Abstract
Ovarian cancer is the leading cause of death among gynecologic diseases in the USA and Europe. High-grade serous carcinoma (HGSC) of the ovary, the most aggressive type of ovarian cancer, is typically diagnosed at advanced stages when the 5-year survival is dismal. Since the cure rate for stage I HGSC is high, early detection of localized initial disease may improve patient outcomes. Serous tubal intraepithelial carcinoma (STIC) is considered to be a precursor lesion of HGSC. Discovery of biomarkers associated with STIC could aid in the development of an HGSC screening algorithm. Using immunohistochemical staining, we have demonstrated overexpression of UCHL1, ADAMTS13, and GAPDH in patients’ STIC lesions, but not in cancer-free fallopian tubes. We additionally demonstrated a marked increase of T cells in perineoplastic stroma surrounding STIC lesions (largely CD4 + cells), but not in normal fallopian tubes and HGSC. FOXP3 + T regulatory cells are absent in STIC lesions but are present in HGSC. These observations indicate the microenvironment surrounding a STIC lesion may be immune promoting in contrast to the immune suppressive microenvironment of invasive carcinoma. In summary, we have identified UCHL1, ADAMTS13, and GAPDH as novel potentially useful markers associated with early stages of HGSC tumorigenesis and possibly contribute to STIC immunogenicity. The lack of immune suppression in the STIC microenvironment indicates that the immune system can still recognize and keep STIC controlled at this stage of the tumor development.
Keywords: Ovarian cancer, high-grade serous carcinoma (HGSC), serous tubal intraepithelial carcinoma (STIC), biomarkers, immune response
1. Introduction
Ovarian cancer (OC) is the second most frequently diagnosed gynecological malignancy in the USA and Europe with the overall mortality higher than any other gynecological cancer [1]. In 2019, there will be more than 22,000 new cases of ovarian cancer diagnosed and more than 14,000 ovarian cancer deaths in the US (https://www.cancer.org). Due to the lack of specific symptoms about 75% of ovarian cancer cases are diagnosed at late stages when the 5-year survival is only 15–20%, although the cure rate for stage I disease is usually > 90% (https://www.cancer.org). These data suggest that early detection of localized initial disease followed by primary surgery and chemotherapy or identification of patients at high risk followed by increased surveillance and chemoprevention should improve patient outcomes.
There are two main types of OC: Type I carcinomas are generally slow-growing indolent neoplasms and include low-grade serous, low-grade endometrioid, mucinous, and clear cell carcinomas [2]. They are thought to develop in a stepwise progression, similar to the adenoma carcinoma sequence in colorectal cancer: from benign lesions via atypia and precursor lesions to noninvasive borderline tumors, and invasive low-grade carcinoma [3]. Typical mutations include KRAS, BRAF, ARID1A, PTEN, PIK3CA, CTNNB1, and PPP2R1A. Type II carcinomas represented by high-grade serous carcinoma (HGSC) that accounts for more than 60% of epithelial ovarian cancers and over 70% of all OC deaths [2,4–6]. Other Type II tumors comprise endometrioid carcinomas, malignant mixed mesodermal tumors (carcinosarcomas), and undifferentiated carcinomas. Over 95% of HGSC harbor TP53 mutation [7,8]. It is well accepted that OC, and particularly HGSC, needs to be diagnosed as early as possible, preferably in preinvasive stages of disease. However, currently, there are no reliable biomarkers for HGSC detection with a lead-time of more than 1 year, when curative intervention is still possible [9–11]. Identification of molecular alterations specific to HGSC or its precursor lesion could help identifying sensitive and specific biomarkers for detection of HGSC at its earliest stages.
The precursor lesions in the ovaries of Type II OC have been described [12] and are easily detected morphologically and histochemically. Originally epithelial OC have been thought to arise from ovarian surface epithelium. However, although numerous studies have explored the ovaries for possible precursor lesions of ovarian cancer, none have been found. Tumors arising from the fallopian tube (FT) had already been described in the 1950s [13], but it was not until the 2000s that the important role of fallopian tube epithelium in ovarian tumors carcinogenesis was further investigated. Recent evidence indicates that at least part of HGSC originates from the FT epithelium [14–16]. Potential precursor lesions of HGSC, including a TP53 mutant single-cell epithelial layer and serous tubal intraepithelial carcinoma (STIC) were identified in the fimbriae of the FT removed as part of prophylactic surgery and in patients with advanced stage sporadic HGSC of the ovary, FT and peritoneum [5,16]. The morphological characteristics of STIC include disorganized, pleomorphic, hyperchromatic, and enlarged epithelial cells with highly atypical nuclei [4]. Regarding to terminology, others prefers the use of the term ‘high-grade tubal intraepithelial neoplasm’ (HGTIN) instead of STIC [17].
STIC lesions are associated with increased risk for HGSC [5,18]. Identification of the biomarkers associated with STIC is therefore critical for identifying the mechanisms of early ovarian tumorigenesis and improving the early detection and prevention of OC. Until now, besides the established markers (TP53, p16, and Ki-67), several proteins were reported to be differentially expressed in STIC lesions including Stathmin 1 [19], signal transducer and activator of transcription 3 (phosphorylated STAT3 Tyr705) and suppression or loss of protein inhibitor of activated STAT3 (PIAS3) [20], Bcl-2 and γH2AX [21], Rsf-1 (HBXAP), cyclin E, and fatty acid synthase (FASN) [22], CD117 and ALDHA1 [23] and HMGA2 [24]. Of these proteins, differential circulating levels of Stathmin 1, FASN, ALDH1 were observed in esophageal, colorectal and lung cancers, and differential levels of Bcl-2 in OC, although none demonstrated high classification power [25–29]. With a better understanding in STIC development, new and better biomarkers for screening purposes can be envisioned.
