Highlights
-
•
As in a previous study, higher GABA concentrations in the dorsolateral prefrontal cortex (DLPFC) were associated with better working memory (WM) in healthy participants.
-
•
Despite no overall group difference in DLPFC GABA concentrations, people with schizophrenia showed significantly different inverse associations, with higher DLPFC GABA associated with worse rather than better WM.
-
•
This opposite pattern of correlations despite a lack of group differences suggests that schizophrenia alters the distribution of different classes of GABAergic interneurons rather than producing a general deficit across the total population of neurons.
Keywords: Functional neuroimaging, Gamma-aminobutyric acid (GABA), Working memory, Psychosis, Magnetic resonance spectroscopy (MRS)
Abstract
Objectives
Gamma-Amiobutyric acid (GABA) is a primary inhibitory neurotransmitter that facilitates neural oscillations that coordinate neural activity between brain networks to facilitate cognition. The present magnetic resonance spectroscopy (MRS) study tests the hypothesis that GABAergic facilitation of working memory is disrupted in people with schizophrenia (PSZ).
Methods
51 healthy participants and 40 PSZ from the UC Davis Early Psychosis Program performed an item and temporal order working memory (WM) task and underwent resting MRS to measure GABA and glutamate concentrations in dorsolateral prefrontal (DLPFC) and anterior cingulate (ACC) regions of interest. MRS was acquired on a 3 Tesla Siemens scanner and GABA and glutamate concentrations were referenced to creatine. Percent correct on the WM task indexed performance and correlation coefficients examined GABAergic or Glutamatergic facilitation of WM, with Fisher's Z transformation testing for group differences.
Results
There were no group differences in GABA or glutamate concentrations, but WM correlations were reversed between groups. In patients, higher DLPFC GABA was associated with worse rather than better WM performance. This pattern was not observed for glutamate or in the ACC. Although under-powered, there was no indication of medication effects.
Conclusions and Relevance
Results cannot be explained by group differences in DLPFC GABA or glutamate concentrations but, instead, indicate that schizophrenia disrupts the GABAergic facilitation of WM seen in healthy individuals. Results appear to parallel post mortem findings in suggesting that schizophrenia alters the distribution of different classes of GABAergic interneurons rather than producing a general deficit across the total population of neurons.
1. Introduction
Gamma-Aminobutyric acid (GABA) is the primary inhibitory neurotransmitter in the brain, and GABA-ergic regulation of neural excitability appears to play a critical role in coordination of activity within and between local brain circuits that support high level cognition (Whittington et al., 1995; Vida et al., 2006). Magnetic resonance spectroscopy (MRS) has emerged as a valuable tool for noninvasively investigating the relationship between GABA and cognition. For example, Porges et al. (2017) found that higher GABA concentrations in frontal but not in posterior cortex predicted better performance on a summary cognitive measure in healthy older adults. In our own laboratory, Yoon and colleagues Yoon et al. (2016) found that higher GABA concentrations in the dorsolateral prefrontal cortex (DLPFC) were correlated with better working memory (WM performance at high load relative to low load task conditions in healthy subjects. Supporting specificity to DLPFC and WM, these associations were not observed in the primary visual cortex, or for other task conditions. These MRS findings converge with the earlier work of Patricia Goldman-Rakic (Rao et al., 2000), who used neural recordings in non-human primates to demonstrate that prefrontal GABAergic interneurons regulate the firing of excitatory glutamatergic neurons in the PFC during WM delay periods.
People with schizophrenia show significant deficits in cognition, and they are particularly impaired at WM tasks that place heavy demands on cognitive control processes dependent on the DLPFC (Barch et al., 2003; Lesh et al., 2011). Given the basic research described above, there is reason to suspect that WM deficits in schizophrenia might reflect disruption of GABAergic function. Indeed, one of the most consistent post-mortem findings (Hyde et al., 2011) in schizophrenia research is deficient GABA synthesis, due to reduced transcription of the GAD67 gene in specific subclasses of interneurons in the DLPFC (Akbarian et al., 1995; Volk et al., 2001), anterior cingulate gyrus (ACC) (Woo et al., 2007), and hippocampus (Benes et al., 2007). This deficit in GAD67 mRNA is most consistently found in a subset of parvalbumin containing GABAergic basket cell inter-neurons, leading to a reduced production of GABA in the cortex of people with schizophrenia (Lewis 2014). These combined human and animal studies make GABAergic inhibition a potential therapeutic target (Vinkers et al., 2010) for improving well-documented WM deficits (Barch and Sheffield 2014) in people with schizophrenia.
Despite the consistency of the post-mortem findings described above, it has been difficult to establish a reproducible in vivo pattern of reduced GABA concentrations in patients relative to controls using MRS. For example, two recent meta-analyses (Egerton et al., 2017; Iwata et al., 2018) failed to find any group differences in GABA concentrations at a meta-analytic level. Although several MRS studies found GABA reductions in patients (Goto et al., 2009; Yoon et al., 2010; Kelemen et al., 2013; Wang et al., 2019), an equal number found either no group differences (Tayoshi et al., 2010; Kegeles et al., 2012; Chen et al., 2014), or increased MRS GABA concentrations in patients relative to controls (Ongur et al., 2010; Rowland et al., 2013). Explanations for this lack of convergence between post-mortem and MRS findings include the often-greater illness progression and lifetime medication exposure of post-mortem individuals (Szulc et al., 2013; Brandt et al., 2016), although there have also been failures to find differences between medicated and un-medicated patients (Wood et al., 2008; Kegeles et al., 2012). An alternative explanation is that GABAergic function in schizophrenia may be specifically disrupted in particular brain regions like the DLPFC—regions that are known to contribute to the kinds of cognitive processes that are impaired in schizophrenia.
