup with recorded R-IPI, THRLBCL was more often in the poor-risk category than DLBCL-NOS (59% versus 49%, respectively). Patients with THRLBCL were also more likely to undergo stem cell transplantation as part of initial therapy (5% versus 2%, respectively).
Table 1.
Characteristics of patients with THRLBCL, compared with DLBCL-NOS and NLPHL, diagnosed in 2010–2015.
| Variable | THRLBCL N=622 |
DLBCL-NOS N=91,588 |
P a | NLPHL N=2,240 |
P a | ||||
|---|---|---|---|---|---|---|---|---|---|
| Age: | |||||||||
| Median | 58 | 68 | <.001 | 47 | <.001 | ||||
| IQR b | 42–70 | 57–77 | 33–60 | ||||||
| Sex, N (%) | |||||||||
| Male | 411 | (66.1) | 50,045 | (54.6) | <.001 | 1,437 | (64.2) | .37 | |
| Female | 211 | (33.9) | 41,543 | (45.4) | 803 | (35.8) | |||
| Male:female ratio | 1.9 | 1.2 | 1.8 | ||||||
| Race/ethnicity, N (%) | |||||||||
| White | 454 | (73.0) | 79,388 | (86.7) | <.001 | 1,566 | (69.9) | .51 | |
| Black | 144 | (23.2) | 7,067 | (7.7) | 574 | (25.6) | |||
| Asian / other | 24 | (3.8) | 5,133 | (5.6) | 100 | (4.5) | |||
| Comorbidity index, N (%) | |||||||||
| 0 | 500 | (80.4) | 66,338 | (72.4) | <.001 | 1,908 | (85.2) | .027 | |
| 1 | 88 | (14.1) | 16,780 | (18.3) | 244 | (10.9) | |||
| 2 | 24 | (3.9) | 4,905 | (5.4) | 68 | (3.0) | |||
| ≥3 | 10 | (1.6) | 3,565 | (3.9) | 20 | (0.9) | |||
| Stage, N (%) | |||||||||
| I/II | 118 | (19.0) | 40,560 | (44.3) | <.001 | 1,475 | (65.8) | <.001 | |
| III/IV or unrecorded c | 504 | (81.0) | 51,028 | (55.7) | 765 | (34.2) | |||
| B symptoms, N (%) | |||||||||
| Absent | 299 | (48.1) | 57,388 | (62.7) | <.001 | 1,776 | (79.3) | <.001 | |
| Present | 276 | (44.4) | 25,525 | (27.9) | 332 | (14.8) | |||
| Unrecorded | 47 | (7.6) | 8,675 | (9.5) | 132 | (5.9) | |||
| Primary site, N (%) | |||||||||
| Nodal | 590 | (94.9) | 59,975 | (65.5) | <.001 | 2,198 | (98.1) | <.001 | |
| Extranodal | 10 | (1.6) | 14,837 | (16.2) | 32 | (1.4) | |||
| High-risk extranodal | 22 | (3.5) | 16,776 | (18.3) | 10 | (0.4) | |||
| Reporting hospital, N (%) | |||||||||
| Academic/research | 292 | (46.9) | 37,240 | (40.7) | .001 | 933 | (41.7) | .018 | |
| Other | 330 | (53.1) | 54,348 | (59.3) | 1,307 | (58.3) | |||
| Chemotherapy, N (%) | |||||||||
| Administered | 558 | (89.7) | 76,083 | (83.1) | <.001 | 1,279 | (57.1) | <.001 | |
| Not administered or unrecorded c | 64 | (10.3) | 15,505 | (16.9) | 961 | (42.9) | |||
| Stem cell transplantation, N (%) | |||||||||
| Yes | 30 | (4.8) | 1,498 | (1.6) | <.001 | <10c | (<1.0) | <.001 | |
| No or unrecorded c | 592 | (95.2) | 90,090 | (98.4) | >2,220c | (>99.0) | |||
| R-IPI, N (%) | |||||||||
| Recorded, N (% total) | 105 | (17.0) | 10,610 | (11.6) | |||||
| Very good | 653 | (6.3) | .043 | ||||||
| Good | 43c | (41.0) | 4,669 | (45.0) | |||||
| Poor | 62 | (59.0) | 5,058 | (48.7) | |||||
IQR: interquartile range; R-IPI: revised International Prognostic Index
P value for univariate comparison with THRLBCL
age range was 18 to ≥90 years for all groups
categories combined or rounded to protect patients’ privacy (NCDB disallows cell sizes smaller than 10); stage was unrecorded in 2.8%, chemotherapy receipt in 1.4%, and transplantation in 0.7% of patients.