In this study, we have identified several candidate biomarkers that were differentially expressed in an in vitro model of STIC lesion and in pre-HGSC serum samples versus controls and validated overexpression of these candidate biomarkers in patients’ STIC lesions. We have additionally assessed immune cells in STIC lesions and correlated their level and location with biochemical markers.
2. Methods
2.1. Patients and clinical data
Tissue samples were obtained from patients with the diagnosis of STIC in fallopian tubes, on either prophylactic salpingectomy, salpingo-oophorectomy or on debulking specimens performed for ovarian cancer at the UPMC Magee-Women’s Hospital between 2014 and 2016. Fallopian tube sections were submitted based recommended SEE-FIM protocol. Formalin-fixed paraffin-embedded tissue sections of fallopian tubes were stained with hematoxylin and eosin and reviewed by an independent pathologist to confirm the presence of invasive carcinoma, STIC or histologically normal tissue (Fig. 1). The presence of STIC was confirmed by positive p53 nuclear staining and by increase in proliferation index by ki67 immunohistochemical stains. A total of 10 patient were identified, however deeper sections for analysis did not contain sufficient tissue for evaluation, leaving a total of 6 final samples.
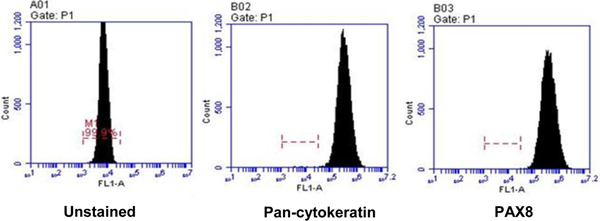
Fig. 1.
Flow cytometry analysis of FT cells. FT epithelial cells were isolated from healthy fallopian tube fimbriae as described in Methods. Newly isolated cells were allowed to reach confluence, were permeabilized and stained for pan-cytokeratin or PAX8 markers. A representative of three cultures is presented.
2.2. Fallopian tube epithelial cells isolation and culture
Fresh, healthy fallopian tube fimbriae were obtained from 3 donors at UPMC Magee-Women’s Hospital. Specimens were collected in a 50 ml of DMEM F12 Ham medium (Sigma-Aldrich) supplemented with 10% FBS (Gibco) and 1% Penn/Strep (Sigma-Aldrich). Upon receipt, FT tissues were processed under sterile tissue culture conditions as described earlier [30,31] and cultured in Prigrow cell culture medium (Applied Biological Materials Inc., catalog #TM004) supplemented with 2% Ultroser G serum substitute (Pall France). To immortalize FT epithelial (FTE) cells, we used lentiviral transduction with SV40 large plus small T antigen (FTE-Tag) that suppresses TP53 signal transduction pathway thus mimicking the genetic makeup of STIC lesion [31]. Unmodified lentivirus was used in control cells. Recombinant lentiviral expression vector (Catalogue #CILV01) and an empty lentiviral expression vector (Catalogue # LV000) were commercially purchased from ALSTEM and used to transduce primary FTE cells in the presence of transduction reagent TransPlus following the manufacturer’s protocol [32–34].
2.3. Flow cytometry
Isolated FT epithelial cells were cultured until they reached confluence. Cells were trypsinized, washed, permeabilized with cytofix/cytoperm buffer (Becton Dickinson), and resuspended with 0.3% saponin (Sigma) in FACS-buffer. Cells were incubated anti-human pan-cytokeratin AE1/AE3 antibody conjugated with Alexa Fluor® 488 (eBiosience) or with anti-PAX8 (PAX8/1492) Alexa Fluor® 488-conjugated antibody (Novus Biologicals) for 30 min at 4°C. Flow cytometric analysis was performed using Becton Dickinson LSR Fortessa I instrument.
2.4. Mass spectrometry
Serum-free culture medium conditioned for 48 hours by SV40 transformed and primary FTE was analyzed using LC-MS/MS on an Orbitrap Fusion system using an Acclaim PepMap 100, 75 μm × 2 cm trap and PepMap RSLC, 75 μm × 25 cm column with a 2-hour gradient. Raw files were submitted to Proteome Discoverer 1.4 using the Sequest and Percolator algorithms and exported to Scaffold for further analysis.
2.5. Study populations for serum biomarkers analysis
We have utilized serum samples collected in the course of a prospective Prostate, Lung, Colorectal, Ovarian (PLCO) clinical trial conducted by the NIH [35–39]. Serum samples were collected from 29 HGSC cases 6–18 months prior to diagnosis and from 203 healthy controls matched by age, race and time of sample draw [35,40]. For all studies, written, informed consents were obtained from each participating subject or from patient’s guardian. All subjects were accrued under protocols approved by each site’s Institutional Review Board. Specimens were transferred in accordance with material transfer agreements between the shipping and receiving institutions regarding transfer of specimens without personal identifiers.
2.6. Luminex analysis
All proteins were analyzed using multiplex bead-based immunoassay using Luminex platform (Luminex, Inc.) as previously described [41]. Kits were purchased from EMD/Millipore.
