Abstract
Phosphatidylserine (PS), a negatively charged phospholipid present predominantly at the inner leaflet of the plasma membrane, has been widely implicated in many cellular processes including membrane trafficking. Along this line, PS has been demonstrated to be important for endocytosis, however, the involved mechanisms remain uncertain. By monitoring clathrin-mediated endocytosis (CME) of single vesicles in mouse chromaffin cells using cell-attached capacitance measurements that offer millisecond time resolution, we demonstrate in the present study that the fission-pore duration is reduced by PS addition, indicating a stimulatory role of PS in regulating the dynamics of vesicle fission during CME. Furthermore, our results show that the PS-mediated effect on the fission-pore duration is Ca2+ dependent and abolished in the absence of synaptotagmin 1 (Syt1), implying that Syt1 is necessary for the stimulatory role of PS in vesicle fission during CME. Consistently, a Syt1 mutant with a defective PS-Syt1 interaction increases the fission-pore duration. Taken together, our study suggests that PS-Syt1 interaction may be critical in regulating fission dynamics during CME.
Keywords: Ca2+, capacitance, clathrin-mediated endocytosis, chromaffin cell, fission, patch clamping, phosphatidylserine, Syt1
Graphical Abstract

In this issue
Phosphatidylserine (PS), a negatively charged phospholipid, may be important for endocytosis, however, the involved mechanisms remain uncertain. By monitoring clathrin-mediated endocytosis (CME) of single vesicles in mouse chromaffin cells using cell-attached capacitance recordings, we demonstrate that the fission-pore duration is reduced by PS addition, indicating a stimulatory role of PS in regulating the dynamics of vesicle fission. Furthermore, the PS-mediated effect is abolished in the absence of synaptotagmin 1 (Syt1) and a Syt1 mutant with a defective PS-Syt1 interaction increases the fission-pore duration. Our study thus suggests an importance of the PS-Syt1 interaction in regulating fission dynamics during CME.
Introduction
Phosphatidylserine (PS), a negatively charged glycerophospholipid enriched in the plasma membrane, is almost exclusively found in the cytoplasmic leaflet of all eukaryotic species (Leventis & Grinstein 2010, Dolis et al. 1997, Bohdanowicz & Grinstein 2013). Due to its location and anionic nature (Yeung et al. 2008), PS has been suggested to contribute to the recruitment and concentration of proteins with important physiological functions (Sezgin et al. 2017, Raghupathy et al. 2015), such as cell polarity, phagosome maturation and cell apoptosis (Fairn et al. 2011, Das et al. 2012, Yeung et al. 2009, Fadok et al. 1992, van Engeland et al. 1998). Notably, the nervous system is particularly enriched in lipids (Bozek et al. 2015). As a negative charged lipid located in the cytosolic face of the plasma membrane, PS plays a key role in organizing the structure of presynaptic terminals and is central to synaptic vesicle cycle (Lauwers et al. 2016, Graham 2004, Sun & Drubin 2012).
Far more abundant than phosphoinositides (PtdIns) in synaptic membranes (Takamori et al. 2006), PS contributes to the regulation of multiple steps during exocytosis. For example, PS may directly interact with C2 domain containing proteins such as synaptotagmin 1 (Syt1) and Doc2-beta during neurotransmitter release (Chapman 2002, Vennekate et al. 2012, Yao et al. 2011a, Brouwer et al. 2015). Amperometric studies in PC12 cells have shown that the electrostatic interaction between PS and Syt1, may modulate the open pore of fusing vesicles as well as the frequency of exocytic events (Zhang et al. 2009, Zhang & Jackson 2010), suggesting that PS may regulate exocytosis via its negative charge (Lauwers et al. 2016). In the case of interaction with VAMP2, it appears that PS may promote the binding between VAMP2 and Syntaxin-1A by shielding positive charges on either protein as they approach to each other during synaptic vesicle docking, thus regulating exocytosis in insulin-secreting beta cells (Williams et al. 2009). Furthermore, the negatively charged PS may control membrane bending via recruiting and activating membrane bending proteins, such as N-BAR domain containing proteins, to specific presynaptic subdomains to regulate exocytosis (Peter et al. 2004, Lauwers et al. 2016).
PS, which is localized to endocytic organelles (Uchida et al. 2011), has been suggested to be important for endocytosis (Bohdanowicz & Grinstein 2013, Ory et al. 2013). A recent study in HeLa cells shows that addition of PS is sufficient to stimulate endocytosis (Hirama et al. 2017), consistent with a previous finding in the K562 cell line (Farge 1995). The emphasis of dynamin, a large GTPase, in vesicle fission during endocytosis has highlighted that vesicle fission may be is a critical and arguably the rate-limiting step in endocytosis (Antonny et al. 2016, Hinshaw 2000). However, the role of anionic PS in vesicle fission during endocytosis remains poorly studied.
By using cell-attached capacitance measurements to detect clathrin-mediated endocytosis (CME) of single vesicles in mouse adrenal chromaffin cells (Yao et al. 2012, Yao et al. 2013), we have established an assay that resolves singular endocytic events. Moreover, we were able to calculate the endocytic fission-pore kinetics through membrane capacitance and membrane conductance (Yao et al. 2012, Wu & Wu 2007, Yao et al. 2013, Debus & Lindau 2000), which were separated by applying a sinusoidal voltage superimposed to the holding potential (Debus & Lindau 2000). In the present study, we investigated the role of PS during CME using the cell-attached capacitance measurements. Our results suggest that PS accelerates vesicle fission during CME in a Ca2+ dependent manner, and that Syt1 may be required for this PS-mediated modulation. Furthermore, a Syt1 mutant with a defective PS-Syt1 interaction disrupts vesicle fission. Our work thus proposes a similar mechanism for the PS-Syt1 interaction in exocytosis (Bhalla et al. 2005, Zhang et al. 2009) and endocytosis.
Materials and Methods
1. Chromaffin Cell Culture
After decapitation of newborn pups (post-natal day 0) under hypothermia as anesthesia of either sex from wildtype (WT), C577BL/6 (IMSR Cat# JAX:000664, RRID: IMSR_JAX:000664), or Syt1 heterozygous (HT) (Yao et al. 2012), B6;129S-Syt1tm1Sud/J (IMSR Cat# JAX:002478, RRID: IMSR_JAX:002478) mouse mating cages. Adrenal glands were isolated according to the guidelines of the National Institutes of Health, as approved by the Animal Care and Use Committee of the University of Illinois at Chicago (approval number: 17–008). Tails were kept for genotyping for newborn pups from Syt1 HT matings. The Syt1 alleles were identified by a three-step polymerase chain reaction (PCR) with the following cycling parameters: 94 °C-2′, 35x [94 °C-30″, 60 °C-30″, 72 °C-45″], 72 °C-5’, held at 4 °C, using Tag DNA polymerase (Catalog number: EP0702, Thermo Scientific, Waltham, MA). The PCR primers are as followings: 5’-GTATTCAGTGCGTCTCAGAGACAGTC-3’ as the forward primer for WT, 5’-GAGCGCGCGCGCCGGAGTTGTTGAC-3’ as the forward primer for KO and 5’-AACTATAATTTGTCACAGGCATTGCCTTTCA-3’ as the shared reverse primer. PCR products were viewed on 1% agarose gels stained with SYBR Safe DNA Gel Stain (Catalog number: S33102, Thermo Scientific, Waltham, MA), Syt1 WT allele is expected to have a band size of 700 bp with a band size of 400 bp for Syt1 KO allele.
Electrophysiological recordings on chromaffin cells in culture from littermate WT and Syt1 KO mice were compared. WT chromaffin cells were typically from WT mating cages, except that those for data presented in Fig. 4 were from Syt1 HT mating cages. The breeding animals were housed in groups of 3 (1 male/2 females) in a transparent polypropylene cage, 7.7” x 12.17” x 5.25”, in a room with controlled temperature and humidity under a 12 h light and 12 h dark cycle and constant access to water and food. Mouse chromaffin cells were prepared and cultured from a pair of adrenal glands from individual pups as described previously (Yao et al. 2012, Yao et al. 2013). Briefly, after incubation with papain solution at 37 °C for 40 min, adrenal glands were titrated gently through a 200 μl pipette tip. Cells from different pups were not pooled before plated on coverslips and incubated at 37 °C in 5% CO2 and utilized within 4 days. Data from pure WT mating cages was pooled from 3–4 cultures, and data from Syt1 HT mating cages were pooled from 4–7 cultures.
Fig. 4.
