Abstract
Objective:
Infection is a common complication of cerebrospinal fluid (CSF) shunts, occurring in 6 to 20 percent of children. Although studies are limited, Staphylococcus aureus is thought to cause more rapid and aggressive infection than coagulase-negative Staphylococcus (CONS) or gram-negative organisms. Our objective was to evaluate the relationship between the causative organisms of CSF shunt infection and the timing of infection.
Methods:
We performed a retrospective cohort study of children who underwent CSF shunt placement at a tertiary care children’s hospital over a nine year period and subsequently developed CSF shunt infection. The primary predictor variable was the causative organism recovered from CSF culture, characterized as S. aureus, CONS, or gram-negative organisms. The primary outcome was time to infection, defined as the number of days from most recent shunt intervention to diagnosis of infection. The association between causative organism and time to infection was visualized via Kaplan Meier curves, with statistical comparison via nonparametric Kruskall-Wallis test.
Results:
Among 103 children who developed CSF shunt infection, the causative organism was CONS in 57 (55%), S. aureus in 19 (18%), and gram-negative organisms in 9 (9%). Median time to infection did not differ (p=0.81) for infections caused by CONS [20 days, interquartile range (IQR) 11–40], S. aureus (26 days, IQR 12–95), and gram-negative organisms (23 days, IQR 17–34).
Conclusions:
No significant difference in time to infection based on the causative organism was observed among children with CSF shunt infection.
Keywords: cerebrospinal, shunt, infection, children
Introduction:
Cerebrospinal fluid (CSF) shunts are critical to the surgical management of hydrocephalus.(12) However, complications are common, with CSF shunt infection occurring in 6 to 20 percent of patients with shunts.(3, 4, 11, 20–22) Despite this high rate of infection, there is limited study of the presentation of shunt infections.
CSF shunt infections are caused by a variety of pathogens, including coagulase negative Staphylococcus (CONS), Staphylococcus aureus, gram-negative organisms, and anaerobes.(2, 15) Among these pathogens, anaerobes and gram-negative organisms are generally thought to cause a more benign and indolent infection, while gram-positive organisms have the potential to cause more aggressive and rapid infections.(24, 28) Among the gram-positive organisms, S. aureus is thought to cause infection of more rapid onset than those caused by CONS.(6, 17) However, despite these commonly held beliefs, studies investigating the association between causative organism and time to infection are limited.
The objective of this study is to evaluate the relationship between the causative organism of CSF shunt infections and the timing of infection. We also evaluate the relationship between the causative organism and clinical features of the infection. Better understanding of these relationships will lead to improved understanding of the etiology and presentation of CSF shunt infections.
Materials and Methods:
Study Design and Setting
As previously described,(22, 23) we conducted a retrospective cohort study among children who received care at Primary Children’s Hospital (PCH), a 252-bed tertiary care children’s hospital serving Utah, Idaho, Wyoming, Nevada, and Montana. The hospital is owned and operated by Intermountain Healthcare, a regional, not-for-profit integrated health care system. PCH has more than 11,000 admissions per year and performs over 95 percent of the pediatric CSF shunt placements in the Intermountain Healthcare System. Institutional review board approval was obtained at the University of Utah and Seattle Children’s Hospital.
The study population included children under 18 years of age who underwent initial CSF shunt placement with a discharge date between 1/1/1997 and 10/12/2006 at PCH who subsequently developed CSF shunt infection,(22, 23) defined as the microbiological determination of bacteria in a culture of CSF. Data from each neurosurgical admission for each cohort member up until the time of CSF shunt infection were collected using Intermountain Healthcare’s database and chart review. The median duration of follow-up from the most recent surgery was 709 days [interquartile range (IQR) 104–1753 days].(22)
Measures
The primary outcome of this study was time to infection. Time to infection was defined as the number of days from the most recent CSF shunt intervention, either initial CSF shunt placement or CSF shunt revision, to the onset of infection, defined as the date on which the first positive CSF culture was collected. Secondary outcomes were clinical and diagnostic features of CSF shunt infection suggested by the 2017 Infectious Diseases Society of America clinical practice guidelines for healthcare-associated ventriculitis and meningitis, including fever, lethargy, headache, nausea, vomiting, abdominal pain, surgical site changes, neurologic changes, and leukocytosis.(26) Surgical site change was defined as documented redness or swelling at the surgical site, leakage of CSF or visible hardware. Neurologic change was defined as documented changes in seizure activity, gait, or visual disturbance, cranial nerve palsy, hemiparesis, or altered level of consciousness. Leukocytosis was defined as white blood cell (WBC) count greater than 15,000 per microliter of blood. Other clinical features we considered as secondary outcomes included demonstration of organisms in CSF Gram stain, leukopenia, pleocytosis, imaging changes, and the intermittent negative cultures. Leukopenia was defined as WBC count less than 5,000 per microliter of blood. CSF pleocytosis was defined as CSF WBC greater than 15 per cubic millimeter for children up to 28 days, greater than 9 per cubic millimeter for children 29 to 90 days, and greater than 6 per cubic millimeter for children greater than 90 days.(13, 25) Imaging changes was defined as a documented change on head computed topography, head magnetic resonance imaging, or shunt series. Intermittent negative culture was defined as documented clearing of the bacteria from culture of the CSF, followed by return of the same organism from culture of the CSF over the course of treatment.