2.7. Immunohistochemistry
Immunohistochemical staining (IHC) was performed on Benchmark Ventana system (Ventana Medical Systems). Staining for ADAMST13 was performed using polyclonal antibody (Pierce ThermoScientific), for AHSG by using polyclonal antibody (Pierce ThermoScientific), for UCHL1 (PGP9.5) by using polyclonal antibody (Cell Marque), and for GAPDH by using polyclonal Ab (Santa Cruz Biotechnology). Immunohistochemical staining recognizing different populations of T cells (CD3, CD4, CD8) was performed using CONFIRM anti-CD3, anti-CD4, and anti-CD8 rabbit monoclonal antibody (Roche Diagnostic) on Benchmark Ventana. The results were evaluated independently by two pathologists. Studies were interpreted in conjunction with appropriate negative (incubation without primary antibodies) controls. Additionally, staining in non-neoplastic epithelium was used an internal negative control.
2.8. Statistical analysis
Descriptive statistics for serum concentrations of each of the tested biomarkers were calculated for each subject group using GraphPad Prism version 6 (GraphPad Software, Inc.). Comparisons of average biomarker concentrations between OC cases and control groups were performed using the Mann-Whitney non-parametric U test. P values less than 0.05 were considered statistically significant.
3. Results
3.1. Biomarker discovery in an in vitro model
Secretory epithelial cells were isolated from healthy control FT obtained from three cancer-free patients at Magee-Women’s Hospital. The FTE nature of FT cells was confirmed by flow cytometry after staining for pan-cytokeratin and PAX8 markers (Fig. 1). From each primary culture we generated isogenic pairs of cells, one transfected with unmodified lentivirus and another- immortalized with SV40 large plus small T antigen (FTE-Tag) that suppresses TP53 signal transduction pathway thus mimicking the genetic makeup of STIC lesion [31]. Transfection with either unmodified or recombinant lentivirus did not change the pan-cytokeratin or PAX8 expression as measured by flow-cytometry (data not shown). We hypothesized that proteins secreted by cells with inactivated TP53 signaling correspond to those secreted by STIC. To test this hypothesis, and to establish a proof-of-principle of pre-neoplastic HGSC biomarker discovery, we analyzed serum-free culture media from 3 isogenic pairs in duplicates by mass spectrometry to identify several proteins whose levels were at least 2-fold higher in immortalized versus primary cells with levels of significance at p < 0.001. In this study, we focused on two proteins, GAPDH, and UCHL1, that displayed greatest differences between SV40 transformed vs. control cells at the highest level of statistical significance (Table 1).
Table 1.
Proteins differentially present in the conditioned media of SV40 transformed vs. control FTE cells
| Control | SV40 | P-value | Fold change | |
|---|---|---|---|---|
| UCHL1 | 9.0 ± 1.58 | 22.2 ± 0.92 | < 0.00001 | 2.5 |
| GAPDH | 39.2 ± 1.49 | 81.2 ± 1.55 | < 0.00001 | 2.1 |
3.2. Biomarker discovery in pre-diagnostic serum
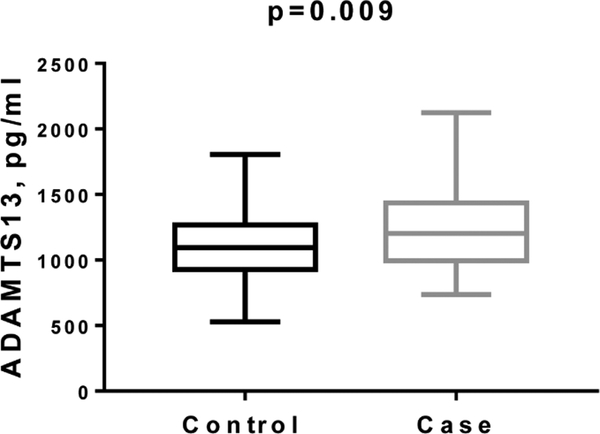
We performed evaluation of 58 serum markers in serum samples from PLCO participants collected 18–84 months before diagnosis with ovarian cancer [35, 40] and found that, along with CA125 and HE4, ADAMTS13 and fibrinogen (FBG) were significantly elevated (Fig. 3 for ADAMTS13).
Fig. 3.
Elevated levels of ADAMTS13 in sera of HGSC cases collected 18–84 months before clinical diagnosis in PLCO prospective study. ADAMTS13 levels were measured using bead-based immunoassay.
3.3. IHC evaluation of GAPDH, ADAMTS13, and UCHL1 in STIC lesions and invasive HGSC
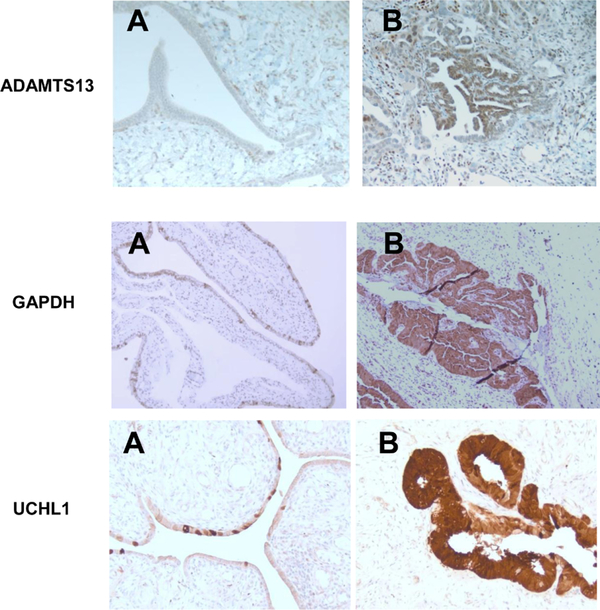
To confirm that STIC cells are the source of proteins identified in an in vitro model and in clinical specimens, we performed IHC in human samples of STIC, normal FT and invasive high-grade serous carcinoma. We found that GAPDH, ADAMTS13 and UCHL1 are significantly overexpressed in malignant cells (both STIC and invasive HGSC), while we did not observe the differential expression of ENO1 and FBG. For ADAMS13, tissue slides showed negative immunostaining in normal epithelium and stroma. In contrast, neoplastic epithelium in STIC and invasive carcinoma showed strong diffuse staining for ADAMTS13 with cytoplasmic and nuclear distribution (Fig. 4). For UCHL1, tissue slides showed weak patchy immunostaining in normal epithelium, while neoplastic epithelium in STIC and invasive carcinoma demonstrated strong diffuse staining for UCHL1 with predominantly cytoplasmic distribution (Fig. 4). The same pattern was observed for GAPDH: weak staining of occasional normal epithelial cells and diffuse strong staining in STIC and invasive carcinoma (Fig. 4).