The PS-mediated effect on the fission-pore duration was abolished in Syt1 KO cells. A. Representative endocytic events, as membrane conductance (Re), membrane capacitance (Im) and the fission-pore conductance (Gp) recorded in the cell-attached configuration at 2 mM [Ca2+]e from a Syt1 KO cell (left) and a Syt1 KO cell treated with 10 μM PS (right). B. PS treatment had no obvious effect on the number of endocytic events within the first 5 min time of cell-attached recordings in Syt1 KO cells, although the number of endocytic events in these 2 group was significantly lower than that in WT cells (WT: 5.10 ± 0.50, n = 82 cells, Control: 2.50 ± 0.34, n = 97 cells, PS: 2.37 ± 0.38, n = 95 cells; one-way ANOVA followed by Tukey’s post hoc test, F(2, 271) = 14.0148, *** p < 0.001, WT vs. Control in Syt1 KO: ** p = 0.0010, WT vs. PS in Syt1 KO: ** p = 0.0010, Control vs PS in Syt1 KO: p = 0.8999). C-D. There was no statistical difference in the capacitance Cv of endocytic vesicles (WT: 0.78 ± 0.06, n = 42 events, Control in Syt1 KO: 0.79 ± 0.06, n = 36 events, PS in Syt1 KO: 0.75 ± 0.05, n = 41 events; one-way ANOVA followed by Tukey’s post hoc test, F(2, 116) = 0.145, p = 0.865, WT vs. Control in Syt1 KO: p = 0.8999, WT vs. PS in Syt1 KO: p = 0.8998, Control vs PS in Syt1 KO: p = 0.8399) (C) and the fission-pore Gp (WT: 178.59 ± 21.79, n = 42 events, Control in Syt1 KO: 180.11 ± 16.42, n = 36 events, PS in Syt1 KO: 183.83 ± 24.10, n = 41 events; one-way ANOVA followed by Tukey’s post hoc test, F(2, 116) = 0.0168, p = 0.9833, WT vs. Control in Syt1 KO: p = 0.8865, WT vs. PS in Syt1 KO: p = 0.8654, Control vs PS in Syt1 KO: p = 0.902) (D) were comparable between Control and PS-treated cells. E. PS mediated reduction of fission-pore duration was abolished in Syt1 KO cells, proposing the requirement of Syt1 for PS to regulate vesicle fission during CME (WT: 188.27 ± 30.16, n = 42 events, Control in Syt1 KO: 474.25 ± 55.98, n = 36 events, PS in Syt1 KO: 451.07 ± 63.96, n = 41 events; one-way ANOVA followed by Tukey’s post hoc test, F(2, 116) = 9.841, *** p = 0.0001, WT vs. Control: ** p = 0.0010, WT vs. PS in Syt1 KO: ** p = 0.0011, Control in Syt1 KO vs PS in Syt1 KO: p = 0.8999). Data was pooled from 7 independent cultures.
Phospholipids such as PS (Sigma, St. Louis, MO, catalog number: P7769), phosphatidylethanolamine (PE) (Sigma, St. Louis, MO, catalog number: P7693), phosphatidylcholine (PC) (Sigma, St. Louis, MO, catalog number: P3556), or fluorescent 1,2-dioleoyl-sn-glycero-3-phospho-L-serine-N-(7-nitro-2–1,3-benzoxadiazol-4-yl (NBD) labeled PS (Avanti Polar Lipids, Alabaster, AL, catalog number: 810198) at 10 μM were added to the culture media for 24 hrs prior to electrophysiological recordings or immunostaining.
Botulinum neurotoxin type A (BoNT/A), which cleaves the last 9 amino acid residues of the c-terminal of SNAP-25 (Pirazzini et al. 2017), was purchased from METABIOLOGIS (Madison, WI). Chromaffin cells were incubated with BoNT/A at either 2 or 10 ng/ml for 45 min prior to electrophysiological recordings.
2. Cell-attached capacitance recordings and the fission-pore analysis
For electrophysiological recordings, cells were bathed in solution with 140 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1mM Mg Cl2, 10 mM HEPES-NaOH, and 10 mM glucose; the pH was adjusted to 7.3 with NaOH, and the osmolarity was ~ 310mmol kg−1. The solution in the cell-attached pipette contained 50 mM NaCl, 100 mM TEACl, 5mM KCl, 2 mM CaCl2, and 10 mM HEPES-NaOH; the pH was adjusted to 7.3 and the osmolarity was adjusted to ~ 290 mmol/kg. Patch pipettes were coated with sticky wax (Freeman supply, Avon, OH) and fire-polished with a typical resistance of ~ 2 MΩ. Cell-attached recordings were performed using an EPC-7 plus patch-clamp amplifier (HEKA-Elektronic, Germany). Changes of membrane capacitance and conductance were measured as described previously (Yao et al. 2012, Yao et al. 2013), with a SR830 lock-in amplifier (Stanford Research Systems, Sunnyvale, CA) using a sinewave with amplitude of 50 mV (root mean square) at a frequency of 20 kHz. The output filter of the lock-in amplifier was set to a 1 ms time constant, 24 dB. The number of endocytic events per patch was counted as the total number of downward capacitance steps with sizes > 0.2 fF within the first 5 min of recordings. WT and knockout (KO) cells were treated with 10 μM PS into cell culture for 24 hrs prior to electrophysiological recordings. The cell-attached recordings were performed at room temperature, and recordings with seal resistance < 2 GΩ were excluded from data analysis.
Endocytic fission-pore closures were analyzed as described previously (Yao et al. 2012, Yao et al. 2013). During fission-pore closure in which a transient increase in the Re signal was associated with a decline in the Im step, the vesicle capacitance (Cv) was determined as the total change in the Im trace for a particular event and the fission-pore conductance (Gp) was calculated using the formula as follows: Gp = (Yao et al. 2012, Yao et al. 2013). The fission-pore duration was defined as the time interval from the first point where the Gp decreased to < 2 nS and the final drop in Gp to zero. This final drop reflects the step response of the low pass filter setting of the lock-in amplifier (1ms, 24 dB). At this setting, 90% of the final value is reached within ~ 7 ms, so the last point of the fission-pore was taken as the time at 7–10 mS before the final drop to zero in the Gp trace. The fission-pore conductance Gp was taken as the average Gp value during the fission-pore duration time interval. Analysis of fission-pore kinetics was restricted to fission-pores with durations > 15 ms because shorter events were distorted by the lock-in amplifier low-pass filter (set to 1 ms, 24 dB).
3. Immunostaining
The incorporation of the fluorescent NBD-PS (McIntyre & Sleight 1991) into chromaffin cells was confirmed by the detection of fluorescence. Briefly, chromaffin cells with 24-hr-incubation of 10 μM NBD-PS in culture media were fixed for 15 minutes in 4% paraformaldehyde in PBS, and then stained for 1 hr with a primary polycolonal antibody against tyrosine hydroxylase (Abcam Cat# ab6211, RRID: AB_2240393), a marker for catecholaminergic cells. Cells were washed and then incubated in secondary TRITC-conjugated goat anti-rabbit antibody (Abcam Cat# ab6718, RRID: AB_955551) for 30 min.
For Annexin V-FITC staining, chromaffin cells were incubated with 10 μM PS (Sigma, St. Louis, MO, product number: P7769) in culture media for 24 hrs and then fixed with a 4% paraformaldehyde for 15 min and then washed 3 times in PBS. The plasma membrane was permeabilized using 0.3% Triton X-100 and washed three more times in PBS. Cells were incubated with Annexin V-FITC (SouthernBiotech, Birmingham, AL, catalog number: 10040–02) in the Annexin-V Binding Buffer (SouthernBiotech, Birmingham, AL, catalog number: 10045–01), with 3 mM CaCl2 for 30 min followed by TH staining as described above.
Cells were mounted with Vectashield mounting medium for fluorescence (Vector Laboratories, Burlingame, CA, catalog number: H-1800), and imaged with a confocal microscope (Fluoview FV10i, Olympus, Center Valley, PA) using a 60x oil immersion objective. Fluorescence intensities of Annexin V-FITC signals were analyzed using NIH ImageJ. All the experiments were conducted at room temperature.
4. Cloning and lentiviral infections
The full-length rat Syt1WT, Syt16DA or Syt1D238N mutant was amplified by PCR using primers: 5’- ATGCGGGCCCATGGTGAGTGCCAGTCATC-3’ and 5’- TGTTGTCGACTTACTTCTTGACAGCCAGCATG-3’, and introduced into a lentiviral expression vector pCDH-EF1-MCS-T2A-copGFP (System Biosciences, catalogue number: CD526A-1) between the ApaI and the SalI sites. Lentiviral infections with Syt1WT, Syt16DA or Syt1D238N in Syt1 KO cells were carried out on chromaffin cells at days in vitro 0, and electrophysiological recordings were carried out 2–3 days after infection as described (Jiang et al. 2019). All the custom-made materials will be shared upon reasonable requests.
