Abstract
Background:
In the US, nearly 30% of liver transplants (LT) are performed for hepatocellular carcinoma (HCC). Although overall long-term survival is highest with LT, there are limited data on the incremental survival benefit of LT versus other curative options (resection or ablation) due to shunting of patients towards LT.
Methods:
We performed a retrospective cohort study of patients aged 50–69 with cirrhosis and HCC in the Veterans Health Administration (population enriched with three curative treatments) from 2008–2016. The cohort was restricted to patients who received LT, resection, or ablation and a calculated model for end-stage liver disease (MELD) score <15 at HCC diagnosis.
Results:
Among 2,129 veterans in the analytic cohort, 658 (26.7%) received LT, 244 (11.5%) underwent resection, and 1,317 (61.59%) received ablation. In multivariable models, patients who underwent resection (HR: 5.42, 95% CI: 4.15–7.08) or ablation (HR: 5.50, 95% CI: 4.51–6.71) had significantly increased hazards of death. However, in absolute terms, the incremental survival benefit of LT over resection or ablation was small, between 0.02–0.03 years at one year, 0.32–0.42 years at three years, and 1.04–1.24 years at five years follow-up. These results were consistent in sensitivity analyses accounting for possible immortal time bias, as well as a cohort restricted to early/intermediate stage HCC.
Conclusion:
Although LT is associated with significantly increased survival compared to resection and ablation, the absolute incremental survival benefit is small over a 5-year time horizon. Optimal selection of patients for LT is critical for maximizing utilization of a scarce resource.
Introduction
The rising prevalence of hepatitis C virus (HCV) and nonalcoholic fatty liver disease (NAFLD) has led to marked increase in the incidence of cirrhosis and hepatocellular carcinoma (HCC).1
For patients with early-stage HCC, there are three curative options—ablation, hepatic resection, and liver transplant (LT).2–4 Since the inception of Model for End-Stage Liver Disease (MELD)-based allocation in 2002, waitlisted patients with HCC within Milan criteria have been eligible for automatic MELD exception points to facilitate LT and decrease the risk of waitlist removal due to tumor progression.5–8 At the level of the individual patient, advocating for transplantation has been considered a strategy that maximizes survival. However, recent data suggest that due to donor organ scarcity, prioritization of HCC patients over those with high laboratory MELD scores has unintentionally resulted in a large reduction in LT-related survival benefit,9 given that nearly 80% of US patients transplanted for HCC have minimal evidence of liver synthetic dysfunction and/or portal hypertension and thus may be candidates for another curative treatment.5–9 However, these data relied solely on a cohort of waitlisted patients, for whom long-term estimates of survival without LT may have been overestimated by inclusion of long-term waitlist survivors.10 There are limited data on the incremental survival benefit of LT versus other curative options (resection or ablation) for HCC patients with preserved liver function, especially in the context of utilizing donor livers for patients with HCC over patients with decompensated cirrhosis without other curative options.
In order to definitively determine the survival benefit of LT vs resection or ablation, one would need to perform a randomized controlled trial of patients eligible for all three treatment modalities. This however is not feasible. The Veterans Health Administration (VHA) is uniquein that it is a natural environment enriched with patients receiving nontransplant treatment due to various limitations in access to LT.11 Thus, the objective of this study was to leverage VHA data to compare the overall survival from HCC diagnosis for those receiving LT, resection, or ablation in order to estimate the incremental survival benefit of LT.
Methods
Study Design and Data Sources
We performed a retrospective cohort study of VHA patients, collected as part of the Veterans Outcomes and Costs Associated with Liver Disease (VOCAL) study group dataset. The VHA is the largest single provider of liver care in the US, and due to limitations in access to LT among this population, the dataset is enriched with patients who received nontransplant HCC therapies.11 The creation of the VOCAL dataset has been previously described, but in brief it is a well-characterized cohort of patients with incident cirrhosis identified between 2008 and 2016.12,13 Transplantation data was obtained from the United Network for Organ Sharing (UNOS) Standard Transplant Analysis and Research (STAR) data file12 and death data were obtained using the Medicare Vital Status File.14
Variable Collection
The VOCAL database provided demographic (age, sex, race), clinical (body mass index [BMI]), comorbidity (hypertension, diabetes, chronic kidney disease, coronary artery disease, congestive heart failure, cerebrovascular accident, atrial fibrillation, pulmonary embolism, decompensated cirrhosis), and laboratory data (sodium, creatinine, international normalized ratio [INR], liver-associated enzymes, platelet count, alpha fetoprotein [AFP]). Comorbidities including prior cirrhosis decompensation were obtained using international classification of disease (ICD)-9 coding algorithms detailed previously.12 Where relevant, all data were screened to identify values closest to the date of HCC diagnosis. AFP was modeled as both a continuous and categorical variable (<50ng/mL, 50–99 ng/mL, 100–499 ng/mL, and ≥500 ng/mL), adapted from literature cut points.15–17 MELD scores were calculated from baseline laboratory parameters, and baseline alcohol use disorders identification test (AUDIT-C) scores were obtained to identify patients with hazardous drinking patterns (positive score≥4 for males and ≥3 for females).18–20 ICD-9 codes, current procedure terminology (CPT) codes, and pharmacy data were also obtained for subsequent exposure ascertainment. Etiology of liver disease was established using a previously validated algorithm21 with the following final classifications: HCV, hepatitis B (HBV), alcoholic liver disease (EtOH), NAFLD, HCV + EtOH, and other.
Ascertainment of Hepatocellular Carcinoma and Patient Selection
Patients with HCC were identified using a validated algorithm based on the presence of one inpatient or two outpatient ICD-9, Clinical Modification codes for malignant neoplasm of the liver (155.0 or 155.2) as a primary or secondary diagnosis.22 We included patients aged 50–69 with cirrhosis and HCC, given that UNOS data demonstrates that only 3.5 % of HCC LT recipients from the VHA during the study period were aged ≥70 years. Importantly, we focused on patients with a calculated MELD score <15 at the time of HCC diagnosis, because data have demonstrated that this is the cutoff for survival benefit, and many consider a calculated MELD score ≥15 as an indication for LT independent of other factors.23 We excluded patients with missing baseline bilirubin, creatinine, and/or INR values as a calculated MELD score was required for inclusion. Because HCC staging was not available for the entire cohort, we restricted our analyses to patients who received LT, resection, or ablation as these are the only three treatment options with a curative intent for HCC.