The primary predictor variable was the causative organism recovered from CSF culture, characterized as S. aureus, CONS, or gram-negative organisms. Subsequent analysis did not focus on other organisms due to the limited number of cases. Covariates of interest included patient risk factors such as demographics (gender, race/ethnicity, insurance); risk factors at the time of initial CSF shunt placement (chronological age, indication for CSF shunt placement, weight at surgery, and complex chronic condition(s) (CCCs));(23) and number of prior shunt revisions.
Data Analysis
Patient characteristics and clinical features of infection were summarized descriptively overall and by causative organism of infection. Binary and categorical variables were described using frequencies and percents, and the continuous variable time to infection was described using medians and interquartile ranges (IQR). Categorical variables were compared using the Fisher’s exact test. Kaplan Meier survival curves were generated for each causative organism. Times to infection associated with each causative organism were compared using the Kruskal-Wallis test. Statistical significance was defined a priori as a p-value < 0.05. Data were analyzed using Stata version 12 (Stata Corporation, College Station, TX).
Results:
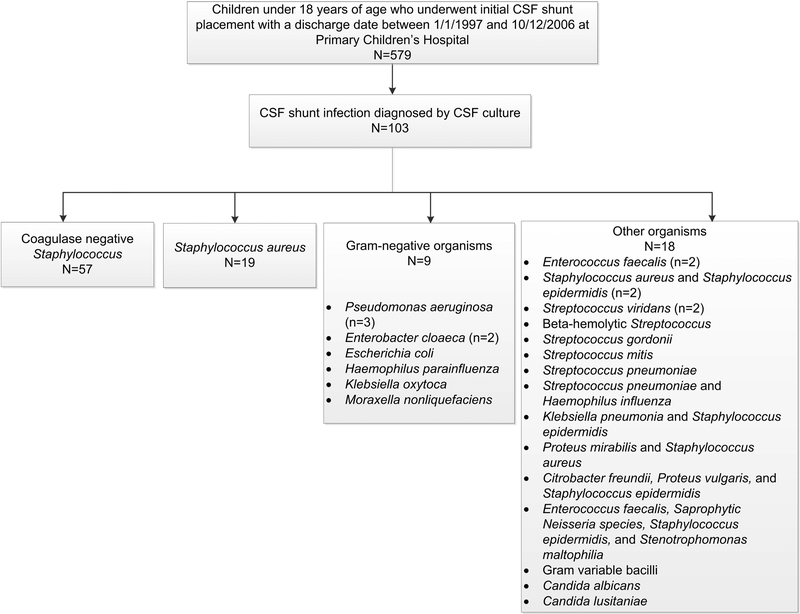
Of 103 children who underwent shunt placement within the study period and subsequently developed CSF shunt infection, the causative organism was CONS in 57 (55%), S. aureus in 19 (18%), gram-negative organisms in 9 (9%), and other organisms in 18 (17%). (Figure 1) Other organisms excluded from subsequent analysis due to heterogeneity and limited number of cases included other gram-positive organisms in 9 (9%), Candida in 2 (2%), and polymicrobial infection in 7 (7%).
Figure 1.