5. Carbon fiber amperometry
Conventional carbon fiber amperometry for catecholamine detection used 5-μm carbon fibers (ALA Scientific Instruments, Farmingdale, NY) as described previously (Gong et al. 2007, Gong et al. 2005, Jiang et al. 2019). The freshly cut tip of the carbon fiber electrode was positioned closely against the cell surface to minimize the diffusion distance from release sites. Exocytosis was elicited by 10 μM Ca2+ in a whole-cell patch pipette, constantly held at −70 mV by an EPC-10 with access resistance in the range of 2–4 MΩ. The intracellular whole-cell patch pipette solution contained 125 mM KCl, 5 mM NaCl, 2 mM MgATP, 0.3 mM Na3GTP, 10 mM HEPES-KOH, and 2 mM EGTA, 2 mM CaCl2, free [Ca2+] 10 μM, pH 7.3 with KOH. The amperometric current, generated by catecholamine oxidation at the exposed tip of the carbon fiber electrode due to stimulation, was measured using an EPC-7 plus amplifier at a holding potential of +700 mV. Amperometric signals were low-pass filtered at 3 kHz and digitized at 5 kHz. Amperometric recordings were collected and then analyzed with a customized macro for Igor software (WaveMetrics, Tigard, OR) (Mosharov & Sulzer 2005) to extract spike information according to the criteria as described (Chow et al. 1992). The following criteria were set in single-spike analysis: (i) only spikes >10 pA were considered for kinetics analysis; (ii) the maximum number of spikes analyzed per cell was set to 100; (iii) foot duration was delimited by the baseline and spike onset as shown previously (Mosharov & Sulzer 2005), and (iv) spikes with a foot duration of < 0.5 ms were excluded for the assays. The number of amperometric spikes was counted as the total number of spikes with an amplitude > 10 pA within 2 min after patch rupture in whole-cell configurations.
6. Experimental design and Statistical Analysis
Our study was not pre-registered prior to experiments design and data analyses. No data points were eliminated, and no statistical methods were engaged to predetermine sample size, instead we based our experimental design on numbers reported in previous studies (Yao et al. 2012, Yao et al. 2013). For each culture prepared from pups with identical genetic background, coverslips with cells, which were assigned with specific numbers, were allocated to each experimental group using a random sequence generator (https://www.random.org/sequences/). The software essentially assigned a coverslip (via its identifier number) to each group, and a randomized number does not contain duplicates and no exclusion criteria were pre-determined (Kim & Shin 2014). Experimenters were constantly blinded with the identities of groups during data analyses but not experiments in this study. Normal distributions were determined by Shapiro-Wilk test before data analysis. Statistical tests were performed with SPSS (V25), with Student’s t-test to compare two groups and one-sided ANOVA for more than two groups. If ANOVAs were significant, we used a post hoc Tukey’s multiple comparisons test to compare groups. Exact p-values are given between 0.0001 and 0.99. Data are presented as means ± SEM. Significance levels were as indicated in figures: * P < 0.05; ** P < 0.01 and *** P < 0.001.
Results
1. Exogenous PS incorporates into chromaffin cells
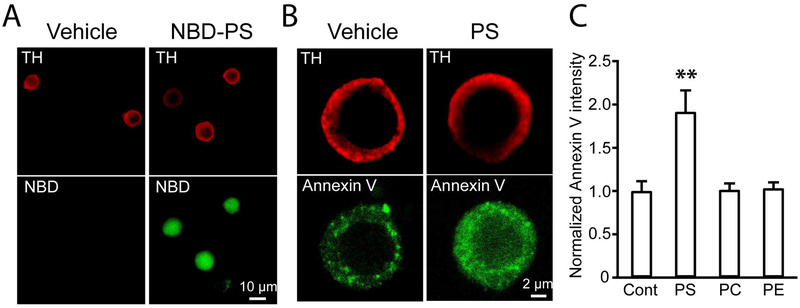
Before determining any potential effects of PS on CME, we explored whether exogenous PS could be incorporated into mouse chromaffin cells. PS insertion into cells was first tested with a fluorescent NBD labeled PS (McIntyre & Sleight 1991). The incorporation of NBD-PS into chromaffin cells was confirmed by the detection of fluorescence signals in cells treated with 10 μM NBD-PS for 24 hrs prior to fixation and visualization (Fig. 1A). We next quantified the cellular PS level by utilizing FITC tagged Annexin V, a PS binding protein. This detection method, commonly used to monitor apoptosis (Janko et al. 2013), has been extensively utilized to mark intracellular PS (Zhang et al. 2009, Montaville et al. 2002). The intensity of Annexin V-FITC signals was significantly increased in PS-treated cells as compared to control cells (p < 0.01) (Fig. 1B and C), indicating an increase in the cellular PS level. Conversely, cells treated at the same concentration of 10 μM PC or PE, displayed no obvious change in Annexin V-FITC intensity (p > 0.05) (Fig. 1C), indicating no obvious PS changes in PC- or PE-treated cells. Collectively, our results indicate that exogenous PS addition can be effectively incorporated into chromaffin cells and thus likely causes an increase in cellular PS levels, which is consistent with previous studies (Zhang et al. 2009, Uchiyama et al. 2007).
Fig. 1.
Exogenous PS addition in culture media increased cellular PS in chromaffin cells A. Images of NBD-PS in mouse chromaffin cells. Images of chromaffin cells treated with vehicle (Left) or NBD-PS (Right). Chromaffin cells were stained with anti-tyrosine hydroxylase (Upper). B. Intensity of Annexin V-FITC signal was increased in PS-incubated cells (Right) alternative to control cells (Left). Chromaffin cells were identified with anti-TH antibody (Upper). C. Normalized intensities of Annexin V-FITC signal indicate increased cellular PS level in PS-treated cells (Control: 1.00 ± 0.11, n = 40 cells, PS: 1.92 ± 0.25, n = 32 cells, PC: 1.01 ± 0.07, n = 36 cells, PE: 1.03 ± 0.07, n = 38 cells; one-way ANOVA followed by Turkey’s post hoc test, F(3, 142) = 10.5436, *** p < 0.001, PS vs. Control: ** p = 0.0010, PC vs. Control: p = 0.8999, PE vs. Control: p = 0.8999). Data was pooled from 3 independent cultures.
2. PS addition reduces the fission-pore duration
Using the cell-attached capacitance recordings, our work has established a detection of single endocytic events in chromaffin cells (Yao et al. 2013, Yao et al. 2012, McAdam et al. 2015, Varga et al. 2014). Furthermore, the occurrence of these endocytic events can be blocked by application of a specific clathrin antibody through a whole cell patch pipette, indicating a predominance of individual CME events in our detection (Yao et al. 2012). Once verified that exogenous PS could be incorporated into chromaffin cells, we explored the potential influence of PS, added at 10 μM, on the kinetics of individual endocytic events in the presence of 2 mM [Ca2+]e (Fig. 2A) through cell attached capacitance measurements. Addition of PS did not alter either the number of endocytic events (Fig. 2B) or the Cv of endocytic vesicles (Fig. 2C) (p > 0.05), indicating that PS may not be critical in controlling the size of endocytic vesicles. Additionally, PS had no obvious effect on the fission-pore conductance (Gp) (Fig. 2D) (p > 0.05), implying that PS may not be essential in determining the geometry of the tubular membrane neck. However, PS addition caused a ~ 50% reduction in the fission-pore duration (p < 0.01) (Fig. 2E), suggesting a stimulatory role of PS in regulating the kinetics of vesicle fission during CME. In contrast to anionic PS, neutral charged PC and PE had no obvious effect on the fission-pore duration (p > 0.05) (Fig. 2E). These results indicate that PS may play a critical role in the kinetics of unitary CME events.
Fig. 2.
PS addition reduced the fission-pore duration of CME events. A. Representative endocytic events, as membrane conductance (Re), membrane capacitance (Im) and the fission-pore conductance (Gp) recorded in the cell-attached configuration at 2 mM [Ca2+]e from a control cell (left) and a cell treated with 10 μM PS (right). B-D. The number of endocytic events within the first 5 min time of cell-attached recordings (Control: 4.22 ± 0.49, n = 152 cells, PS: 3.63 ± 0.54, n = 118 cells, PC: 3.72 ± 0.51, n = 129 cells, PE: 4.42 ± 0.64, n = 91 cells; one-way ANOVA followed by Turkey’s post hoc test, F(3, 486) = 0.4680, p = 0.7047) (B), the capacitance Cv of endocytic vesicles (Control: 0.77 ± 0.05, n = 61 events, PS: 0.82 ± 0.06, n = 46 events, PC: 0.78 ± 0.05, n = 56 events, PE: 0.75 ± 0.05, n = 52 events; one-way ANOVA followed by Turkey’s post hoc test, F(3, 211) = 0.2309, p = 0.8748) (C), and the fission-pore Gp (Control: 164.25 ± 13.37, n = 61 events, PS: 175.98 ± 19.03, n = 46 events, PC: 153.75 ± 16.98, n = 56 events, PE: 143.71 ± 14.36, n = 52 events; one-way ANOVA followed by Turkey’s post hoc test, F(3, 211) = 0.7097, p = 0.5472) (D) were statistically comparable between Control and PS-treated cells. E. The fission-pore duration was significantly reduced by ~ 50% in PS-treated cells, indicating a critical role of PS in vesicle fission during CME (Control: 180.72 ± 21.67, n = 61 events, PS: 95.50 ± 19.08, n = 46 events, PC: 170.21 ± 22.98, n = 56 events, PE: 182.25 ± 27.37, n = 52 events; one-way ANOVA followed by Turkey’s post hoc test, F(3, 211) = 4.8245, ** p = 0.0029, PS vs. Control: ** p = 0.0056, PC vs. Control: p = 0.8999, PE vs. Control: p = 0.8999). Data was pooled from 4 independent cultures.
