Abstract
As reviewed in Part 1 of this two part review, birth prior to full term is a substantial public health issue. In the US, ~400, 000 babies per year are born preterm (< 37 weeks), while > 1 million are early term (37–386/7 weeks) and remarkable racial disparities in shortened gestation are observed among African Americans as compared to Whites. Biomechanisms linking stressor exposures with birth outcomes are increasingly being explicated. The current paper reviews the mechanistic role of maternal biological functioning in the link between behavioral exposures and birth outcomes. These include the inter-related roles of neuroendocrine function, inflammatory regulation, biological aging, and the microbiome. An integrative approach which addresses both behavioral and biological factors within the same study, carefully considers the role of race/ethnicity, and rigorously defines birth outcomes (e.g., spontaneous versus medically-indicated and inclusive of early term birth) is needed to move research in this field toward better mechanistic understanding and clinical application.
Keywords: Inflammation, Neuroendocrine, Social genomics, Transcriptomics, Microbiome, Psychoneuroimmunology, Stress, Pregnancy, Birth outcomes, Racial disparities
1. Introduction
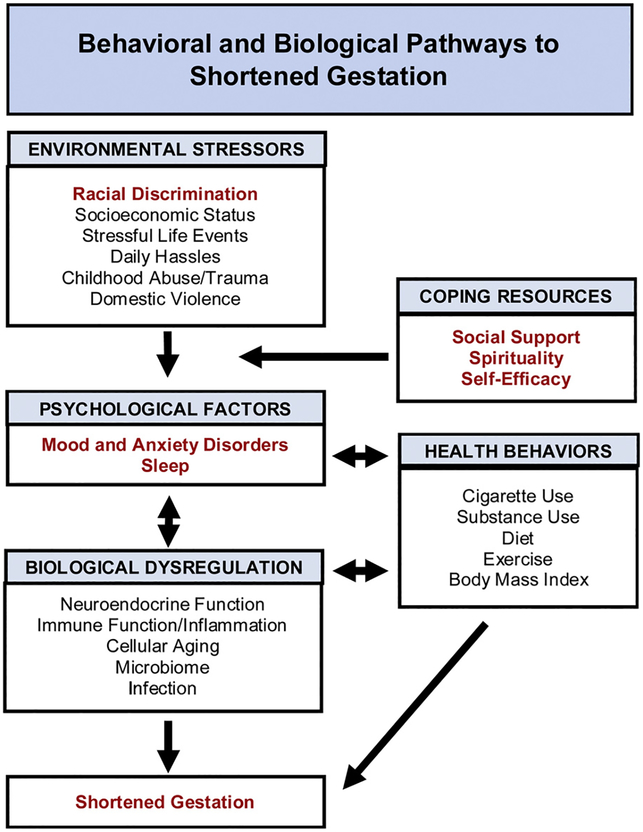
As reviewed in Part 1, there are considerable data linking depression and anxiety, and to a lesser extent sleep, with shortened gestation. However, as detailed in this companion paper, biological mechanisms require greater attention. Neuroendocrine and inflammatory mechanisms are implicated; however, there are significant gaps in these data. Moreover, enhanced inflammatory activity is commonly indicative of underlying biological aging. Although chronological age is a known risk factor for shortened gestation (Heffner, 2004; Laopaiboon et al., 2014), limited data are available regarding the role of individual differences in biological aging. In addition, increasing data demonstrates the value of social genomics for examining stress and perinatal health. Finally, data on the role of the microbiome is growing at a remarkable pace; however, studies are predominately in animal models, limiting ability to address issues relevant to exposure to stressors such as racial discrimination. Key focal points of this review are highlighted in Fig. 1.
Fig. 1.
Behavioral and Biological Pathways to Shortened Gestation. Racial discrimination and other objective stressors are psychosocial exposures that increase the risk for shortened gestation. These are not necessarily clinically modifiable at the individual level. However, as reviewed herein, biological sequelae (neuroendocrine function, immune function, cellular aging, microbiome) present promising targets for identifying risk and targeting intervention. These may directly impact delivery length, contributing to spontaneous occurrence of shortened gestation. In addition, these pathways can contribute to medically-indicated shortened gestation by increasing risk for clinical conditions including pre-eclampsia and gestational diabetes. Pathways bolded in red print are emphasized in the current review.
2. Neuroendocrine regulation
During pregnancy, the functioning of the hypothalamic-pituitary-adrenal (HPA) axis is substantially altered and neuroendocrine mediators including corticotrophin releasing hormone (CRH), cortisol, and brain derived neurotrophic factor (BDNF) are implicated in fetal growth and delivery timing. Critical to these relationships, the placenta becomes an active organ within the HPA axis during gestation. During pregnancy, the placenta synthesizes the vast majority of CRH found in the maternal bloodstream, resulting in CRH concentrations 1,000–10,000 times higher than those seen in non-pregnant women (Thomson, 2013). A primary function of placental CRH is to regulate and stimulate the development of the fetal HPA axis (Mastorakos and Ilias, 2003). With predictive value for birth timing, CRH has been dubbed a “placental clock” (Mclean et al., 1995; Sandman et al., 2006). CRH has repeatedly been linked with perinatal stress, particularly pregnancy-specific anxiety. For example, in a prospective study of 337 pregnant Latina and non-Latina White women, pregnancy anxiety was predictive of CRH in late pregnancy as well as increases in CRH over time. Notably, in predicting length of gestation, moderating mediation effects were observed whereby pregnancy anxiety contributed to shorter gestation via CRH only among Latina women (Ramos et al., 2018). The importance of considering race/ethnicity is highlighted by other research showing that the association between CRH and length of gestation is race-specific when examining African American versus White women, because, overall African American women tend to exhibit lower CRH than Whites (Chen et al., 2010; Holzman et al., 2001).
Driven by placental production of CRH, which stimulates both the pituitary and adrenal glands, maternal cortisol increases by 2 to 3-fold by late gestation (Duthie and Reynolds, 2013; Mastorakos and Ilias, 2000). Increasing maternal cortisol further stimulates CRH production, in a feed forward loop. Although considerable heterogeneity has been observed across studies, attributable to differences in sampling time-point, maternal characteristics, and other factors, a meta-analysis of nine studies including a total of 1606 maternal-fetal dyads concluded that elevated maternal cortisol during pregnancy is consistently negatively correlated with birth weight (Cherak et al., 2018).