Ascertainment of Nontransplant HCC Therapies (Exposures)
We identified nontransplant therapies through a combination of relational database queries and manual expression searches using STATA 15.1/IC (College Station, TX), adapted from prior literature.22 Resection/partial hepatectomy events were identified using structured query language (SQL) searches for relevant CPT codes, excluding orthotopic liver transplantation and liver biopsy (Table S1, SDC, http://links.lww.com/TP/B757). Ablation therapy included microwave ablation, radiofrequency ablation, cryo-ablation, and ethanol ablation. Treatment group (LT, resection, or ablation) was initially assigned based on a ranking of highest survival rate in the following order: transplant > resection > ablation. That is, if a patient received resection followed by ablation, their treatment group was classified as “resection” throughout the duration of follow-up.
Treatment group was subsequently coded as a time updating variable, where patients could be reclassified if they received multiple therapies (e.g., ablation followed by LT).
Outcome
The primary outcome was time-to-event survival, measured from date of HCC diagnosis. The time horizons we focused on were 1–5 years from diagnosis. We chose to begin follow-up from the time of diagnosis, as literature demonstrates that beginning follow-up at the time of treatment leads to biased estimates of risk in favor of treatment exposures with longer time intervals from diagnosis (immortal time).24,25 Rather, we elected to measure survival time from HCC diagnosis and model time-updating treatment status through Cox regression, as detailed below. This approach also reflects an ‘intention to treat’ analysis whereby the decision to pursue a curative treatment path was made at the time of HCC diagnosis, accounting for multiple treatments and crossovers as described.
Statistical Analysis
Descriptive statistics were computed based on the highest level of treatment received. Pearson chi-squared and Wilcoxon rank-sum tests were performed for categorical and continuous variables, respectively. We used univariate Cox regression analysis to select predictors for testing in multivariable regression. We considered all variables shown in Table 1, and used an alpha=0.15 threshold. Subsequently, we performed multivariable Cox regression analysis using elements of reverse stepwise and clinician-driven modeling approach, with an alpha=0.05 threshold used for variable retention. We used minimized Bayesian Information Criterion (BIC) to select final models. Because of potential immortal time bias induced by establishing time of HCC diagnosis as the start of follow-up, we evaluated several models. The full model included age, AST, ALT, alkaline phosphatase, albumin, platelet count, AFP category, and decompensation status. The immortal model adjusted for the same covariates, but also incorporated time-updated treatment exposure variables to mitigate potential immortal time bias, consistent with literature recommendations.24–26 This allowed for accurate risk adjustment for unexposed time periods. It also properly accounted for patients who received multiple treatments, including possible neoadjuvant, or “bridging” therapies. For example, a patient might have an unexposed period which was followed by ablation and later LT. Importantly, owing to a violation in the proportional hazards assumption caused by alpha fetoprotein level, we treated this as a continuously time-varying exposure. We produced adjusted coefficient plots and survival curves for each model, stratified by treatment group, and also reported predicted probabilities of survival. In order to determine relative survival benefit of transplant, we integrated the adjusted survival curves to calculate expected survival over time as a function of treatment group, and subsequently took the difference in expected survival between LT and resection or ablation, respectively, similar to previously-described methods.27,28 This was done for each model, with the results plotted and point estimates reported at 1–5 years.
Table 1 –
Demographic and Clinical Characteristics by Treatment Group
| Factor | Liver Transplant (n = 568) | Hepatectomy (n = 244) | Ablation (n = 1317) | p-value |
|---|---|---|---|---|
| Age category | <0.001 | |||
| 50–64 | 489 (86.1%) | 197 (80.7%) | 1032 (78.4%) | |
| 65–69 | 79 (13.9%) | 47 (19.3%) | 285 (21.6%) | |
| Female sex | 10 (1.8%) | 4 (1.6%) | 16 (1.2%) | 0.62 |
| Race | <0.001 | |||
| White | 333 (58.6%) | 113 (46.3%) | 655 (49.7%) | |
| Black | 73 (12.9%) | 76 (31.1%) | 308 (23.4%) | |
| Hispanic | 58 (10.2%) | 16 (6.6%) | 139 (10.6%) | |
| Asian | 21 (3.7%) | 4 (1.6%) | 32 (2.4%) | |
| Other/Unknown | 83 (14.6%) | 35 (14.3%) | 183 (13.9%) | |
| Body mass index | <0.001 | |||
| Underweight (<18.5) | 7 (1.2%) | 10(4.1%) | 44 (3.3%) | |
| Normal (18.5–24.9) | 143 (25.2%) | 86 (35.2%) | 417 (31.7%) | |
