Highlights
-
•
We explored a potential relation between trait and chronic pain-induced impulsivity.
-
•
Low trait impulsivity rats with neuropathic pain perform similarly to controls.
-
•
High trait impulsivity rats are delay intolerant in chronic pain conditions.
-
•
Trait characteristics influence chronic pain comorbid manifestations.
Keywords: Decision-making, Delay tolerance, Neuropathic pain, Variable delay-to-signal, High impulsivity, Low impulsivity
Abstract
Preclinical studies on impulsive decision-making in chronic pain conditions are sparse and often contradictory. Outbred rat populations are heterogeneous regarding trait impulsivity manifestations and therefore we hypothesized that chronic pain-related alterations depend on individual traits. To test this hypothesis, we used male Wistar-Han rats in two independent experiments. Firstly, we tested the impact of spared nerve injury (SNI) in impulsive behavior evaluated by the variable delay-to-signal test (VDS). In the second experiment, SNI impact on impulsivity was again tested, but in groups previously categorized as high (HI) and low (LI) trait impulsivity in the VDS.
Results showed that in an heterogenous population SNI-related impact on motor impulsivity and delay intolerance cannot be detected. However, when baseline impulsivity was considered, HI showed a significantly higher delay intolerance than the respective controls more prevalent in left-lesioned animals and appearing to result from a response correction on prematurity from VDS I to VDS II, which was present in Sham and right-lesioned animals.
In conclusion, baseline differences should be more often considered when analyzing chronic pain impact. While this study pertained to impulsive behavior, other reports indicate that this can be generalized to other behavioral dimensions and that trait differences can influence not only the manifestation of comorbid behaviors but also pain itself in a complex and not totally understood manner.
1. Introduction
Impulsivity is defined as a predisposition toward rapid and unplanned actions without regard to their negative consequences. It is classically divided in two main dimensions: motor and choice impulsivity, the former related with the incapacity to suppress actions and the latter reflecting delay intolerance, characterized, for instance, as a preference for small but immediate instead of larger but delayed rewards (Bari and Robbins, 2013, Dalley et al., 2011). Impulsivity can be advantageous in competitive environments, where riskier and quicker actions can result in positive outcomes. However, impulsivity can be maladaptive, manifesting in conditions such as attention deficit and hyperactivity disorder (ADHD), addiction and substance abuse (Dalley and Robbins, 2017, Patros et al., 2016, Smith et al., 2014) and even in neurodegenerative disorders (Gleichgerrcht et al., 2010). On the other hand, evidence suggests that trait impulsivity is a predisposing factor for the development of maladaptive behaviors – see for instance (de Wit, 2009).
Around 10% of chronic pain patients develop addictive behaviors and are highly predisposed to opioid misuse (Vowles et al., 2015). However, little is known about the relationship between impulsivity and chronic pain. Some studies indicate that chronic pain patients and healthy subjects present similar scores in the Barret Impulsivity Scale (BIS) (Berger et al., 2014, Margari et al., 2014). Similarly, no differences were observed in the Go/No-Go and Stroop tasks (Glass et al., 2011, Jongsma et al., 2011, Pidal-Miranda et al., 2019, Veldhuijzen et al., 2012). On the other hand, chronic pain patients with comorbid opioid misuse score higher on BIS than chronic pain patients without this problem (Margari et al., 2014, Marino et al., 2013, Tompkins et al., 2016). Additionally, urgency and sensation-seeking dimensions of the urgency, premeditation, perseverance and sensation-seeking (UPPS) impulsivity scale can predict this misuse (Vest et al., 2016), suggesting an importance of trait impulsivity on analgesic-related addiction.
In rodent models of chronic pain, data is scarce and conflicting – contrast for instance (Pais-Vieira et al., 2009, Leite-Almeida et al., 2012, Higgins et al., 2015). Recently, we have analyzed a large cohort of rats in the variable delay-to-signal (VDS) and found a high variability in trait impulsivity related with sex, age and even strain (Soares et al., 2018). Considering the conflicting results in chronic pain conditions and the heterogeneity observed in outbred animals, we hypothesized that baseline impulsivity can influence the impact of chronic pain on impulsive behavior. For that we first compared impulsive behavior of controls and neuropathic rats on the VDS paradigm. Next, we evaluate the impact of the neuropathic lesion on animals with high and low levels of impulsivity at the baseline. We show that the neuropathy exacerbates impulsivity in animals that present high trait impulsivity at baseline.
2. Materials and methods
2.1. Experimental design
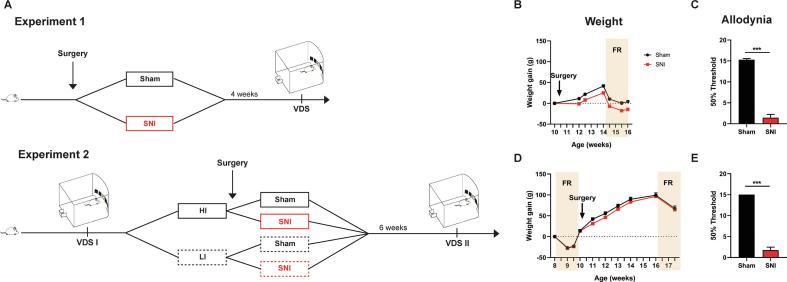
Two independent experiments were performed in this study (Fig. 1A). In the first experiment we studied the impact of chronic pain on impulsivity. For that, a group of rats performed the VDS paradigm 4 weeks after the spared nerve injury (SNI) or the Sham surgery. In the second experiment, we studied the influence of baseline impulsivity on chronic pain-related impulsivity. For that, a group of naïve rats was divided in high (HI) and low impulsive (LI) according to their performance in the VDS (VDS I). Then, SNI was performed in 2/3 of the animals from each group and Sham surgery on the other 1/3. 6 weeks after the surgery, animals were tested again in the VDS paradigm (VDS II).
Fig. 1.
Experimental Design. Experimental design for experiments 1 and 2 (A). Weight evolution of Sham and SNI was similar in experiment 1 (B) and 2 (D); average difference between groups was at its maximum <4% of controls’ weight. SNI animals present increased allodynia to the Von Frey monofilaments in comparison to controls in experiment 1 (C) and 2 (E). Data presented as mean ± SEM. *** p < 0.001. FR – Food restriction; HI – High impulsive; LI – Low Impulsive; SNI – Spared Nerve Injury; VDS – Variable delay-to-signal.