The goal of this study is to examine the impact of schizophrenia on the association between DLPFC GABA and WM performance using single-voxel proton MRS. Data were also obtained in an ACC ROI as a control region, and with a conventional PRESS acquisition to obtain glutamate concentrations (Glu) to provide tissue measurements of both excitatory and inhibitory neurotransmitters. To examine these associations, we utilized a WM task that has been shown to elicit DLPFC activity (Roberts et al., 2018) and neural oscillations that depend on inhibitory neurotransmission in PFC (Hsieh et al., 2011). We chose this task because our previous functional MRI research (Ragland et al., 2012; Ragland et al., 2015) found that people with schizophrenia had a disproportionate deficit in relational versus item-specific working and long-term memory that was accompanied by a reduction in DLPFC activation for relational but not item-specific task conditions. We predicted that patients would show a regionally specific pattern of disrupted GABAergic facilitation of WM in a DLPFC but not in an ACC ROI. Additionally, our paradigm allowed us to separately test relationships between GABA and a WM task that involved maintenance of temporal order information and a WM task that required maintenance of detailed visual information about items.
2. Methods
2.1. Participants
Data are reported on 51 controls and 40 patients with schizophrenia. Patients were early in their illness (within 5 years of illness onset). Groups were matched for age, gender, handedness and parental education (Table 1). As expected, participant education and an estimate of premorbid intellectual ability (WTAR) was lower in patients. All but 9 patients were receiving medication (all atypical antipsychotics) and were clinically stable with mild to moderate symptomatology (Table 1). Subjects were excluded for substance dependence or a positive urine drug screen, a neurological illness, head trauma leading to unconsciousness, low IQ (WAIS-R Total score < 70), corrected vision that does not achieve 20/30, pregnancy, ferrous metal in any part of body, serious medical conditions, and claustrophobia. Data collection began after participants provided written informed consent following Institutional Review Board approval.
Table 1.
Participant demographics.
| Healthy control group (n =51) | People with schizophrenia (n =40) | p-value | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age (years) | 24.2 | 4.7 | 23.5 | 4.6 | ns |
| Gender (% male) | 67 | 70 | ns | ||
| Handedness (% right) | 94 | 95 | ns | ||
| WTAR | 41.5 | 5.5 | 38.3 | 8.3 | p < .05 |
| Education (years) | 15.0 | 2.1 | 13.6 | 1.8 | p < .005 |
| Parental Education (years) | 13.9 | 2.4 | 14.6 | 2.7 | ns |
| SANS (total) | – | – | 20.4 | 12.5 | – |
| SAPS (total) | – | – | 6.0 | 8.0 | – |
| BPRS (total) | – | – | 37.5 | 8.3 | – |
Note: ns = no significant group difference at p<.05, two-tailed; WTAR = Wechsler Test of Adult Reading; Parental Education = average number of years across parents
2.2. Procedures
After informed consent, each participant performed a resting-state (eyes open) MRS study and a Temporal-Order and Item Maintenance WM paradigm (Hsieh et al., 2011) that was administered separately during EEG, which will be reported in a separate manuscript. Studies were performed within 2-weeks of each other, and patients did not experience any changes in medication or clinical status between the two sessions.
2.3. MRS acquisition
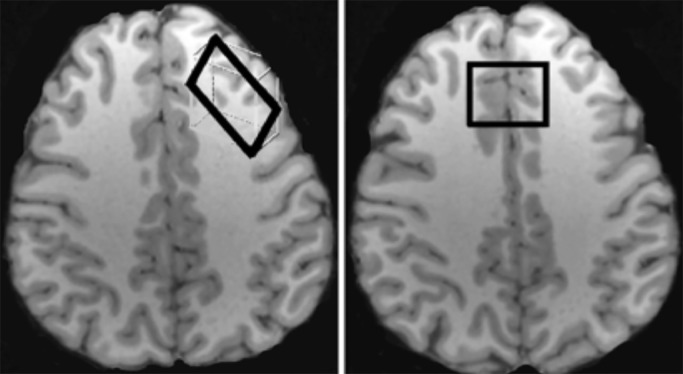
Participants were scanned with proton MRS using a Siemens TIM Trio 3T MRI system with a 32-channel head coil. An anatomical MRI was acquired and used for placement of DLPFC and ACC voxels (Fig. 1). As in our previous study (Yoon et al., 2016), the DLPFC voxel was placed in the left middle frontal gyrus over Brodmann areas 9 and 46, and angled parallel to the brain surface, with dimensions of 30 mm x 15 mm x 35 mm (volume 15.75 cm3). The ACC voxel was un-rotated and placed midline over Brodmann area 32, approximately 1 cm above the upper extent of the body of the lateral ventricles, with the posterior extent abutting the corpus callosum, and voxel dimensions of 30 mm x 25 mm x 25 mm (volume 18.75 cm3).
Fig. 1.
Placement of DLPFC (left) and ACC voxels (right).
After voxel placement, we acquired GABA measurements using a Mescher-Garwood point resolved spectroscopy (MEGA-PRESS) GABA editing sequence with water suppression (Mescher et al., 1998; Mullins et al., 2014). This single voxel J-difference spectral editing sequence enables the GABA resonance at 2.99 ppm to be distinguished from the overlapping creatine singlet, as well as removing other resonances. This is achieved through the subtraction of two independently acquired spectra that have GABA J-coupled resonances of opposite polarity at 2.99 ppm. MRS spectra were acquired with the following parameters: TR/TE: 1500/68; acquisition duration: 852 ms; 16 step EXOR phase cycling; data points: 1024; receiver bandwidth: 1200 Hz; edit pulse on resonance frequency: 1.9 ppm; edit pulse off resonance frequency: 7.5 ppm; delta frequency: 1.7 ppm; edit pulse bandwidth: 45 Hz. Acquisitions in which the spectral editing pulse was applied on resonance were interleaved with those in which the pulse was applied off resonance for a total of 144 acquisitions in each of four consecutive 3.6 min subscans in the 2 brain regions studied (DLPFC and ACC). The water frequency was adjusted before each 3.6 min scan. The subscans were later combined off-line. GABA content measured by this method includes signal from macromolecules and homocarnosine that coedit with GABA. Total GABA acquisition time for each voxel location was 14.4 min, and the order of DLPFC versus ACC voxel acquisition was randomized across subjects. To measure DLPFC glutamate content, we also obtained a PRESS acquisition from the DLPFC voxel using the following parameters: TR/TE = 1500/80 ms, delta frequency = −1.7 ppm, NEX = 160, duration = 4.0 min.