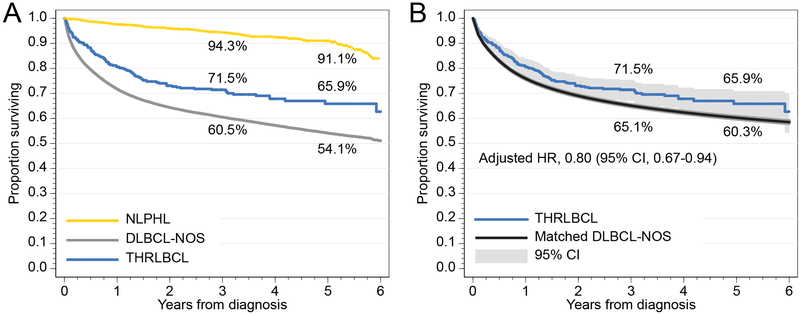
Fig. 1.
(A) Overall survival of patients with THRLBCL, DLBCL-NOS, and NLPHL; (B) overall survival in THRLBCL, compared with DLBCL-NOS matched by age, sex, race, stage, presence of B symptoms, extranodal site, comorbidity index, income, and type of treating hospital.
Median follow-up time for the entire cohort was 4.5 years (95% CI, 4.4–4.8 years). Median OS was not reached, but unadjusted 5-year OS was higher for THRLBCL (65.9%, 95%CI, 60.2–70.9) than for DLBCL-NOS (54.1%, 95%CI, 53.7–54.6; Fig. 1A). In a multivariate survival model adjusting for differences in age, sex, race, stage, B symptoms, extranodal site, comorbidities, income, and type of hospital, diagnosis of THRLBCL was still associated with a better OS (adjusted HR, 0.80; 95%CI, 0.67–0.94; P=0.007; Fig. 1B). This association was similar in academic and community hospitals (P for interaction = 0.52). We consistently observed better OS for THRLBCL in models limited to stage III/IV lymphomas only (adjusted HR, 0.72; 95%CI 0.60–0.87; P=0.0006), patients treated with multi-agent chemotherapy (adjusted HR, 0.72; 95%CI, 0.59–0.89, P=0.002), or in a model adjusted by the R-IPI (adjusted HR, 0.55; 95%CI, 0.32–0.94; P=0.030).
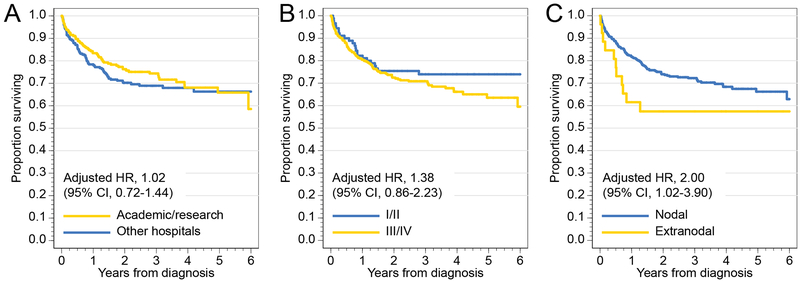
We then evaluated prognostic factors for OS and RS in the THRLBCL cohort (Table 2). Multivariable models revealed increasing age, higher number of comorbidities, and extranodal origin as significant high-risk factors (Fig. 2A). In contrast, treatment in an academic hospital or advanced stage were not prognostic (Fig. 2B/C). The associations were consistent in the subgroup of patients who received multi-agent chemotherapy (data not shown).
Table 2.