In terms of psychological factors, numerous studies have linked cortisol with stress, anxiety, and mood in pregnancy (e.g., Evans et al., 2008; Kane et al., 2014; Pluess et al., 2010). In particular, higher pregnancy anxiety has been associated with steeper increases in cortisol trajectories across pregnancy (Kane et al., 2014). Building upon these findings, we found that higher cortisol observed in women pregnant for the first time (primiparous) versus those who have previously given birth (multiparous) during pregnancy is partially mediated by higher pregnancy-specific anxiety among the primiparous women (Gillespie et al., 2018). In addition, among African American women, exposure to childhood stress predicted shorter gestation, an effect partially mediated by elevations in maternal cortisol (Gillespie et al., 2017). Overall, the literature on psychosocial factors and maternal cortisol regulation is characterized by wide variability in measurement approaches, including a range of psychological assessment instruments and approaches to cortisol assessment including via serum, saliva, and hair levels and with focus on a single assessment versus diurnal slopes or cortisol awakening responses. However, despite inconsistencies (including reports of blunting versus heightened effects) and null findings, evidence suggests that maternal psychological functioning is associated with neuroendocrine adaptation in pregnancy (Entringer et al., 2015; Glover et al., 2010). Moreover, data indicate that psychological factors alter the regulation of 11 beta-hydroxysteroid dehydrogenase 2 (11b-HSD2), the enzyme that metabolizes cortisol and largely determines the degree to which maternal cortisol is transferred to the fetus (Glover et al., 2009). Therefore, there exists more than one pathway via which neuroendocrine disruption may ultimately impact birth outcomes and fetal development.
Of methodological and clinical importance, our data demonstrate that race is associated with differential cortisol adaptation in pregnancy. We found that, compared to demographically similar White women, African American women exhibited less robust increases in serum cortisol in the 2nd and 3rd trimesters of pregnancy (Christian et al., 2016). These findings correspond with those of Glynn et al. (2007), who reported lower cortisol among African American women in late pregnancy as compared to Whites, as well as higher levels of adrenocorticotrophic hormone (ACTH) and lower CRH.
Differential cortisol adaptation by race may directly affect fetal development and birth outcomes, and also exert indirect effects, via related processes including production of brain derived neurotrophic factor (BDNF) - a neurotrophin involved not only in fetal brain development, but also in placental function. In correspondence with racial differences in cortisol adaptation across pregnancy, we observed associations between higher cortisol levels and lower serum levels of BDNF (Christian et al., 2016). Further, lower serum BDNF in late pregnancy predicted risk for delivering a low birth weight baby. While this effect was observed in both African Americans and Whites, a moderating effect of race was observed, whereby the prediction line among African Americans was shifted upward due to paradoxically higher BDNF in this group. These data speak to the complex interplay amongst neuroendocrine mediators – the functioning of a given biomarker is dependent on the overall milieu in which it exerts its effects. Altogether, data on CRH, cortisol, and BNDF highlight the importance of considering moderating effects of race and ethnicity.
3. Inflammation
Intricately linked with HPA functioning, the maternal immune system changes substantially in parallel to support fetal development. Localized inflammatory responses at the site of implantation appear essential to successful pregnancy (Mor et al., 2011). Moreover, systemically, elevations in some serum inflammatory markers (e.g., IL-6, TNF-α) are observed across the course of pregnancy (e.g., Christian and Porter, 2014). However, excessive inflammation is incompatible with healthy pregnancy; preterm compared to term delivery has repeatedly been linked with elevations in proinflammatory cytokines in maternal serum and amniotic fluid in the context of infection as well as idiopathic cases (Cappelletti et al., 2016; Muglia and Katz, 2010). Inflammation can promote early delivery by triggering contractions, cervical ripening, and rupture of the membranes.
Relationships between psychological functioning (i.e., depression, anxiety), and inflammation are seen in adults across the life span (Kiecolt-Glaser et al., 2015; Segerstrom and Miller, 2004; Toker et al., 2005). Accumulating evidence from our group and others show that, despite substantial immune changes, effects of anxiety and depression on inflammation are observed in pregnancy, with elevations in serum proinflammatory cytokines and exaggerated proinflammatory cytokine production among women reporting higher perceived stress, depressive symptoms, or history of trauma (for review see Christian, 2014; Osborne and Monk, 2013). For example, our data link higher depressive symptoms during pregnancy to elevations in serum IL-6 and TNF-α (Christian et al., 2009), as well as exaggerated inflammatory responses to the in vivo immune challenge of seasonal influenza virus vaccination (Christian et al., 2010). Together, studies in this area indicate that pregnant women with particular psychological risk factors may experience higher daily exposure to inflammatory mediators, as well as exaggerated responses to biological challenges.
Closely tied with mood and anxiety, effects of sleep on inflammatory processes also warrant attention. Poor sleep – as operationalized by short sleep duration (objectively and subjectively assessed) and poor subjective sleep quality – has been linked with inflammation in numerous studies throughout the lifespan (Irwin et al., 2016). Notably, the deleterious inflammatory effects of poor sleep appear to be stronger among women than men (Irwin et al., 2016). The effects of sleep on inflammation in pregnancy are only beginning to be explored (e.g., Blair et al., 2015; Okun and Coussons-Read, 2007, 2006). In a racially diverse sample of 138 pregnant women, we found that, among African American women only, poor subjective sleep quality predicted elevations in serum IL-8, which corresponded to risk for shortened gestation (Blair et al., 2015). Importantly, in this cohort, no significant differences in sleep quality were observed by race. These data suggest that, in the context of similar exposure to poor sleep, African American women have heightened vulnerability to experience adverse physiological sequelae. Subsequent data from our lab confirmed a similar effect in a different cohort assessed at early postpartum. In this study of 69 women (32 African American, 37 White), poorer subjective sleep quality predicted greater ex vivo LPS-stimulated production of both IL-6 and IL-8 by PBMCs among African American women, but not Whites (Christian et al., 2018). Coupled with greater overall exposure to sleep disturbances and short sleep, racial differences in susceptibility to sleep-induced immune dysregulation may contribute to marked racial disparities in perinatal health outcomes in the US.
Of relevance in the context of inflammation, infections including bacterial vaginosis and chorioamniotitis contribute considerably to risk for spontaneous preterm birth (Cappelletti et al., 2016; Goldenberg et al., 2008, 2000). While infection is rarely a causal factor in births occurring at > = 34 weeks, it does contribute to most cases of birth at < 30 weeks (Goldenberg et al., 2000). These types of infections are more common among African Americans than Whites. Psychosocial stress has been associated with increased risk for bacterial vaginosis during pregnancy (Nansel et al., 2006). As described earlier, stress is also associated with exaggerated inflammatory responses to biological challenges (Christian et al., 2010; Coussons-Read et al., 2007). Thus, racial differences in stressor exposure may contribute to both occurrence and response to infection during pregnancy.