2.4. Working memory assessment
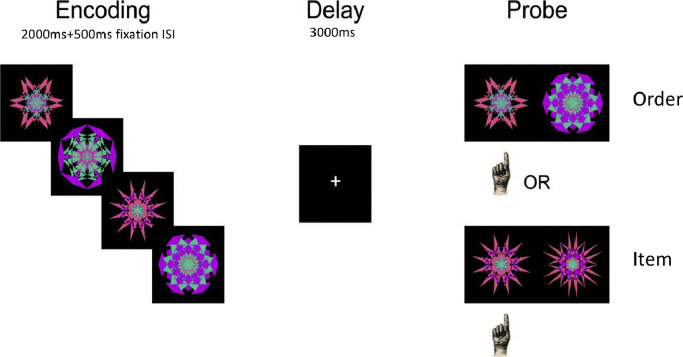
The Temporal-Order and Item Maintenance task (Hsieh et al., 2011) is illustrated in Fig. 2. On each trial, subjects first see an instruction word, either “ITEM” or “ORDER”, followed by a memory set of four sequentially presented kaleidoscopic (fractal) images. Following a 3-s retention delay, a test probe is shown. On “order” trials, the probe consists of two fractals from the previous sequence, and subjects were asked to make a left-handed button-press if the object on the left was seen first, and a right-handed button press if the object on the right was seen first. On “item” trials, the test probe consisted of one previously presented fractal along with another fractal that was never presented, and subjects were instructed to make a left handed button-press if the object on the left is old, and a right handed response if the object on the right is old. During each run, blocks of 10 order trials and 10 item trials were presented in a counter-balanced order, and the sequence was repeated for 10 runs, requiring about 60 min to complete. Participants were given a practice task to ensure comprehension and instructed to respond as quickly and accurately as possible.
Fig. 2.
Illustration of Temporal Order and Item Maintenance Task.
2.5. Data analysis and hypothesis testing
2.5.1. MRS processing
Using jMRUI 4.0 software, all pairs of on- and off-resonance spectra from the MEGA-PRESS acquisitions were phase aligned with reference to creatine, zero filled to 4096, apodized with a 4 Hz Gaussian filter, and frequency aligned to creatine (Cr) at 3.02 ppm. Difference spectra were processed in parallel fashion, with the phase correction derived from each pair of on and off spectra applied to each difference spectrum. Peak integration was used to quantify GABA (2.99 ± 0.12 ppm) in the difference spectra and Cr (3.02 ± 0.09 ppm) in the summed on and off spectra, as described previously (Yoon et al., 2010). The ratio of GABA/Cr signal averaged across all subscans in each brain region for each subject was used for hypothesis testing. Normalizing to Cr reduces intersubject variance attributable to differences in global signal strength and CSF fraction within the voxel and has been shown to yield reliable GABA values (Greenhouse et al., 2016). A large, multicenter study has shown that measures of GABA normalized to creatine were reliable and exhibited strong agreement across sites (Mikkelsen et al., 2017). All final difference spectra were inspected for water or lipid contamination.
PRESS spectra were analyzed with LCModel software (v6.3 1-L) (Provencher 2001). Metabolite values were fit using an analysis window from 4.0 to 1.7 ppm and a simulated basis set provided with LCModel that included the following metabolites: glutamate, glutamine, glutathione, creatine, phosphocreatine, n-acetylaspartate, n-acetylaspartylglutamate, phosphocholine, glycerophosphorylcholine, myo-inositol, scyllo-inositol, aspartate, taurine, GABA, and glucose. Glutamate signal (Glu) was normalized to the LCModel fits for creatine plus phosphocreatine (Cr). The Glu/Cr ratio was used for hypothesis testing. All GABA/Cr and Glu/Cr distributions were inspected for extreme outliers, defined as a modified Z score > 4.0 by the method of Iglewicz and Hoaglin (Iglewicz and Hoaglin 1993).
Data quality assessment (QA) was performed by examining signal to noise (SNR) and spectroscopic line width resolution (FWHM) for both the PRESS and the MEGA-PRESS acquisitions. In addition, the Cramer–Rao lower bound (CRLB; Kreis 2004) provided an index of the reliability of the glutamate estimates from the PRESS spectra. As can be seen in Table 2, QA metrics were excellent across both groups and regions.
Table 2.
Mean (±SD) Quality Assessment (QA) metrics for MRS acquisitions.
| SNRa | FWHM (ppm)b | CRLB (glu only)c | |
|---|---|---|---|
| Off-res MPRESS DLPFCd | |||
| Patients | 39.0 (4.1) | 0.036 (0.007) | |
| Controls | 39.3 (4.8) | 0.038 (0.010) | |
| Off-res MPRESS ACCe | |||
| Patients | 36.9 (5.9) | 0.040 (0.013) | |
| Controls | 37.0 (6.0) | 0.042 (0.013) | |
| PRESS DLPFCf | |||
| Patients | 33.2 (3.7) | 0.041 (0.006) | 5.7% (0.7) |
| Controls | 33.0 (4.8) | 0.042 (0.008) | 5.8% (0.7) |
Abbreviations:.
SNR = signal to noise ratio.
FWHM = full width half max line width spectral resolution.
CRLB = Cramer–Rao lower bound.
Off-res MPRESS DLPFC = off-resonance Mega-PRESS GABA acquisition in dorsolateral prefrontal cortex voxel.