Study cohort
Characteristics of the overall cohort and patients with infection caused by CONS, S. aureus, and gram-negative organisms are shown in Table 1. The median age at initial shunt placement was 9 weeks (IQR, 1–29 weeks). The median age at CSF shunt infection was 22 weeks (IQR, 6–101 weeks). There was no significant difference in age at initial shunt placement, gender, ethnicity, insurance, indication for shunt placement, presence of complex chronic conditions, distal shunt location, number of shunt revisions prior to first CSF shunt infection, or age at CSF shunt infection in relation to the causative organism of infection.
Table 1.
Patient factors for the overall cohort and by causative organism
| Overall Cohort (n=103) | CONS (n=57) | S. aureus (n=19) | Gram-negative organisms (n=9) | |
|---|---|---|---|---|
| Age at initial placement, n (%) | ||||
| 0–30 days | 39 (38) | 20 (35) | 6 (32) | 5 (56) |
| 1–6 month | 39 (38) | 26 (46) | 6 (32) | 1 (11) |
| 7–48 months | 13 (13) | 6 (11) | 2 (11) | 2 (22) |
| > 48 months | 12 (11) | 5 (9) | 5 (26) | 1 (11) |
| Age at most recent surgery, n (%) | ||||
| 0–30 days | 28 (27) | 13 (23) | 5 (26) | 4 (44) |
| 1–6 month | 34 (33) | 21 (37) | 5 (26) | 1 (11) |
| 7–48 months | 19 (18) | 13 (23) | 2 (11) | 3 (33) |
| > 48 months | 22 (21) | 10 (18) | 7 (37) | 1 (11) |
| Gender (male), n (%) | 64 (62) | 36 (63) | 13 (68) | 5 (56) |
| Ethnicity, n (%) | ||||
| Caucasian | 82 (80) | 47 (82) | 17 (89) | 4 (44) |
| Hispanic | 9 (9) | 4 (7) | 1 (5) | 2 (22) |
| Other | 12 (12) | 6 (11) | 1 (5) | 3 (33) |
| Insurance, n (%) | ||||
| Private | 62 (60) | 34 (60) | 11 (58) | 6 (67) |
| Medicaid | 39 (38) | 22 (39) | 8 (42) | 2 (22) |
| Self pay | 2 (2) | 1 (2) | 0 (0) | 1 (11) |
| Indication for shunt placement, n (%) | ||||
| Post-IVH due to prematurity | 22 (21) | 14 (25) | 4 (21) | 0 (0) |
| Aqueductal stenosis | 16 (16) | 11 (19) | 3 (16) | 1 (11) |
| Cyst (posterior fossa, intracranial) | 15 (15) | 6 (11) | 4 (21) | 3 (33) |
| Congenital | 13 (13) | 7 (12) | 3 (16) | 0 (0) |
| Myelomeningocele | 12 (12) | 6 (11) | 0 (0) | 2 (22) |
| Tumor | 7 (7) | 4 (7) | 1 (5) | 1 (11) |
| Spontaneous ICH/IVH/SAH | 5 (5) | 3 (5) | 2 (11) | 0 (0) |
| Post-infectious | 5 (5) | 3 (5) | 0 (0) | 1 (11) |
| Post-head injury | 5 (5) | 3 (5) | 0 (0) | 0 (0) |
| Other | 3 (3) | 0 (0) | 2 (11) | 1 (11) |
| Complex chronic conditions, n (%) | ||||
| None (except hydrocephalus) | 85 (83) | 47 (82) | 14 (74) | 9 (100) |
| One | 10 (10) | 4 (7) | 3 (16) | 0 (0) |
| Two or more | 8 (8) | 6 (11) | 2 (11) | 0 (0) |
| Distant shunt locations, n (%) | ||||
| Peritoneal | 97 (94) | 53 (93) | 19 (100) | 9 (100) |
| Atrial | 6 (6) | 4 (7) | 0 (0) | 0 (0) |
| Revisions before first infection, n (%) | ||||
| Zero | 60 (58) | 35 (61) | 11 (58) | 4 (44) |
| One | 28 (27) | 14 (25) | 4 (21) | 0 (0) |