Across studies of psychological factors, contrasting results are seen with regard to which specific constructs (e.g., perceived stress, depression, trauma) are related to inflammation. Differing results may be attributable, in part, to differences in study samples including race and socioeconomic background. In addition, other exposures, such as obesity (Cassidy-Bushrow et al., 2012) and sleep disturbance (Okun et al., 2013) may serve as moderators which can obscure or heighten observed effects. Thus, while literature in this area is growing, understanding is far from complete. As in the literature linking psychological factors to birth outcomes, literature in relation to inflammatory regulation lacks a focus on clinical diagnoses (e.g., clinical depression, insomnia) and objective assessment of sleep per actigraphy. In addition, given the potential for confounding as well as moderating effects, simultaneous assessment of psychological functioning and sleep health would greatly forward understanding.
Finally, in terms of clinical applicability, the ultimate physical health implications of stress and sleep-related inflammatory dysregulation in pregnancy remain largely unknown. One study has linked maternal anxiety with shortened gestation via inflammatory mechanisms; among 173 women (67% Hispanic, 14% White, 11% African American), the association between anxiety and gestational age at birth was mediated by serum IL-6 & TNF-α (Coussons-Read et al., 2012). As described earlier, our data from 132 pregnant women (79 African American) demonstrated that elevations in serum IL-8 mediated the relationship between poor subjective sleep quality and shortened gestation in African American women (Blair et al., 2015). Altogether, data on inflammation as a mediator linking psychological health or sleep with perinatal health outcomes is very limited.
4. Cellular aging
Enhanced inflammatory activity often corresponds with underlying cellular aging. The risk for adverse perinatal health outcomes increases with maternal chronological age. Importantly from a prediction and clinical intervention standpoint, the role of biological indicators of aging is not fully delineated, even though women of the same chronological age exhibit variability in cellular indicators of aging (Müezzinler et al., 2013). Psychological stress and sleep disturbance may increase risk for adverse perinatal health outcomes in part by promoting biological aging.
The length of telomeres, DNA-protein complexes located at the end of chromosomes, is a critical indicator of biological aging. Likened to the aglets – plastic protective tips – on shoelaces that prevent unraveling, telomeres play a crucial role in the protection of genetic stability (Blackburn, 2005). Telomeres shorten every time that a cell replicates. When shortened to a critical length, genomic instability occurs, along with cessation of cellular replication and cell senescence - the end state of the cell when it no longer divides (Campisi and di Fagagna, 2007). Senescent cells promote further cellular aging via the release of inflammatory mediators and stimulating inflammatory processes (Freund et al., 2010; O’Donovan et al., 2011).
As a sensitive indicator of cellular aging, shorter telomere length has predictive value in relation to numerous age-related health outcomes, including myocardial infarction, stroke, cancer, and all-cause mortality (D’Mello et al., 2015; Marioni et al., 2016; Rode et al., 2015). Importantly, there is a considerable literature indicating that the placenta has a finite lifespan. In pregnant women, placental senescence as indicated by shorter telomere length and other indicators of biological aging in placental cells has been linked with preeclampsia, intrauterine growth restriction, and obstetric complications including increased risk for fetal death at late term (Biron-Shental et al., 2016; Cox and Redman, 2017; Davy et al., 2009; Maiti et al., 2017; Sultana et al., 2018; Toutain et al., 2013). Moreover, racial differences in placental aging have been implicated in racial disparities in birth outcomes (Jones et al., 2017). From a clinical application standpoint, placental measures of biological aging such as telomere length can only be obtained invasively, with considerable risk, or after delivery.
Data on telomere length in maternal cells (e.g., circulating leukocytes) in relation to perinatal health outcomes is relatively limited. However, available data suggest that shorter maternal leukocyte telomere length is observed in gestational diabetes, recurrent miscarriages, and idiopathic pregnancy loss (Hanna et al., 2009; Harville et al., 2010) suggesting accelerated reproductive aging may drive pregnancy complications. For example, in a case-control study, women with history of recurrent miscarriage (n = 95) had shorter age-adjusted telomeres than women from the general population or those with healthy pregnancy after 37 years of age (Hanna et al., 2009). Overall, this is an untapped area which presents a clear potential path to clinical application.
In relation to psychological health, peripheral blood telomere length has been examined in relation to depression, anxiety, and other types of exposure to subjective and objective stress in numerous studies (Adler et al., 2013; Carroll et al., 2013a; Carroll et al., 2013b; Entringer et al., 2011; Epel et al., 2004; Hanssen et al., 2017; Kiecolt-Glaser et al., 2011; Mitchell et al., 2018; Needham et al., 2015; Puterman et al., 2015; Ridout et al., 2015a, b; Schutte and Malouff, 2015; Shalev et al., 2013; Vance et al., 2018). Altogether, these data suggest the presence of effects, which are moderated by specific risk factors (e.g., age, sex, symptom severity). For example, in a population-based study of 1290 adults, shorter telomere length was observed among women with generalized anxiety disorder or panic disorder, an effect not observed in men (Needham et al., 2015). Further, shorter telomeres were observed among those with major depressive disorder, but only among those who were treated pharmacologically, suggesting effects only in the context of more severe symptomatology (Needham et al., 2015).
In addition, poor sleep quality and shorter sleep duration have been linked with shortened leukocyte telomeres in numerous studies (Carroll et al., 2016b; Cribbet et al., 2014; Jackowska et al., 2012; Prather et al., 2015, 2011). Data from our group demonstrated that early life exposure to socioeconomic adversity as well as current social support predicted differences in PBMC telomere length in women assessed during pregnancy and postpartum (Mitchell et al., 2018). However, the extent to which maternal anxiety, mood, and sleep health may affect risk for adverse birth outcomes through cellular aging remains to be determined.
Also of note, in our study of perinatal women, we observed a trend for African Americans to exhibit longer telomeres than Whites, after accounting for chronological age (p = 0.10) (Mitchell et al., 2018). This effect of race is consistent with prior research suggesting that longer telomeres are observed in African Americans (Hunt et al., 2008). This difference may be accounted for by racial differences in biological factors, particularly differential rates of replication of hematopoietic stem cells (Hunt et al., 2008). Regardless of the cause, the predictive value of telomere length for health outcomes may be moderated by race. Data from samples of sufficient size and racial diversity are needed to permit statistical power to empirically determine if race-specific associations are present. In addition, as described earlier, shortened gestation shows intergenerational transmission of risk; women who were born preterm or low birth weight have increased risk for having preterm and low birth weight babies themselves – particularly African American women (Smid et al., 2017). The potential role of prenatal programming through biological aging pathways remains to be explicated.