Off-res MPRESS ACC = off-resonance Mega-PRESS GABA acquisition in anterior cingulate cortex voxel.
PRESS DLPFC = PRESS glutamate acquisition in dorsolateral prefrontal cortex voxel.
2.5.2. Working memory processing
Percent correct performance (out of 100 item and 100 order trials) was calculated separately for each task condition to provide a measure of WM accuracy. Data were inspected to insure that all participants were performing above chance (i.e., > 50% accuracy), and no participants had to be excluded for below-chance performance. Percent correct performance was entered into a two (patient, control) by two (item, order) repeated-measures analysis of variance (ANOVA). Associations with MRS GABA and Glutamate concentrations were examined with separate Pearson Product Moment correlations, and a Fisher's Z transformation was performed to test for significant group differences in correlational results. Significance level was set at p < .05, two-tailed for all analyses.
3. Results
3.1. Gray and white matter concentrations
Given potential anatomical differences between groups, we first examined gray and white matter concentrations in DLPFC and ACC MRS voxels to rule-out any group differences that could influence MRS findings. Gray and white matter proportions were estimated for each subject and each voxel utilizing their segmented MPRAGE image, with MRS voxels applied as a mask. T-test analyses did not reveal any group differences in percent gray (p-value =.17) or white matter (p-value =.26) in the ACC and also failed to reveal any group differences in the DLPFC for either gray (p-value =.19) or white matter (p-value =.28).
3.2. GABA
DLPFC GABA data were excluded for two subjects: one control subject whose spectra were excluded due to excessive lipid contamination, and one patient whose GABA/Cr value was an extreme outlier (modified Z score > 4). MRS spectra for the remaining 50 control subjects and 39 patients are illustrated in Fig. 3. Group comparisons revealed that DLPFC GABA/Cr values were similar for patients (0.147 ± 0.013) and controls (0.145 ± 0.011; p-value =.62) and did not differ between medicated (0.147 ± 0.013) and un-medicated patients (0.144 ± 0.014; p-value =.54). Because analysis of medication effects was under-powered, we also examined effect sizes, which revealed small differences in DLPFC GABA/Cr ratios between medicated and unmedicated patients (Cohen's d = 0.22). ACC GABA data were not obtained in two control subjects and two patients. In the remaining 49 control subjects and 38 patients, ACC GABA/Cr values were similar for patients (0.156 ± 0.022) and controls (0.151 ± 0.024; p-value =.28) and did not differ between medicated (0.151 ± 0.013) and un-medicated patients (0.154 ± 0.015; p-value =.64; Cohen's d = 0.21).
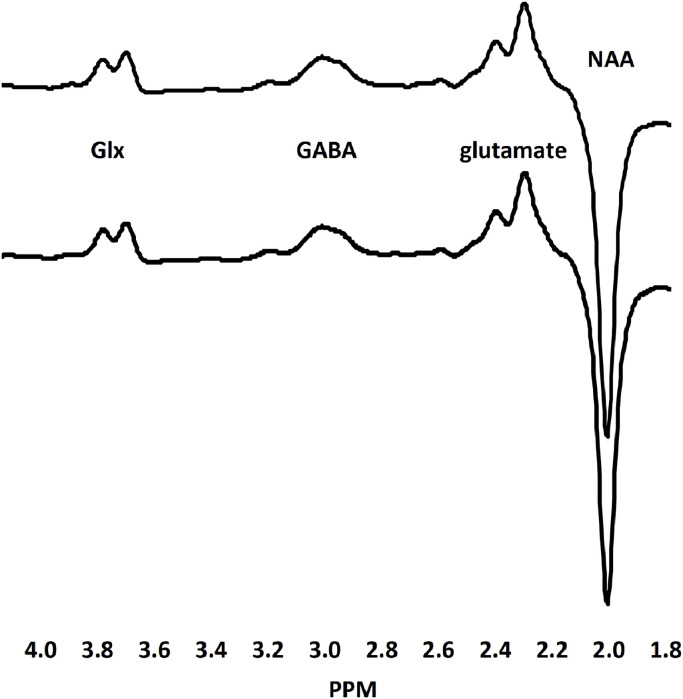
Fig. 3.
Creatine-normalized grand mean difference spectra for people with schizophrenia (top tracing) and healthy controls (bottom tracing). The edited resonances for GABA, glutamate and Glx (glutamate plus glutamine) and the inverted NAA peak are indicated.
3.3. Glutamate
DLPFC Glu/Cr values were excluded for two control subjects and two patients whose PRESS spectra did not meet our LCModel FWHM and SNR inclusion thresholds (≤ 0.063 ppm and ≥ 20 respectively). In the remaining 49 control subjects and 38 patients, FWHM values ranged from 0.028 to 0.063 ppm (mean = 0.042) and SNR values ranged from 24 to 43 (mean = 33.0). In these subjects, DLPFC Glu/Cr values were similar for patients (0.894 ± 0.104) and controls (0.906 + +0.091; p-value =.54) and did not significantly differ between medicated (0.892 ± 0.093) and unmedicated patients (0.900 ± 0.134; p-value =.82; Cohen's d = 0.07).
3.4. Working memory
Accuracy was generally higher on Item (80 ± 11) than on Order (75 ± 14) trials [F(1,86) =14.7, p < .001], and overall accuracy was lower for patients than controls [F(1,86) =30.0, p < .0001] on both Item (73 ± 12 vs. 84 ± 8) and Order tasks (67 ± 14 vs. 80 ± 10). There was no effect of medication on WM performance (t-values <1.0, p > .05; Cohen's d = 0.23).