| Overweight (25–29.9) | 248 (43.7%) | 87 (35.7%) | 476 (36.2%) | |
| Obese (≥30) | 169 (29.8%) | 61 (25.0%) | 379 (28.8%) | |
| Cause of liver disease | 0.013 | |||
| Hepatitis C | 176 (31.0%) | 57 (23.4%) | 297 (22.6%) | |
| Hepatitis B | 11 (1.9%) | 6 (2.5%) | 28 (2.1%) | |
| Alcoholic liver disease | 49 (8.6%) | 34 (13.9%) | 179 (13.6%) | |
| HCV + EtOH | 272 (47.9%) | 119 (48.8%) | 680 (51.6%) | |
| NAFLD | 49 (8.6%) | 24 (9.8%) | 108 (8.2%) | |
| Other | 11 (1.9%) | 4 (1.6%) | 25 (1.9%) | |
| MELD, median (IQR) | 9 (8,12) | 8 (7, 9) | 9 (7, 11) | <0.001 |
| AST, median (IQR) | 76 (47,112) | 62 (41, 96) | 73 (47,113) | 0.003 |
| ALT, median (IQR) | 63 (41,102) | 64 (38, 94) | 64 (39, 98) | 0.66 |
| Alk Phos, median (IQR) | 106 (81, 143) | 88 (69, 122) | 105 (81, 142) | <0.001 |
| Albumin, median (IQR) | 3 (3, 4) | 4 (3, 4) | 4 (3, 4) | <0.001 |
| Platelet count, median (IQR) | 90 (64,134) | 158 (124, 202) | 109 (75, 153) | <0.001 |
| AFP, median (IQR) | 13 (5, 38) | 12 (5, 73) | 16 (7, 55) | 0.005 |
| AFP category (ng/mL) | <0.001 | |||
| <50 | 444 (79.4%) | 174 (71.6%) | 975 (74.0%) | |
| 50–99 | 45 (8.1%) | 16 (6.6%) | 113 (8.6%) | |
| 100–499 | 52 (9.3%) | 27 (11.1%) | 163 (12.4%) | |
| ≥500 | 18 (3.2%) | 26 (10.7%) | 66 (5.0%) | |
| AUDIT-C positive | 46 (8.1%) | 47 (19.3%) | 261 (19.8%) | <0.001 |
| Decompensated cirrhosis | 100 (17.6%) | 7 (2.9%) | 162 (12.3%) | <0.001 |
| Coronary artery disease | 55 (9.7%) | 32 (13.1%) | 188 (14.3%) | 0.024 |
| Congestive heart failure | 10 (1.8%) | 6 (2.5%) | 62 (4.7%) | 0.004 |
| Cerebrovascular accident | 5 (0.9%) | 1 (0.4%) | 31 (2.4%) | 0.019 |
| Atrial fibrillation | 8 (1.4%) | 6 (2.5%) | 55 (4.2%) | 0.006 |
| Pulmonary embolism | 0 (0.0%) | 2 (0.8%) | 4 (0.3%) | 0.13 |
| Diabetes mellitus | 317 (55.8%) | 118 (48.4%) | 674 (51.2%) | 0.084 |
| Hypertension | 453 (79.8%) | 231 (94.7%) | 1235 (93.8%) | <0.001 |
HCV = hepatitis C; EtOH = alcoholic liver disease; NAFLD = non-alcoholic fatty liver disease; MELD = model for end-stage liver disease; IQR = interquartile range; AST = aspartate aminotransferase; ALT = alanine aminotransferase; Alk Phos = alkaline phosphatase; AFP = alpha fetoprotein; AUDIT-C = alcohol use disorders identification test
HCC Staging Subgroup Analysis
A subgroup of ~20% of the analytic cohort was randomly chosen for manually chart review in order to ascertain HCC staging data at the time of HCC diagnosis, as previously described.22
This involved review of clinical documentation and radiology reports. Adherence to the Milan criteria was defined as one lesion ≤5cm or no more than 3 lesions all ≤3cm.29 We obtained tumor number, largest tumor diameter (cm), and total tumor diameter (cm), and patients were also classified according to the Barcelona Clinic Liver Cancer (BCLC) staging system (stage 0, A, B, C, or D).4 We used this staging to confirm that this cohort primarily consisted of BCLC stage patients for whom hepatectomy, ablation, or transplant would be potentially reasonable pathways. Recent data suggest that BCLC-B (intermediate stage) candidates are a heterogeneous group with potentially comparable outcomes with ablation or resection as compared to chemoembolization.30–34 As such, we performed a subgroup analysis restricted to patients with BCLC stage 0, A, or B at HCC diagnosis (early/intermediate stage). This staging model followed the same variable selection process as described previously, and adjusted for albumin and platelet count. Of note, tumor characteristics were tested but not found to be significant predictors in this model.
Results
Patient Characteristics
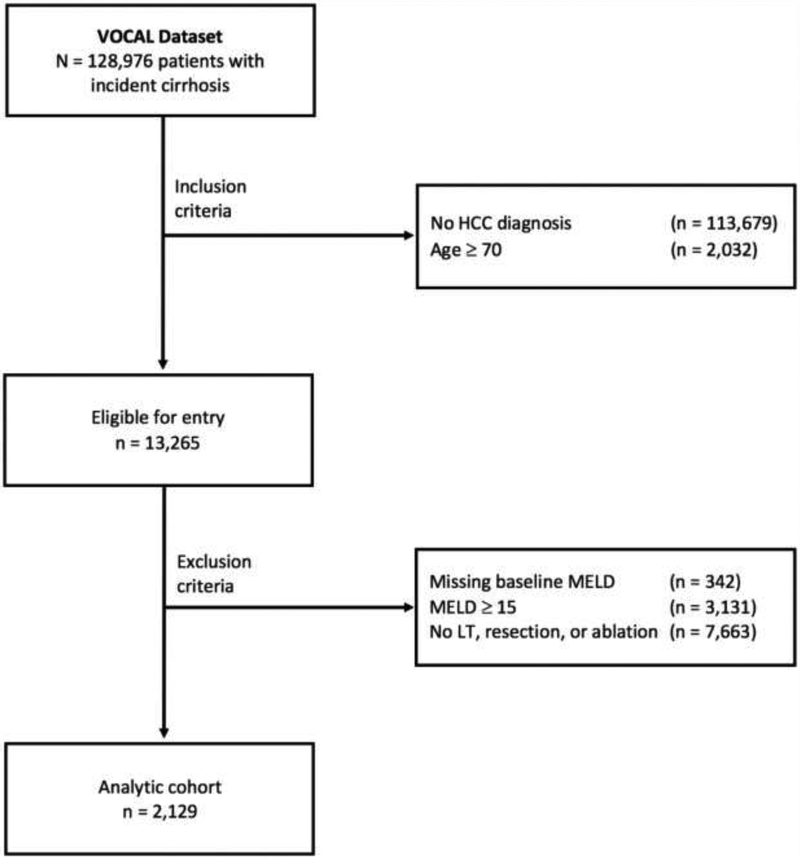
After selection criteria, 2,129 veterans with cirrhosis and HCC were included in the analytic cohort (Figure 1), with median follow-up time 2.90 years (interquartile range [IQR] 1.82–4.59 years). The patient population was primarily male, with a racial distribution reflective of the overall population (Table 1). Of the three curative options, ablation was most common as the highest level of therapy (61.9%), followed by LT (26.7%) and hepatic resection (11.5%). From HCC diagnosis, the median time to therapy was longest for LT (1.25 years, IQR 0.70–1.98 years), followed by ablation (median 0.25 years, IQR 0.11–0.75 years) and resection (median 0.17 years, IQR 0.10–0.41). LT was most common among patients aged 50–64, with less invasive treatments (ablation and resection) more common in the 65–69 age group. LT patients were less likely to have isolated alcohol-induced liver disease and/or active alcohol use (measured by AUDIT-C), while they were more likely to have a lower platelet count and decompensated cirrhosis. Patients who received ablation or resection more cardiovascular comorbidities (i.e., coronary artery disease, congestive heart failure, hypertension, and atrial fibrillation) relative to those who underwent LT.