5. Social genomics
As evidenced by expanding data in this area, transcriptomics approaches hold promise for evaluating both inflammation and cellular aging. As compared to circulating indicators of inflammation, whole genome transcriptional analysis provides a comprehensive and unbiased assessment of the inflammatory pathways with unique advantages in mechanistic understanding at the cellular level as well as greater precision and statistical power. There is growing interest in applying transcriptomics approaches to understanding risk for mood and anxiety disorders (Redei and Mehta, 2015). Relatedly, social genomics approaches in a small but growing literature have demonstrated that psychosocial stress is linked with a specific pattern of gene expression characterized by 1) up-regulation of genes indicative of inflammation, and 2) down-regulation of genes involved in glucocorticoid activity (Cole, 2009; Cole et al., 2011). This pattern has been coined the “conserved transcriptional response to adversity” (CTRA) (Cole, 2009). Similarly, in transcriptomics studies, sleep disturbance has been linked with inflammatory and cell-stress pathways implicated in biological aging (e.g., Carroll et al., 2016a).
In relation to transcriptomics and birth timing, a reasonably large literature has developed. However, it is characterized by notable gaps. First, although spontaneous early births are both much more common and difficult to predict, a systematic review of transcriptomics in preterm birth studies found that 76% focused on medically-indicated cases, predominately preeclampsia (Eidem et al., 2015). Second, despite the remarkable racial disparity observed in early birth in the US, data on African American women are lacking. Third, data inclusive of births occurring between 37–38 weeks gestation (early term births) are limited due to the relatively recent recognition of adverse outcomes associated with this timing. Finally, the vast majority of existing transcriptomics data focus on the placenta, myometrium, or fetal membranes (Eidem et al., 2015). While such data hold great promise for identifying and addressing mechanistic pathways to early birth, they do not provide clinical utility for prediction because they cannot be examined non-invasively during pregnancy. Two published studies (lacking African Americans or behavioral measures) have examined peripheral gene expression and shortened gestation (Enquobahrie et al., 2009; Heng et al., 2016). These data suggest that pregnancies that result in preterm birth are characterized by greater proinflammatory gene expression and differences in cell cycle activity and metabolism indicative of cell stress. Moreover, emerging data link exposure to life stress with inflammatory gene expression in pregnant women (Ross et al., 2018). Further research in this area using racially diverse samples, with a focus on peripheral markers, and representation of spontaneous occurrence of shortened gestation (inclusive of early term birth) is needed to advance this line of inquiry.
A key pathway by which behavioral exposures alter gene expression is via epigenetic modifications. Of particular relevance in the context of perinatal health, such modifications are documented to underlie intergenerational transmission of adversity (Lester et al., 2018; Meaney, 2001). One of several epigenetic mechanisms controlling gene expression, DNA methylation is affected by differential environmental exposures. In mice, chronic and unpredictable maternal separation at 1–14 days increases depressive-like behaviors in adults with accompanying alterations in DNA methylation, affecting gene expression (Franklin et al., 2010). Moreover, these differences in behavior and DNA methylation are, in part, transmitted to subsequent generations (Franklin et al., 2010). Another epigenetic mechanism is altered microRNA (miRNA) expression. Animal models show that stress-induced differences in length of gestation correspond with changes in miRNA expression and these effects are transmitted to subsequent generations (Yao et al., 2014). Thus, complementary data, particularly in animal models, which provide the ability to carefully control environments and study multiple generations, is necessary to explicate the mechanisms underlying differential gene expression.
6. Microbiome
A rapidly expanding literature links the microbiome – particularly the gut microbiome – with mood and behavior. To-date, these studies have overwhelming been conducted in rodent models, and data specific to the perinatal period is limited. For example, a recent systematic review of the literature on microbiota-brain axis in perinatal mood and anxiety disorders identified only 17 peer-reviewed publications for inclusion, ten of which were conducted in mice (Rackers et al., 2018). In contrast to data on mental health, there is a more sizable literature linking the maternal microbiome, including the vaginal, gut, oral, cervical and even possibly the placental microbiome to birth outcomes and fetal development (Christian et al., 2015; Galley et al., 2014; Hyman et al., 2014b; Lamont et al., 2011; Paropkari et al., 2016; Vinturache et al., 2016). In particular, literature on the vaginal microbiome and birth outcomes is substantial and growing (Hyman et al., 2014a; Prince et al., 2014; Ravel et al., 2011; Stout et al., 2017). Racial differences in the maternal microbiome and relevance to health outcomes are also increasingly being explicated (Human Microbiome Project, C, 2012), however, these data lack integration with the larger literature on mood/anxiety as well as racial disparities in stressor exposure.
Beyond birth outcomes, our own data link temperament in toddlers (18–27 months of age) with differences in gut microbiome composition; in particular greater phylogenetic diversity is observed in association with higher maternal ratings of Surgency\Extraversion (Christian et al., 2015). Our data also link maternal obesity with differences in the gut microbiome in toddlers (Galley et al., 2014), supporting intergenerational transmission of risk. Rodent models support similar intergenerational transmission of risk in relation to behavioral factors; prenatally stressed dams exhibit differences in the gut microbiome which correspond with differences in the gut microbiome and behavior in offspring (Rackers et al., 2018). In addition, the mode of delivery (vaginal versus C-section), considerably impacts the composition of the infant microbiome across multiple body locations (Dominguez-Bello et al., 2010), with potential long-term implication for physical (e.g., obesity) and mental health. Of note, maternal depression and insomnia have been associated with increased risk for delivery by C-section.
In sum, offspring physical, cognitive, and physiological development may be affected via 1) direct effects of the maternal microbiome on the establishment of the offspring microbiome, 2) effects of the maternal microbiome on birth outcomes (birth weight, length of gestation), thereby affecting long-term child health, and 3) effects of maternal psychosocial stress, sleep, and other behavioral factors on mode of delivery – ultimately affecting the composition of the infant microbiome with corresponding health effects. The microbiome is intimately and bi-directionally linked with HPA axis functioning, autonomic nervous system, and immune system, providing compelling avenues of investigation in relation to well-developed lines of research described above. It also provides a compelling target for intervention. Thus, research in this line of inquiry will continue to expand rapidly.