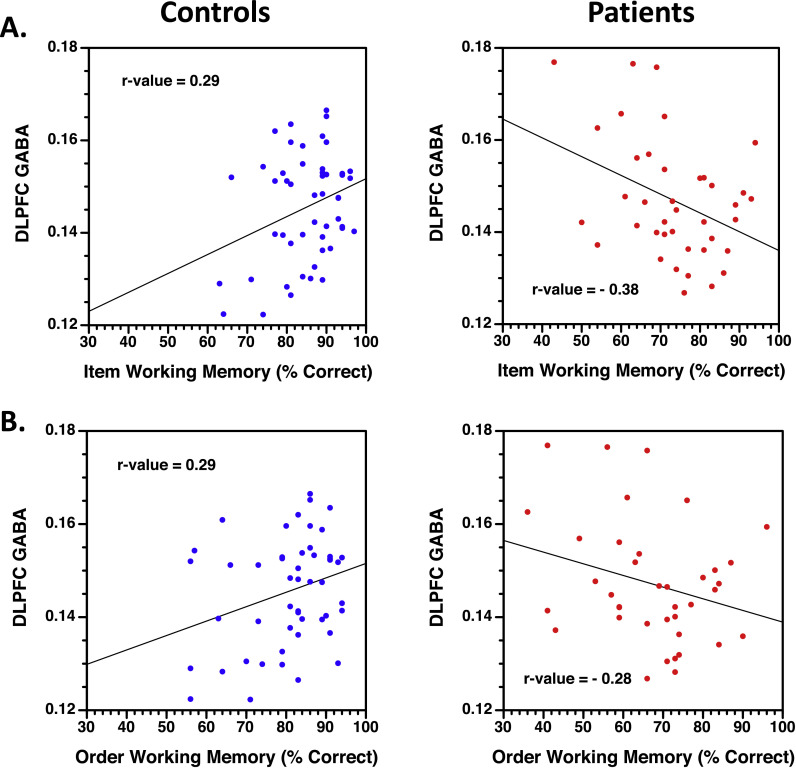
3.5. GABA correlations with working memory
For healthy controls, higher DLPFC GABA/Cr concentrations were correlated with better WM on both Item (r-value =0.29, p < .05; Fig. 4A) and Order tasks (r-value =0.29, p < .05; Fig. 4B). Conversely, higher GABA/Cr values correlated with worse WM in patients for the Item task (r-value =−0.38, p < .005), with a similar trend evident for the Order task (r-value =−0.27, p =.09). Crucially, these correlations differed between groups for both Item (Fisher's Z =3.15, p < .005) and Order conditions (Fisher's Z =2.60, p < .005), with no medication effect (all Fisher's Z < 0.1). There were no significant correlations between WM performance and either ACC GABA/Cr or DLPFC Glu/Cr values.
Fig. 4.
Scatter plots of correlations between DLPFC GABA/Cr ratios (y-axis) and percent correct working memory performance for Item (1A) and Order task conditions (1B). Healthy controls presented on the left (blue symbols) and people with schizophrenia on right (red symbols). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
The goal of this study was to examine relationships between prefrontal GABA levels and WM performance in schizophrenia. We replicated and extended our previous findings (Yoon et al., 2016) showing that higher GABA concentrations in the DLPFC are associated with better WM performance in healthy participants. Our results additionally show that healthy controls with higher DLPFC GABA were better able to maintain object and temporal order information in WM. This facilitation effect appeared to be both regionally specific – as it was not observed in the anterior cingulate, and specific to inhibitory (GABA) and not excitatory (glutamate) transmitter levels. Our prediction of disrupted GABAergic modulation of WM in people with schizophrenia was also supported. Patients produced negative correlations between DLPFC GABA and WM performance that were significantly different that the positive correlations evidenced by healthy controls. Moreover, these results did not appear to be secondary to group differences in gray or white matter tissue concentrations, were not affected by medication status and were, again, specific to the DLPFC rather than the ACC and to GABA rather than glutamate.
Although our results revealed significant disruption of the relationship between DLPFC GABA levels and WM performance in schizophrenia we found no evidence of overall GABA deficits in patients either for DLPFC or ACC. Previous meta-analyses of the MRS literature have not shown reliable group differences in MRS GABA concentrations across many regions of the brain (medial and dorsolateral PFC; temporal, parietal and occipital cortex; basal ganglia, hippocampus and thalamus) (Egerton et al., 2017; Iwata et al., 2018). Previous MRS studies have not consistently found significant medication effects (Wood et al., 2008; Kegeles et al., 2012) and, in our sample, GABA concentrations values in DLPFC and ACC were almost identical across medicated and unmedicated patients, with small effect sizes of medication status. Conversely, results from a recent positron emission tomography (PET) study converged with post-mortem findings and found that antipsychotic treatment appears to normalize GABA signaling that is typically reduced in patients prior to treatment (Frankle et al., 2015). The consistency of post-mortem findings and inconsistency of MRS GABA results deserves further scrutiny as our unmedicated sample was very small.
As previously noted, post-mortem analyses reveal that GAD67 is reduced only in sub-populations of GABAergic inter-neurons in people with schizophrenia (Akbarian et al., 1995; Lewis 2012), whereas MRS measures GABA in all intra- and extra-cellular compartments across all populations of brain cells within the ROI. This global MRS value may, therefore, reduce sensitivity to group differences in a smaller population of parvalbumin containing inter-neurons. The absence of group differences in the current study contrasts with consistent prior reports of a schizophrenia-related reduction in GABA concentration in the early visual cortex (Yoon et al., 2010; Kelemen et al., 2013). This may reflect a regional difference in how the disease process affects global GABA levels as measurable with MRS. For example, post-mortem studies show a normal gradient in GABA neurotransmission, with a decrease in GABA transcript levels from the visual cortex to the DLPFC (Hoftman et al., 2018). It is also possible that the greater signal-to-noise (SNR) in the posterior cortex where the skull is in direct contact with MRI head coil leads to better detection of group differences in early visual cortex. Use of higher-field scanners increases SNR and may also improve detection of group differences. However, recent 7 Tesla studies have been both successful (Marsman et al., 2014; Wang et al., 2019) and unsuccessful (Reid et al., 2018) in detecting reduced GABA concentrations between psychosis patients and healthy controls.