Multivariable prognostic model for overall and relative survival in THRLBCL.
| Variable | Overall survival | Relative survival a | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Age: 18–50 years | Ref. | <0.001 | Ref. | <0.001 | |||
| 51–60 years | 1.39 | (0.77–2.48) | 1.26 | (0.66–2.42) | |||
| 61–70 years | 2.27 | (1.35–3.83) | 2.11 | (1.18–3.79) | |||
| 71–80 years | 2.74 | (1.57–4.79) | 2.48 | (1.32–4.65) | |||
| >80 years | 7.46 | (4.27–13.04) | 6.28 | (3.30–11.95) | |||
| Sex: male | Ref. | 0.41 | Ref. | 0.35 | |||
| Female | 1.16 | (0.82–1.64) | 1.21 | (0.81–1.79) | |||
| Race/ethnicity: white | Ref. | 0.52 | Ref. | 0.53 | |||
| Black | 1.24 | (0.79–1.95) | 1.27 | (0.77–2.08) | |||
| Asian / other | 0.65 | (0.16–2.67) | 0.65 | (0.14–2.95) | |||
| Comorbidity index: 0 | Ref. | 0.024 | Ref. | 0.018 | |||
| 1 | 0.78 | (0.47–1.30) | 0.82 | (0.46–1.45) | |||
| 2 | 1.20 | (0.55–2.64) | 1.29 | (0.54–3.07) | |||
| ≥3 | 3.17 | (1.39–7.20) | 3.62 | (1.53–8.52) | |||
| Stage: I/II | Ref. | 0.18 | Ref. | 0.23 | |||
| III/IV | 1.38 | (0.86–2.23) | 1.42 | (0.80–2.51) | |||
| B symptoms: present | Ref. | 0.06 | Ref. | 0.044 | |||
| Absent | 1.45 | (0.99–2.14) | 1.58 | (1.01–2.46) | |||
| Primary site: nodal | Ref. | 0.043 | Ref. | 0.024 | |||
| Extranodal | 2.00 | (1.02–3.90) | 2.31 | (1.12–4.79) | |||
| Reporting hospital: other | Ref. | 0.91 | Ref. | 0.99 | |||
| Academic/research | 1.02 | (0.72–1.44) | 1.00 | (0.67–1.49) | |||
CI: confidence interval; HR: hazard ratio; Ref.: reference level.
Relative survival adjusts for baseline mortality rate according to age, sex, race, and calendar year based on US national statistics, thus measuring lymphoma-related excess mortality.
Fig. 2.
Overall survival of patients with THRLBCL, stratified by (A) type of reporting hospital, (B) stage, and (C) nodal/extranodal primary site.
To further examine the association between THRLBCL and prior NLPHL we used the SEER data to study 305 cases of THRLBCL and 33,273 contemporary cases of DLBCL-NOS. Socio-demographic characteristics of THRLBCL patients were similar as in the NCDB (median age, 56 years, 75% men, 20% black patients). Nine percent of THRLBCL cases and 15% of DLBCL-NOS cases had any pre-existing cancers (P=.002), which reflected different age distribution. Conversely, pre-existing NLPHL, while overall rare, was more frequent in THRLBCL (2.3% versus 0.1%, respectively, P<0.001). Adjusting for differences in age, sex, and race, the prevalence of other cancers was similar for THRLBCL and DLBCL-NOS, but the odds of prior NLPHL were 18.2 times higher in THRLBCL (95% CI, 7.2–45.7; Table 3).
Table 3.
Association between THRLBCL histology (relative to DLBCL-NOS) and antecedent cancers in the SEER data, 2010–2015.
| Antecedent cancer |
Unadjusted models |
Models adjusting for age, sex, and race |
|||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | ||
| Any histology | 0.56 | (0.38–0.84) | 0.005 | 0.74 | (0.49–1.10) | 0.14 | |
| Solid tumor | 0.44 | (0.26–0.76) | 0.003 | 0.62 | (0.36–1.07) | 0.09 | |
| Any lymphoma | 0.90 | (0.54–1.52) | 0.70 | 1.02 | (0.61–1.73) | 0.93 | |
| NLPHL | 35.5 | (15.1–83.7) | <0.001 | 18.2 | (7.2–45.7) | <0.001 | |
CI: confidence interval; NLPHL: nodular lymphocyte-predominant Hodgkin lymphoma.