7. Advancing understanding and moving toward clinical translation
Although efforts to integrate findings across the medical and psychology literatures are increasing, to-date these literatures remain largely distinct. Anxiety, depression, and sleep have received limited consideration in studies of biological pathways, and vice versa. Integration of these literatures is needed to fully inform data interpretation and clinical application, as well as provide for appropriate measurement/control of confounds and moderators, clarifying understanding. In addition, the existing literature focuses disproportionately on medically-indicated cases; spontaneous occurrence of shortened gestation is both more prevalent and unpredictable. Finally, given the remarkable racial disparities in perinatal health, and evidence for moderating effects of race in relation to stress physiology, better representation of African American women as well as addressing the unique considerations of women of Hispanic ethnicity (e.g., the role of acculturation) is needed in studies linking behavior, biology, and birth outcomes moving forward. With a rigorous transdisciplinary approach addressing these gaps, the next decade of investigation holds great promise for clinical impact in addressing racial disparities as well as ameliorating effects of stress on perinatal health more broadly across women of all races/ethnicities.
Acknowledgements
The studies described by Christian et al., and preparation of this manuscript, were supported by by NICHD (R21HD067670and R21HD061644) and NINR (R01NR013661). The studies by Christian et al., were also supported by Award Number UL1R001070 from the National Center For Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Financial disclosures
Dr. Christian reported no biomedical financial interests or potential conflicts of interest.
References
- Adler N, Pantell MS, O’Donovan A, Blackburn E, Cawthon R, Koster A, Opresko P, Newman A, Harris TB, Epel E, 2013. Educational attainment and late life telomere length in the health, aging and body composition study. Brain Behav. Immun 27, 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biron-Shental T, Sadeh-Mestechkin D, Amiel A, 2016. Telomere homeostasis in IUGR placentas–a review. Placenta 39, 21–23. [DOI] [PubMed] [Google Scholar]
- Blackburn EH, 2005. Telomeres and telomerase: their mechanisms of action and the effects of altering their functions. FEBS Lett 579, 859–862. [DOI] [PubMed] [Google Scholar]
- Blair LM, Porter K, Leblebicioglu B, Christian LM, 2015. Poor sleep quality and associated inflammation predict preterm birth: heightened risk among African Americans. Sleep 38, 1259–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J, di Fagagna FD, 2007. Cellular senescence: when bad things happen to good cells. Nat. Rev. Mol. Cell Biol 8, 729–740. [DOI] [PubMed] [Google Scholar]
- Cappelletti M, Della Bella S, Ferrazzi E, Mavilio D, Divanovic S, 2016. Inflammation and preterm birth. J. Leukoc. Biol Suppl 99, 67–78. [DOI] [PubMed] [Google Scholar]
- Carroll JE, Diez-Roux AV, Adler NE, Seeman TE, 2013a. Socioeconomic factors and leukocyte telomere length in a multi-ethnic sample: findings from the multi-ethnic study of atherosclerosis (MESA). Brain Behav. Immun 28, 108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JE, Roux AVD, Fitzpatrick AL, Seeman T, 2013b. Low social support is associated with shorter leukocyte telomere length in late life: multi-ethnic study of atherosclerosis (MESA). Psychosom. Med 75, 171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JE, Cole SW, Seeman TE, Breen EC, Witarama T, Arevalo JM, Ma J, Irwin MR, 2016a. Partial sleep deprivation activates the DNA damage response (DDR) and the senescence-associated secretory phenotype (SASP) in aged adult humans. Brain Behav. Immun 51, 223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JE, Esquivel S, Goldberg A, Seeman TE, Effros RB, Dock J, Olmstead R, Breen EC, Irwin MR, 2016b. Insomnia and telomere length in older adults. Sleep 39, 559–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy-Bushrow AE, Peters RM, Johnson DA, Templin TN, 2012. Association of depressive symptoms with inflammatory biomarkers among pregnant African-American women. J. Reprod. Immunol 94, 202–209. [DOI] [PubMed] [Google Scholar]
- Chen Y, Holzman C, Chung H, Senagore P, Talge NM, Siler-Khodr T, 2010. Levels of maternal serum corticotropin-releasing hormone (CRH) at midpregnancy in relation to maternal characteristics. Psychoneuroendocrinology 35, 820–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherak SJ, Giesbrecht GF, Metcalfe A, Ronksley PE, Malebranche ME, 2018. The effect of gestational period on the association between maternal prenatal salivary cortisol and birth weight: a systematic review and meta-analysis. Psychoneuroendocrinology 94, 49–62. [DOI] [PubMed] [Google Scholar]
- Christian LM, 2014. Effects of stress and depression on inflammatory immune parameters in pregnancy. Am. J. Obstet. Gynecol 211, 275–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian LM, Porter K, 2014. Longitudinal changes in serum proinflammatory markers across pregnancy and postpartum: effects of maternal body mass index. Cytokine 70, 134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian LM, Franco A, Glaser R, Iams J, 2009. Depressive symptoms are associated with elevated serum proinflammatory cytokines among pregnant women. Brain Behav. Immun 23, 750–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian LM, Franco A, Iams JD, Sheridan J, Glaser R, 2010. Depressive symptoms predict exaggerated inflammatory response to in vivo immune challenge during human pregnancy. Brain Behav. Immun 24, 49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian LM, Galley JD, Hade EM, Schoppe-Sullivan S, Dush CK, Bailey MT, 2015. Gut microbiome composition is associated with temperament during early childhood. Brain Behav. Immun 45, 118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian LM, Mitchell AM, Gillespie SL, Palettas M, 2016. Serum brain-derived neurotrophic factor (BDNF) across pregnancy and postpartum: associations with race, depressive symptoms, and low birth weight. Psychoneuroendocrinology 74, 69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian LM, Kowalsky JM, Mitchell AM, Porter K, 2018. Associations of postpartum sleep, stress, and depressive symptoms with LPS-stimulated cytokine production among African American and White women. J. Neuroimmunol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, 2009. Social regulation of human gene expression. Curr. Dir. Psychol. Sci 18, 132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Hawkley LC, Arevalo JMG, Cacioppo JT, 2011. Transcript origin analysis identifies antigen-presenting cells as primary targets of socially regulated gene expression in leukocytes. Proc. Natl. Acad. Sci. U. S. A 108, 3080–3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussons-Read ME, Okun ML, Nettles CD, 2007. Psychosocial stress increases inflammatory markers and alters cytokine production across pregnancy. Brain Behav. Immun 21, 343–350. [DOI] [PubMed] [Google Scholar]