| Two | 15 (15) | 8 (14) | 4 (21) | 5 (56) |
| Age at CSF shunt infection, n (%) | ||||
| 0–30 days | 13 (13) | 8 (14) | 1 (5) | 1 (11) |
| 1–6 months | 43 (42) | 22 (39) | 8 (42) | 4 (44) |
| 7–48 months | 30 (29) | 21 (37) | 4 (21) | 3 (33) |
| > 48 months | 17 (17) | 6 (11) | 6 (32) | 1 (11) |
p<0.05
CONS coagulase-negative Staphylococcus, CSF cerebrospinal fluid, ICH intracerebral hemorrhage, IVH intraventricular hemorrhage, SAH subarachnoid hemorrhage
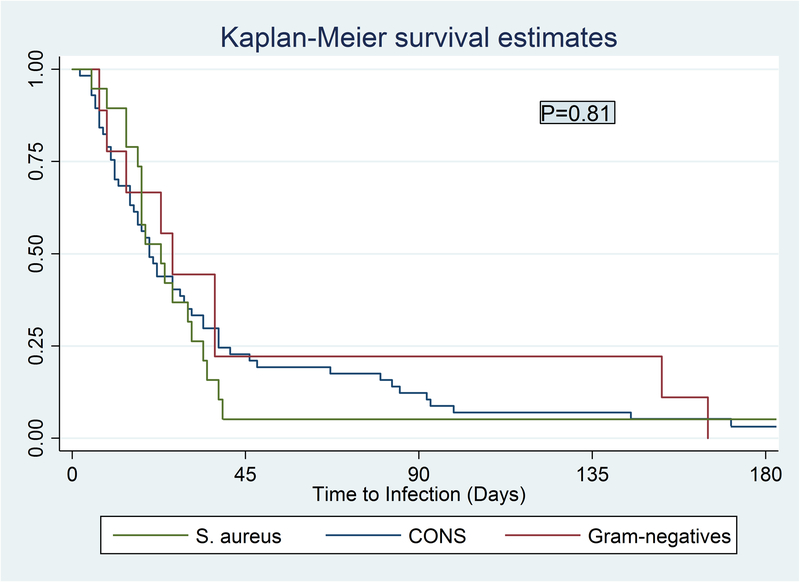
Kaplan Meier survival curves for time to infection are shown in Figure 2. Median time to infection for the total cohort was 20 days (IQR, 11–38 days). Median time to CSF shunt infection was not significantly different (p=0.81) among infections caused by CONS (median 20 days, IQR 11–40), S. aureus (median 26 days, IQR 12–95), and gram-negative organisms (median 23 days, IQR 17–34). Two infections caused by CONS and one caused by S. aureus had time to infection greater than 180 days and are not depicted in Figure 2. Three other infection occurring beyond 180 days were caused by Streptococcus gordonii, Streptococcus viridans, and Beta-hemolytic Streptococcus. The heterogeneous group of infections excluded from the Kaplan Meier curves and Kruskal-Wallis analysis had a median time to infection of 17 days (IQR 8–67).
Figure 2.
Time to infection by causative organism
Clinical features of infection by causative organism are shown in Table 2. Children with infection caused by S. aureus were more likely to present with surgical site changes (p=0.04) and leukocytosis (p=0.03) at the time of diagnosis than children with infection caused by CONS or gram-negative organisms. Children with infection caused by CONS were less likely to present with leukopenia (p=0.03).
Table 2.
Clinical features of infection for the overall cohort and by causative organism
| Overall Cohort (n=103) | CONS (n=57) | S. aureus (n=19) | Gram-negative organisms (n=9) | |
|---|---|---|---|---|
| Fever, n (%) | 83 (81) | 44 (77) | 16 (84) | 8 (89) |
| Lethargy, n (%) | 28 (27) | 10 (18) | 7 (37) | 3 (33) |
| Headache, n (%) | 10 (10) | 3 (5) | 3 (16) | 1 (11) |
| Nausea or vomiting, n (%) | 57 (55) | 30 (53) | 7 (37) | 6 (67) |
| Abdominal pain, n (%) | 31 (30) | 15 (26) | 7 (37) | 3 (33) |
| Surgical site changes, n (%) * | 60 (58) | 27 (47) | 15 (79) | 6 (67) |
| CSF leak | 24 (23) | 14 (25) | 7 (37) | 3 (33) |
| Neurologic changes, n (%) | 25 (24) | 14 (25) | 6 (32) | 2 (22) |
| Leukocytosis, n (%) * | 49 (48) | 29 (51) | 12 (63) | 1 (11) |
| Leukopenia, n (%) * | 6 (6) | 1 (2) | 2 (11) | 2 (22) |
| Positive CSF gram stain, n (%) | 51 (62) | 32 (67) | 6 (50) | 6 (67) |
| CSF pleocytosis, n (%) | 98 (95) | 56 (98) | 17 (89) | 9 (100) |
| Imaging changes, n (%) | 20 (27) | 13 (30) | 1 (11) | 3 (43) |
| Intermittent negative culture, n (%) | 13 (13) | 6 (11) | 4 (21) | 1 (11) |
p<0.05.