Figure 1 –
Patient Flow Diagram
Primary Cox Regression Analysis
In the full model, resection and ablation had significantly higher hazards of death relative to LT (hazard ratio [HR] 5.42, 95% confidence interval [CI] 4.15–7.08; and HR 5.50, 95% CI 4.51–6.71, respectively; Table 2 and Figure 2A). When adjusting for time-varying treatment exposures (immortal model), the hazards for each increased slightly (resection HR 6.18, 95% CI 4.80–7.97; ablation HR 5.79, 95% CI 5.27–6.37), however overall survival was improved in all groups (Figure 2B). In both models, higher age, baseline MELD, alkaline phosphatase, AST, AFP category, and decompensated cirrhosis status were positively associated with poorer survival.
Table 2 –
Multivariable Cox Regression Models‡
| Full Model | Immortal Model* | Staging Model† | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Treatment (ref = LT) | ||||||
| Resection | 5.42(4.15–7.08) | <0.001 | 6.18(4.80–7.97) | <0.001 | 4.54(2.80–7.36) | <0.001 |
| Ablation | 5.50(4.51–6.71) | <0.001 | 5.79(5.27–6.37) | <0.001 | 4.88(3.44–6.92) | <0.001 |
| Age (per 5 years) | 1.12(1.04–1.20) | 0.002 | 1.11(1.09–1.12) | <0.001 | . | . |
| AST (per 10) | 1.02(1.00–1.03) | 0.011 | 1.02(1.01–1.02) | <0.001 | . | . |
| ALT(per 10) | 0.97(0.95–0.99) | 0.001 | 0.97(0.97–0.97) | <0.001 | . | . |
| Alk phos (per 10) | 1.02(1.01–1.03) | <0.001 | 1.02(1.02–1.02) | <0.001 | . | . |
| Albumin | 0.75(0.66–0.84) | <0.001 | 0.75(0.70–0.80) | <0.001 | 0.73(0.59–0.91) | 0.005 |
| Platelets (per 10) | 0.98(0.97–0.99) | 0.001 | 0.98(0.98–0.98) | <0.001 | 0.97(0.95–0.99) | 0.014 |
| AFP(ref<50ng/mL)§ | ||||||
| 50–100 | 1.35(1.06–1.72) | 0.015 | 1.23(1.13–1.35) | <0.001 | . | . |
| 101–499 | 2.30(1.77–2.99) | <0.001 | 2.15(1.97–2.34) | <0.001 | . | . |
| ≥500 | 2.54(1.73–3.73) | <0.001 | 2.51(2.05–3.06) | <0.001 | . | . |
| Decompensation | 1.57(1.31–1.89) | <0.001 | 1.54(1.49–1.60) | <0.001 | . | . |
LT = liver transplantation; AST = aspartate aminotransferase; ALT = alanine aminotransferase; Alk Phos = alkaline phosphatase; AFP = alpha fetoprotein
Model adjusts for time-varying exposure status
Model restricted to patients with BCLC staging data (N = 432), and specifically those with BCLC stage 0, A, or B (n = 414)
The following variables were evaluated but not retained in final models: sex, race, body mass index, hazardous alcohol use, model for end-stage liver disease score, etiology of liver disease, coronary artery disease, congestive heart failure, cerebrovascular accident, atrial fibrillation, pulmonary embolism, diabetes mellitus, and hypertension
Treated as a continuously time-varying exposure