- Coussons-Read ME, Lobel M, Carey JC, Kreither MO, D’Anna K, Argys L, Ross RG, Brandt C, Cole S, 2012. The occurrence of preterm delivery is linked to pregnancy-specific distress and elevated inflammatory markers across gestation. Brain Behav. Immun 26, 650–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LS, Redman C, 2017. The role of cellular senescence in ageing of the placenta. Placenta 52, 139–145. [DOI] [PubMed] [Google Scholar]
- Cribbet MR, Carlisle M, Cawthon RM, Uchino BN, Williams PG, Smith TW, Gunn HE, Light KC, 2014. Cellular aging and restorative processes: subjective sleep quality and duration moderate the association between age and telomere length in a sample of middle-aged and older adults. Sleep 37, 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Mello MJ, Ross SA, Briel M, Anand SS, Gerstein H, Paré G, 2015. Association between shortened leukocyte telomere length and cardiometabolic outcomes systematic review and meta-analysis. Circ. Cardiovasc. Genet 8, 82–90. [DOI] [PubMed] [Google Scholar]
- Davy P, Nagata M, Bullard P, Fogelson N, Allsopp R, 2009. Fetal growth restriction is associated with accelerated telomere shortening and increased expression of cell senescence markers in the placenta. Placenta 30, 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, Knight R, 2010. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. U. S. A 107, 11971–11975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duthie L, Reynolds RM, 2013. Changes in the maternal hypothalamic-pituitaryadrenal axis in pregnancy and postpartum: influences on maternal and fetal outcomes. Neuroendocrinology 98, 106–115. [DOI] [PubMed] [Google Scholar]
- Eidem HR, Ackerman WE, McGary KL, Abbot P, Rokas A, 2015. Gestational tissue transcriptomics in term and preterm human pregnancies: a systematic review and meta-analysis. BMC Med. Genomics 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enquobahrie DA, Williams MA, Qiu CF, Muhie SY, Slentz-Kesler K, Ge ZP, Sorenson T, 2009. Early pregnancy peripheral blood gene expression and risk of preterm delivery: a nested case control study. Bmc Preg. Childb 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, Epel ES, Kumsta R, Lin J, Hellhammer DH, Blackburn EH, Wüst S, Wadhwa PD, 2011. Stress exposure in intrauterine life is associated with shorter telomere length in young adulthood. Proc. Natl. Acad. Sci 108, E513–E518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, Buss C, Wadhwa PD, 2015. Prenatal stress, development, health and disease risk: a psychobiological perspective-2015 Curt Richter Award Paper. Psychoneuroendocrinology 62, 366–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM, 2004. Accelerated telomere shortening in response to life stress. Proc. Natl. Acad. Sci. U. S. A 101, 17312–17315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans LM, Myers MM, Monk C, 2008. Pregnant women’s cortisol is elevated with anxiety and depression—but only when comorbid. Arch. Womens Ment. Health 11, 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TB, Russig H, Weiss IC, Graff J, Linder N, Michalon A, Vizi S, Mansuy IM, 2010. Epigenetic transmission of the impact of early stress across generations. Biol. Psychiatry 68, 408–415. [DOI] [PubMed] [Google Scholar]
- Freund A, Orjalo AV, Desprez P-Y, Campisi J, 2010. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol. Med 16, 238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galley JD, Bailey M, Kamp Dush C, Schoppe-Sullivan S, Christian LM, 2014. Maternal obesity is associated with alterations in the gut microbiome in toddlers. PLoS One 9, e113026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie SL, Christian LM, Alston AD, Salsberry PJ, 2017. Childhood stress and birth timing among African American women: cortisol as biological mediator. Psychoneuroendocrinology 84, 32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie SL, Mitchell AM, Kowalsky JM, Christian LM, 2018. Maternal parity and perinatal cortisol adaptation: the role of pregnancy-specific distress and implications for postpartum mood. Psychoneuroendocrinology 97, 86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover V, Bergman K, Sarkar P, O’Connor TG, 2009. Association between maternal and amniotic fluid cortisol is moderated by maternal anxiety. Psychoneuroendocrinology 34, 430–435. [DOI] [PubMed] [Google Scholar]
- Glover V, O’Connor TG, O’Donnell K, 2010. Prenatal stress and the programming of the HPA axis. Neurosci. Biobehav. Rev 35, 17–22. [DOI] [PubMed] [Google Scholar]
- Glynn LM, Schetter CD, Chicz-DeMet A, Hobel CJ, Sandman CA, 2007. Ethnic differences in adrenocorticotropic hormone, cortisol and corticotropin-releasing hormone during pregnancy. Peptides 28, 1155–1161. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Hauth JC, Andrews WW, 2000. Intrauterine infection and preterm delivery. N. Engl. J. Med 342, 1500–1507. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Culhane JF, Iams JD, Romero R, 2008. Epidemiology and causes of preterm birth. Lancet 371, 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna CW, Bretherick KL, Gair JL, Fluker MR, Stephenson MD, Robinson WP, 2009. Telomere length and reproductive aging. Hum. Reprod 24, 1206–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanssen LM, Schutte NS, Malouff JM, Epel ES, 2017. The relationship between childhood psychosocial stressor level and telomere length: a meta-analysis. Health Psychol. Res 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harville EW, Williams MA, Qiu CF, Mejia J, Risques RA, 2010. Telomere length, pre-eclampsia, and gestational diabetes. BMC Res. Notes 3, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffner LJ, 2004. Advanced maternal age - how old is too old? New England J. Med. Surg. Collat. Branches Sci. 351, 1927–1929. [DOI] [PubMed] [Google Scholar]
- Heng YJJ, Pennell CE, McDonald SW, Vinturache AE, Xu JX, Lee MWF, Briollais L, Lyon AW, Slater DM, Bocking AD, de Koning L, Olson DM, Dolan SM, Tough SC, Lye SJ, 2016. Maternal whole blood gene expression at 18 and 28 weeks of gestation associated with spontaneous preterm birth in asymptomatic women. PLoS One 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzman C, Jetton J, Siler-Khodr T, Fisher R, Rip T, 2001. Second trimester corticotropin-releasing hormone levels in relation to preterm delivery and ethnicity. Obstet. Gynecol 97, 657–663. [DOI] [PubMed] [Google Scholar]