CONS coagulase-negative Staphylococcus, CSF cerebrospinal fluid
Discussion:
This comprehensive retrospective cohort study of children with first CSF shunt infection examined the relationship of the causative organism of CSF shunt infection with time to infection and clinical features of infection. We found no significant difference in time to infection between CONS, S. aureus, and gram-negative organisms. Children with shunt infection caused by S. aureus were more likely to have surgical site changes and leukocytosis at the time of presentation.
Numerous review articles addressing CSF shunt infections have noted that CONS, gram-negative organisms, and anaerobes are associated with more indolent infection than S. aureus.(1, 7, 14, 17) However, the presence of primary literature supporting these assertions is limited. In a small study of 12 shunt infections occurring beyond 180 days from shunt placement, the most commonly identified organisms were Staphylococcus epidermidis and Propionibacterium acnes.(18) Arnell, et al. also suggested that infection caused by P. acnes was more delayed than infection caused by other organisms, though this result did not reach statistical significance.(2) In a study of 59 CSF shunt infections in children, Odio et al suggested that infection was “often more acute in staphylococcal disease than in gram-negative infection,” but this result also did not reach statistical significance.(15) This current study, the largest cohort of children to date to evaluate the relationship of causative organism of CSF shunt infection and time to infection, suggest that there is no difference in time to infection between CONS, S. aureus, and gram-negatives. Previous authors have suggested that delayed onset of infection caused by gram-negative organisms may be due to inoculation with gram-negative organisms after the time of initial surgery as compared with gram-positive organisms which are introduced at the time of surgery; however, the results of this current study suggests that this may not be the case.(14)
Previous articles have suggested that infections caused by CONS are generally more benign than infection caused by S. aureus, while the discussion regarding the morbidity of gram-negative organisms is more conflicting.(1, 7, 14) Early studies of CSF shunt infection had suggested that gram-negative infection was associated with significant morbidity and a mortality rate as high as 80%.(8, 16, 19) By contrast, a more recent analysis of CSF shunt infections caused by gram-negative organisms suggested that gram-negative organisms are not associated with excessive morbidity and mortality when treated appropriately.(24) The authors attributed the improved outcomes of this cohort when compared with previous literature to decreased prevalence of ventriculoatrial shunts, changing microbiology, increased removal of infected shunts, and decreased use of intraventricular antibiotics. Among children in our cohort, those with infection caused by S. aureus were more likely to present with surgical site changes and leukocytosis; however, no other significant differences were noted in the clinical features of infection based on the causative organism of infection.
This study had several limitations. First, data regarding clinical features of infection and neurological outcomes was collected retrospectively limiting the availability of clinical signs and symptoms to that which was documented in the medical record. Second, the single center design may limit the generalizability of these findings. Third, the limited number of infections may limit our ability to find differences, in both time to infection and clinical features of infection. Assuming an underlying log-normal distribution, typical time to event data, and the observed group sizes (57, 19, 9), we anticipate that this study was adequately powered to detect an effect size 1 (pairwise power >90%, >75%, >65%, respectively).(5, 9) Fourth, CSF cultures, most often via pre-operative shunt tap, were obtained at the discretion of the neurosurgeon. If an earlier CSF shunt revision had occurred and intraoperative CSF cultures were not obtained, the earlier revision may have represented an occult infection; however, multiple CSF shunt revisions were observed in a minority (15%) of the cohort. Additionally, culturing practices likely limited the ability to identify certain organisms, particularly anaerobic organisms, as this cohort included no identified P. acnes infections. As described above, prior studies have suggested that P acnes shunt infections may be associated with delayed presentation. Unfortunately, the absence of P acnes infection in this cohort limits our ability to evaluate the time to infection by this causative organism. Use of specific culture media and prolonged monitoring of cultures may increase the identification of anaerobic organisms,(2, 27) and the 2017 Infectious Diseases Society of America clinical practice guidelines for healthcare-associated ventriculitis and meningitis recommend that cultures be monitored for 10 days to increase the identification of organisms such as P. acnes.(26) We are hopeful that these revised guidelines will lead to greater identification of P acnes infections and better understanding of their epidemiology. Fifth, while detection bias in time to infection is possible given the retrospective design, we do not believe it was substantial given our prolonged duration of follow-up. Finally, the cohort is comprised of children with CSF shunt placement occurring between 1997 and 2006; while unlikely to impact the timing or clinical features of infection by causative organism, it is plausible that there has been changes in the microbiology of CSF shunt infections since the time of data collection that may further limit the generalizability of these results. Of note, antibiotic impregnated catheters were not part of hospital practice at this institution during the time of this study except for a 9 month period;(10) their use may have an impact on the epidemiology of shunt infection and is area of active ongoing investigation. Despite these limitations, this study has the strength of a comprehensive cohort of children with rich demographic and clinical information about first CSF shunt infection.