2.2. Experimental subjects
All procedures were approved by the Portuguese National Authority for Animal Health (Direção Geral de Alimentação e Veterinária – DGAV) and in accordance with the guidelines of the European Communities Council Directive 2010/63/EU. Efforts were made to ensure the well-being of the animals used. Sixty (30 + 30) male Wistar-Han rats with 7 weeks of age at the beginning of the experiments were used. Animals were kept in Specific Pathogen Free (SPF) conditions, in a room with controlled temperature (22 °C ± 1 °C) and humidity (50–60%) under a 12 h light/dark cycle (lights on at 8 am), and randomly housed in pairs in plastic cages with food (4RF21; Mucedola, SRL, Settimo Milanese, Italy) and water available ad libitum, except during the VDS protocol, in which food availability was restricted to 1 h/day. Weight was controlled along the entire experiment time course, particularly during the food restriction period to prevent drops below 15% of baseline values.
2.3. Variable delay-to-signal
The VDS was performed in 5-hole operant chambers (25 × 25 cm; TSE Systems, Germany) as previously described (Leite-Almeida et al., 2013). Briefly, the VDS test comprised 3 phases: habituation to the chamber and rewards (4 days), training, and VDS test. The training consisted of 10 sessions (1 per day) with a maximum of 100 trials or 30 min each. Animals which repeated the VDS, performed only 6 sessions of training in the VDS II. Trials started with the lightning of the house light for 3 s (delay period) followed by the light stimulus (3 W) in the response aperture (central hole) for 60 s (response period). Nosepokes during the response period were rewarded with the delivery of a sugar pellet (45 mg, Bioserv Inc., New Jersey, EUA). Omissions and responses in the delay period (premature responses) were punished with a timeout in complete darkness (3 s) and no reward. The order in which animals performed the training was changed along the days to exclude timing effects. The test session was composed by 120 trials similar to training. This session started with an initial block of 25 trials with 3 s-delay trials (3si) followed by 70 trials of 6- and 12-seconds delays (6 s and 12 s, respectively) presented in a random order. The VDS test session terminated with a final block with 25 trials of 3 s delays (3sf). In the test, premature responses were registered but not punished.
Motor impulsivity was evaluated by the number of premature responses along training, while choice impulsivity was evaluated by the number of premature responses in the test during and after the exposure to 6 s and 12 s delays (delay intolerance). Prematurity rate (PR) was defined as the number of premature responses per time of available delay and PRratio as the PR corrected for baseline responsiveness (3si):
2.4. Spared nerve injury
Chronic neuropathic pain was induced using the SNI (Decosterd and Woolf, 2000). Rats were anesthetized via intraperitoneal administration of 1:1.5 mix (1 ml/kg) of Sededorm® (Medetomidine, 1 mg/mL – VetPharma Animal Health, Spain) and Ketamidor® (Ketamine, 100 mg/mL – Richter Pharma AG, Austria), respectively (Esteves et al., 2019). A blunt incision was then performed to expose the three branches of sciatic nerve: common peroneal, tibial and sural nerves. A unilateral ligation and subsequent distal axotomy of the tibial and common peroneal nerves were then performed, leaving the sural nerve (spared nerve) intact. In Sham animals all the nerves were exposed but left intact. Anaesthesia was reversed with a subcutaneous injection of Antisedan® (Atipamezole Hydrochloride, ORION Corporation, Finland). In both experiments, 10 animals were used as Sham controls and 20 as SNI, 10 lesioned in the left (SNI-L) and 10 in the right hindpaw (SNI-R). In the second experiment, animals were ranked by their PRratio in VDS I and distributed according to the following sequence: Sham, SNI-L, SNI-R, SNI-R, SNI-L, Sham. After SNI, animals were left to recover in their cages and monitored for open wounds and signs of inflammation. No major problems were observed in the study.
2.5. Mechanical allodynia
Mechanical allodynia was assessed as previously described (Guimarães et al., 2018) using the up-and-down method (Chaplan et al., 1994). Briefly, Von Frey monofilaments of different forces were used: 15.0 g, 8.0 g, 6.0 g, 4.0 g, 2.0 g, 1.0 g, 0.6 g and 0.4 g (North Coast Medical Inc., USA). Each measure started with the central filament (2.0 g) and advanced upward if no response was elicited or downward if a brisk paw withdrawn was observed, until 6 measures around the turning point were obtained or the limits of the scale were reached. 50% threshold was then calculated using the formula
where Xf = value (in log units) of the final von Frey filament; k = tabular value corresponding to pattern of positive and negative responses; δ = mean difference (in log units) between stimuli (0.224).
2.6. Statistics
Statistical analysis was done in the JASP 0.9.2 software (JASP Team (2019), Netherlands) and graphs were obtained through GraphPad PRISM 8.0 software (GraphPad software, Inc., USA). In the second experiment, 2 animals (1 Sham HI and 1 Sham LI) were excluded from the VDS test analysis because they were considered extreme outliers according to Tukey’s criterion (higher than Q3 + 3(Q3 – Q1), being Q1 and Q3, 1st and 3rd quartile, respectively) in the 6 s and in the 3si and 3sf intervals, respectively. Repeated measures ANOVA was used to analyze body weight, VDS training, VDS test and re-test effects. Independent t-tests were used to compare SNI and Sham allodynia and PR or its ratios, at the different intervals, whenever a difference between the groups was revealed in the ANOVA. Mauchly’s test was used to evaluate sphericity, and Levene’s test to evaluate equality of variances. The Greenhouse-Geisser and the Welch corrections were used when sphericity and equality of variances were rejected, respectively. Bonferroni was used as post-hoc test. Cohens’ d (d) and eta square(ɳ2) were used as effect size measures in t-tests and ANOVA’s, respectively. Data was considered significant if p < 0.05. All results are represented as mean ± SEM.