Figure 2 –
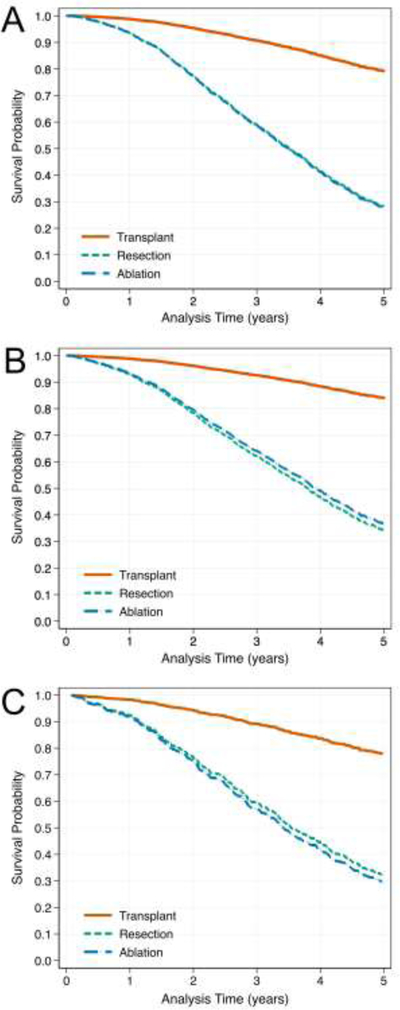
Adjusted Survival Curves by Treatment for Full (A), Immortal (B), and Staging (C) Models
Staging Subgroup Analysis
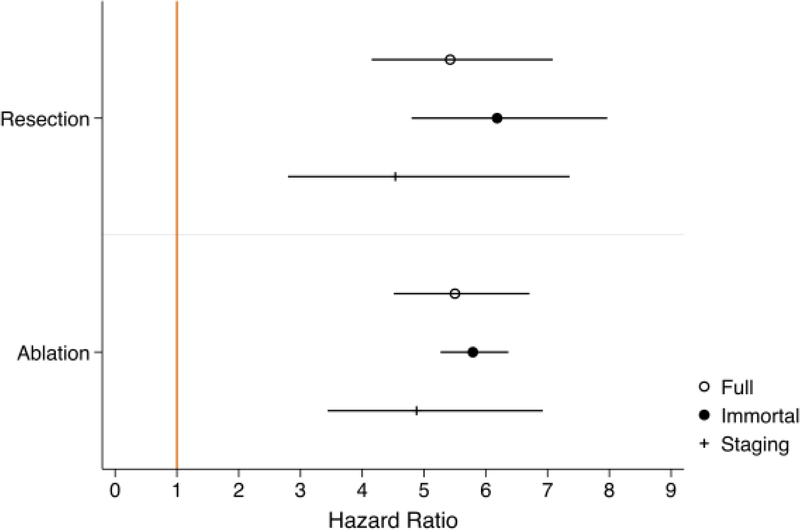
Of the 2,129 patients in the analytic cohort, a total 432 (20.3%) were manually reviewed for staging data. Of these patients, 78.5% were within Milan criteria at the time of HCC diagnosis. A total 16.4% were BCLC stage 0, 62.0% stage A, 17.4% stage B, 2.3% stage C, and 1.9% stage D. When isolating the cohort to only those with early/intermediate stage HCC (as defined by BCLC stage 0, A, or B), the increased hazard of death relative to LT with resection or ablation was somewhat attenuated (resection HR 4.54, 95% CI 2.80–7.36; ablation HR 4.88, 95% CI 3.44–6.92; staging model in Table 2). Tumor characteristics of this subcohort are shown in Table 3, where patients who received resection had a significantly larger maximum tumor diameter and total tumor diameter versus LT or ablation patients (median 3.45cm versus 2.5–2.6cm, and 3.45cm versus 2.7cm, respectively; p<0.001 and p=0.031). However, neither of these factors was retained in the multivariable staging model. Survival distributions by treatment (Figure 2C) were similar to those estimated in the full model. Hazard ratios for resection and ablation relative to LT, for each model, are summarized in Figure 3.
Table 3 –
Tumor Characteristics of the Staging Subgroup
| Factor | Liver Transplant (n = 98) | Hepatectomy (n = 48) | Ablation (n = 268) | p-value |
|---|---|---|---|---|
| Number of tumors (median, IQR) | 1 (1,1) | 1 (1,1) | 1 (1, 2) | 0.002 |
| Largest tumor diameter (cm; median, IQR) | 2.6 (2.0, 3.5) | 3.45 (2.2, 4.7) | 2.5 (2.0, 3.4) | <0.001 |
| Total tumor diameter (cm; median, IQR) | 2.7(2.1,3.6) | 3.45(2.2,4.85) | 2.7(2.0,4.1) | 0.031 |
Figure 3 –
Hazard Ratios for Mortality Associated with Treatment Group, Relative to Liver Transplantation
Survival Benefit of Transplant
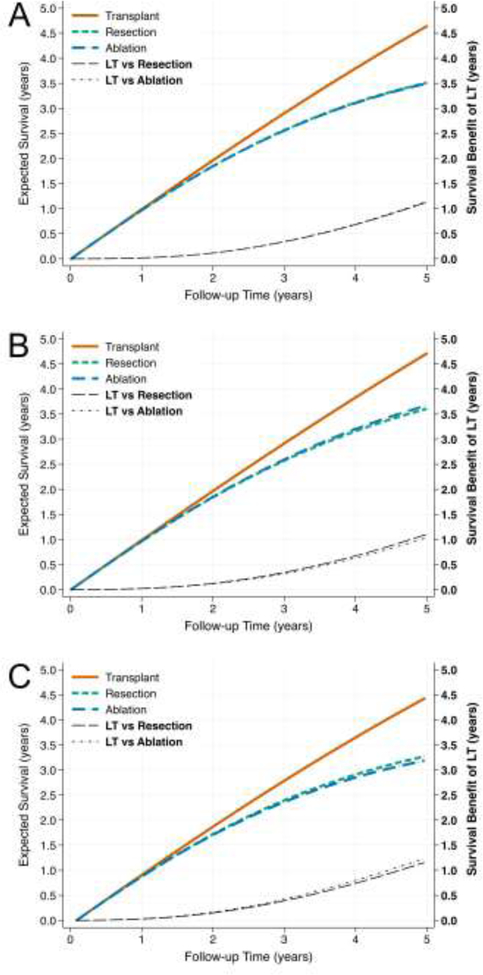
Predicted probabilities of survival at 1–5 years, stratified by treatment modality, are reported in Table 4. By contrast, the expected survival over time and survival benefit relative to LT for each treatment group are shown in Figure 4, with plots produced for each model. Although LT yielded the highest expected survival in all models, the degree of survival benefit was small, especially at short follow-up intervals. For example, in all models, the survival benefit of LT over resection or ablation at one year was only 0.02–0.03 years. At five years, the survival benefit was between 1.04 and 1.24 years (Table 5).