- Human Microbiome Project, C, 2012. Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt SC, Chen W, Gardner JP, Kimura M, Srinivasan SR, Eckfeldt JH, Berenson GS, Aviv A, 2008. Leukocyte telomeres are longer in African Americans than in whites: the national heart, lung, and blood institute family heart study and the Bogalusa heart study. Aging Cell 7, 451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman RW, Fukushima M, Jiang H, Fung E, Rand L, Johnson B, Vo KC, Caughey AB, Hilton JF, Davis RW, 2014a. Diversity of the vaginal microbiome correlates with preterm birth. Reprod. Sci 21, 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman RW, Fukushima M, Jiang H, Fung E, Rand L, Johnson B, Vo KC, Caughey AB, Hilton JF, Davis RW, Giudice LC, 2014b. Diversity of the vaginal microbiome correlates with preterm birth. Reprod. Sci 21, 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R, Carroll JE, 2016. Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol. Psychiatry 80, 40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackowska M, Hamer M, Carvalho LA, Erusalimsky JD, Butcher L, Steptoe A, 2012. Short sleep duration is associated with shorter telomere length in healthy men: findings from the whitehall II cohort study. PLoS One 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CW, Gambala C, Esteves KC, Wallace M, Schlesinger R, O’Quinn M, Kidd L, Theall KP, Drury SS, 2017. Differences in placental telomere length suggest a link between racial disparities in birth outcomes and cellular aging. Am. J. Obstet. Gynecol 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane HS, Dunkel Schetter C, Glynn LM, Hobel CJ, Sandman CA, 2014. Pregnancy anxiety and prenatal cortisol trajectories. Biol. Psychol 100, 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Gouin J-P, Weng N. p., Malarkey WB, Beversdorf DQ, Glaser R, 2011. Childhood adversity heightens the impact of later-life caregiving stress on telomere length and inflammation. Psychosom. Med 73, 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Derry HM, Fagundes CP, 2015. Inflammation: depression fans the flames and feasts on the heat. A. J. Psychiatry 172, 1075–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont RF, Sobel JD, Akins RA, Hassan SS, Chaiworapongsa T, Kusanovic JP, Romero R, 2011. The vaginal microbiome: new information about genital tract flora using molecular based techniques. Bjog-Int. J. Obstet. Gynaecol 118, 533–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laopaiboon M, Lumbiganon P, Intarut N, Mori R, Ganchimeg T, Vogel JP, Souza JP, Gulmezoglu AM, Network, W.H.O.M.S.o.M.N.H.R, 2014. Advanced maternal age and pregnancy outcomes: a multicountry assessment. BJOG 121 (Suppl 1), 49–56. [DOI] [PubMed] [Google Scholar]
- Lester BM, Conradt E, LaGasse LL, Tronick EZ, Padbury JF, Marsit CJ, 2018. Epigenetic programming by maternal behavior in the human infant. Pediatrics 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiti K, Sultana Z, Aitken RJ, Morris J, Park F, Andrew B, Riley SC, Smith R, 2017. Evidence that fetal death is associated with placental aging. Am. J. Obstet. Gynecol 217 (441) e441–441 e414. [DOI] [PubMed] [Google Scholar]
- Marioni RE, Harris SE, Shah S, Mcrae AF, von Zglinicki T, Martin-Ruiz C, Wray NR, Visscher PM, Deary IJ, 2016. The epigenetic clock and telomere length are independently associated with chronological age and mortality. Int. J. Epidemiol 45, 424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastorakos G, Ilias I, 2000. Maternal hypothalamic-pituitary-adrenal axis in pregnancy and the postpartum period. Postpartum-related disorders. Ann. N. Y. Acad. Sci 900, 95–106. [DOI] [PubMed] [Google Scholar]
- Mastorakos G, Ilias I, 2003. Maternal and fetal hypothalamic-pituitary-adrenal axes during pregnancy and postpartum. Women’s Health Dis.: Gynecol. Reprod. Issues 997, 136–149. [DOI] [PubMed] [Google Scholar]
- Mclean M, Bisits A, Davies J, Woods R, Lowry P, Smith R, 1995. A placental clock controlling the length of human-pregnancy. Nat. Med 1, 460–463. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, 2001. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu. Rev. Neurosci 24, 1161–1192. [DOI] [PubMed] [Google Scholar]
- Mitchell AM, Kowalsky JM, Epel ES, Lin J, Christian LM, 2018. Childhood adversity, social support, and telomere length among perinatal women. Psychoneuroendocrinology 87, 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor G, Cardenas I, Abrahams V, Guller S, 2011. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann. N. Y. Acad. Sci 1221, 80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müezzinler A, Zaineddin AK, Brenner H, 2013. A systematic review of leukocyte telomere length and age in adults. Ageing Res. Rev 12, 509–519. [DOI] [PubMed] [Google Scholar]
- Muglia LJ, Katz M, 2010. Current concepts the enigma of spontaneous preterm birth. New England J. Med. Surg. Collat. Branches Sci 362, 529–535. [DOI] [PubMed] [Google Scholar]
- Nansel TR, Riggs MA, Yu KF, Andrews WW, Schwebke JR, Klebanoff MA, 2006. The association of psychosocial stress and bacterial vaginosis in a longitudinal cohort. Am. J. Obstet. Gynecol 194, 381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham BL, Mezuk B, Bareis N, Lin J, Blackburn EH, Epel ES, 2015. Depression, anxiety and telomere length in young adults: evidence from the National Health and Nutrition Examination Survey. Mol. Psychiatry 20, 520–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, Pantell MS, Puterman E, Dhabhar FS, Blackburn EH, Yaffe K, Cawthon RM, Opresko PL, Hsueh WC, Satterfield S, Newman AB, Ayonayon HN, Rubin SM, Harris TB, Epel ES, Study HABC, 2011. Cumulative inflammatory load is associated with short leukocyte telomere length in the health, aging and body composition study. PLoS One 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun ML, Coussons-Read MF, 2006. Sleep quantity and quality during pregnancy is associated with changes in in vitro cytokine production. Sleep 29, A122–A123. [Google Scholar]
- Okun ML, Coussons-Read ME, 2007. Sleep disruption during pregnancy: how does it influence serum cytokines? J. Reprod. Immunol 73, 158–165. [DOI] [PubMed] [Google Scholar]