Discussion
In this large nationwide cohort, we leveraged newly available epidemiologic data to describe outcomes of patients with THRLBCL diagnosed in the US in 2010–2015, revealing several novel observations. First, the socio-demographic profile of patients with THRLBCL significantly differs from DLBCL-NOS, but resembles NLPHL, thus supporting the hypothesis that THRLBCL and NLPHL may be related. Despite higher prevalence of unfavorable risk factors in THRLBCL, when matched by all relevant characteristics, THRLBCL actually showed better OS than DLBCL-NOS, and 5-year OS estimate (66%) was more favorable than in historical case series. Finally, we have identified extranodal primary site (usually high-risk, like liver or bone marrow) as an important, albeit rare, risk factor, more prognostic than advanced stage. THRLBCL involving extranodal sites may thus have uniquely unfavorable biology, potentially explaining poor outcomes reported in prior case series.
The relationship between THRLBCL and NLPHL has been described from the histopathologic, clinical, and molecular viewpoints, although the aggressive nature and poor survival in THRLBCL contrasted with the indolent clinical course of NLPHL [6–8, 10, 11, 31]. Our findings provide a novel epidemiologic perspective, indicating that THRLBCL and NLPHL share socio-demographic characteristics as well as predominantly nodal origin, and that THRLBCL is associated with 18 times higher odds of prior NLPHL compared with DLBCL-NOS. NLPHL is known to have familial predisposition,[32] and its high incidence among males or black patients suggests a biologic underpinning, which may extend to THRLBCL as well, and may involve host immunity.[13, 33–35] Male predominance is striking in prior case series and clinical trials in both THRLBCL and NLPHL.[3, 6, 9, 13, 14, 33, 36–38] THRLBCL shows not only morphologic, but also transcriptional features indicating strong dependence on tolerogenic host immune response.[33] Conversely, primary mediastinal B-cell lymphoma, another large B-cell lymphoma highly dependent on immune evasion mechanisms, is much more frequent among women. These observations suggest that sex-specific immune milieu factors might influence predisposition to lymphoma subtypes. The 11-year difference in median age between NLPHL and THRLBCL, and high prevalence of advanced-stage disease, suggests that THRLBCL might occur as a progression of occult NLPHL with a latency of about a decade. This hypothesis is consistent with prior case series of NLPHL, showing median interval from diagnosis to histologic transformation of 8 years.[9, 39] Interestingly, DLBCL-like chemotherapy in NLPHL may help avert the risk of future transformation.[37] However, nearly 98% of THRLBCL cases in the US are diagnosed de novo, without a pre-existing NLPHL. Furthermore, one study reported a lower number of genomic imbalances in THRLBCL than in NLPHL (median 4.7 and 10.8, respectively), questioning the theory of transformation in favor of a common precursor hypothesis.[11]
We have observed better OS in THRLBCL than in matched DLBCL-NOS, contrary to reports from the pre-rituximab era, which described THRLBCL as an aggressive, disseminated lymphoma associated with 3-year OS less than 50%.[3–5, 40] Recently, Kommalapati et al. estimated 3-year OS of 72% among 270 THRLBCL cases from the SEER registry, similar to 540 cases of DLBCL-NOS matched by age and stage.[12] One potential explanation for these discrepancies between modern registries and prior literature could be that historical case series were biased towards more aggressive extranodal THRLBCL with marrow involvement. Alternatively, NLPHL might be misclassified as THRLBCL in the community, particularly in extranodal biopsies that lack the nodal architecture.[10, 36] However, most THRLBCL cases in our analysis were nodal, and the extranodal cases had worse rather than better prognosis. Additionally, disseminated THRLBCL cases diagnosed from bone marrow biopsy may be designated as DLBCL-NOS by a pathologist uncomfortable with identifying THRLBCL outside of the lymph node. Lack of central histopathologic review is a significant limitation of all large-scale registries, but at the same time registry-based studies reflect treatments and outcomes among patients diagnosed in the community according to published criteria. In one case series, 18% of THRLBCL cases referred to an academic center were reclassified.[4] We found no difference in THRLBCL survival between academic and community hospitals, and HR for THRLBCL relative to DLBCL-NOS was similar in those two types of facilities. Because academic/research centers largely rely on expert hematopathologists, misdiagnosis may be less likely in those hospitals, although it appears that molecular tools going beyond morphology and immunophenotyping are needed to confidently differentiate NLPHL, THRLBCL, and DLBCL-NOS in clinical practice. Therefore, our data raise hypotheses that application of consistent diagnostic criteria has uncovered more cases of THRLBCL with less aggressive features, or that the THRLBCL prognosis has markedly improved with modern rituximab-based immunochemotherapy. Recent studies emphasize distinct genomic and molecular features of THRLBCL, which does not cluster with other subtypes of DLBCL-NOS, and shows overexpression of immune checkpoint antigens like PD-L1.[38, 41–44] We observed a higher rate of stem cell transplantation in THRLBCL, suggesting a more aggressive approach in some institutions guided by historical data, or, alternatively, a possible biologic heterogeneity of THRLBCL, with an aggressive subset refractory to standard treatment.