4.1. Conclusions
In the present study, in a large sample of people with schizophrenia, we found no evidence for an overall change in DLPFC GABA or glutamate concentrations in either the DLPFC or the ACC. Instead, our results showed significant group differences in the relationship between GABA levels and WM performance. Unlike controls, higher GABA/Cr ratios were correlated with worse rather than better cognitive performance in the patient sample. Previous MRS studies have also found inverse correlations between cognition and GABA levels (Marsman et al., 2014; Reid et al., 2018). However, Rowland et al. (2013) found that ACC GABA was positively correlated with a coding test of attention in a pooled sample of schizophrenia patients and healthy volunteers. Recent developments in performing functional task-based MRS studies (Jelen et al., 2018) may help to improve understanding of these brain-behavior relationships.
Given that overall prefrontal GABA concentrations were relatively normal in the early psychosis sample, altered relationships between GABA levels and cognition could reflect differences in the distribution of GABA across different classes of GABAergic interneurons, which might, in turn, lead to alterations in neural activity in circuitry associated with WM. Changes in the relative functioning of parvalbumin-containing Interneurons (Lewis 2012) and cholecystokinin expressing (CCKb) basket cells (Hartwich et al., 2009), might produce network-level imbalances that alter interactions between DLPFC and posterior cortical regions, and the hippocampus.
Financial disclosures
Dr. Ragland has received research grants from the NIH, NARSAD and the Robert Wood Johnson Foundation.
Dr. Maddock has received research grants from the NIH.
Ms. Hurtado has no currently active grant or contract support from private or public sources.
Dr. Tanase and dr. Lesh do not report any conflicts.
Dr. Niendam has received research grants from the NIH, NARSAD, the Robert Wood Johnson Foundation, and the UC Davis Behavioral Health Center of Excellence.
Dr. Carter has received research grants from the NIMH, NIDA, and the Robert Wood Johnson Foundation.
Dr. Ranganath has received research grants from the NIH, the Department of Defense, and has been an external consultant for Helicon Pharmaceuticals.
CRediT authorship contribution statement
J.D. Ragland: Conceptualization, Funding acquisition, Investigation, Writing - original draft, Writing - review & editing, Project administration. R.J. Maddock: Methodology, Resources, Writing - review & editing. M.Y. Hurtado: Investigation, Data curation. C. Tanase: Methodology, Resources. T.A. Lesh: Writing - review & editing. T.A. Niendam: Resources, Writing - review & editing. C.S. Carter: Resources, Writing - review & editing. C. Ranganath: Funding acquisition, Methodology, Resources, Writing - review & editing.
Acknowledgments
This research was supported by the National Institute of Health [grant: R01 MH105411].
We thank Scott Martin, Jerry Sonico and Dennis Thompson at the Imaging Research Center for their technical assistance, and our participants for their time, energy and cooperation.
References
- Akbarian S., Huntsman M.M., Kim J.J., Tafazzoli A., Potkin S.G., Bunney W.E., Jr, Jones E.G. GABA a receptor subunit gene expression in human prefrontal cortex: comparison of schizophrenics and controls. Cereb. Cortex. 1995;5(6):550–560. doi: 10.1093/cercor/5.6.550. [DOI] [PubMed] [Google Scholar]
- Barch D.M., Sheffield J.M. Cognitive impairments in psychotic disorders: common mechanisms and measurement. World Psychiatry. 2014;13(3):224–232. doi: 10.1002/wps.20145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch D.M., Sheline Y.I., Csernansky J.G., Snyder A.Z. Working memory and prefrontal cortex dysfunction: specificity to schizophrenia compared with major depression. Biol. Psychiatry. 2003;53(5):376–384. doi: 10.1016/s0006-3223(02)01674-8. [DOI] [PubMed] [Google Scholar]
- Benes F.M., Lim B., Matzilevich D., Walsh J.P., Subburaju S., Minns M. Regulation of the GABA cell phenotype in hippocampus of schizophrenics and bipolars. Proc. Natl. Acad. Sci. USA. 2007;104(24):10164–10169. doi: 10.1073/pnas.0703806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt A.S., Unschuld P.G., Pradhan S., Lim I.A., Churchill G., Harris A.D., Hua J., Barker P.B., Ross C.A., van Zijl P.C., Edden R.A., Margolis R.L. Age-related changes in anterior cingulate cortex glutamate in schizophrenia: a (1)H MRS study at 7 tesla. Schizophr. Res. 2016;172(1–3):101–105. doi: 10.1016/j.schres.2016.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.M., Stanford A.D., Mao X., Abi-Dargham A., Shungu D.C., Lisanby S.H., Schroeder C.E., Kegeles L.S. GABA level, gamma oscillation, and working memory performance in schizophrenia. Neuroimage Clin. 2014;4:531–539. doi: 10.1016/j.nicl.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerton A., Modinos G., Ferrera D., McGuire P. Neuroimaging studies of GABA in schizophrenia: a systematic review with meta-analysis. Transl. Psychiatry. 2017;7(6):e1147. doi: 10.1038/tp.2017.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankle W.G., Cho R.Y., Prasad K.M., Mason N.S., Paris J., Himes M.L., Walker C., Lewis D.A., Narendran R. In vivo measurement of GABA transmission in healthy subjects and schizophrenia patients. Am. J. Psychiatry. 2015;172(11):1148–1159. doi: 10.1176/appi.ajp.2015.14081031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto N., Yoshimura R., Moriya J., Kakeda S., Ueda N., Ikenouchi-Sugita A., Umene-Nakano W., Hayashi K., Oonari N., Korogi Y., Nakamura J. Reduction of brain gamma-aminobutyric acid (GABA) concentrations in early-stage schizophrenia patients: 3T Proton MRS study. Schizophr. Res. 2009;112(1–3):192–193. doi: 10.1016/j.schres.2009.04.026. [DOI] [PubMed] [Google Scholar]