Table 4 –
Predicted Probabilities of Survival, Stratified by Treatment Modality
| Full Model | Immortal Model* | Staging Model† | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 years | 99.1% | 94.5% | 94.4% | 99.0% | 93.8% | 94.2% | 98.3% | 92.4% | 91.8% |
| 2 years | 96.6% | 80.3% | 80.1% | 96.6% | 80.9% | 82.0% | 94.3% | 76.5% | 75.0% |
| 3 years | 93.1% | 63.5% | 63.1% | 93.5% | 66.0% | 67.8% | 89.2% | 59.4% | 57.1% |
| 4 years | 88.8% | 47.1% | 46.6% | 89.8% | 51.5% | 53.7% | 83.4% | 44.6% | 41.9% |
| 5 years | 84.2% | 33.6% | 33.1% | 85.9% | 39.2% | 41.6% | 77.9% | 32.3% | 29.6% |
LT = liver transplantation
Model adjusts for time-varying exposure status
Model restricted to patients with BCLC staging data (N = 432), and specifically those with BCLC stage 0, A, or B (n = 414)
Figure 4 –
Expected Survival and Survival Benefit by Treatment for Full (A), Immortal (B), and Staging (C) Model
Table 5 –
Estimated Survival Benefit of Liver Transplant versus Resection or Ablation (in years)
| Full Model | Immortal Model* | Staging Model† | ||||
|---|---|---|---|---|---|---|
| 1 years | 0.02 | 0.02 | 0.02 | 0.02 | 0.03 | 0.03 |
| 2 years | 0.11 | 0.12 | 0.12 | 0.11 | 0.15 | 0.16 |
| 3 years | 0.34 | 0.34 | 0.34 | 0.32 | 0.38 | 0.42 |
| 4 years | 0.68 | 0.69 | 0.67 | 0.63 | 0.72 | 0.78 |
| 5 years | 1.13 | 1.14 | 1.10 | 1.04 | 1.15 | 1.24 |
LT = liver transplantation
Model adjusts for time-varying exposure status
Model restricted to patients with BCLC staging data (N = 432), and specifically those with BCLC stage 0, A, or B (n = 414)
Discussion
Unfortunately, due to a scarcity of donor organs, only one in six patients in need of a liver transplant is waitlisted each year, and up to 20% die on the waitlist each year.5 Because of the persistent mismatch between organ supply and demand, transplant physicians must balance the responsibility of advocating for individual patients with the overarching need to equitably and efficiently allocate scarce donor organs to those in greatest need who derive the greatest benefit from transplantation. In this analysis of patients with cirrhosis and HCC with low MELD scores, we demonstrated that LT is associated with significantly increased survival compared to resection and ablation.11−12 However, the incremental survival benefit in absolute terms is small at five and three years, and negligible at one year. Through multiple strategies of adjusted modeling, at five years from HCC diagnosis, the survival benefit of LT was uniformly less than 1.25 years over resection or ablation. Depending on one’s perspective (individual patient versus population health), this survival benefit of LT may or may not be considered sufficient to justify the use of a deceased donor liver that could otherwise be used for a patient with decompensated cirrhosis who lacks other curative options.
The concept of survival benefit of transplant was first introduced in the context of MELD score thresholds.23 More recently, due to the increasing number of patients transplanted for HCC, the issue of transplant-related survival benefit for HCC has come into question.9 In 2016, Berry et al. evaluating waitlisted patients from 2002–2013 to estimate the survival benefit of LT for HCC and non-HCC patients by calculating the difference of estimated 5-year post-LT and pre-LT survival.23 Based on this, the authors suggested there was a net negative survival benefit for HCC LT recipients with a calculated MELD score <14, which included 60% of all LT recipients with HCC during the study period. For patients with a MELD score of 14–21, the estimated 5-year survival benefit of LT was less than one year.9 Thus, the authors suggested that for the 90% of LT recipients with HCC with a MELD score ≤21, the survival benefit of LT was negligible, and potentially associated with a net negative survival benefit.10,23 Critics argued that this underestimated the survival benefit of LT because waitlist data are insufficient to estimate long-term survival without LT as the natural history of a patient’s clinical course cannot be evaluated when more than 80% of the patients with HCC are transplanted within two years of being waitlisted. It is for this reason that we sought to compare LT survival with resection and ablation in the VHA population, where LT is less frequently achieved.
In the US, HCC patients are more likely to be waitlisted than patients with decompensated cirrhosis, despite data suggesting a smaller relative survival benefit. Consideration of transplant for these patients should be viewed relative to the expected survival for patients undergoing other curative options (resection and ablation), and also relative to non-HCC patients with decompensated cirrhosis without other curative options. The relative survival benefit of transplant five years after HCC diagnosis compared to resection and ablation was between 1.04 and 1.24 years, but was less than 0.5 years over a three-year time horizon, and less than 0.05 years over a one-year time horizon. This reflects the fact that while all three options can be curative for a tumor or tumors at a specific time point, resection and ablation leave a diseased liver in place, with a risk of recurrent and/or de-novo HCC, while LT removes the liver, serving to dramatically reduce the risk of recurrence and mortality. Importantly, our estimates of survival benefit were similar when accounting for time-varying treatment exposure to address immortal time bias, and when isolating the cohort to early/intermediate stage HCC patients through manual chart review. In fact, when accounting for different times to therapy (immortal model), the survival benefit of LT was even lower (i.e., 1.04–1.10 years of survival benefit at five years). This intuitively makes sense, as the median time to transplant (1.25 years) was much longer than the median times to ablation (0.25 years) or resection (0.17 years).
A key consideration is whether a survival benefit of 1.04–1.24 years over five years clinically justifies transplant over other curative options. We must consider the increased risks and resources required for LT, as well as long-term health risk of immunosuppression, and quality of life after transplant and other procedures. When cost is added to the equation, data suggest that resection is the most cost-effective approach for early-stage HCC, with LT as the least cost-effective.35 Additionally, the magnitude of survival benefit is dependent on the availability of an organ; if the waiting period is too long, depending on tumor growth pattern, the survival benefit provided by transplantation may be dampened by the risks that patients face while waiting.36
Importantly, these considerations are for patients without a potential living liver donor, who are competing against patients with decompensated cirrhosis for a potential deceased donor organ. In the setting of living donor liver transplantation, our data would suggest that undergoing a living donor liver transplant is the preferred strategy, as it maximizes the chance for long-term survival without impacting the deceased donor organ pool. However, in the context of deceased donation, which mandates maximal utilization of a scarce resource,37,38 our data suggest that deceased donor LT for a low MELD HCC patient, rather than a patient with decompensated cirrhosis, fails to optimally utilize this scarce resource.