- Okun ML, Luther JF, Wisniewski SR, Wisner KL, 2013. Disturbed sleep and inflammatory cytokines in depressed and nondepressed pregnant women: an exploratory analysis of pregnancy outcomes. Psychosom. Med 75, 670–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne LM, Monk C, 2013. Perinatal depression–the fourth inflammatory morbidity of pregnancy?: theory and literature review. Psychoneuroendocrinology 38, 1929–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paropkari AD, Leblebicioglu B, Christian LM, Kumar PS, 2016. Smoking, pregnancy and the subgingival microbiome. Sci. Rep.-Uk 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluess M, Bolten M, Pirke KM, Hellhammer D, 2010. Maternal trait anxiety, emotional distress, and salivary cortisol in pregnancy. Biol. Psychol 83, 169–175. [DOI] [PubMed] [Google Scholar]
- Prather AA, Puterman E, Lin J, O’Donovan A, Krauss J, Tomiyama AJ, Epel ES, Blackburn EH, 2011. Shorter leukocyte telomere length in midlife women with poor sleep quality. J. Aging Res 2011, 721390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather AA, Gurfein B, Moran P, Daubenmier J, Acree M, Bacchetti P, Sinclair E, Lin J, Blackburn E, Hecht FM, Epel ES, 2015. Tired telomeres: poor global sleep quality, perceived stress, and telomere length in immune cell subsets in obese men and women. Brain Behav. Immun 47, 155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince AL, Antony KM, Chu DM, Aagaard KM, 2014. The microbiome, parturition, and timing of birth: more questions than answers. J. Reprod. Immunol 104, 12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puterman E, Lin J, Krauss J, Blackburn EH, Epel ES, 2015. Determinants of telomere attrition over 1 year in healthy older women: stress and health behaviors matter. Mol. Psychiatry 20, 529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rackers HS, Thomas S, Williamson K, Posey R, Kimmel MC, 2018. Emerging literature in the microbiota-brain Axis and perinatal mood and anxiety disorders. Psychoneuroendocrinology 95, 86–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos IF, Guardino CM, Mansolf M, Glynn LM, Sandman CA, Hobel CJ, Dunkel Schetter C, 2018. Pregnancy anxiety predicts shorter gestation in Latina and non-Latina white women: the role of placental corticotrophin-releasing hormone. Psychoneuroendocrinology 99, 166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, Brotman RM, Davis CC, Ault K, Peralta L, Forney LJ, 2011. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. U. S. A 108 (Suppl. 1), 4680–4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redei EE, Mehta NS, 2015. Blood transcriptomic markers for major depression: from animal models to clinical settings. Trans. Neurosci. Psychiatry 1344, 37–49. [DOI] [PubMed] [Google Scholar]
- Ridout S, Ridout KK, Price LH, Sen S, Tyrka AR, 2015a. Depression and telomere length: a meta-analysis. Biol. Psychiatry 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridout SJ, Ridout KK, Kao H-T, Carpenter LL, Philip NS, Tyrka AR, Price LH, 2015b. Telomeres, Early-life Stress and Mental Illness, Clinical Challenges in the Biopsychosocial Interface. Karger Publishers, pp. 92–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rode L, Nordestgaard BG, Bojesen SE, 2015. Peripheral blood leukocyte telomere length and mortality among 64 637 individuals from the general population. Jnci-J. Natl. Cancer I 107. [DOI] [PubMed] [Google Scholar]
- Ross KM, Cole SW, Carroll JE, Dunkel Schetter C, 2018. Elevated pro-inflammatory gene expression in the third trimester of pregnancy in mothers who experienced stressful life events. Brain Behav. Immun [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman CA, Glynn L, Schetter CD, Wadhwa P, Garite T, Chicz-DeMet A, Hobel C, 2006. Elevated maternal cortisol early in pregnancy predicts third trimester levels of placental corticotropin releasing hormone (CRH): priming the placental clock. Peptides 27, 1457–1463. [DOI] [PubMed] [Google Scholar]
- Schutte NS, Malouff JM, 2015. The association between depression and leukocyte telomere length: a meta‐analysis. Depress. Anxiety 32, 229–238. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, Miller GE, 2004. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol. Bull 130, 601–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev I, Entringer S, Wadhwa PD, Wolkowitz OM, Puterman E, Lin J, Epel ES, 2013. Stress and telomere biology: a lifespan perspective. Psychoneuroendocrinology 38, 1835–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smid MC, Lee JH, Grant JH, Miles G, Stoddard GJ, Chapman DA, Manuck TA, 2017. Maternal race and intergenerational preterm birth recurrence. Am. J. Obstet. Gynecol 217 (480) e481–480 e489. [DOI] [PubMed] [Google Scholar]
- Stout MJ, Zhou Y, Wylie KM, Tarr PI, Macones GA, Tuuli MG, 2017. Early pregnancy vaginal microbiome trends and preterm birth. Am. J. Obstet. Gynecol 217 (356), e351–356 e318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana Z, Maiti K, Dedman L, Smith R, 2018. Is there a role for placental senescence in the genesis of obstetric complications and fetal growth restriction? Am. J. Obstet. Gynecol 218, S762–S773. [DOI] [PubMed] [Google Scholar]
- Thomson M, 2013. The physiological roles of placental corticotropin releasing hormone in pregnancy and childbirth. J. Physiol. Biochem 69, 559–573. [DOI] [PubMed] [Google Scholar]
- Toker S, Shirom A, Shapira I, Berliner S, Melamed S, 2005. The association between burnout, depression, anxiety, and inflammation biomarkers: C-reactive protein and fibrinogen in men and women. J. Occup. Health Psychol 10, 344. [DOI] [PubMed] [Google Scholar]
- Toutain J, Prochazkova-Carlotti M, Cappellen D, Jarne A, Chevret E, Ferrer J, Idrissi Y, Pelluard F, Carles D, Maugey-Laulon B, 2013. Reduced placental telomere length during pregnancies complicated by intrauterine growth restriction. PLoS One 8, e54013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance MC, Bui E, Hoeppner SS, Kovachy B, Prescott J, Mischoulon D, Walton ZE, Dong M, Nadal MF, Worthington JJ, Hoge EA, Cassano P, Orr EH, Fava M, de Vivo I, Wong KK, Simon NM, 2018. Prospective association between major depressive disorder and leukocyte telomere length over two years. Psychoneuroendocrinology 90, 157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinturache AE, Gyamfi-Bannerman C, Hwang J, Mysorekar IU, Jacobsson B, Preterm Birth International, C, 2016. Maternal microbiome - a pathway to preterm birth. Semin. Fetal Neonatal Med 21, 94–99. [DOI] [PubMed] [Google Scholar]
- Yao YL, Robinson AM, Zucchi FCR, Robbins JC, Babenko O, Kovalchuk O, Kovalchuk I, Olson DM, Metz GAS, 2014. Ancestral exposure to stress epigenetically programs preterm birth risk and adverse maternal and newborn outcomes. BMC Med 12. [DOI] [PMC free article] [PubMed] [Google Scholar]