Fig. 4.
Immunohistochemical staining for ADAMS13, UCHL1, and GAPDH in normal fallopian epithelium (A) and in STIC (B), 200x magnification, A representative of 36 sections from 6 patients is presented.
3.4. Immune cells IHC
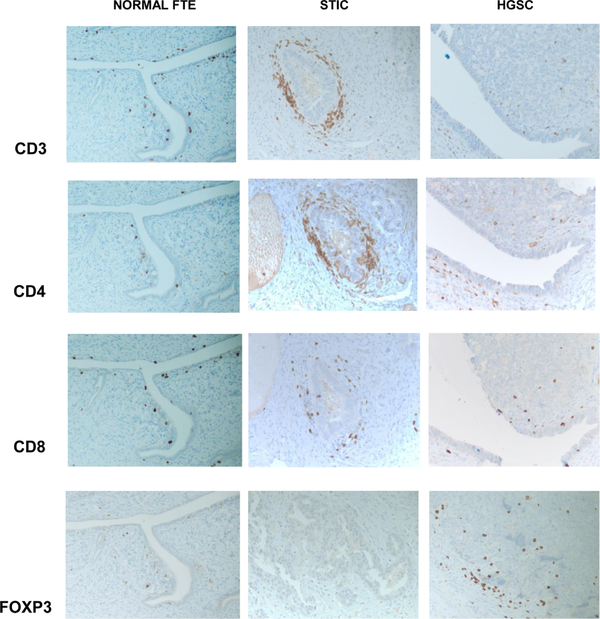
Since the aberrant protein expression may alter immune cells profiling and homing and because lymphocyte infiltrates have been observed in pre-neoplastic lesions [42,43], we raised the question whether immune cells may recognize the emerging malignant cells of STIC lesion and infiltrate the early lesions. To that end we studied different subsets of T cells in normal FT, STIC, and invasive carcinoma by immunohistochemistry. Tissue analysis revealed occasional CD3 positive T lymphocytes in benign tubular tissue, associated with epithelial layer and surrounding stroma. There is a mild predominance of CD8 positive subset of T cells. In STIC, there is a marked increase of CD3 positive T lymphocyte associated mainly with peri-neoplastic stroma. Both subtypes (CD8 and CD4 positive) of T cells were increased in STIC tissue. In contrast, the numbers of tumor-associated T lymphocytes were not increased in invasive carcinoma. There were occasional CD3 positive T cells seen in the tumor mass, as well as in peri-tumoral stroma. It appears that there is a predominance of CD4 positive cells (Fig. 4). Immunostaining for FOXP3, T regulatory cell marker, reveals their increase in invasive carcinoma, compared to benign FT and STIC (Fig. 5).
Fig. 5.
Immunohistochemical staining for CD3, CD4, CD8, and FOXP3 in normal FT, STIC, and HGSC. 200x magnification, A representative of 36 sections from 6 patients is presented.
4. Discussion
We have used two different approaches, Luminex analysis of pre-diagnostic HGSC serum samples and MS analysis of proteins secreted by cultured STIC-like cells, to ascertain whether these proteins might originate from patients’ STIC lesions. In this study, we report five proteins elevated in either pre-diagnostic sera or conditioned medium, ADAMTS13, FBG, GAPDH, ENO1, UCHL1. Three of them – ADAMTS13, GAPDH, and UCHL1, were overexpressed by IHC in both STIC lesions and invasive carcinoma. At the same time, no differential expression of FBG and ENO1 was observed indicating that these two proteins could originated from sources other than ovarian malignant cells or associated stromal cells.
Aberrant protein expression in early ovarian tumorigenesis may induce immune response as the immune contexture plays a key role in cancer development, prognosis, and treatment [44]. Thus, we have assessed immune cells homing in STIC samples in comparison with normal FT and invasive HGSC. Our findings show a marked increase of T cells in peri-neoplastic stroma surrounding STIC lesions, which indicates the immunogenic properties of neoplastic cells, with both CD4 positive and CD8 positive cells being present. In contrast, T cells are sparse in invasive carcinoma, indicative of immunosuppressive microenvironment. In addition, immunostaining for FOXP3, T regulatory cell marker, reveals the absence of these cells in benign FT and, most importantly in STIC lesions, and their presence in invasive carcinoma, again confirming the immune promoting microenvironment of STIC and immune suppressive or tolerogenic microenvironment of invasive carcinoma. This is an important observation since it provides an opportunity to distinguish between immunological profile of premalignant and malignant lesions and establish a marker for prognostic and diagnostics predictions. Additional studies are needed to confirm the relationship between the immune cell subtypes associated with histologic progression/persistence/regression of STIC, including dendritic cells, macrophages, MDSC, B and NK cells.