Conclusions:
Contrary to the hypothesized relationship between causative organism and time to infection, this study revealed no significant difference in time to infection between CONS, S. aureus, and gram-negative organisms. Children with infection caused by S. aureus were more likely to present with surgical site changes and leukocytosis than children with infection caused by other organisms.
Funding:
This work was supported by a PCH Innovative Research Grant and the Children’s Health Research Center at the University of Utah which provided salary support to ML. Subsequent support for investigators included Award K23NS062900 from the National Institute of Neurological Disorders And Stroke and Seattle Children’s Center for Clinical and Translational Research and CTSA Grant Number ULI RR025014 from the National Center for Research Resources, a component of the National Institutes of Health to TDS and KW. None of the sponsors participated in design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the National Center for Research Resources or National Institutes of Health.
Footnotes
Portions of this work were presented in poster form at the Pediatric Academic Societies, San Francisco, California, United States, May 7, 2017.
References
- 1.Adams DJ, Rajnik M: Microbiology and treatment of cerebrospinal fluid shunt infections in children. Curr Infect Dis Rep 16: 427, 2014. [DOI] [PubMed] [Google Scholar]
- 2.Arnell K, Cesarini K, Lagerqvist-Widh A, Wester T, Sjolin J: Cerebrospinal fluid shunt infections in children over a 13-year period: anaerobic cultures and comparison of clinical signs of infection with Propionibacterium acnes and with other bacteria. J Neurosurg Pediatr 1: 366–372, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Borgbjerg BM, Gjerris F, Albeck MJ, Borgesen SE: Risk of infection after cerebrospinal fluid shunt: an analysis of 884 first-time shunts. Acta Neurochir (Wien) 136: 1–7, 1995. [DOI] [PubMed] [Google Scholar]
- 4.Cochrane DD, Kestle JR: The influence of surgical operative experience on the duration of first ventriculoperitoneal shunt function and infection. Pediatr Neurosurg 38: 295–301, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Cohen J: Statistical Power Analysis for the Behavioral Sciences. New York, NY: Lawrence Erlbaum Associates, Publishers, 1988. [Google Scholar]
- 6.Conen A, Walti LN, Merlo A, Fluckiger U, Battegay M, Trampuz A: Characteristics and treatment outcome of cerebrospinal fluid shunt-associated infections in adults: a retrospective analysis over an 11-year period. Clin Infect Dis 47: 73–82, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Duhaime AC: Evaluation and management of shunt infections in children with hydrocephalus. Clin Pediatr (Phila) 45: 705–713, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Groover RV, Sutherland JM, Landing BH: Purulent meningitis of newborn infants. Eleven-year experience in the antibiotic era. N Engl J Med 264: 1115–1121, 1961. [DOI] [PubMed] [Google Scholar]
- 9.Hedges LV, Olkin I: Statistical Methods for Meta-Analysis. Orlando: Academic Press, 1985. [Google Scholar]
- 10.Kan P, Kestle J: Lack of efficacy of antibiotic-impregnated shunt systems in preventing shunt infections in children. Childs Nerv Syst 23(7): 773–777, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Kestle J, Drake J, Milner R, Sainte-Rose C, Cinalli G, Boop F, et al. : Long-term follow-up data from the Shunt Design Trial. Pediatr Neurosurg 33: 230–236, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Kestle JR: Pediatric hydrocephalus: current management. Neurol Clin 21: 883–895, vii, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Kim KS: Bacterial meningitis beyond the neonatal period, in Cherry JD, Demmler-Harrison GJ, Kaplan SL, Steinbach WJ, Hotez PJ (ed): Feigin and Cherry’s Textbook of Pediatric Infectious Diseases. Philadelphia: Elsevier Saunders, 2014. [Google Scholar]