3. PS regulates the fission-pore duration in a Ca2+ dependent manner
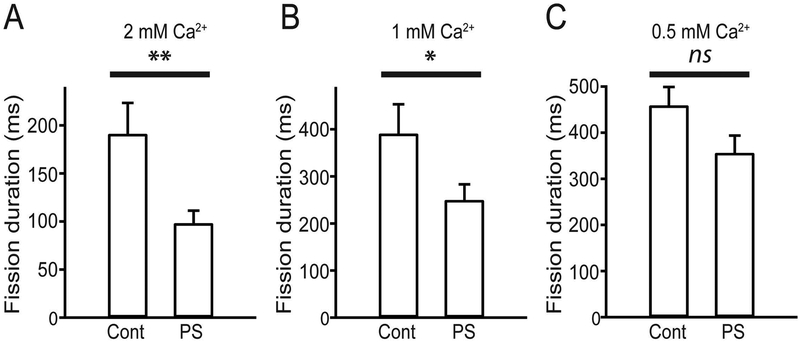
Ca2+ has been reported to be important for PS-related physiological events at the plasma membrane (Roux & Bloom 1991, Sinn et al. 2006, Martin-Molina et al. 2012, Onishi & Ito 1974, Takatsu et al. 2017). Considering recent evidence outlining the central importance of Ca2+ in endocytosis including vesicle fission during CME (Yao et al. 2017, Wu & Wu 2014, Yao et al. 2012), we explored whether the observed regulation of PS on the fission-pore duration is Ca2+ dependent. Our findings revealed that PS addition consistently caused a ~ 50% and ~36% reduction in the fission-pore duration at 2 mM [Ca2+]e (p < 0.01) (Fig. 3A) and 1 mM [Ca2+]e (p < 0.05) (Fig. 3B), respectively. However, PS addition had no detectable effect on the fission-pore duration when [Ca2+]e was reduced to 0.5 mM (p > 0.05) (Fig. 3C). Thus, our data indicates a necessary role of Ca2+ in the PS-mediated effect on vesicle fission during CME.
Fig. 3.
The PS-mediated effect on the fission-pore duration was Ca2+ dependent. A-B. Quantifications of the fission-pore duration from endocytic events recorded in the cell-attached configuration show that 10 μM PS treatment significantly accelerated the dynamics of vesicle fission during CME at 2 mM [Ca2+]e (Control: 191.14 ± 31.82, n = 37 events, PS: 98.13 ± 12.87, n = 39 events; unpaired Student’s t-test, t(74) = 2.7591, ** p = 0.0073) (A) and 1 mM [Ca2+]e (Control: 390.89 ± 61.62, n = 36 events, PS: 249.76 ± 32.78, n = 37 events; unpaired Student’s t-test, t(71) = 2.0378, * p =0.0453) (B). C. Quantification indicates that low [Ca2+]e at 0.5 mM prevents the acceleration of vesicle fission by PS (Control: 459.26 ± 39.34, n = 39 events, PS: 356.03 ± 38.17, n = 37 events; unpaired Student’s t-test, t(74) = 1.8803, p = 0.0640), suggesting a critical role of Ca2+ in mediating the modulation of PS on fission dynamics during CME. Data was pooled from 3–4 independent cultures.
4. Syt1 is required for the PS-induced effect on the fission-pore duration
Anionic PS is highly implicated in the direct binding with C2 domain containing proteins, particularly Syt1 (Wang et al. 2016, Chapman & Davis 1998, Chae et al. 1998). It has been shown that the hydrophobic residues in the top of the Ca2+ binding loops of both C2A and C2B domains of Syt1 will insert into PS-containing membrane lipids upon Ca2+ binding (Fernandez et al. 2001, Chapman & Davis 1998). Furthermore, the Ca2+ dependent PS-Syt1 interaction is crucial for the Ca2+ sensing role of Syt1 in excitation-secretion coupling (Fernandez-Chacon et al. 2001, Rhee et al. 2005). Given the Ca2+ dependent PS-mediated modulation of the fission-pore duration (Fig. 3) and the defect in the fission-pore duration in Syt1 KO cells previously revealed by our lab (Yao et al. 2012), we next examined whether Syt1 is required for the PS-mediated effect on the fission-pore duration using Syt1 KO cells (Fig. 4A). Consistent with those in WT cells (Fig. 2B–D), the number of endocytic events (Fig. 4B), the Cv of endocytic vesicles (Fig. 4C) and the fission-pore Gp (Fig. 4D) remained unaltered in Syt1 KO cells treated with PS (p > 0.05). In contrast to WT cells, PS addition failed to reduce the fission-pore duration in Syt1 KO cells (p > 0.05) (Fig. 4E). Collectively, these results demonstrate that Syt1 is required for the PS-mediated effect on the fission-pore duration, suggesting that the PS-Syt1 interaction may be critical for fission kinetics during CME.
It is of note that the remaining fraction of endocytic events with slower kinetics in Syt1 KO cells (Fig. 4B & E) could be mediated by other Syt isoforms with weaker or no Ca2+ affinity or even complexin, an obligatory cofactor of Syt1 as the Ca2+ sensor for exocytosis (Sudhof 2012). Indeed, many Syt isoforms that are expressed in neuroendocrine cells (Moghadam & Jackson 2013). In chromaffin cells, Syt1 is responsible for the fast component of exocytosis while Syt7, a Syt isoform with slower Ca2+ binding kinetics (Bhalla et al. 2005), is responsible for the slow component of exocytosis (Rao et al. 2014, Schonn et al. 2008). Interestingly, Syt1-mediated fast synchronous release is coupled to fast endocytosis with the Syt7-mediated slow asynchronous release coupled to slow endocytosis (Li et al. 2017), which may alternatively explain slow endocytic kinetics we observed in Syt1 KO chromaffin cells.
5. A Syt1 mutant with no Ca2+ binding affinity slows down vesicle fission
Since there were reductions in the number of exocytic and endocytic events, in addition to an increase in the fission-pore duration, in the Syt1 KO cells (Yao et al. 2012), an increase in the fission-pore duration could be a simple consequence of defects in exocytic and/or endocytic event number. To explore this possibility, we next examined effects of BoNT/A, which cleaves the last 9 amino acids of SNAP-25 C-terminus (Pirazzini et al. 2017). Carbon fiber recordings showed that the number of amperometrical spikes is reduced by treatments of BoNT/A in a concentration dependent manner (p < 0.01) (Fig. S1), consistent with a critical role of SNAP-25 in exocytosis (Sollner 2003). Furthermore, BoNT/A treatment increased the duration of the foot signal (p < 0.05) (Table S1), which is in line with a previous study (Fang et al. 2008), supporting a key role of SNAP-25 in fusion during exocytosis (Sollner 2003). In contrast, cell-attached recordings observed that BoNT/A treatment had no detectable effects on the fission-pore duration (P > 0.05), regardless of a reduction in the number of endocytic events (p < 0.01) (Fig. S2). Thus, these results demonstrate that in our experiments, the fission-pore duration represents an independent parameter for endocytic events, which will unlikely be affected by any changes in other exocytic or endocytic parameters, such as the number of exocytic or endocytic events as we have examined in BoNT/A experiments.
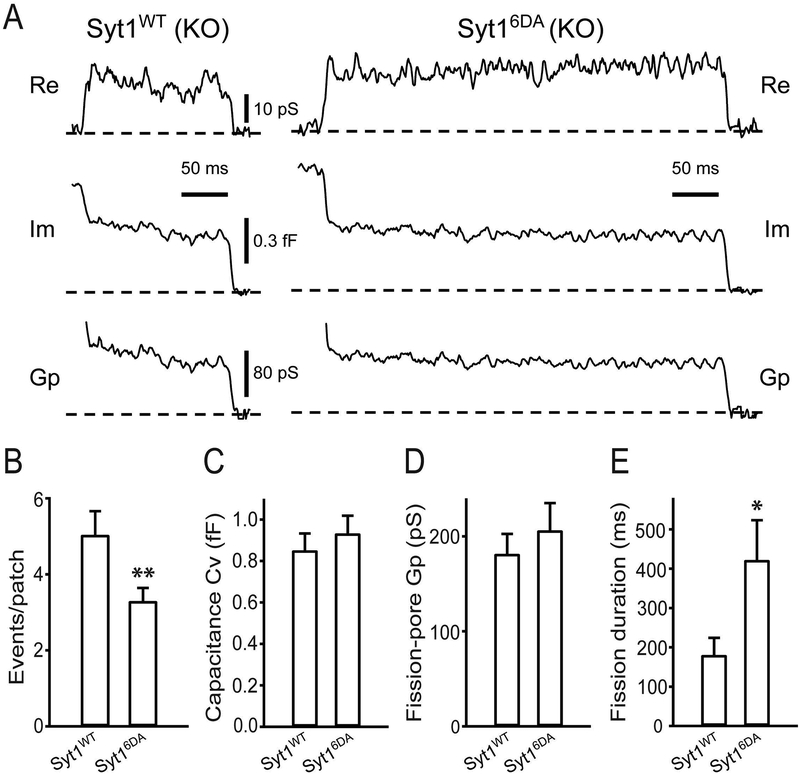
To understand the PS-Syt1 interaction in vesicle fission during CME, we next examined effects of the Syt16DA mutant (C2A: D178A, D230A, D232A; C2B: D309A, D363A and D365A), which eliminates Syt1 Ca2+-binding capacity (Xu et al. 2009), on kinetics of single vesicle endocytosis using the cell-attached capacitance recordings, since Ca2+ binding to Syt1 is essential for the PS-Syt1 interaction (Chapman 2008, Pang & Sudhof 2010). Experiments were carried out in Syt1 KO cells expressing either Syt1WT or Syt16DA (Fig. 5A). Expressions of Syt1WT rescued the number of endocytic events and the fission-pore duration in Syt1 KO cells to a level that is comparable to those in WT cells (Fig. 4B & E), while the number of endocytic events was smaller in KO cells expressing Syt16DA as compared to Syt1WT (p < 0.01) (Fig. 5B). We observed no difference in the Cv of endocytic vesicles (p > 0.05) (Fig. 5C) and the fission-pore Gp (p > 0.05) (Fig. 5D), however, the fission-pore duration was significantly increased in Syt16DA-expressing KO cells as compared to Syt1WT (p < 0.05) (Fig. 5E). This data thus indicates that the Ca2+ binding affinity of Syt1 is essential for its role in regulating vesicle fission during CME.