Although this is an observational study and does not include mechanistic experiments, the overexpression of ADAMTS13, GAPDH, and UCHL1 in women’s STIC lesion has not been previously reported. In addition, these data demonstrate that (i) cancer-associated elevated blood levels of some proteins (in this case ADAMTS13) could results from the premalignant lesions, and that (ii) in vitro modelling of early stage of HGSC tumorigenesis by inactivating p53 in normal FTE could be a useful tool for discovery of mechanisms of early HGSC development and potentially identifying early biomarkers of HGSC.
Altered STIC expression of some proteins could be directly upregulated in response to TP53 knockdown. Tumor suppressor, TP53, that is mutated in > 95% HGSC [7,8], controls a wide range of intracellular pathways including proliferation, apoptosis, senescence, differentiation, response to DNA damage, energy metabolism, and others pathways [45–50]. Therefore, the primary event of TP53 knockdown could potentially result in upregulation of some pathways and potential biomarkers in STIC lesions. Relevant to this report, TP53 was reported to directly upregulate GAPDH [51] and glycolytic pathway [52–54], and UCHL1 directly interacts with TP53 and stabilizes p53 through the ubiquitination pathway [55,56].
ADAMTS13 is mainly known for prevention of microvascular thrombus formation by decreasing thrombotic activity through proteolysis of its only known substrate, von Willebrand factor (VWF) [57–59]. Recent studies demonstrate that ADAMTS13 also plays a role in the down-regulation of inflammation, regulation angiogenesis, and degradation of extracellular matrix [60–62]. There is not enough experimental evidence to speculate on possible functional effects of ADAMTS13 overexpression in malignant ovarian cells.
Ubiquitin carboxyl terminal hydrolase 1 (UCHL1) has two opposing biologic functions, functioning as both a ubiquitin carboxyl-terminal hydrolase and ligase [63]. It has been reported as either an oncogene or a tumor suppressor [56,64–70]. Knockdown of UCHL1 in OC cell lines promoted cell proliferation and reduced cell apoptosis [66] UCHL1 also promotes prostate cancer metastasis through EMT induction [71]. Conversely, in other publications, UCHL1 was described as a tumor suppressor and its overexpression was attributed to a response to tumor growth [56,66,68,72]. In this role, UCHL1 forms a complex with p53/MDM2/ARF to promote p53 signaling and mediate apoptosis through stabilizing p53 [56, 73,74]. Thus, an experimental strategy has to be devised to ascertain a role of UCHL1 expression in STIC lesions for HGSC progression.
GAPDH was initially identified as a glycolytic enzyme, but emerging evidence indicates that GAPDH is implicated in several other functions including regulation of apoptosis (reviewed in [75]) GAPDH is frequently upregulated in various cancers. TP53 not only modulates GAPDH gene expression but also affects its functions (reviewed in [75]). High GAPDH expression indicate early disease progression in advanced serous ovarian cancer [76,77].
Currently, there is lack of efficient markers to identify pre-invasive lesions at highest risk of progression to invasive carcinoma. The development of validated tools to determine markers of disease progression will identify patients at high-risk, suggest novel OC chemoprevention and immunoprevention agents, and provide clinically relevant cellular and molecular biomarkers for monitoring outcome in OC prevention trials. Our results here offer novel potentially effective and useful markers associated with high-grade histology and malignant lesion progression.
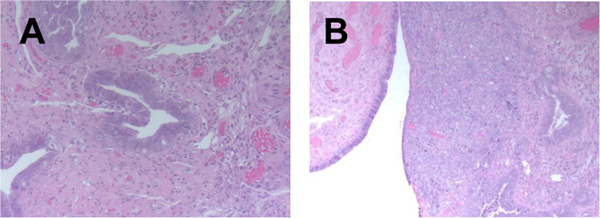
Fig. 2.
H&E staining of STIC lesion with adjacent superficially invasive tumor (A – STIC, B – invasive carcinoma); 200x magnification, A representative of 36 sections from 6 patients is presented.