Our study is limited by reliance on de-identified cancer registry data, precluding analysis of detailed histologic or clinical features, laboratory values, or more in-depth evaluation of therapy. We analyzed only adult cases, but NLPHL has a higher incidence among children and adolescents, so epidemiology of THRLBCL in pediatric population would also be of interest. We have overcome at least partly the lack of direct pathology review by demonstrating consistent results in the subset of academic centers. Absent data on response to treatment, rate of recurrence or progression-free survival, we used RS as an indirect measure of lymphoma-related mortality. Only a small subset of cases had a recorded IPI, so we could not assess the value of R-IPI in THRLBCL. We also note that the NCDB is a hospital-based rather than a population-based registry, so we could not calculate incidence rates or other population-based measures. These have been recently described using SEER data.[12]
In summary, we have provided an epidemiologic perspective on the close relationship between THRLBCL and NLPHL, and suggest that in the era of rituximab-based immunochemotherapy, THRLBCL may have a more favorable prognosis than DLBCL-NOS. Similar diagnostic patterns in academic and community hospitals indicate a fairly reliable histology designation, which will allow future analysis of outcomes based on cancer registry records in this rare disease. Further research should evaluate potential biologic and clinical heterogeneity of THRLBCL, which may encompass aggressive subcategories potentially correlating with extranodal involvement.[45] Increasing recognition that THRLBCL and NLPHL are characterized by ineffective immune response, possibly mediated by sex- or ethnicity-related host factors and/or tumor-induced modulation of T-cell immunity, raises a question whether THRLBCL might be one DLBCL subtype particularly amenable to checkpoint inhibitor-based immunotherapy.
Acknowledgements
The data used in the study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator. Preliminary data from this research have been presented at the AACR International Meeting on Advances in Malignant Lymphoma, Boston, June 22–26, 2018.
Funding:
This work was supported by the American Cancer Society under Grant 128608-RSGI-15-211-01-CPHPS, and the National Institute of General Medical Sciences at National Institutes of Health under Grant U54GM115677.
Footnotes
Disclosure of interest
T.A.O. reports no conflict of interest; A.J.O. reports research funding from Genentech and Spectrum Pharmaceuticals; J.L.R. reports consultancy for Teva.
References:
- 1.Swerdlow SH, World Health Organization, International Agency for Research on Cancer WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: International Agency for Research on Cancer; 2017. 585 pages p. [Google Scholar]
- 2.Pittaluga S, Jaffe ES. T-cell/histiocyte-rich large B-cell lymphoma. Haematologica 2010; 95: 352–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Achten R, Verhoef G, Vanuytsel L, et al. T-cell/histiocyte-rich large B-cell lymphoma: a distinct clinicopathologic entity. J Clin Oncol 2002; 20: 1269–1277. [DOI] [PubMed] [Google Scholar]
- 4.El Weshi A, Akhtar S, Mourad WA, et al. T-cell/histiocyte-rich B-cell lymphoma: Clinical presentation, management and prognostic factors: report on 61 patients and review of literature. Leuk Lymphoma 2007; 48: 1764–1773. [DOI] [PubMed] [Google Scholar]
- 5.Bouabdallah R, Mounier N, Guettier C, et al. T-cell/histiocyte-rich large B-cell lymphomas and classical diffuse large B-cell lymphomas have similar outcome after chemotherapy: a matched-control analysis. J Clin Oncol 2003; 21: 1271–1277. [DOI] [PubMed] [Google Scholar]
- 6.Boudova L, Torlakovic E, Delabie J, et al. Nodular lymphocyte-predominant Hodgkin lymphoma with nodules resembling T-cell/histiocyte-rich B-cell lymphoma: differential diagnosis between nodular lymphocyte-predominant Hodgkin lymphoma and T-cell/histiocyte-rich B-cell lymphoma. Blood 2003; 102: 3753–3758. [DOI] [PubMed] [Google Scholar]