3. Results
3.1. Body weight and mechanical allodynia
Body weight was controlled frequently to ensure animals’ well-being and to regulate weight loss during food restriction. None of the animals lost more than 15% of its initial weight. In the first experiment, SNI rats were more affected by the surgery and weight gain remain below the levels of controls throughout the experiment (F(1, 28) = 21.61, p > 0.001, ɳ2 = 0.436) (Fig. 1B). Importantly, after the first week, the weight evolution was identical between the 2 groups (F(1, 28) = 2.045, p = 0.164, ɳ2 = 0.068) and, at its maximum, average weight difference between the two groups was <4% of controls weight. In the second experiment, no differences between the groups were found (Lesion: F(1, 28) = 2.438, p = 0.130, ɳ2 = 0.080; Lesion*Time: F(1.976, 55.340) = 1.290, p = 0.283, ɳ2 = 0.002) (Fig. 1D).
As expected, SNI animals developed mechanical allodynia after the installation of the model in the first (t(28) = −12.678, p > 0.001, d = −4.910) (Fig. 1C) and second experiment (t(19) = 18.52, p > 0.001, d = −5.026) (Fig. 1E).
3.2. Experiment 1 – chronic pain does not affect impulsivity
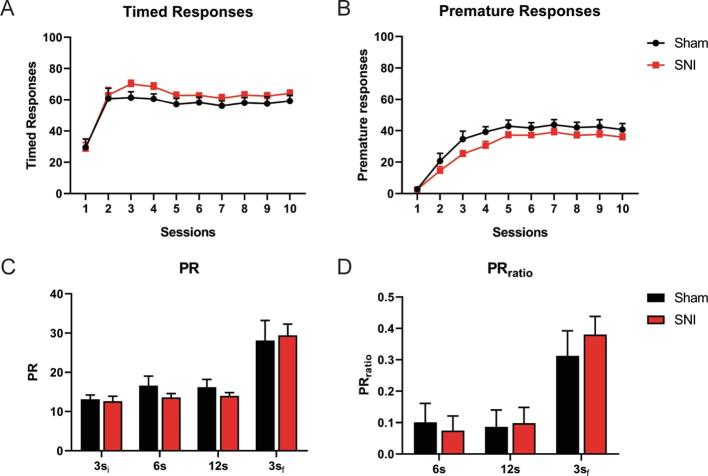
The potential impact of chronic pain on impulsivity was accessed using the VDS test (Fig. 1A, Experiment 1).
All animals learned the task equally (Lesion: F(1, 28) < 0.001, p > 0.999, ɳ2 < 0.001; Lesion*Time: F(9, 252) = 0.097, p > 0.999, ɳ2 = 0.001) (Fig. 2A) as observed by the evolution of the timed responses. Also, no significant differences between groups were observed in the number of premature responses throughout training (Lesion: F(1, 28) = 3.654, p = 0.066, ɳ2 = 0.115; Lesion*Time: F(3.606, 100.978) = 0.646, p = 0.615, ɳ2 = 0.007) (Fig. 2B) or in the test sessions (PR – Lesion: F(1, 28) = 0.193, p = 0.064, ɳ2 = 0.007; Lesion*Time: F(1.400, 39.203) = 0.643, p = 0.478, ɳ2 = 0.010; PRratio – Lesion: F(1, 27) = 0.046, p = 0.831, ɳ2 = 0.002; Lesion * Time: F(1.374, 37.104) = 1.103, p = 0.322, ɳ2 = 0.005) (Fig. 2C-D).
Fig. 2.
Experiment 1: impact of chronic pain on impulsive behavior. Both groups learned the task equally (A) and no differences on impulsive behavior were found between the groups during VDS training (B) nor test (C, D). Data presented as mean ± SEM. 3si – initial trials with 3 s’ delay; 6 s – trials with 6 s’ delay; 12 s – trials with 12 s’ delay; 3sf – final trials with 3 s’ delay; PR – Prematurity rate; SNI – Spared Nerve Injury; VDS – Variable delay-to-signal.
3.3. Experiment 2
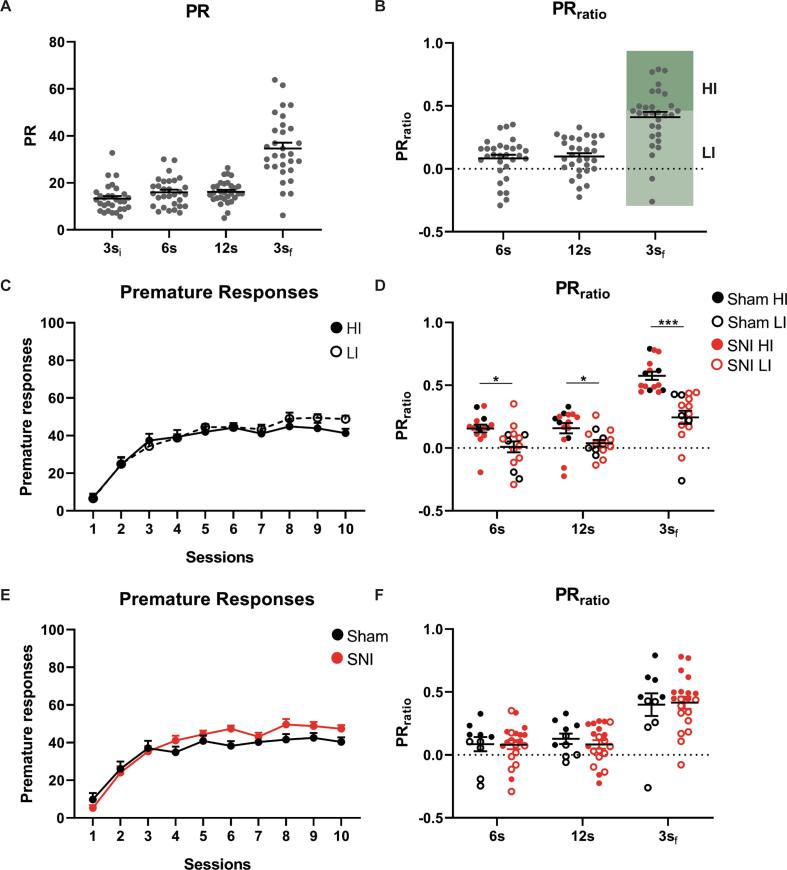
3.3.1. VDS I – determination of baseline impulsivity
To test for potential effects of baseline impulsivity, we evaluated, in a second experiment, impulsive behavior before pain onset (Fig. 1A, Experiment 2). The group presented high heterogeneity regarding PR and PRratio on the 3sf of the VDS test (Fig. 3A-B). Based on the 3sf PRratio animals were divided in two groups: half were considered HI and half LI (Fig. 3B). 2/3 of each group were ascribed to SNI surgery while the remaining 1/3 to Sham surgery. In a retrospective analysis accounting for the newly formed groups, no differences were observed in the learning curves for LI vs HI comparisons (F(1, 28) = 0.439, p = 0.513, ɳ2 = 0.015) (Fig. 3C) and Sham vs SNI comparisons (F(1, 28) = 1.341, p = 0.257, ɳ2 = 0.046) (Fig. 3E). Expectedly, HI presented a significantly higher PRratio than LI in all phases of the VDS test (F(1, 28) = 19.09, p < 0.001, ɳ2 = 0.450; 6 s: t(28) = 2.706, p = 0.011, d = 0.988; 12 s: t(28) = 2.454, p = 0.021, d = 0.896; 3sf: t(28) = 5.387, p < 0.001, d = 1.967) (Fig. 3D). No differences were however found between the animals ascribed to be Sham or SNI (F(1, 28) = 0.031, p = 0.861, ɳ2 = 0.001) (Fig. 3F).