Fig. 5.
The Ca2+-binding affinity of Syt1 is important in regulating vesicle fission during CME. A. Representative endocytic events, as membrane conductance (Re), membrane capacitance (Im) and the fission-pore conductance (Gp) in the cell-attached configuration at 2 mM [Ca2+]e from Syt1 KO cells expressing either Syt1WT (left) or Syt16DA (right). B. The number of endocytic events within the first 5 min time of recordings was reduced in KO cells expressing Syt16DA as compared to Syt1WT (Syt1WT: 5.04 ± 0.62, n = 99 cells, Syt16DA: 3.31 ± 0.34, n = 101 cells; unpaired Student’s t-test, t(198) = 3.0115, ** p = 0.0029). C-D. There was no significant difference between these 2 group in the Cv of endocytic vesicles (Syt1WT: 0.85 ± 0.08, n = 36 events, Syt16DA: 0.93 ± 0.08, n = 32 events; unpaired Student’s t-test, t(66) = 0.0439, p = 0.9651) (C), and the fission-pore Gp (Syt1WT: 181.58 ± 21.04, n = 36 events, Syt16DA: 206.30 ± 28.59, n = 32 events; unpaired Student’s t-test, t(66) = 0.7638, p = 0.4477) (D). E. The fission-pore duration was statistically increased in Syt16DA-expressing KO cells (Syt1WT: 180.65 ± 43.42, n = 36 events, Syt16DA: 421.89 ± 101.28, n = 32 events; unpaired Student’s t-test, t(66) = 2.3311, * p = 0.02281). Data was pooled from 6 independent cultures.
6. A Syt1 mutant with a weaker interaction to phospholipids disrupts vesicle fission
Upon Ca2+ binding, Syt1 interacts with negatively charged phospholipids (Chapman 2008, Pang & Sudhof 2010). As the most abundant negatively charged phospholipids in eukaryotic membrane (Leventis & Grinstein 2010), PS interacts directly with Syt1, which is essential for the role of Syt1 in exocytosis as the Ca2+ sensor (Chapman 2008, Pang & Sudhof 2010). Confirming the importance of this PS-Syt1 interaction in exocytosis, a previous work by Pang et al, has shown that a Syt1D238N mutant, harboring a mutation in Syt1 C2A domain, specifically disrupts its interaction to phospholipids but leaves the interaction to SNARE proteins intact (Pang et al. (2006). We therefore analyzed the effects of the Syt1D238N mutant on the kinetics of single vesicle endocytosis in Syt1 KO cells (Fig. 6A) to further understand the potential role of the PS-Syt1 interaction in CME. While there was no detectable difference in the number of endocytic events (p > 0.05) (Fig. 6B), the Cv of endocytic vesicles (p > 0.05) (Fig. 5C), and the fission-pore Gp (p > 0.05) (Fig. 6D), the fission-pore duration was significantly increased in Syt1D238N-expressing KO cells (p < 0.05) (Fig. 5A & E). These experiments indicate that the PS-Syt1 interaction may be essential for the kinetics of vesicle fission during CME.
Fig. 6.
A Syt1D238N mutant with a defective PS-Syt1 interaction disrupts vesicle fission during CME. A. Representative endocytic events, as membrane conductance (Re), membrane capacitance (Im) and the fission-pore conductance (Gp) in the cell-attached configuration at 2 mM [Ca2+]e from Syt1 KO cells expressing either Syt1WT (left) or Syt1D238N (right). B-D. There was no detectable difference between there 2 groups in the number of endocytic events within the first 5 min time of cell-attached recordings (Syt1WT: 4.92 ± 0.59, n = 98 cells, Syt1D238N: 5.30 ± 0.59, n = 102 cells; unpaired Student’s t-test, t(198) = 0.631, p = 0.5287 ) (B), the Cv of endocytic vesicles (Syt1WT: 0.85 ± 0.09, n = 42 events, Syt1D238N: 0.84 ± 0.09, n = 39 events; unpaired Student’s t-test, t(79) = 0.01474, p = 0.9883) (C), and the fission-pore Gp (Syt1WT: 171.78 ± 18.94, n = 42 events, Syt1D238N: 169.33 ± 24.30, n = 39 events; unpaired Student’s t-test, t(79) = 0.1065, p = 0.9155) (D). E. The fission-pore duration was significantly increased in KO cells expressing Syt1D238N as compared to Syt1WT (Syt1WT: 179.20 ± 39.08, n = 42 events, Syt1D238N: 376.20 ± 111.41, n = 39 events; unpaired Student’s t-test, t(79) = 2.0308, * p =0.04564). Data was pooled from 4 independent cultures.
Discussion
Vesicle fission, the rate-limiting step in endocytosis of synaptic vesicles, is key to maintain a sustainable and precise synaptic transmission (Haucke et al. 2011, Truckenbrodt et al. 2018, Kononenko & Haucke 2015). While most studies on the molecular mechanisms of vesicle fission have been centered on proteins such as Syt1 (Yao et al. 2012, McAdam et al. 2015), dynamin (Antonny et al. 2016, Roux et al. 2006) and endophilin (Milosevic et al. 2011, Hohendahl et al. 2017), it has recently been shown that phospholipids play critical roles in vesicle fission (Pinot et al. 2014, Manni et al. 2018, Pannuzzo et al. 2018). Presently, our data have revealed that PS addition reduces the fission-pore duration in WT cells, indicating a stimulatory role of PS in vesicle fission during CME. Furthermore, it has been proposed that PS may also facilitate endocytic invagination by enhancing membrane bending (Zha et al. 2001, Hirama et al. 2017) and it has been shown that the presence of PS is required for the in vitro binding to phospholipid bilayers of F-BAR protein (Itoh et al. 2005), a protein essential for endocytic invagination (Itoh & De Camilli 2006). Finally, it is implied that PS may enhance membrane curvature in the endocytic pathway between the trans-Golgi network and early endosome (Xu et al. 2013). Taken together, our work adds to the growing research in support of PS function throughout the endocytic process, including invagination and vesicle fission during CME.
It is worth noting that a previous study in yeast demonstrates a reduction in the endocytic site in PS deficient mutants (Sun & Drubin 2012), which predicts a likely reduction in ‘effective’ endocytic events. However, our study using mice chromaffin cells proves that PS addition does not affect the number of single CME events (Fig. 2B), indicating that PS may not be critical for the endocytic site initiation in mammalian cells. In parallel to differentiated roles of PS, phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2], another anionic phospholipid, is critical for CME (Di Paolo & De Camilli 2006), also exhibits varied functions in CME between yeast and mammalian cells. In contrast, PI(4,5)P2 is crucial for the initiation of endocytic sites in mammalian cells (Abe et al. 2008, Zoncu et al. 2007) but not in budding yeast (Sun & Drubin 2012). Therefore, it is possible that, opposite to PI(4,5)P2, PS may be obligatory for the initiation of endocytic sites in yeast but not in mammalian cells. Furthermore, our data demonstrates that PS-induced effects on vesicle fission is abolished in Syt1 KO chromaffin cells, indicative of Syt1 as the molecular mediator for the role of PS in vesicle fission. Along this line, it is speculative that PIP2 may regulate vesicle fission in a similar manner, since PS and PI(4,5)P2, the most abundant anionic phospholipids in the plasma membranes (Takamori et al. 2006), interact with Syt1 synergistically to regulate fusion during exocytosis (Bhalla et al. 2005, Zhang et al. 2009, Courtney et al. 2018, Bello et al. 2018).