References
- [1].Siegel R, Ma J, Zou Z and Jemal A, Cancer statistics 2014, CA Cancer J Clin 64 (2014), 9–29. [DOI] [PubMed] [Google Scholar]
- [2].Lim D and Oliva E, Precursors and pathogenesis of ovarian carcinoma, Pathology 45 (2013), 229–242. [DOI] [PubMed] [Google Scholar]
- [3].Zeppernick F, Meinhold-Heerlein I and Shih Ie M, Precursors of ovarian cancer in the fallopian tube: Serous tubal intraepithelial carcinoma – an update, J Obstet Gynaecol Res 41 (2015), 6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kurman RJ, Origin and molecular pathogenesis of ovarian high-grade serous carcinoma, Ann Oncol 10(24 Suppl) (2013), x16–x21. [DOI] [PubMed] [Google Scholar]
- [5].Kim J, Park EY, Kim O et al. , Cell origins of high-Grade serous ovarian cancer, Cancers (Basel) (2018), 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Peres LC, Cushing-Haugen KL, Kobel M et al. , Invasive epithelial ovarian cancer survival by histotype and disease stage, J Natl Cancer Inst 111 (2019), 60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kurman RJ and Shih Ie M, Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer–shifting the paradigm, Hum Pathol 42 (2011), 918–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ahmed AA, Etemadmoghadam D, Temple J et al. , Driver mutations in TP53 are ubiquitous in high grade serous carcinoma of the ovary, J Pathol 221 (2010), 49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yang WL, Lu Z and Bast RC Jr., The role of biomarkers in the management of epithelial ovarian cancer, Expert Rev Mol Diagn 17 (2017), 577–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gloss BS and Samimi G, Epigenetic biomarkers in epithelial ovarian cancer, Cancer Lett 342 (2014), 257–263. [DOI] [PubMed] [Google Scholar]
- [11].Nolen BM and Lokshin AE, Protein biomarkers of ovarian cancer: The forest and the trees, Future Oncol 8 (2012), 55–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Koshiyama M, Matsumura N and Konishi I, Recent concepts of ovarian carcinogenesis: type I and type II, Biomed Res Int 2014 (2014), 934261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hu CY, Taymor ML and Hertig AT, Primary carcinoma of the fallopian tube, Am J Obstet Gynecol 59 (1950), 58–67. [DOI] [PubMed] [Google Scholar]
- [14].Gockley AA and Elias KM, Fallopian tube tumorigenesis and clinical implications for ovarian cancer risk-reduction, Cancer Treat Rev 69 (2018), 66–71. [DOI] [PubMed] [Google Scholar]
- [15].Nishida N, Murakami F and Higaki K, Detection of serous precursor lesions in resected fallopian tubes from patients with benign diseases and a relatively low risk for ovarian cancer, Pathol Int 66 (2016), 337–342. [DOI] [PubMed] [Google Scholar]
- [16].Soong TR, Kolin DL, Teschan NJ and Crum CP, Back to the future? The fallopian tube precursor escape and a dualistic model of high-grade serous carcinogenesis, Cancers (Basel) (2018), 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Conner JR, Meserve E, Pizer E et al. , Outcome of unexpected adnexal neoplasia discovered during risk reduction salpingo-oophorectomy in women with germ-line BRCA1 or BRCA2 mutations, Gynecol Oncol 132 (2014), 280–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Visvanathan K, Shaw P, May BJ et al. , Fallopian tube lesions in women at high risk for ovarian cancer: A multicenter study, Cancer Prev Res (Phila) 11 (2018), 697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Novak M, Lester J, Karst AM et al. , Stathmin 1 and p16(INK4A) are sensitive adjunct biomarkers for serous tubal intraepithelial carcinoma, Gynecol Oncol 139 (2015), 104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Saini U, Suarez AA, Naidu S et al. , STAT3/PIAS3 levels serve as “Early signature” genes in the development of high-Grade serous carcinoma from the fallopian tube, Cancer Res 78 (2018), 1739–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chene G, Cayre A, Raoelfils I, Lagarde N, Dauplat J and Penault-Llorca F, Morphological and immunohistochemical pattern of tubo-ovarian dysplasia and serous tubal intraepithelial carcinoma, Eur J Obstet Gynecol Reprod Biol 183 (2014), 89–95. [DOI] [PubMed] [Google Scholar]
- [22].Sehdev AS, Kurman RJ, Kuhn E and Shih Ie M, Serous tubal intraepithelial carcinoma upregulates markers associated with high-grade serous carcinomas including rsf-1 (HBXAP) cyclin E and fatty acid synthase, Mod Pathol 23 (2010), 844–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chene G, Ouellet V, Rahimi K et al. , Expression of stem cell markers in preinvasive tubal lesions of ovarian carcinoma, Biomed Res Int 2015 (2015), 808531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wei JJ, Wu J, Luan C et al. , HMGA2: A potential biomarker complement to p53 for detection of early-stage high-grade papillary serous carcinoma in fallopian tubes, Am J Surg Pathol 34 (2010), 18–26. [DOI] [PubMed] [Google Scholar]
- [25].Yan L, Dong X, Gao J et al. , A novel rapid quantitative method reveals stathmin-1 as a promising marker for esophageal squamous cell carcinoma, Cancer Med 7 (2018), 1802–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chudecka-Glaz AM, Cymbaluk-Ploska AA, Menkiszak JL, Sompolska-Rzechula AM, Toloczko-Grabarek AI and Rzepka-Gorska IA, Serum HE4 CA125 YKL-40 bcl-2 cathepsin-L and prediction optimal debulking surgery response to chemotherapy in ovarian cancer, J Ovarian Res 7 (2014), 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tas F, Duranyildiz D, Oguz H, Camlica H, Yasasever V and Topuz E, The value of serum bcl-2 levels in advanced epithelial ovarian cancer, Med Oncol 23 (2006), 213–217. [DOI] [PubMed] [Google Scholar]