Fig. 3.
Experiment 2, VDS I: determination of baseline impulsivity. Naïve rats show different levels of impulsivity in the VDS test (A). Based on the 3sf PRratio (B), animals were divided in half in HI and LI. HI rats are more impulsive on the test (D) but not during training (C). Rats selected for SNI or Sham surgeries, presented similar impulsive behavior (E, F). Data presented as mean ± SEM. * p < 0.05 *** p < 0.001. 3si – initial trials with 3 s’ delay; 6 s – trials with 6 s’ delay; 12 s – trials with 12 s’ delay; 3sf – final trials with 3 s’ delay; HI – High impulsive; LI – Low Impulsive; PR – Prematurity rate; SNI- Spared Nerve Injury; VDS – Variable delay-to-signal.
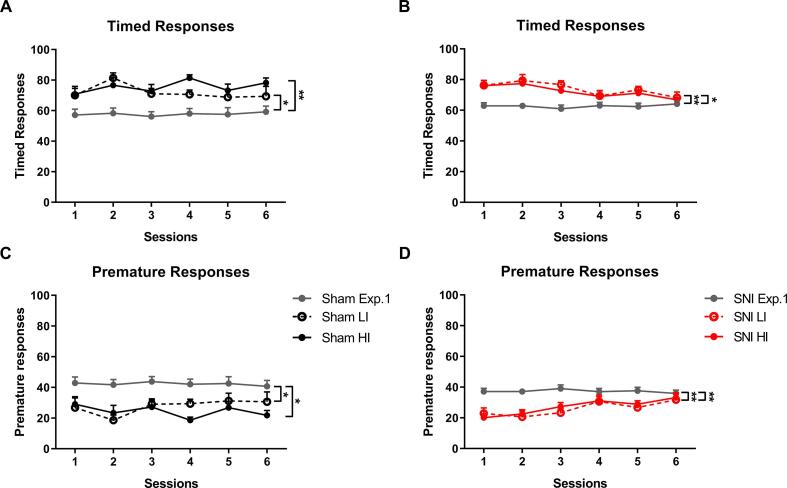
3.3.2. VDS II – CP effects on impulsivity are dependent on the baseline
After the definition of the groups, HI and LI (3.2.1), SNI surgery was performed. At 6 weeks post-surgery, animals performed 6 VDS training sessions, presenting a moderate reduction on premature responses and consequently an increase in the number of timed responses, in comparison with rats of the same groups from experiment 1 (Timed responses – Sham: F(2, 17) = 7.398, p = 0.005, ɳ2 = 0.465; Exp 1 vs HI: t = −3.453, p = 0.009, d = −0.772; Exp 1 vs LI: t = −2.749, p = 0.041, d = −0.615; HI vs LI: t = 0.610, p > 0.999, d = 0.137 (Fig. 4A); SNI: F(2, 37) = 8.450, p < 0.001, ɳ2 = 0.314; Exp 1 vs HI: t = −3.062, p = 0.012, d = −0.484; Exp 1 vs LI: t = −3.607, p = 0.003, d = −0.570; HI vs LI: t = −0.471, p > 0.999, d = −0.075 (Fig. 4B); Premature responses – Sham: F(2, 17) = 7.391, p = 0.005, ɳ2 = 0.465; Exp 1 vs HI: t = 3.411, p = 0.010, d = 0.763; Exp 1 vs LI: t = 2.810, p = 0.036, d = 0.628; HI vs LI: t = −0.520, p > 0.999, d = −0.116 (Fig. 4C); SNI: F(2, 37) = 8.500, p < 0.001, ɳ2 = 0.315; Exp 1 vs HI: t = 3.171, p = 0.009, d = 0.501; Exp 1 vs LI: t = 3.542, p = 0.003, d = 0.560; HI vs LI: t = 0.321, p > 0.999, d = 0.051 (Fig. 4D)), possibly reflecting a learning and/or re-test effect. Importantly, all groups presented no differences in timed (Group: F(3, 26) = 0.117, p = 0.949, ɳ2 = 0.013; Group*Time: F(10.437, 90.458) = 1.557, p = 0.129, ɳ2 = 0.063) or premature responses (Group: F(3, 26) = 0.168, p = 0.917, ɳ2 = 0.019). A group*time interaction was observed in the premature responses (F(11.802, 102.284) = 2.711, p = 0.003, ɳ2 = 0.083) but post-hoc tests did not reveal any differences between the groups.
Fig. 4.
Re-test effects. Both Sham and SNI increased the number of timed responses (A, B) and decrease prematurity levels (C, D) when compared with the same groups from experiment 1. No differences were found in the number of timed and premature responses between the four groups. HI – High impulsive; LI – Low Impulsive; SNI – Spared Nerve Injury.