- Greenhouse I., Noa S., Maddock R.J., Ivry R.B. Individual differences in GABA content are reliable but are not uniform across the human cortex. Neuroimage. 2016;139:1–7. doi: 10.1016/j.neuroimage.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwich K., Pollak T., Klausberger T. Distinct firing patterns of identified basket and dendrite-targeting interneurons in the prefrontal cortex during hippocampal theta and local spindle oscillations. J. Neurosci. 2009;29(30):9563–9574. doi: 10.1523/JNEUROSCI.1397-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoftman G.D., Dienel S.J., Bazmi H.H., Zhang Y., Chen K., Lewis D.A. Altered gradients of glutamate and gamma-aminobutyric acid transcripts in the cortical visuospatial working memory network in schizophrenia. Biol. Psychiatry. 2018;83(8):670–679. doi: 10.1016/j.biopsych.2017.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh L.T., Ekstrom A.D., Ranganath C. Neural oscillations associated with item and temporal order maintenance in working memory. J. Neurosci. 2011;31(30):10803–10810. doi: 10.1523/JNEUROSCI.0828-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde T.M., Lipska B.K., Ali T., Mathew S.V., Law A.J., Metitiri O.E., Straub R.E., Ye T., Colantuoni C., Herman M.M., Bigelow L.B., Weinberger D.R., Kleinman J.E. Expression of GABA signaling molecules KCC2, NKCC1, and GAD1 in cortical development and schizophrenia. J. Neurosci. 2011;31(30):11088–11095. doi: 10.1523/JNEUROSCI.1234-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglewicz B., Hoaglin D. ASQC Quality Press; Milwaukee: 1993. How to Detect and Handle Outliers. [Google Scholar]
- Iwata Y., Nakajima S., Plitman E., Mihashi Y., Caravaggio F., Chung J.K., Kim J., Gerretsen P., Mimura M., Remington G., Graff-Guerrero A. Neurometabolite levels in antipsychotic-naive/free patients with schizophrenia: a systematic review and meta-analysis of (1)H-MRS studies. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2018;86:340–352. doi: 10.1016/j.pnpbp.2018.03.016. [DOI] [PubMed] [Google Scholar]
- Jelen L.A., King S., Mullins P.G., Stone J.M. Beyond static measures: a review of functional magnetic resonance spectroscopy and its potential to investigate dynamic glutamatergic abnormalities in schizophrenia. J. Psychopharmacol. 2018;32(5):497–508. doi: 10.1177/0269881117747579. [DOI] [PubMed] [Google Scholar]
- Kegeles L.S., Mao X., Stanford A.D., Girgis R., Ojeil N., Xu X., Gil R., Slifstein M., Abi-Dargham A., Lisanby S.H., Shungu D.C. Elevated prefrontal cortex gamma-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Arch. Gen. Psychiatry. 2012;69(5):449–459. doi: 10.1001/archgenpsychiatry.2011.1519. [DOI] [PubMed] [Google Scholar]
- Kelemen O., Kiss I., Benedek G., Keri S. Perceptual and cognitive effects of antipsychotics in first-episode schizophrenia: the potential impact of GABA concentration in the visual cortex. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2013;47:13–19. doi: 10.1016/j.pnpbp.2013.07.024. [DOI] [PubMed] [Google Scholar]
- Kreis R. Issues of spectral quality in clinical 1H-magnetic resonance spectroscopy and a gallery of artifacts. NMR Biomed. 2004;17:361–381. doi: 10.1002/nbm.891. [DOI] [PubMed] [Google Scholar]
- Lesh T.A., Niendam T.A., Minzenberg M.J., Carter C.S. Cognitive control deficits in schizophrenia: mechanisms and meaning. Neuropsychopharmacol. 2011;36(1):316–338. doi: 10.1038/npp.2010.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D.A. Cortical circuit dysfunction and cognitive deficits in schizophrenia – implications for preemptive interventions. Eur. J. Neurosci. 2012;35(12):1871–1878. doi: 10.1111/j.1460-9568.2012.08156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D.A. Inhibitory neurons in human cortical circuits: substrate for cognitive dysfunction in schizophrenia. Curr. Opin. Neurobiol. 2014;26:22–26. doi: 10.1016/j.conb.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsman A., Mandl R.C.W., Klomp D.W.J., Bohlken M.M., Boer V.O., Andreychenko A., Cahn W., Kahn R.S., Luijten P.R., H Pol H.E. GABA and glutamate in schizophrenia: a 7 T 1H-MRS study. NeuroImage. 2014;6:398–407. doi: 10.1016/j.nicl.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher M., Merkle H., Kirsch J., Garwood M., Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998;11(6):266–272. doi: 10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Mikkelsen M., Barker P.B., Bhattacharyya P.K. Big GABA: edited MR spectroscopy at 24 research sites. Neuroimage. 2017;159:32–45. doi: 10.1016/j.neuroimage.2017.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins P.G., McGonigle D.J., O'Gorman R.L., Puts N.A., Vidyasagar R., Evans C.J., M. R. S. o. G. Cardiff Symposium on. Edden R.A. Current practice in the use of Mega-Press spectroscopy for the detection of GABA. Neuroimage. 2014;86:43–52. doi: 10.1016/j.neuroimage.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D., Prescot A.P., McCarthy J., Cohen B.M., Renshaw P.F. Elevated gamma-aminobutyric acid levels in chronic schizophrenia. Biol. Psychiatry. 2010;68(7):667–670. doi: 10.1016/j.biopsych.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges E.C., Woods A.J., Edden R.A., Puts N.A., Harris A.D., Chen H., Garcia A.M., Seider T.R., Lamb D.G., Williamson J.B., Cohen R.A. Frontal gamma-aminobutyric acid concentrations are associated with cognitive performance in older adults. Biol. Psychiatry Cognit. Neurosci. Neuroimaging. 