Fission-pore dynamics are typically quantified with the lifetime of the fission-pore (the fission-pore duration) and the fission-pore Gp (Flores et al. 2014, Yao et al. 2013). Interestingly, PS addition only alters the fission-pore duration (Fig. 2E) and has no obvious effect on the fission-pore Gp (Fig. 2D). Dynamin, a key mechano-chemical enzyme for vesicle fission during CME (Slepnev & De Camilli 2000, Antonny et al. 2016), is regulated by PS through stimulation of its helix assembly (Sweitzer & Hinshaw 1998) or GTPase activity (Rasmussen et al. 1998). It is thus implied that dynamin could be the potential molecular mediator for the PS-mediated effect on the fission-pore duration we have observed. Yet, our previous work has indicated that disturbing the function of dynamin actually results in increases in both the fission-pore Gp and duration (Yao et al. 2013), a phenotype more complicated than the one revealed in our current research. Our lab did confirm that Syt1 deletion causes a prolonged fission-pore duration but no change in the fission-pore Gp (Yao et al. 2012). Consistently, the PS-induced effect on the fission-pore duration was diminished in Syt1 KO cells (Fig. 4E), indicating Syt1 rather than dynamin as the key mediator for the PS-mediated modulation of fission pore dynamics during CME.
Our present study shows that the effect of PS on fission pore dynamics, which is Ca2+ dependent (Fig. 3), is abolished in Syt1 KO cells (Fig. 4), thus extending a crucial importance of the PS-Syt1 interaction in vesicle fission, the last step, of CME. Consistently, the Syt16DA mutant, with abolished Ca2+ binding affinities (Fig. 5), or the Syt1D238N mutant, with specific defective interaction to phospholipids (Fig. 6), disrupts fission pore dynamics during CME. It is acknowledged that when bound to Ca2+, Syt1 binds PS in the plasma membrane to facilitate exocytosis (Chapman 2008, Pang & Sudhof 2010) and this PS-Syt1 interaction may be involved in the formation of a fusion pore, the last step during exocytosis (Bhalla et al. 2005, Zhang et al. 2009). Therefore, our study points out a functional similarity of the PS-Syt1 interaction in exocytosis and endocytosis, which is consistent with Ca2+-sensing role of Syt1 in both exocytosis (Chapman 2008, Pang & Sudhof 2010) and endocytosis (Yao et al. 2011b) of synaptic vesicles in neurons.
Vesicle fission is a step for the central role of dynamin as the mechano-chemical enzyme in CME (Doherty & McMahon 2009). It is proposed that dynamin functions in vesicle fission by twisting the tubular neck of the endocytic vesicles in the rotatory movement (Roux et al. 2006). More importantly, the constricting efficiency of dynamin in vesicle fission is dependent on the longitudinal tension of the tubular neck of the endocytic vesicle (Roux et al. 2006). Here, our study indicates that PS-Syt1 interaction is critical for vesicle fission during CME. Therefore, it is speculated that the trans binding of Syt1 C2 domains, to the PS-enriched plasma membrane, as proposed during exocytosis (Chapman 2008, Pang & Sudhof 2010), could potentially enhance the longitudinal tension of the tubular neck of the endocytic vesicle, which will in turn facilitate the function of dynamin in vesicle fission.
Finally, PS, a key negative charged phospholipid present in both presynaptic membrane and vesicles, has been extensively studied for its involvements in neurological disorders (Bozek et al. 2015, Takamori et al. 2006, Bader Lange et al. 2008, Naftelberg et al. 2016, Bennett et al. 2013). Our results highlight a significance of PS in vesicle fission during CME in chromaffin cells, which implies a potential involvement of PS in synaptic vesicle endocytosis, since there are many similar aspects in molecular mechanisms of vesicle exocytosis and endocytosis between neuroendocrine cells and neurons (Murthy & De Camilli 2003, Neher 2018). Interestingly, many neurological diseases are tightly linked to abnormalities in endocytosis, particularly CME (Keating et al. 2006, Wu & Yao 2009, Schreij et al. 2016). Our work may thus provide a new insight into pathological mechanisms of PS-related neurological diseases.
Supplementary Material
Acknowledgments:
This work is supported by NIH (R01NS110533) to L.-W.G. Syt16DA and Syt1D238N mutants were kindly provided by Dr. Z.P. Pang at Robert Wood Johnson Medical School.
Abbreviations:
- BoNT/A
Botulinum neurotoxin type A
- CME
clathrin-mediated endocytosis
- Cv
capacitance size
- FITC
Fluorescein isothiocyanate
- Gp
fission-pore conductance
- HT
heterozygous
- Im
patch capacitance
- KO
knockout
- NBD
1,2-dioleoyl-sn-glycero-3-phospho-L-serine-N-(7-nitro-2–1,3-benzoxadiazol-4-yl
- PBS
phosphate-buffered saline
- PC
phosphatidylcholine
- PE
phoshatidylethanolamine
- PI(4,5)P2
phosphatidylinositol; 4,5-bisphosphate
- PS
phosphatidylserine
- PtdIns
phosphoinositides
- Re
patch conductance
- Syt1
synaptotagmin 1
- WT
wildtype
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
References
- Abe N, Inoue T, Galvez T, Klein L and Meyer T (2008) Dissecting the role of PtdIns(4,5)P2 in endocytosis and recycling of the transferrin receptor. Journal of cell science, 121, 1488–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonny B, Burd C, De Camilli P et al. (2016) Membrane fission by dynamin: what we know and what we need to know. The EMBO journal, 35, 2270–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader Lange ML, Cenini G, Piroddi M, Abdul HM, Sultana R, Galli F, Memo M and Butterfield DA (2008) Loss of phospholipid asymmetry and elevated brain apoptotic protein levels in subjects with amnestic mild cognitive impairment and Alzheimer disease. Neurobiol Dis, 29, 456–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello OD, Jouannot O, Chaudhuri A, Stroeva E, Coleman J, Volynski KE, Rothman JE and Krishnakumar SS (2018) Synaptotagmin oligomerization is essential for calcium control of regulated exocytosis. Proc Natl Acad Sci U S A, 115, E7624–E7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett SA, Valenzuela N, Xu H, Franko B, Fai S and Figeys D (2013) Using neurolipidomics to identify phospholipid mediators of synaptic (dys)function in Alzheimer’s Disease. Front Physiol, 4, 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla A, Tucker WC and Chapman ER (2005) Synaptotagmin isoforms couple distinct ranges of Ca2+, Ba2+, and Sr2+ concentration to SNARE-mediated membrane fusion. Molecular biology of the cell, 16, 4755–4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohdanowicz M and Grinstein S (2013) Role of phospholipids in endocytosis, phagocytosis, and macropinocytosis. Physiol Rev, 93, 69–106. [DOI] [PubMed] [Google Scholar]
- Bozek K, Wei Y, Yan Z et al. (2015) Organization and evolution of brain lipidome revealed by large-scale analysis of human, chimpanzee, macaque, and mouse tissues. Neuron, 85, 695–702. [DOI] [PubMed] [Google Scholar]
- Brouwer I, Giniatullina A, Laurens N, van Weering JR, Bald D, Wuite GJ and Groffen AJ (2015) Direct quantitative detection of Doc2b-induced hemifusion in optically trapped membranes. Nat Commun, 6, 8387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae YK, Abildgaard F, Chapman ER and Markley JL (1998) Lipid binding ridge on loops 2 and 3 of the C2A domain of synaptotagmin I as revealed by NMR spectroscopy. The Journal of biological chemistry, 273, 25659–25663. [DOI] [PubMed] [Google Scholar]
- Chapman ER (2002) Synaptotagmin: a Ca(2+) sensor that triggers exocytosis? Nat Rev Mol Cell Biol, 3, 498–508. [DOI] [PubMed] [Google Scholar]
- Chapman ER (2008) How does synaptotagmin trigger neurotransmitter release? Annual review of biochemistry, 77, 615–641. [DOI] [PubMed] [Google Scholar]
- Chapman ER and Davis AF (1998) Direct interaction of a Ca2+-binding loop of synaptotagmin with lipid bilayers. The Journal of biological chemistry, 273, 13995–14001. [DOI] [PubMed] [Google Scholar]
- Chow RH, von Ruden L and Neher E (1992) Delay in vesicle fusion revealed by electrochemical monitoring of single secretory events in adrenal chromaffin cells. Nature, 356, 60–63. [DOI] [PubMed] [Google Scholar]
- Courtney NA, Briguglio JS, Bradberry MM, Greer C and Chapman ER (2018) Excitatory and Inhibitory Neurons Utilize Different Ca(2+) Sensors and Sources to Regulate Spontaneous Release. Neuron, 98, 977–991 e975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Slaughter BD, Unruh JR, Bradford WD, Alexander R, Rubinstein B and Li R (2012) Flippase-mediated phospholipid asymmetry promotes fast Cdc42 recycling in dynamic maintenance of cell polarity. Nature cell biology, 14, 304–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debus K and Lindau M (2000) Resolution of patch capacitance recordings and of fusion pore conductances in small vesicles. Biophysical journal, 78, 2983–2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo G and De Camilli P (2006) Phosphoinositides in cell regulation and membrane dynamics. Nature, 443, 651–657. [DOI] [PubMed] [Google Scholar]
- Doherty GJ and McMahon HT (2009) Mechanisms of endocytosis. Annual review of biochemistry, 78, 857–902. [DOI] [PubMed] [Google Scholar]