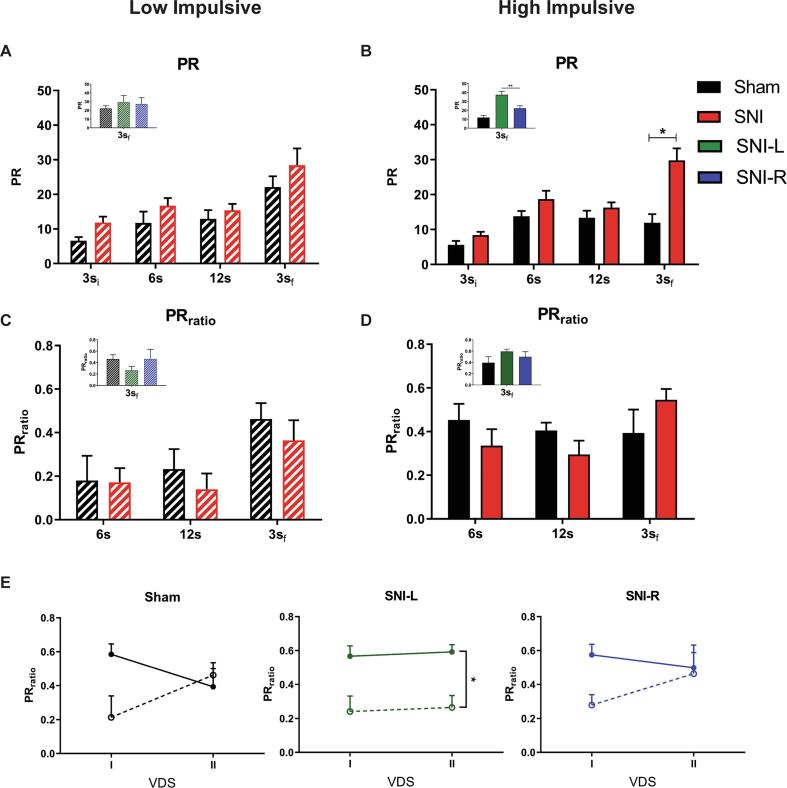
In the VDS test, SNI animals showed a tendency to perform more premature responses than Sham (PR: F(1,26) = 3.887, p = 0.059, ɳ2 = 0.130) (Supplementary data, Fig. 1). Considering trait impulsivity in these animals, we observed that while LI animals were not affected by SNI (PR: F(1,12) = 0.584, p = 0.459, ɳ2 = 0.046; PRratio: F(1,13) = 0.344, p = 0.568, ɳ2 = 0.026) (Fig. 5A, C), HI SNI rats were more impulsive than the corresponding Sham controls on the 3sf, (PR: F(1,12) = 4.928, p = 0.046, ɳ2 = 0.291; 3si: t(12) = 1.671, p = 0.121, d = 0.988; 6 s: t(12) = 0.827, p = 0.424, d = 0.489; 12 s: t(12) = 0.884, p = 0.394, d = 0.523; 3sf: t(12) = 2.951, p = 0.012, d = 1.746) (Fig. 5B). These results show that only animals that are more impulsive at the baseline are affected after a neuropathic lesion. Interestingly, differences between Sham and SNI in the PR on the 3sf trials are mainly dependent of SNI-L (F(2,11) = 4.027, p = 0.049, ɳ2 = 0.423; Sham vs SNI-L: t = −2.824, p = 0.050, d = −0.755) (Fig. 5D inset; supplementary data, Fig. 2). No effects were seen in the PRratio (F(1,13) = 0.073, p = 0.792, ɳ2 = 0.006) (Fig. 5D). However, comparing the evolution of PRratio from VDS I to VDS II in all groups, only SNI-L LI and HI are different between each other (F(5,24) = 3.644, p = 0.014, ɳ2 = 0.432; SNI-L HI vs SNI-L LI: t = 3.474, p = 0.029, d = 0.634) (Fig. 5E). It appears that while Sham and SNI-R animals corrected their impulsivity levels in the second VDS, SNI-L animals maintained them (Fig. 5E).
Fig. 5.
Experiment 2, VDS II: Effect of chronic pain on HI and LI animals. HI rats with chronic pain, mainly SNI-L, are more impulsive than the corresponding controls in the PR (B) although not in the PRratio (D). LI animals are not affected (A, C). Sham and SNI-R animals corrected the levels of impulsivity from VDS I to VDS II, but in SNI-L, HI and LI maintained their PRratio (E). Data presented as mean ± SEM. * p < 0.05, ** p < 0.01. 3si – initial trials with 3 s’ delay; 6 s – trials with 6 s’ delay; 12 s – trials with 12 s’ delay; 3sf – final trials with 3 s’ delay; HI – High impulsive; L – left; LI – Low Impulsive; PR – Prematurity rate; R – right; SNI – Spared Nerve Injury; VDS – Variable delay-to-signal.
4. Discussion
In this work we studied SNI impact on impulsive behavior in an heterogeneous population of outbred rats (experiment 1) and in a previously characterized population regarding trait impulsivity (experiment 2). We observed no differences between lesioned and control groups in any aspect of impulsive behavior. When considering impulsivity baseline, HI SNI animals were more intolerant to delay than the respective controls while LI were not affected by the neuropathy. Also, while Sham animals adjusted their behavior between the 2 VDS assessments, SNI animals failed to do so.
Results reported here are in line with a previous study of the group in which at the 3sf VDS block Sham and SNI behaved similarly (Leite-Almeida et al., 2012), even though in that report SNI-R presented a higher PRratio during the longest delay trials that was not observed here. Results shown here are also in accordance with the work from Higgins and colleagues that tested SNI animals in the 5-choice serial reaction time task (5-csrtt) which bears some resemblance with the VDS training protocol (Higgins et al., 2015). On the contrary, Pais-Vieira and colleagues reported a decrease in impulsivity on the 5-csrtt (Pais-Vieira et al., 2009), in a monoarthritic inflammatory model of chronic pain, initiated in the day after chronic pain induction, suggesting some specificity regarding the type or duration of pain.
VDS II revealed, in the training, a re-test effect characterized by an increase in the number of timed responses and a decrease in motor impulsivity. Importantly, this effect was observed independently of lesion and trait impulsivity. In the test, HI SNI animals (mainly left-lesioned) were more intolerant to delay than the respective controls and when VDS I and VDS II were compared, we observed that both LI and HI Sham and SNI-R adjusted their behavior but not SNI-L. This maintenance of impulsivity levels might result from a complex interaction of re-test and/or learning effects.