2017;2(1):38–44. doi: 10.1016/j.bpsc.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencher S.W. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001;14(4):260–264. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- Ragland J.D., Blumenfeld R.S., Ramsay I.S., Yonelinas A., Yoon J., Solomon M., Carter C.S., Ranganath C. Neural correlates of relational and item-specific encoding during working and long-term memory in schizophrenia. Neuroimage. 2012;59(2):1719–1726. doi: 10.1016/j.neuroimage.2011.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland J.D., Ranganath C., Harms M.P., Barch D.M., Gold J.M., Layher E., Lesh T.A., MacDonald A.W., 3rd, Niendam T.A., Phillips J., Silverstein S.M., Yonelinas A.P., Carter C.S. Functional and neuroanatomic specificity of episodic memory dysfunction in schizophrenia: a functional magnetic resonance imaging study of the relational and item-specific encoding task. JAMA Psychiatry. 2015;72(9):909–916. doi: 10.1001/jamapsychiatry.2015.0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S.G., Williams G.V., Goldman-Rakic P.S. Destruction and creation of spatial tuning by disinhibition: GABA(A) blockade of prefrontal cortical neurons engaged by working memory. J. Neurosci. 2000;20(1):485–494. doi: 10.1523/JNEUROSCI.20-01-00485.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid M.A., Salibi N., White D.M., Gawne T.J., Denney T.S., Lahti A.C. 7T proton magnetic resonance spectroscopy of the anterior cingulate cortex in first-episode schizophrenia. Schizophr. Bull. 2018;45(1):180–189. doi: 10.1093/schbul/sbx190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts B.M., Libby L.A., Inhoff M.C., Ranganath C. Brain activity related to working memory for temporal order and object information. Behav. Brain Res. 2018;354:55–63. doi: 10.1016/j.bbr.2017.05.068. [DOI] [PubMed] [Google Scholar]
- Rowland L.M., Edden R.A., Kontson K., Zhu H., Barker P.B., Hong L.E. GABA predicts inhibition of frequency-specific oscillations in schizophrenia. J. Neuropsychiatry Clin. Neurosci. 2013;25(1):83–87. doi: 10.1176/appi.neuropsych.11120368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szulc A., Konarzewska B., Galinska-Skok B., Lazarczyk J., Waszkiewicz N., Tarasow E., Milewski R., Walecki J. Proton magnetic resonance spectroscopy measures related to short-term symptomatic outcome in chronic schizophrenia. Neurosci. Lett. 2013;547:37–41. doi: 10.1016/j.neulet.2013.04.051. [DOI] [PubMed] [Google Scholar]
- Tayoshi S., Nakataki M., Sumitani S., Taniguchi K., Shibuya-Tayoshi S., Numata S., Iga J., Ueno S., Harada M., Ohmori T. GABA concentration in schizophrenia patients and the effects of antipsychotic medication: a proton magnetic resonance spectroscopy study. Schizophr. Res. 2010;117(1):83–91. doi: 10.1016/j.schres.2009.11.011. [DOI] [PubMed] [Google Scholar]
- Vida I., Bartos M., Jonas P. Shunting inhibition improves robustness of gamma oscillations in hippocampal interneuron networks by homogenizing firing rates. Neuron. 2006;49(1):107–117. doi: 10.1016/j.neuron.2005.11.036. [DOI] [PubMed] [Google Scholar]
- Vinkers C.H., Mirza N.R., Olivier B., Kahn R.S. The inhibitory GABA system as a therapeutic target for cognitive symptoms in schizophrenia: investigational agents in the pipeline. Expert Opin. Investig. Drugs. 2010;19(10):1217–1233. doi: 10.1517/13543784.2010.513382. [DOI] [PubMed] [Google Scholar]
- Volk D., Austin M., Pierri J., Sampson A., Lewis D. GABA transporter-1 mRNA in the prefrontal cortex in schizophrenia: decreased expression in a subset of neurons. Am. J. Psychiatry. 2001;158(2):256–265. doi: 10.1176/appi.ajp.158.2.256. [DOI] [PubMed] [Google Scholar]
- Wang A.M., Pradhan S., Coughlin J.M., Trivedi A., DuBois S.L., C.rawford J.L., Sedlak T.W., Nucifora F.C., Nestadt G., Nucifora L.G., Schretlen D.J., Sawa A., Barker P.B. Assessing brain metabolism with 7-T proton magnetic resonance spectroscopy in patients with first episode psychosis. JAMA Psychiatry. 2019;76(3):314–323. doi: 10.1001/jamapsychiatry.2018.3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington M.A., Traub R.D., Jefferys J.G. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature. 1995;373(6515):612–615. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]
- Woo T.U., Shrestha K., Amstrong C., Minns M.M., Walsh J.P., Benes F.M. Differential alterations of kainate receptor subunits in inhibitory interneurons in the anterior cingulate cortex in schizophrenia and bipolar disorder. Schizophr. Res. 2007;96(1–3):46–61. doi: 10.1016/j.schres.2007.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood S.J., Berger G.E., Wellard R.M., Proffitt T., McConchie M., Velakoulis D., McGorry P.D., Pantelis C. A 1H-MRS investigation of the medial temporal lobe in antipsychotic-naïve and early-treated first episode psychosis. Schizophr. Res. 2008;102:163–170. doi: 10.1016/j.schres.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Yoon J.H., Grandelis A., Maddock R.J. Dorsolateral prefrontal cortex GABA concentration in humans predicts working memory load processing capacity. J. Neurosci. 2016;36(46):11788–11794. doi: 10.1523/JNEUROSCI.1970-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J.H., Maddock R.J., Rokem A., Silver M.A., Minzenberg M.J., Ragland J.D., Carter C.S. GABA concentration is reduced in visual cortex in schizophrenia and correlates with orientation-specific surround suppression. J. Neurosci. 2010;30(10):3777–3781. doi: 10.1523/JNEUROSCI.6158-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]