- Dolis D, Moreau C, Zachowski A and Devaux PF (1997) Aminophospholipid translocase and proteins involved in transmembrane phospholipid traffic. Biophys Chem, 68, 221–231. [DOI] [PubMed] [Google Scholar]
- Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL and Henson PM (1992) Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. Journal of immunology, 148, 2207–2216. [PubMed] [Google Scholar]
- Fairn GD, Hermansson M, Somerharju P and Grinstein S (2011) Phosphatidylserine is polarized and required for proper Cdc42 localization and for development of cell polarity. Nature cell biology, 13, 1424–1430. [DOI] [PubMed] [Google Scholar]
- Fang Q, Berberian K, Gong LW, Hafez I, Sorensen JB and Lindau M (2008) The role of the C terminus of the SNARE protein SNAP-25 in fusion pore opening and a model for fusion pore mechanics. Proceedings of the National Academy of Sciences of the United States of America, 105, 15388–15392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farge E (1995) Increased vesicle endocytosis due to an increase in the plasma membrane phosphatidylserine concentration. Biophys J, 69, 2501–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez I, Arac D, Ubach J, Gerber SH, Shin O, Gao Y, Anderson RG, Sudhof TC and Rizo J (2001) Three-dimensional structure of the synaptotagmin 1 C2B-domain: synaptotagmin 1 as a phospholipid binding machine. Neuron, 32, 1057–1069. [DOI] [PubMed] [Google Scholar]
- Fernandez-Chacon R, Konigstorfer A, Gerber SH et al. (2001) Synaptotagmin I functions as a calcium regulator of release probability. Nature, 410, 41–49. [DOI] [PubMed] [Google Scholar]
- Flores JA, Balseiro-Gomez S, Cabeza JM, Acosta J, Ramirez-Ponce P and Ales E (2014) A new role for myosin II in vesicle fission. PLoS One, 9, e100757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong LW, de Toledo GA and Lindau M (2007) Exocytotic catecholamine release is not associated with cation flux through channels in the vesicle membrane but Na+ influx through the fusion pore. Nature cell biology, 9, 915–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong LW, Di Paolo G, Diaz E, Cestra G, Diaz ME, Lindau M, De Camilli P and Toomre D (2005) Phosphatidylinositol phosphate kinase type I gamma regulates dynamics of large dense-core vesicle fusion. Proceedings of the National Academy of Sciences of the United States of America, 102, 5204–5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham TR (2004) Flippases and vesicle-mediated protein transport. Trends Cell Biol, 14, 670–677. [DOI] [PubMed] [Google Scholar]
- Haucke V, Neher E and Sigrist SJ (2011) Protein scaffolds in the coupling of synaptic exocytosis and endocytosis. Nature reviews. Neuroscience, 12, 127–138. [DOI] [PubMed] [Google Scholar]
- Hinshaw JE (2000) Dynamin and its role in membrane fission. Annual review of cell and developmental biology, 16, 483–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirama T, Lu SM, Kay JG, Maekawa M, Kozlov MM, Grinstein S and Fairn GD (2017) Membrane curvature induced by proximity of anionic phospholipids can initiate endocytosis. Nat Commun, 8, 1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohendahl A, Talledge N, Galli V, Shen PS, Humbert F, De Camilli P, Frost A and Roux A (2017) Structural inhibition of dynamin-mediated membrane fission by endophilin. Elife, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T and De Camilli P (2006) BAR, F-BAR (EFC) and ENTH/ANTH domains in the regulation of membrane-cytosol interfaces and membrane curvature. Biochimica et biophysica acta, 1761, 897–912. [DOI] [PubMed] [Google Scholar]
- Itoh T, Erdmann KS, Roux A, Habermann B, Werner H and De Camilli P (2005) Dynamin and the actin cytoskeleton cooperatively regulate plasma membrane invagination by BAR and F-BAR proteins. Developmental cell, 9, 791–804. [DOI] [PubMed] [Google Scholar]
- Janko C, Jeremic I, Biermann M, Chaurio R, Schorn C, Munoz LE and Herrmann M (2013) Cooperative binding of Annexin A5 to phosphatidylserine on apoptotic cell membranes. Physical biology, 10, 065006. [DOI] [PubMed] [Google Scholar]
- Jiang ZJ, Delaney TL, Zanin MP et al. (2019) Extracellular and intracellular sphingosine-1-phosphate distinctly regulates exocytosis in chromaffin cells. Journal of neurochemistry, 149, 729–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating DJ, Chen C and Pritchard MA (2006) Alzheimer’s disease and endocytic dysfunction: clues from the Down syndrome-related proteins, DSCR1 and ITSN1. Ageing Res Rev, 5, 388–401. [DOI] [PubMed] [Google Scholar]
- Kim J and Shin W (2014) How to do random allocation (randomization). Clinics in orthopedic surgery, 6, 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kononenko NL and Haucke V (2015) Molecular mechanisms of presynaptic membrane retrieval and synaptic vesicle reformation. Neuron, 85, 484–496. [DOI] [PubMed] [Google Scholar]
- Lauwers E, Goodchild R and Verstreken P (2016) Membrane Lipids in Presynaptic Function and Disease. Neuron, 90, 11–25. [DOI] [PubMed] [Google Scholar]
- Leventis PA and Grinstein S (2010) The distribution and function of phosphatidylserine in cellular membranes. Annual review of biophysics, 39, 407–427. [DOI] [PubMed] [Google Scholar]
- Li YC, Chanaday NL, Xu W and Kavalali ET (2017) Synaptotagmin-1- and Synaptotagmin-7-Dependent Fusion Mechanisms Target Synaptic Vesicles to Kinetically Distinct Endocytic Pathways. Neuron, 93, 616–631 e613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manni MM, Tiberti ML, Pagnotta S, Barelli H, Gautier R and Antonny B (2018) Acyl chain asymmetry and polyunsaturation of brain phospholipids facilitate membrane vesiculation without leakage. Elife, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Molina A, Rodriguez-Beas C and Faraudo J (2012) Effect of calcium and magnesium on phosphatidylserine membranes: experiments and all-atomic simulations. Biophys J, 102, 2095–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdam RL, Varga KT, Jiang Z, Young FB, Blandford V, McPherson PS, Gong LW and Sossin WS (2015) The juxtamembrane region of synaptotagmin 1 interacts with dynamin 1 and regulates vesicle fission during compensatory endocytosis in endocrine cells. Journal of cell science, 128, 2229–2235. [DOI] [PubMed] [Google Scholar]
- McIntyre JC and Sleight RG (1991) Fluorescence assay for phospholipid membrane asymmetry. Biochemistry, 30, 11819–11827. [DOI] [PubMed] [Google Scholar]
- Milosevic I, Giovedi S, Lou X et al. (2011) Recruitment of endophilin to clathrin-coated pit necks is required for efficient vesicle uncoating after fission. Neuron, 72, 587–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghadam PK and Jackson MB (2013) The functional significance of synaptotagmin diversity in neuroendocrine secretion. Frontiers in endocrinology, 4, 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaville P, Neumann JM, Russo-Marie F, Ochsenbein F and Sanson A (2002) A new consensus sequence for phosphatidylserine recognition by annexins. The Journal of biological chemistry, 277, 24684–24693. [DOI] [PubMed] [Google Scholar]
- Mosharov EV and Sulzer D (2005) Analysis of exocytotic events recorded by amperometry. Nature methods, 2, 651–658. [DOI] [PubMed] [Google Scholar]
- Murthy VN and De Camilli P (2003) Cell biology of the presynaptic terminal. Annual review of neuroscience, 26, 701–728. [DOI] [PubMed] [Google Scholar]
- Naftelberg S, Abramovitch Z, Gluska S et al. (2016) Phosphatidylserine Ameliorates Neurodegenerative Symptoms and Enhances Axonal Transport in a Mouse Model of Familial Dysautonomia. PLoS Genet, 12, e1006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E (2018) Neurosecretion: what can we learn from chromaffin cells. Pflugers Archiv : European journal of physiology, 470, 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi S and Ito T (1974) Calcium-induced phase separations in phosphatidylserine--phosphatidylcholine membranes. Biochemistry, 13, 881–887. [DOI] [PubMed] [Google Scholar]
- Ory S, Ceridono M, Momboisse F et al. (2013) Phospholipid scramblase-1-induced lipid reorganization regulates compensatory endocytosis in neuroendocrine cells. The Journal of neuroscience : the official journal of the Society for Neuroscience, 33, 3545–3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang ZP and Sudhof TC (2010) Cell biology of Ca2+-triggered exocytosis. Current opinion in cell biology, 22, 496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannuzzo M, McDargh ZA and Deserno M (2018) The role of scaffold reshaping and disassembly in dynamin driven membrane fission. Elife, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter BJ, Kent HM, Mills IG, Vallis Y, Butler PJ, Evans PR and McMahon HT (2004) BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science, 303, 495–499. [DOI] [PubMed] [Google Scholar]
- Pinot M, Vanni S, Pagnotta S et al. (2014) Lipid cell biology. Polyunsaturated phospholipids facilitate membrane deformation and fission by endocytic proteins. Science, 345, 693–697. [DOI] [PubMed] [Google Scholar]