Trait impulsivity differences manifest in other behavioral domains (Hayward et al., 2016). For instance, it has been observed that HI animals perform worse in working memory tasks (James et al., 2007, Renda et al., 2014) and present increased anxiety-like behavior(Stein et al., 2015) though some conflicting evidence has also been reported (Velázquez-Sánchez et al., 2014). Furthermore, HI predicts nicotine and cocaine self-administration (Anker et al., 2009, Dalley et al., 2007, Diergaarde et al., 2008, Perry et al., 2008) and increases seeking behavior for sucrose and palatable food (Diergaarde et al., 2009, Velázquez-Sánchez et al., 2014).
Transcriptional differences between HI and LI rats have been found in the Nucleus Accumbens (NAc), ventral tegmental area, dorsomedial striatum and orbitofrontal cortex at basal conditions (Besson et al., 2013, Caprioli et al., 2014, Moloney et al., 2019). In the NAc, HI animals present a decreased availability of D1 and D2/3 receptors and of dopamine transporter (DAT) (Caprioli et al., 2015, Jupp et al., 2013) as well as a reduction in grey matter density (Caprioli et al., 2014). Interestingly, deep brain stimulation in the NAc decreases impulsivity particularly in HI animals (Schippers et al., 2017). The NAc has been involved in chronic pain – see for review (Benarroch, 2016, Mitsi and Zachariou, 2016) – and appears as a prime candidate to mediate decision-making and motivational alterations observed in chronic pain models. Moreover, it has been described in HI rats a reduction of gray matter density as well as a reduction of D2/3 receptor, glutamate decarboxylase (GAD)65/67, microtubule-associated protein 2 (MAP2) and spinophilin in the left (but not right) NAc (Caprioli et al., 2015, Caprioli et al., 2014) which might explain some of the lateralized effects observed in animal models of chronic pain. Indeed, in this and in previous works from the group we observed lesion-side specific impairments on behavior, namely increased anxious-like behavior in SNI-L and cognitive flexibility deficits in SNI-R (Leite-Almeida et al., 2014, Leite-Almeida et al., 2012). Interestingly, lateralized effects of chronic pain have also been observed in chronic pain patients (Gagliese et al., 1995).
In conclusion, chronic pain preclinical models manifest comorbid behaviors such as depressive- and anxiety-like behaviors, and cognitive deficits (Leite-Almeida et al., 2015, Low, 2013, Yalcin et al., 2014). These have been shown to depend on a number of factors including experimental subject age (Leite-Almeida et al., 2009), pain duration (Yalcin et al., 2011) and injury location (Leite-Almeida et al., 2014, Leite-Almeida et al., 2012). In addition, our results indicate that trait manifestations should also be considered in the complex relation between chronic pain and comorbid behaviors.
Funding
This work has been funded by the European Regional Development Fund (FEDER), through the Competitiveness Factors Operational Programme (COMPETE) and the Northern Portugal Regional Operational Programme (NORTE 2020) under the Portugal 2020 Partnership Agreement (project NORTE-01-0145-FEDER-000023). It was also funded by National and International funds, through the Foundation for Science and Technology (FCT), under the scope of the projects POCI-01-0145-FEDER-007038 and PTDC/NEU-SCC/5301/2014 and by the IASP Early Career Research Grant 2015. Researchers were supported by FCT grant numbers SFRH/BD/109111/2015 (AMC via PhD Program in Health Sciences), PD/BD/114117/2015 (MRG via Inter-University Doctoral Programme in Ageing and Chronic Disease, PhDOC) and SFRH/BD/52291/2013 (ME via PhDOC). J. P-M integrated the Master Programme in Health Sciences of the School of Medicine, University of Minho.
Authors contributions
A.M.C., M.E., J.P-M. and M.R.G. acquired data. AMC did the statistical analysis. A.M.C. and H.L-A. designed the experiment and wrote the first version of the manuscript. All authors discussed and interpreted the data and revised and approved the final version of the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynpai.2019.100042.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Anker J.J., Perry J.L., Gliddon L.A., Carroll M.E. Impulsivity predicts the escalation of cocaine self-administration in rats. Pharmacol. Biochem. Behav. 2009;93:343–348. doi: 10.1016/j.pbb.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A., Robbins T.W. Inhibition and impulsivity: behavioral and neural basis of response control. Neurobiol. Prog. 2013 doi: 10.1016/j.pneurobio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Benarroch E.E. Involvement of the nucleus accumbens and dopamine system in chronic pain. Neurology. 2016;87:1720–1726. doi: 10.1212/WNL.0000000000003243. [DOI] [PubMed] [Google Scholar]
- Berger S.E., Baria A.T., Baliki M.N., Mansour A., Herrmann K.M., Torbey S., Huang L., Parks E.L., Schnizter T.J., Apkarian A.V. Risky monetary behavior in chronic back pain is associated with altered modular connectivity of the nucleus accumbens. BMC Res. Notes. 2014;7:739. doi: 10.1186/1756-0500-7-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson M., Pelloux Y., Dilleen R., Theobald D.E., Lyon A., Belin-Rauscent A., Robbins T.W., Dalley J.W., Everitt B.J., Belin D. Cocaine modulation of frontostriatal expression of Zif 268, D2, and 5-HT2c receptors in high and low impulsive rats. Neuropsychopharmacology. 2013;38:1963–1973. doi: 10.1038/npp.2013.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprioli D., Jupp B., Hong Y.T., Sawiak S.J., Ferrari V., Wharton L., Williamson D.J., McNabb C., Berry D., Aigbirhio F.I., Robbins T.W., Fryer T.D., Dalley J.W. Dissociable rate-dependent effects of oral methylphenidate on impulsivity and D2/3 receptor availability in the striatum. J. Neurosci. 