There are several limitations that we acknowledge in this work. First, the VHA system may not represent the overall population in terms of sex or socioeconomic conditions. However, the VHA is the largest single provider of liver care in the US, and importantly, we would not expect estimates of the survival benefit of LT relative to resection or ablation to differ substantially as a function of sex or socioeconomic status (indeed, these covariates were not retained in any multivariable models). Thus, this should not have biased our results. Second, it has been shown for the VOCAL database that among patients with potentially curable HCC (based on BCLC staging; n=1419), only 25% received potentially curative therapies such as resection, transplantation, or ablative therapy; even among patients with potentially curable HCC and good performance status (ECOG 1–2), 13% of patients did not receive active HCC therapy.22 Thus our analyses included a restricted cohort, which may limit external validity, but enhance internal validity by addressing a highly-selected population. Third, HCC staging data was not available for the entire cohort, which would have enabled more precision in isolating a similarly-staged cohort, in addition to narrowing confidence intervals for parameter estimates. However, in our subgroup analysis of patients with staging data, the overall study results were not substantively different. This implies that the analytic cohort that we identified was primarily comprised of patients with early/intermediate-stage HCC who could conceivably be candidates for LT, resection, or ablation. Fourth, we did not look beyond a five-year survival horizon, largely due to the available follow-up data. However, the focus of most survival benefit studies in LT range from one to five years, so our study is consistent with published literature. That being said, the benefit of LT over a longer-term is likely to be even greater. Fifth, we measured survival time from diagnosis, rather than from treatment. As noted before, this was an intentional decision to minimize bias, and we accounted for different times to treatment through time-updating modeling techniques. Additionally, our goal was to account not only for the time a patient undergoes treatment, but for the transplant recipient, the time they were evaluated and waitlisted which reflects a potentially more important measure. Sixth, there is likely some degree of residual confounding in our regression models. For example, we could not account for differing tumor locations, where certain modalities might not be a viable option. However, in circumstances where resection is infeasible based on location, ablation is typically an option. Thus, our goal of informing decisions when more than one curative option is available is still reflected by the data presented. Finally, we only included patients that were waitlisted and transplanted due to the nature of the data merge, and could not ascertain which patients were waitlisted but not transplanted. This is a small fraction of waitlisted patients, as 86.6% of veterans waitlisted patients with HCC in the MELD era are transplanted (according to OPTN/UNOS data as of 4/30/2018), and this exclusion would serve to overestimate the survival benefit of pursuing LT as a treatment option by excluding those who died without a LT.
In summary, these data demonstrate that over the short-term, LT offers minimal survival benefit compared to resection or ablation, but over a five-year time horizon, confers a survival benefit of 1.04–1.24 years. These data and others presented herein are important for contextualizing how to best allocate deceased donor organs, as well as counseling patients about their treatment options when diagnosed with early-stage HCC. Further data are needed to validate these results outside the VHA, however this work represents an important step in optimizing the care of patients with HCC while also remaining responsible stewards of the scarce resource of deceased donor organs.
Supplementary Material
Acknowledgments:
This work was supported by resources and facilities available through the Philadelphia VA Healthcare System as well as the central data repositories maintained by the VA Information Resource Center. The views expressed in this article do not reflect position or policy of the Department of Veterans Affairs or the United States government.
Financial Support: This work was supported by unrestricted research funds from Bayer Healthcare Pharmaceuticals and the VA HIV, Hepatitis and Public Health Pathogens Programs in the Office of Public Health/Clinical Public Health. This funding source had no role in data acquisition, analysis or interpretation of data. Nadim Mahmud is supported by a National Institutes of Health T32 grant (2-T32-DK007740–21A1).
Conflicts of interest: The views expressed in this article are those of the authors and do not necessarily represent the views of the U.S. Department of Veterans Affairs of the U.S. Government. This work was supported in part by Health Resources and Services Administration contract 234-2005-37011C. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. The authors have no additional disclosures.
Abbreviations:
- LT:
Liver transplant
- HCC:
Hepatocellular carcinoma
- HCV:
Hepatitis C virus
- NAFLD:
Nonalcoholic fatty liver disease
- MELD:
Model for End-Stage Liver Disease
- VHA:
Veterans Health Administration
- VOCAL:
Veterans Outcomes and Costs Associated with Liver Disease
- BMI:
Body mass index
- AFP:
Alpha fetoprotein
- HBV:
Hepatitis B virus
References
- 1.Goldberg D, Ditah IC, Saeian K, et al. Changes in the prevalence of hepatitis C virus infection, nonalcoholic steatohepatitis, and alcoholic liver disease among patients with cirrhosis or liver failure on the waitlist for liver transplantation. Gastroenterology. 2017;152(5):1090–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723–750. [DOI] [PubMed] [Google Scholar]
- 3.European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. [DOI] [PubMed] [Google Scholar]
- 4.Bruix J, Sherman M, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldberg D, French B, Newcomb C, et al. Patients With Hepatocellular Carcinoma Have Highest Rates of Wait-listing for Liver Transplantation Among Patients With End-Stage Liver Disease. Clin Gastroenterol Hepatol. 2016;14(11):1638–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldberg D, French B, Abt P, et al. Increasing disparity in waitlist mortality rates with increased model for end-stage liver disease scores for candidates with hepatocellular carcinoma versus candidates without hepatocellular carcinoma. Liver Transpl. 2012;18(4):434–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bittermann T, Niu B, Hoteit MA, et al. Waitlist priority for hepatocellular carcinoma beyond milan criteria: a potentially appropriate decision without a structured approach. Am J Transplant. 2014;14(1):79–87. [DOI] [PubMed] [Google Scholar]