- [28].Long QQ, Yi YX, Qiu J, Xu CJ and Huang PL, Fatty acid synthase (FASN) levels in serum of colorectal cancer patients: Correlation with clinical outcomes, Tumour Biol 35 (2014), 3855–3859. [DOI] [PubMed] [Google Scholar]
- [29].Cao YT, Li JH, Wang YT, Fu YW and Xu J, Serum ALDH1A1 is a tumor marker for the diagnosis of non-small cell lung cancer, Tumori 100 (2014), 214–218. [DOI] [PubMed] [Google Scholar]
- [30].Karst AM and Drapkin R, Primary culture and immortalization of human fallopian tube secretory epithelial cells, Nat Protoc 7 (2012), 1755–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Karst AM, Levanon K and Drapkin R, Modeling high-grade serous ovarian carcinogenesis from the fallopian tube, Proc Natl Acad Sci U S A 108 (2011), 7547–7552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Jazaeri AA, Bryant JL, Park H et al. , Molecular requirements for transformation of fallopian tube epithelial cells into serous carcinoma, Neoplasia 13 (2011), 899–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sherman-Baust CA, Kuhn E, Valle BL et al. , A genetically engineered ovarian cancer mouse model based on fallopian tube transformation mimics human high-grade serous carcinoma development, J Pathol 233 (2014), 228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Szabova L, Yin C, Bupp S et al. , Perturbation of rb p53 and brca1 or brca2 cooperate in inducing metastatic serous epithelial ovarian cancer, Cancer Res 72 (2012), 4141–4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Cramer DW, Bast RC Jr., Berg CD et al. , Design of the prostate, lung, colorectal and ovarian (PLCO) cancer screening trial, Control Clin Trials 4 (2011), 365–374. [Google Scholar]
- [36].Prorok PC, Andriole GL, Bresalier RS et al. , Design of the prostate lung colorectal and ovarian (PLCO) cancer screening trial, Control Clin Trials 21 (2000), 273S–309S. [DOI] [PubMed] [Google Scholar]
- [37].Gohagan JK, Prorok PC, Hayes RB, Kramer BS, L.C. Prostate and Ovarian Cancer Screening Trial Project T, The prostate lung colorectal and ovarian (PLCO) cancer screening trial of the national cancer institute: History organization and status, Control Clin Trials 21 (2000), 251S–272S. [DOI] [PubMed] [Google Scholar]
- [38].Jacobs IJ, Menon U, Ryan A et al. , Ovarian cancer screening and mortality in the UK collaborative trial of ovarian cancer screening (UKCTOCS): A randomised controlled trial, Lancet 387 (2016), 945–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Menon U, Gentry-Maharaj A, Ryan A et al. , Recruitment to multicentre trials–lessons from UKCTOCS: descriptive study, BMJ 337 (2008), a2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zhu CS, Pinsky PF, Cramer DW et al. , A framework for evaluating biomarkers for early detection: Validation of biomarker panels for ovarian cancer, Cancer Prev Res (Phila) 4 (2011), 375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Yurkovetsky Z, Skates S, Lomakin A et al. , Development of a multimarker assay for early detection of ovarian cancer, J Clin Oncol 28 (2010), 2159–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Monnier-Benoit S, Mauny F, Riethmuller D et al. , Immunohistochemical analysis of CD4+ and CD8+ t-cell subsets in high risk human papillomavirus-associated pre-malignant and malignant lesions of the uterine cervix, Gynecol Oncol 102 (2006), 22–31. [DOI] [PubMed] [Google Scholar]
- [43].Abdulrahman Z, Kortekaas KE, De Vos Van Steenwijk PJ, Van Der Burg SH and Van Poelgeest MI, The immune microenvironment in vulvar (pre)cancer: Review of literature and implications for immunotherapy, Expert Opin Biol Ther 18 (2018), 1223–1233. [DOI] [PubMed] [Google Scholar]
- [44].Taube JM, Galon J, Sholl LM et al. , Implications of the tumor immune microenvironment for staging and therapeutics, Mod Pathol 31 (2018), 214–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zheng R, Yao Q, Du S et al. , The status of p53 in cancer cells affects the role of autophagy in tumor radiosensitisation, J Buon 19 (2014), 336–341. [PubMed] [Google Scholar]
- [46].Krell J, Frampton AE, Colombo T et al. , The p53 miRNA interactome and its potential role in the cancer clinic, Epigenomics 5 (2013), 417–428. [DOI] [PubMed] [Google Scholar]
- [47].Sun Y, Holley AK and St Clair DK, p53 regulation of energy metabolism and mitochondria regulation of p53 in cancer cells: an insight into the role of manganese superoxide dismutase, Curr Pharm Biotechnol 14 (2013), 261–273. [DOI] [PubMed] [Google Scholar]
- [48].Goh AM, Coffill CR and Lane DP, The role of mutant p53 in human cancer, J Pathol 223 (2011), 116–126. [DOI] [PubMed] [Google Scholar]
- [49].Corney DC, Flesken-Nikitin A, Choi J and Nikitin AY, Role of p53 and rb in ovarian cancer, Adv Exp Med Biol 622 (2008), 99–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Hickman ES, Moroni MC and Helin K, The role of p53 and pRB in apoptosis and cancer, Curr Opin Genet Dev 12 (2002), 60–66. [DOI] [PubMed] [Google Scholar]