2015;35:3747–3755. doi: 10.1523/JNEUROSCI.3890-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprioli D., Sawiak S.J., Merlo E., Theobald D.E.H., Spoelder M., Jupp B., Voon V., Carpenter T.A., Everitt B.J., Robbins T.W., Dalley J.W. Gamma aminobutyric acidergic and neuronal structural markers in the nucleus accumbens core underlie trait-like impulsive behavior. Biol. Psychiatry. 2014;75:115–123. doi: 10.1016/j.biopsych.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan S.R., Bach F.W., Pogrel J.W., Chung J.M., Yaksh T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Dalley J.W., Everitt B.J., Robbins T.W. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011 doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Dalley J.W., Fryer T.D., Brichard L., Robinson E.S.J., Theobald D.E.H., Lääne K., Peña Y., Murphy E.R., Shah Y., Probst K., Abakumova I., Aigbirhio F.I., Richards H.K., Hong Y., Baron J.-C., Everitt B.J., Robbins T.W. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley J.W., Robbins T.W. Fractionating impulsivity: neuropsychiatric implications. Nat. Rev. Neurosci. 2017 doi: 10.1038/nrn.2017.8. [DOI] [PubMed] [Google Scholar]
- de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict. Biol. 2009;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decosterd I., Woolf C.J. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149–158. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- Diergaarde L., Pattij T., Nawijn L., Schoffelmeer A.N.M., De Vries T.J. Trait impulsivity predicts escalation of sucrose seeking and hypersensitivity to sucrose-associated stimuli. Behav. Neurosci. 2009;123:794–803. doi: 10.1037/a0016504. [DOI] [PubMed] [Google Scholar]
- Diergaarde L., Pattij T., Poortvliet I., Hogenboom F., de Vries W., Schoffelmeer A.N.M., De Vries T.J. Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biol. Psychiatry. 2008;63:301–308. doi: 10.1016/j.biopsych.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Esteves M., Almeida A.M., Silva J., Silva Moreira P., Carvalho E., Pêgo J.M., Almeida A., Sotiropoulos I., Sousa N., Leite-Almeida H. MORPhA scale: behavioral and electroencephalographic validation of a rodent anesthesia scale. J. Neurosci. Methods. 2019;324 doi: 10.1016/j.jneumeth.2019.05.013. [DOI] [PubMed] [Google Scholar]
- Gagliese L., Schiff B.B., Taylor A. Differential consequences of left- and right-sided chronic pain. Clin. J. Pain. 1995;11:201–207. [PubMed] [Google Scholar]
- Glass J.M., Williams D.A., Fernandez-Sanchez M.L., Kairys A., Barjola P., Heitzeg M.M., Clauw D.J., Schmidt-Wilcke T. Executive function in chronic pain patients and healthy controls: different cortical activation during response inhibition in fibromyalgia. J. Pain. 2011;12:1219–1229. doi: 10.1016/j.jpain.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleichgerrcht E., Ibáñez A., Roca M., Torralva T., Manes F. Decision-making cognition in neurodegenerative diseases. Nat. Rev. Neurol. 2010;6:611–623. doi: 10.1038/nrneurol.2010.148. [DOI] [PubMed] [Google Scholar]
- Guimarães M.R., Soares A.R., Cunha A.M., Esteves M., Borges S., Magalhães R., Moreira P.S., Rodrigues A.J., Sousa N., Almeida A., Leite-Almeida H. Evidence for lack of direct causality between pain and affective disturbances in a rat peripheral neuropathy model. Genes, Brain Behav. 2018 doi: 10.1111/gbb.12542. [DOI] [PubMed] [Google Scholar]
- Hayward A., Tomlinson A., Neill J.C. Low attentive and high impulsive rats: A translational animal model of ADHD and disorders of attention and impulse control. Pharmacol. Ther. 2016 doi: 10.1016/j.pharmthera.2015.11.010. [DOI] [PubMed] [Google Scholar]
- Higgins G.A., Silenieks L.B., Van Niekerk A., Desnoyer J., Patrick A., Lau W., Thevarkunnel S. Enduring attentional deficits in rats treated with a peripheral nerve injury. Behav. Brain Res. 2015;286:347–355. doi: 10.1016/j.bbr.2015.02.050. [DOI] [PubMed] [Google Scholar]
- James A.S., Groman S.M., Seu E., Jorgensen M., Fairbanks L.A., Jentsch J.D. Dimensions of impulsivity are associated with poor spatial working memory performance in monkeys. J. Neurosci. 2007;27:14358–14364. doi: 10.1523/JNEUROSCI.4508-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongsma M.L.A., Postma S.A.E., Souren P., Arns M., Gordon E., Vissers K., Wilder-Smith O., van Rijn C.M., van Goor H. Neurodegenerative properties of chronic pain: cognitive decline in patients with chronic pancreatitis. PLoS One. 2011;6 doi: 10.1371/journal.pone.0023363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jupp B., Caprioli D., Saigal N., Reverte I., Shrestha S., Cumming P., Everitt B.J., Robbins T.W., Dalley J.W. Dopaminergic and GABA-ergic markers of impulsivity in rats: evidence for anatomical localisation in ventral striatum and prefrontal cortex. Eur. J. Neurosci. 2013;37:1519–1528. doi: 10.1111/ejn.12146. [DOI] [PubMed] [Google Scholar]
- Leite-Almeida H., Almeida-Torres L., Mesquita A.R., Pertovaara A., Sousa N., Cerqueira J.J., Almeida A. The impact of age on emotional and cognitive behaviours triggered by experimental neuropathy in rats. Pain. 2009;144:57–65. doi: 10.1016/j.pain.2009.02.024. [DOI] [PubMed] [Google Scholar]
- Leite-Almeida H., Cerqueira J.J., Wei H., Ribeiro-Costa N., Anjos-Martins H., Sousa N., Pertovaara A., Almeida A. Differential effects of left/right neuropathy on rats’ anxiety and cognitive behavior. Pain. 2012;153:2218–2225. doi: 10.1016/j.pain.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Leite-Almeida H., Guimarães M.R., Cerqueira J.J., Ribeiro-Costa N., Anjos-Martins H., Sousa N., Almeida A. Asymmetric c-fos expression in the ventral orbital cortex is associated with impaired reversal learning in a right-sided neuropathy. Mol. Pain. 2014;10:41. doi: 10.1186/1744-8069-10-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite-Almeida H., Melo A., Pêgo J.M., Bernardo S., Milhazes N., Borges F., Sousa N., Almeida A., Cerqueira J.J. Variable delay-to-signal: a fast paradigm for assessment of aspects of impulsivity in rats. Front. Behav. Neurosci. 2013;7:154. doi: 10.3389/fnbeh.2013.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite-Almeida H., Pinto-Ribeiro F., Almeida A. Animal models for the study of comorbid pain and psychiatric disorders. Trends Pharmacopsychiatry. 2015 doi: 10.1159/000435929. [DOI] [PubMed] [Google Scholar]