- 14.Naradzay JF, Browne BJ, Rolnick MA, Doherty RJ: Cerebral ventricular shunts. J Emerg Med 17: 311–322, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Odio C, McCracken GH Jr., Nelson JD: CSF shunt infections in pediatrics. A seven-year experience. Am J Dis Child 138: 1103–1108, 1984. [DOI] [PubMed] [Google Scholar]
- 16.Overall JC Jr: Neonatal bacterial meningitis. Analysis of predisposing factors and outcome compared with matched control subjects. J Pediatr 76: 499–511, 1970. [DOI] [PubMed] [Google Scholar]
- 17.Sandoe JA, Longshaw CM: Ventriculoperitoneal shunt infection caused by Staphylococcus lugdunensis. Clin Microbiol Infect 7: 385–387, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Schiff SJ, Oakes WJ: Delayed cerebrospinal-fluid shunt infection in children. Pediatr Neurosci 15: 131–135, 1989. [DOI] [PubMed] [Google Scholar]
- 19.Sells CJ, Shurtleff DB, Loeser JD: Gram-negative cerebrospinal fluid shunt-associated infections. Pediatrics 59: 614–618, 1977. [PubMed] [Google Scholar]
- 20.Simon TD, Butler J, Whitlock KB, Browd SR, Holubkov R, Kestle JR, et al. : Risk factors for first cerebrospinal fluid shunt infection: findings from a multi-center prospective cohort study. J Pediatr 164: 1462–1468 e1462, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simon TD, Hall M, Riva-Cambrin J, Albert JE, Jeffries HE, Lafleur B, Dean JM,et al. : Infection rates following initial cerebrospinal fluid shunt placement across pediatric hospitals in the United States. Clinical article. J Neurosurg Pediatr 4: 156–165, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simon TD, Whitlock KB, Riva-Cambrin J, Kestle JR, Rosenfeld M, Dean JM, et al. : Revision surgeries are associated with significant increased risk of subsequent cerebrospinal fluid shunt infection. Pediatr Infect Dis J 31: 551–556, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simon TD, Whitlock KB, Riva-Cambrin J, Kestle JR, Rosenfeld M, Dean JM, et al. : Association of intraventricular hemorrhage secondary to prematurity with cerebrospinal fluid shunt surgery in the first year following initial shunt placement. J Neurosurg Pediatr 9: 54–63, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stamos JK, Kaufman BA, Yogev R: Ventriculoperitoneal shunt infections with gram-negative bacteria. Neurosurgery 33: 858–862, 1993. [DOI] [PubMed] [Google Scholar]
- 25.Thomson J, Sucharew H, Cruz AT, Nigrovic LE, Freedman SB, Garro AC, et al. : Cerebrospinal Fluid Reference Values for Young Infants Undergoing Lumbar Puncture. Pediatrics [epub ahead of print Feb 2 2018. doi: 10.1542/peds.2017-3405]. [DOI] [PubMed] [Google Scholar]
- 26.Tunkel AR, Hasbun R, Bhimraj A, Byers K, Kaplan SL, Michael Scheld W, et al. : 2017. Infectious Diseases Society of America’s Clinical Practice Guidelines for Healthcare-Associated Ventriculitis and Meningitis. Clin Infect Dis [epub ahead of print Feb 14 2017. doi: 10.1093/cid/ciw861]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Viraraghavan R, Jantausch B, Campos J: Late-onset central nervous system shunt infections with Propionibacterium acnes: diagnosis and management. Clin Pediatr (Phila) 43: 393–397, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Wells DL, Allen JM: Ventriculoperitoneal shunt infections in adult patients. AACN Adv Crit Care 24: 6–12; quiz 13–14, 2013. [DOI] [PubMed] [Google Scholar]