- Pirazzini M, Rossetto O, Eleopra R and Montecucco C (2017) Botulinum Neurotoxins: Biology, Pharmacology, and Toxicology. Pharmacological reviews, 69, 200–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghupathy R, Anilkumar AA, Polley A et al. (2015) Transbilayer lipid interactions mediate nanoclustering of lipid-anchored proteins. Cell, 161, 581–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao TC, Passmore DR, Peleman AR, Das M, Chapman ER and Anantharam A (2014) Distinct fusion properties of synaptotagmin-1 and synaptotagmin-7 bearing dense core granules. Molecular biology of the cell, 25, 2416–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen RK, Rusak J, Price G, Robinson PJ, Simpson RJ and Dorow DS (1998) Mixed-lineage kinase 2-SH3 domain binds dynamin and greatly enhances activation of GTPase by phospholipid. The Biochemical journal, 335 ( Pt 1), 119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee JS, Li LY, Shin OH, Rah JC, Rizo J, Sudhof TC and Rosenmund C (2005) Augmenting neurotransmitter release by enhancing the apparent Ca2+ affinity of synaptotagmin 1. Proceedings of the National Academy of Sciences of the United States of America, 102, 18664–18669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux A, Uyhazi K, Frost A and De Camilli P (2006) GTP-dependent twisting of dynamin implicates constriction and tension in membrane fission. Nature, 441, 528–531. [DOI] [PubMed] [Google Scholar]
- Roux M and Bloom M (1991) Calcium binding by phosphatidylserine headgroups. Deuterium NMR study. Biophys J, 60, 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonn JS, Maximov A, Lao Y, Sudhof TC and Sorensen JB (2008) Synaptotagmin-1 and −7 are functionally overlapping Ca2+ sensors for exocytosis in adrenal chromaffin cells. Proceedings of the National Academy of Sciences of the United States of America, 105, 3998–4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreij AM, Fon EA and McPherson PS (2016) Endocytic membrane trafficking and neurodegenerative disease. Cellular and molecular life sciences : CMLS, 73, 1529–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sezgin E, Levental I, Mayor S and Eggeling C (2017) The mystery of membrane organization: composition, regulation and roles of lipid rafts. Nat Rev Mol Cell Biol, 18, 361–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinn CG, Antonietti M and Dimova R (2006) Binding of calcium to phosphatidylcholine-phosphatidylserine membranes. Colloid Surface A, 282, 410–419. [Google Scholar]
- Slepnev VI and De Camilli P (2000) Accessory factors in clathrin-dependent synaptic vesicle endocytosis. Nature reviews. Neuroscience, 1, 161–172. [DOI] [PubMed] [Google Scholar]
- Sollner TH (2003) Regulated exocytosis and SNARE function (Review). Molecular membrane biology, 20, 209–220. [DOI] [PubMed] [Google Scholar]
- Sudhof TC (2012) Calcium control of neurotransmitter release. Cold Spring Harbor perspectives in biology, 4, a011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y and Drubin DG (2012) The functions of anionic phospholipids during clathrin-mediated endocytosis site initiation and vesicle formation. Journal of cell science, 125, 6157–6165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweitzer SM and Hinshaw JE (1998) Dynamin undergoes a GTP-dependent conformational change causing vesiculation. Cell, 93, 1021–1029. [DOI] [PubMed] [Google Scholar]
- Takamori S, Holt M, Stenius K et al. (2006) Molecular anatomy of a trafficking organelle. Cell, 127, 831–846. [DOI] [PubMed] [Google Scholar]
- Takatsu H, Takayama M, Naito T, Takada N, Tsumagari K, Ishihama Y, Nakayama K and Shin HW (2017) Phospholipid flippase ATP11C is endocytosed and downregulated following Ca(2+)-mediated protein kinase C activation. Nat Commun, 8, 1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truckenbrodt S, Viplav A, Jahne S, Vogts A, Denker A, Wildhagen H, Fornasiero EF and Rizzoli SO (2018) Newly produced synaptic vesicle proteins are preferentially used in synaptic transmission. EMBO J, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida Y, Hasegawa J, Chinnapen D et al. (2011) Intracellular phosphatidylserine is essential for retrograde membrane traffic through endosomes. Proc Natl Acad Sci U S A, 108, 15846–15851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama Y, Maxson MM, Sawada T, Nakano A and Ewing AG (2007) Phospholipid mediated plasticity in exocytosis observed in PC12 cells. Brain research, 1151, 46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Engeland M, Nieland LJ, Ramaekers FC, Schutte B and Reutelingsperger CP (1998) Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry, 31, 1–9. [DOI] [PubMed] [Google Scholar]
- Varga KT, Jiang Z and Gong LW (2014) Methods for cell-attached capacitance measurements in mouse adrenal chromaffin cell. J Vis Exp, e52024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennekate W, Schroder S, Lin CC, van den Bogaart G, Grunwald M, Jahn R and Walla PJ (2012) Cis- and trans-membrane interactions of synaptotagmin-1. Proc Natl Acad Sci U S A, 109, 11037–11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Li Y and Ma C (2016) Synaptotagmin-1 C2B domain interacts simultaneously with SNAREs and membranes to promote membrane fusion. Elife, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D, Vicogne J, Zaitseva I, McLaughlin S and Pessin JE (2009) Evidence that electrostatic interactions between vesicle-associated membrane protein 2 and acidic phospholipids may modulate the fusion of transport vesicles with the plasma membrane. Mol Biol Cell, 20, 4910–4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F and Yao PJ (2009) Clathrin-mediated endocytosis and Alzheimer’s disease: an update. Ageing Res Rev, 8, 147–149. [DOI] [PubMed] [Google Scholar]
- Wu W and Wu LG (2007) Rapid bulk endocytosis and its kinetics of fission pore closure at a central synapse. Proceedings of the National Academy of Sciences of the United States of America, 104, 10234–10239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XS and Wu LG (2014) The yin and yang of calcium effects on synaptic vesicle endocytosis. The Journal of neuroscience : the official journal of the Society for Neuroscience, 34, 2652–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Pang ZP, Shin OH and Sudhof TC (2009) Synaptotagmin-1 functions as a Ca2+ sensor for spontaneous release. Nature neuroscience, 12, 759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Baldridge RD, Chi RJ, Burd CG and Graham TR (2013) Phosphatidylserine flipping enhances membrane curvature and negative charge required for vesicular transport. The Journal of cell biology, 202, 875–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao CK, Liu YT, Lee IC, Wang YT and Wu PY (2017) A Ca2+ channel differentially regulates Clathrin-mediated and activity-dependent bulk endocytosis. PLoS biology, 15, e2000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Gaffaney JD, Kwon SE and Chapman ER (2011a) Doc2 is a Ca2+ sensor required for asynchronous neurotransmitter release. Cell, 147, 666–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Kwon SE, Gaffaney JD, Dunning FM and Chapman ER (2011b) Uncoupling the roles of synaptotagmin I during endo- and exocytosis of synaptic vesicles. Nature neuroscience, 15, 243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao LH, Rao Y, Bang C et al. (2013) Actin Polymerization Does Not Provide Direct Mechanical Forces for Vesicle Fission during Clathrin-Mediated Endocytosis. The Journal of neuroscience : the official journal of the Society for Neuroscience, 33, 15793–15798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao LH, Rao Y, Varga K, Wang CY, Xiao P, Lindau M and Gong LW (2012) Synaptotagmin 1 is necessary for the Ca2+ dependence of clathrin-mediated endocytosis. The Journal of neuroscience : the official journal of the Society for Neuroscience, 32, 3778–3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung T, Gilbert GE, Shi J, Silvius J, Kapus A and Grinstein S (2008) Membrane phosphatidylserine regulates surface charge and protein localization. Science, 319, 210–213. [DOI] [PubMed] [Google Scholar]
- Yeung T, Heit B, Dubuisson JF, Fairn GD, Chiu B, Inman R, Kapus A, Swanson M and Grinstein S (2009) Contribution of phosphatidylserine to membrane surface charge and protein targeting during phagosome maturation. J Cell Biol, 185, 917–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha X, Genest J Jr. and McPherson R (2001) Endocytosis is enhanced in Tangier fibroblasts: possible role of ATP-binding cassette protein A1 in endosomal vesicular transport. The Journal of biological chemistry, 276, 39476–39483. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Hui E, Chapman ER and Jackson MB (2009) Phosphatidylserine regulation of Ca2+-triggered exocytosis and fusion pores in PC12 cells. Mol Biol Cell, 20, 5086–5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z and Jackson MB (2010) Membrane bending energy and fusion pore kinetics in Ca(2+)-triggered exocytosis. Biophys J, 98, 2524–2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoncu R, Perera RM, Sebastian R, Nakatsu F, Chen H, Balla T, Ayala G, Toomre D and De Camilli PV (2007) Loss of endocytic clathrin-coated pits upon acute depletion of phosphatidylinositol 4,5-bisphosphate. Proceedings of the National Academy of Sciences of the United States of America, 104, 3793–3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.