- 7.Rudiger T, Gascoyne RD, Jaffe ES, et al. Workshop on the relationship between nodular lymphocyte predominant Hodgkin’s lymphoma and T cell/histiocyte-rich B cell lymphoma. Ann Oncol 2002; 13 Suppl 1: 44–51. [DOI] [PubMed] [Google Scholar]
- 8.Hartmann S, Doring C, Vucic E, et al. Array comparative genomic hybridization reveals similarities between nodular lymphocyte predominant Hodgkin lymphoma and T cell/histiocyte rich large B cell lymphoma. Br J Haematol 2015; 169: 415–422. [DOI] [PubMed] [Google Scholar]
- 9.Al-Mansour M, Connors JM, Gascoyne RD, et al. Transformation to aggressive lymphoma in nodular lymphocyte-predominant Hodgkin’s lymphoma. J Clin Oncol 2010; 28: 793–799. [DOI] [PubMed] [Google Scholar]
- 10.Hartmann S, Doring C, Jakobus C, et al. Nodular lymphocyte predominant hodgkin lymphoma and T cell/histiocyte rich large B cell lymphoma--endpoints of a spectrum of one disease? PLoS One 2013; 8: e78812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franke S, Wlodarska I, Maes B, et al. Comparative genomic hybridization pattern distinguishes T-cell/histiocyte-rich B-cell lymphoma from nodular lymphocyte predominance Hodgkin’s lymphoma. Am J Pathol 2002; 161: 1861–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kommalapati A, Tella SH, Go RS, et al. T cell/histiocyte-rich large B cell lymphoma: incidence, demographic disparities, and long-term outcomes. Br J Haematol 2018: doi: 10.1111/bjh.15391. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 13.Olszewski AJ, Shrestha R, Cook NM. Race-specific features and outcomes of nodular lymphocyte-predominant Hodgkin lymphoma: Analysis of the National Cancer Data Base. Cancer 2015; 121: 3472–3480. [DOI] [PubMed] [Google Scholar]
- 14.Shenoy P, Maggioncalda A, Malik N, et al. Incidence patterns and outcomes for hodgkin lymphoma patients in the United States. Adv Hematol 2011; 2011: 725219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sehn LH, Berry B, Chhanabhai M, et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood 2007; 109: 1857–1861. [DOI] [PubMed] [Google Scholar]
- 16.Boffa DJ, Rosen JE, Mallin K, et al. Using the National Cancer Database for Outcomes Research: A Review. JAMA Oncol 2017; 3: 1722–1728. [DOI] [PubMed] [Google Scholar]
- 17.Bilimoria KY, Stewart AK, Winchester DP, et al. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol 2008; 15: 683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldstein JS, Nastoupil LJ, Han X, et al. Disparities in survival by insurance status in follicular lymphoma. Blood 2018; 132: 1159–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olszewski AJ, Shrestha R, Castillo JJ. Treatment selection and outcomes in early-stage classical Hodgkin lymphoma: analysis of the National Cancer Data Base. J Clin Oncol 2015; 33: 625–633. [DOI] [PubMed] [Google Scholar]
- 20.Olszewski AJ, Ollila T, Reagan JL. Time to treatment is an independent prognostic factor in aggressive non-Hodgkin lymphomas. Br J Haematol 2018; 181: 495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992; 45: 613–619. [DOI] [PubMed] [Google Scholar]
- 22.The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med 1993; 329: 987–994. [DOI] [PubMed] [Google Scholar]
- 23.Olszewski AJ, Winer ES, Castillo JJ. Validation of clinical prognostic indices for diffuse large B-cell lymphoma in the National Cancer Data Base. Cancer Causes Control 2015; 26: 1163–1172. [DOI] [PubMed] [Google Scholar]
- 24.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, November 2017 Sub (1973–2015 varying), National Cancer Institute, DCCPS, Surveillance Research Program, released April 2018, based on the November 2017 submission.; 2018. [Google Scholar]
- 25.Zhou Z, Sehn LH, Rademaker AW, et al. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood 2014; 123: 837–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Royston P, Ambler G, Sauerbrei W. The use of fractional polynomials to model continuous risk variables in epidemiology. Int J Epidemiol 1999; 28: 964–974. [DOI] [PubMed] [Google Scholar]