- 8.Bittermann T, Hoteit MA, Abt PL, et al. Waiting time and explant pathology in transplant recipients with hepatocellular carcinoma: a novel study using national data. Am J Transplant. 2014;14(7):1657–1663. [DOI] [PubMed] [Google Scholar]
- 9.Berry K, Ioannou GN. Comparison of Liver Transplant-Related Survival Benefit in Patients With Versus Without Hepatocellular Carcinoma in the United States. Gastroenterology. 2015;149(3):669–680. [DOI] [PubMed] [Google Scholar]
- 10.Mehta N, Heimbach J, Hirose R, et al. Minimal Transplant Survival Benefit for Hepatocellular Carcinoma: Is it Real or an Overestimation of Waitlist Life Expectancy? Gastroenterology. 2016;150(2):533–534. [DOI] [PubMed] [Google Scholar]
- 11.Goldberg DS, French B, Forde KA, et al. Association of distance from a transplant center with access to waitlist placement, receipt of liver transplantation, and survival among US veterans. JAMA. 2014;311(12):1234–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaplan DE, Dai F, Aytaman A, et al. Development and Performance of an Algorithm to Estimate the Child-Turcotte-Pugh Score From a National Electronic Healthcare Database. Clin Gastroenterol Hepatol. 2015;13(13):2333–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahmud N, Kaplan DE, Taddei TH, et al. Incidence and Mortality of Acute-on-Chronic Liver Failure using Two Definitions in Patients with Compensated Cirrhosis. Hepatology. 2019;69(5):2150–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sohn M-W, Arnold N, Maynard C, et al. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr. 2006;4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehta N, Heimbach J, Harnois DM, et al. Validation of a risk estimation of tumor recurrence after transplant (RETREAT) score for hepatocellular carcinoma recurrence after liver transplant. JAMA Oncol. 2017;3(4):493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahmud N, Shaked A, Olthoff KM, et al. Differences in Posttransplant Hepatocellular Carcinoma Recurrence by Etiology of Liver Disease. Liver Transpl. 2019;25(3):388–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ioannou GN, Perkins JD, Carithers RL Jr. Liver transplantation for hepatocellular carcinoma: impact of the MELD allocation system and predictors of survival. Gastroenterology. 2008;134(5):1342–1351. [DOI] [PubMed] [Google Scholar]
- 18.Eyawo O, McGinnis KA, Justice AC, et al. Alcohol and Mortality: Combining Self-Reported (AUDIT-C) and Biomarker Detected (PEth) Alcohol Measures Among HIV Infected and Uninfected. J Acquir Immune Defic Syndr. 2018;77(2):135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Justice AC, Smith RV, Tate JP, et al. AUDIT-C and ICD codes as phenotypes for harmful alcohol use: association with ADH1B polymorphisms in two US populations. Addiction. 2018;113(12):2214–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGinnis KA, Tate JP, Williams EC, et al. Comparison of AUDIT-C collected via electronic medical record and self-administered research survey in HIV infected and uninfected patients. Drug Alcohol Depend. 2016;168:196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beste LA, Leipertz SL, Green PK, et al. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001–2013. Gastroenterology. 2015;149(6):1471–1482. [DOI] [PubMed] [Google Scholar]
- 22.Serper M, Taddei TH, Mehta R, et al. Association of Provider Specialty and Multidisciplinary Care With Hepatocellular Carcinoma Treatment and Mortality. Gastroenterology. 2017;152(8):1954–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Merion RM, Schaubel DE, Dykstra DM, et al. The survival benefit of liver transplantation. Am J Transplant. 2005;5(2):307–313. [DOI] [PubMed] [Google Scholar]
- 24.Liu J, Weinhandl ED, Gilbertson DT, et al. Issues regarding ‘immortal time’in the analysis of the treatment effects in observational studies. Kidney Int. 2012;81(4):341–350. [DOI] [PubMed] [Google Scholar]
- 25.Liu J, Weinhandl E, Peter WS. Immortal time bias must be considered in observational studies Paper presented at: American Society of Nephrology Conference; November 4–9, 2008; Philadelphia, PA. [Google Scholar]
- 26.Jones M, Fowler R. Immortal time bias in observational studies of time-to-event outcomes. J Crit Care. 2016;36:195–199. [DOI] [PubMed] [Google Scholar]
- 27.Uno H, Claggett B, Tian L, et al. Moving beyond the hazard ratio in quantifying the between-group difference in survival analysis. J Clin Oncol. 2014;32(22):2380–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sung RS, Zhang M, Schaubel DE, et al. A reassessment of the survival advantage of simultaneous kidney-pancreas versus kidney-alone transplantation. Transplantation. 2015;99(9):1900–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334(11):693–699. [DOI] [PubMed] [Google Scholar]
- 30.Lin CT, Hsu KF, Chen TW, et al. Comparing hepatic resection and transarterial chemoembolization for Barcelona Clinic Liver Cancer (BCLC) stage B hepatocellular carcinoma: change for treatment of choice? World J Surg. 2010;34(9):2155–2161. [DOI] [PubMed] [Google Scholar]
- 31.Ciria R, López-Cillero P, Gallardo AB, et al. Optimizing the management of patients with BCLC stage-B hepatocellular carcinoma: modern surgical resection as a feasible alternative to transarterial chemoemolization. Eur J Surg Oncol. 2015;41(9):1153–1161. [DOI] [PubMed] [Google Scholar]
- 32.Zhong JH, Xiang BD, Gong WF, et al. Comparison of long-term survival of patients with BCLC stage B hepatocellular carcinoma after liver resection or transarterial chemoembolization. PloS One. 2013;8(7):e68193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galle PR, Tovoli F, Foerster F, et al. The treatment of intermediate stage tumours beyond TACE: From surgery to systemic therapy. J Hepatol. 2017;67(1):173–183. [DOI] [PubMed] [Google Scholar]
- 34.Bolondi L, Burroughs A, Dufour JF, et al. Heterogeneity of patients with intermediate (BCLC B) Hepatocellular Carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis. 2012;32(4):348–359. [DOI] [PubMed] [Google Scholar]
- 35.Shaya FT, Breunig IM, Seal B, et al. Comparative and cost effectiveness of treatment modalities for hepatocellular carcinoma in SEER-Medicare. Pharmacoeconomics. 2014;32(1):63–74. [DOI] [PubMed] [Google Scholar]
- 36.Sarasin FP, Giostra E, Mentha G, et al. Partial hepatectomy or orthotopic liver transplantation for the treatment of resectable hepatocellular carcinoma? A cost-effectiveness perspective. Hepatology. 1998;28(2):436–442. [DOI] [PubMed] [Google Scholar]
- 37.Ioannou GN. Transplant-related survival benefit should influence prioritization for liver transplantation especially in patients with hepatocellular carcinoma. Liver Transpl. 2017;23(5):652–662. [DOI] [PubMed] [Google Scholar]
- 38.Schaubel DE, Guidinger MK, Biggins SW, et al. Survival benefit-based deceased-donor liver allocation. Am J Transplant. 2009;9(4 Pt 2):970–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.