- [51].Chen RW, Saunders PA, Wei H, Li Z, Seth P and Chuang DM, Involvement of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and p53 in neuronal apoptosis: evidence that GAPDH is upregulated by p53, J Neurosci 19 (1999), 9654–9662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Zhang C, Liu J, Wu R et al. , Tumor suppressor p53 negatively regulates glycolysis stimulated by hypoxia through its target RRAD, Oncotarget 5 (2014), 5535–5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Yeung SJ, Pan J and Lee MH, Roles of p53 MYC and HIF-1 in regulating glycolysis - the seventh hallmark of cancer, Cell Mol Life Sci 65 (2008), 3981–3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Bensaad K, Tsuruta A, Selak MA et al. , TIGAR, a p53-inducible regulator of glycolysis and apoptosis, Cell 126 (2006), 107–120. [DOI] [PubMed] [Google Scholar]
- [55].Yu J, Tao Q, Cheung KF et al. , Epigenetic identification of ubiquitin carboxyl-terminal hydrolase l1 as a functional tumor suppressor and biomarker for hepatocellular carcinoma and other digestive tumors, Hepatology 48 (2008), 508–518. [DOI] [PubMed] [Google Scholar]
- [56].Xiang T, Li L, Yin X et al. , The ubiquitin peptidase UCHL1 induces g0/G1 cell cycle arrest and apoptosis through stabilizing p53 and is frequently silenced in breast cancer, PLoS One 7 (2012), e29783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Budde U and Schneppenheim R, Interactions of von willebrand factor and ADAMTS13 in von willebrand disease and thrombotic thrombocytopenic purpura, Hamostaseologie 34 (2014), 215–225. [DOI] [PubMed] [Google Scholar]
- [58].George JN, The role of ADAMTS13 in the pathogenesis of thrombotic thrombocytopenic purpura-hemolytic uremic syndrome, Clin Adv Hematol Oncol 3 (2005), 627–632. [PubMed] [Google Scholar]
- [59].Zheng XL, ADAMTS13 and von willebrand factor in thrombotic thrombocytopenic purpura, Annu Rev Med 66 (2015), 211–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Feng Y, Li X, Xiao J et al. , ADAMTS13: more than a regulator of thrombosis, Int J Hematol 104 (2016), 534–539. [DOI] [PubMed] [Google Scholar]
- [61].Tsai HM, ADAMTS13 and microvascular thrombosis, Expert Rev Cardiovasc Ther 4 (2006), 813–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Zheng X, Majerus EM and Sadler JE, ADAMTS13 and TTP, Curr Opin Hematol 9 (2002), 389–394. [DOI] [PubMed] [Google Scholar]
- [63].Liu Y, Fallon L, Lashuel HA, Liu Z, Lansbury PT Jr, The UCH-L1 gene encodes two opposing enzymatic activities that affect alpha-synuclein degradation and parkinson’s disease susceptibility, Cell 111 (2002), 209–218. [DOI] [PubMed] [Google Scholar]
- [64].Abdelmaksoud-Dammak R, Saadallah-Kallel A, Miladi-Abdennadher I et al. , CpG methylation of ubiquitin carboxyl-terminal hydrolase 1 (UCHL1) and p53 mutation pattern in sporadic colorectal cancer, Tumour Biol 37 (2016), 1707–1714. [DOI] [PubMed] [Google Scholar]
- [65].Gu YY, Yang M, Zhao M et al. , The de-ubiquitinase UCHL1 promotes gastric cancer metastasis via the akt and erk1/2 pathways, Tumour Biol 36 (2015), 8379–8387. [DOI] [PubMed] [Google Scholar]
- [66].Jin C, Yu W, Lou X et al. , UCHL1 is a putative tumor suppressor in ovarian cancer cells and contributes to cisplatin resistance, J Cancer 4 (2013), 662–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Trifa F, Karray-Chouayekh S, Jmaa ZB et al. , Frequent cpG methylation of ubiquitin carboxyl-terminal hydrolase 1 (UCHL1) in sporadic and hereditary tunisian breast cancer patients: clinical significance, Med Oncol 30 (2013), 418. [DOI] [PubMed] [Google Scholar]
- [68].Ummanni R, Jost E, Braig M et al. , Ubiquitin carboxyl-terminal hydrolase 1 (UCHL1) is a potential tumour suppressor in prostate cancer and is frequently silenced by promoter methylation, Mol Cancer 10 (2011), 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Wang G, Zhang W, Zhou B et al. , The diagnosis value of promoter methylation of UCHL1 in the serum for progression of gastric cancer, Biomed Res Int 2015 (2015), 741030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Zhong J, Zhao M, Ma Y et al. , UCHL1 acts as a colorectal cancer oncogene via activation of the beta-catenin/TCF pathway through its deubiquitinating activity, Int J Mol Med 30 (2012), 430–436. [DOI] [PubMed] [Google Scholar]
- [71].Jang MJ, Baek SH and Kim JH, UCH-L1 promotes cancer metastasis in prostate cancer cells through EMT induction, Cancer Lett 302 (2011), 128–135. [DOI] [PubMed] [Google Scholar]
- [72].Liu Y, Lashuel HA, Choi S et al. , Discovery of inhibitors that elucidate the role of UCH-L1 activity in the h1299 lung cancer cell line, Chem Biol 10 (2003), 837–846. [DOI] [PubMed] [Google Scholar]
- [73].Reddy SS, Shruthi K, Reddy VS et al. , Altered ubiquitin-proteasome system leads to neuronal cell death in a spontaneous obese rat model, Biochim Biophys Acta 1840 (2014), 2924–2934. [DOI] [PubMed] [Google Scholar]
- [74].Li L, Tao Q, Jin H et al. , The tumor suppressor UCHL1 forms a complex with p53/MDM2/ARF to promote p53 signaling and is frequently silenced in nasopharyngeal carcinoma, Clin Cancer Res 16 (2010), 2949–2958. [DOI] [PubMed] [Google Scholar]
- [75].Zhang JY, Zhang F, Hong CQ et al. , Critical protein GAPDH and its regulatory mechanisms in cancer cells, Cancer Biol Med 12 (2015), 10–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Hjerpe E, Egyhazi Brage S, Carlson J et al. , Metabolic markers GAPDH PKM2 ATP5B and BEC-index in advanced serous ovarian cancer, BMC Clin Pathol 13 (2013), 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Hansen CN, Ketabi Z, Rosenstierne MW, Palle C, Boesen HC and Norrild B, Expression of CPEB GAPDH and u6snRNA in cervical and ovarian tissue during cancer development, APMIS 117 (2009), 53–59. [DOI] [PubMed] [Google Scholar]