- 27.Qunaj L, Castillo JJ, Olszewski AJ. Survival of patients with CD20-negative variants of large B-cell lymphoma: an analysis of the National Cancer Data Base. Leuk Lymphoma 2018; 59: 1375–1383. [DOI] [PubMed] [Google Scholar]
- 28.Royston P, Lambert PC. Flexible parametric survival analysis using Stata : beyond the Cox model. College Station, TX: Stata Press; 2011. xxvi, 347 p. p. [Google Scholar]
- 29.Rutherford MJ, Dickman PW, Lambert PC. Comparison of methods for calculating relative survival in population-based studies. Cancer Epidemiol 2012; 36: 16–21. [DOI] [PubMed] [Google Scholar]
- 30.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med 2011; 30: 377–399. [DOI] [PubMed] [Google Scholar]
- 31.Delabie J, Vandenberghe E, Kennes C, et al. Histiocyte-rich B-cell lymphoma. A distinct clinicopathologic entity possibly related to lymphocyte predominant Hodgkin’s disease, paragranuloma subtype. Am J Surg Pathol 1992; 16: 37–48. [PubMed] [Google Scholar]
- 32.Saarinen S, Pukkala E, Vahteristo P, et al. High familial risk in nodular lymphocyte-predominant Hodgkin lymphoma. J Clin Oncol 2013; 31: 938–943. [DOI] [PubMed] [Google Scholar]
- 33.Van Loo P, Tousseyn T, Vanhentenrijk V, et al. T-cell/histiocyte-rich large B-cell lymphoma shows transcriptional features suggestive of a tolerogenic host immune response. Haematologica 2010; 95: 440–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monti S, Savage KJ, Kutok JL, et al. Molecular profiling of diffuse large B-cell lymphoma identifies robust subtypes including one characterized by host inflammatory response. Blood 2005; 105: 1851–1861. [DOI] [PubMed] [Google Scholar]
- 35.Hartmann S From a pathologist’s point of view: Histiocytic cells in Hodgkin lymphoma and T cell/histiocyte rich large B cell lymphoma. Pathol Res Pract 2015; 211: 901–904. [DOI] [PubMed] [Google Scholar]
- 36.Hartmann S, Eichenauer DA, Plutschow A, et al. The prognostic impact of variant histology in nodular lymphocyte-predominant Hodgkin lymphoma: a report from the German Hodgkin Study Group (GHSG). Blood 2013; 122: 4246–4252. [DOI] [PubMed] [Google Scholar]
- 37.Fanale MA, Cheah CY, Rich A, et al. Encouraging activity for R-CHOP in advanced stage nodular lymphocyte-predominant Hodgkin lymphoma. Blood 2017; 130: 472–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kunder C, Cascio MJ, Bakke A, et al. Predominance of CD4+ T Cells in T-Cell/Histiocyte-Rich Large B-Cell Lymphoma and Identification of a Subset of Patients With Peripheral B-Cell Lymphopenia. Am J Clin Pathol 2017; 147: 596–603. [DOI] [PubMed] [Google Scholar]
- 39.Ali S, Olszewski AJ. Disparate survival and risk of secondary non-Hodgkin lymphoma in histologic subtypes of Hodgkin lymphoma: a population-based study. Leuk Lymphoma 2014; 55: 1570–1577. [DOI] [PubMed] [Google Scholar]
- 40.Greer JP, Macon WR, Lamar RE, et al. T-cell-rich B-cell lymphomas: diagnosis and response to therapy of 44 patients. J Clin Oncol 1995; 13: 1742–1750. [DOI] [PubMed] [Google Scholar]
- 41.Kiyasu J, Miyoshi H, Hirata A, et al. Expression of programmed cell death ligand 1 is associated with poor overall survival in patients with diffuse large B-cell lymphoma. Blood 2015; 126: 2193–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004; 103: 275–282. [DOI] [PubMed] [Google Scholar]
- 43.Chapuy B, Stewart C, Dunford AJ, et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat Med 2018; 24: 679–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen BJ, Chapuy B, Ouyang J, et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res 2013; 19: 3462–3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ollila TA, Olszewski AJ. Extranodal Diffuse Large B Cell Lymphoma: Molecular Features, Prognosis, and Risk of Central Nervous System Recurrence. Curr Treat Options Oncol 2018; 19: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]