- Low L.A. The impact of pain upon cognition: what have rodent studies told us? PAIN®. 2013;154:2603–2605. doi: 10.1016/j.pain.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margari F., Lorusso M., Matera E., Pastore A., Zagaria G., Bruno F., Puntillo F., Margari L. Aggression, impulsivity, and suicide risk in benign chronic pain patients – a cross-sectional study. Neuropsychiatr. Dis. Treat. 2014;10:1613–1620. doi: 10.2147/NDT.S66209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino E.N., Rosen K.D., Gutierrez A., Eckmann M., Ramamurthy S., Potter J.S. Impulsivity but not sensation seeking is associated with opioid analgesic misuse risk in patients with chronic pain. Addict. Behav. 2013;38:2154–2157. doi: 10.1016/j.addbeh.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsi V., Zachariou V. Modulation of pain, nociception, and analgesia by the brain reward center. Neuroscience. NIH Public Access. 2016 doi: 10.1016/j.neuroscience.2016.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moloney G.M., van Oeffelen W.E.P.A., Ryan F.J., van de Wouw M., Cowan C., Claesson M.J., Schellekens H., Dinan T.G., Cryan J.F. Differential gene expression in the mesocorticolimbic system of innately high- and low-impulsive rats. Behav. Brain Res. 2019;364:193–204. doi: 10.1016/j.bbr.2019.01.022. [DOI] [PubMed] [Google Scholar]
- Pais-Vieira M., Lima D., Galhardo V. Sustained attention deficits in rats with chronic inflammatory pain. Neurosci. Lett. 2009;463:98–102. doi: 10.1016/j.neulet.2009.07.050. [DOI] [PubMed] [Google Scholar]
- Patros C.H.G., Alderson R.M., Kasper L.J., Tarle S.J., Lea S.E., Hudec K.L. Choice-impulsivity in children and adolescents with attention-deficit/hyperactivity disorder (ADHD): A meta-analytic review. Clin. Psychol. Rev. 2016;43:162–174. doi: 10.1016/j.cpr.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Perry J.L., Nelson S.E., Carroll M.E. Impulsive choice as a predictor of acquisition of IV cocaine self-administration and reinstatement of cocaine-seeking behavior in male and female rats. Exp. Clin. Psychopharmacol. 2008;16:165–177. doi: 10.1037/1064-1297.16.2.165. [DOI] [PubMed] [Google Scholar]
- Pidal-Miranda M., González-Villar A.J., Carrillo-de-la-Peña M.T. Pain expressions and inhibitory control in patients with fibromyalgia: behavioral and neural correlates. Front. Behav. Neurosci. 2019;12:323. doi: 10.3389/fnbeh.2018.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renda C.R., Stein J.S., Madden G.J. Impulsive choice predicts poor working memory in male rats. PLoS One. 2014;9 doi: 10.1371/journal.pone.0093263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schippers M.C., Bruinsma B., Gaastra M., Mesman T.I., Denys D., De Vries T.J., Pattij T. Deep brain stimulation of the nucleus accumbens core affects trait impulsivity in a baseline-dependent manner. Front. Behav. Neurosci. 2017;11:52. doi: 10.3389/fnbeh.2017.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.L., Mattick R.P., Jamadar S.D., Iredale J.M. Deficits in behavioural inhibition in substance abuse and addiction: a meta-analysis. Drug Alcohol Depend. 2014;145:1–33. doi: 10.1016/j.drugalcdep.2014.08.009. [DOI] [PubMed] [Google Scholar]
- Soares A.R., Esteves M., Moreira P.S., Cunha A.M., Guimarães M.R., Carvalho M.M., Raposo-Lima C., Morgado P., Carvalho A.F., Coimbra B., Melo A., Rodrigues A.J., Salgado A.J., Pêgo J.M., Cerqueira J.J., Costa P., Sousa N., Almeida A., Leite-Almeida H. Trait determinants of impulsive behavior: a comprehensive analysis of 188 rats. Sci. Rep. 2018;8:17666. doi: 10.1038/s41598-018-35537-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein J.S., Renda C.R., Barker S.M., Liston K.J., Shahan T.A., Madden G.J. Impulsive choice predicts anxiety-like behavior, but not alcohol or sucrose consumption, in male Long-Evans rats. Alcohol. Clin. Exp. Res. 2015;39:932. doi: 10.1111/acer.12713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins D.A., Johnson P.S., Smith M.T., Strain E.C., Edwards R.R., Johnson M.W. Temporal preference in individuals reporting chronic pain: discounting of delayed pain-related and monetary outcomes. Pain. 2016;157:1724–1732. doi: 10.1097/j.pain.0000000000000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velázquez-Sánchez C., Ferragud A., Moore C.F., Everitt B.J., Sabino V., Cottone P. High trait impulsivity predicts food addiction-like behavior in the rat. Neuropsychopharmacology. 2014;39:2463–2472. doi: 10.1038/npp.2014.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuijzen D.S., Sondaal S.F.V., Oosterman J.M. Intact cognitive inhibition in patients with fibromyalgia but evidence of declined processing speed. J. Pain. 2012;13:507–515. doi: 10.1016/j.jpain.2012.02.011. [DOI] [PubMed] [Google Scholar]
- Vest N., Reynolds C.J., Tragesser S.L. Impulsivity and risk for prescription opioid misuse in a chronic pain patient sample. Addict. Behav. 2016;60:184–190. doi: 10.1016/j.addbeh.2016.04.015. [DOI] [PubMed] [Google Scholar]
- Vowles K.E., McEntee M.L., Julnes P.S., Frohe T., Ney J.P., van der Goes D.N. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 2015;156:569–576. doi: 10.1097/01.j.pain.0000460357.01998.f1. [DOI] [PubMed] [Google Scholar]
- Yalcin I., Barthas F., Barrot M. Emotional consequences of neuropathic pain: Insight from preclinical studies. Neurosci. Biobehav. Rev. 2014;47:154–164. doi: 10.1016/j.neubiorev.2014.08.002. [DOI] [PubMed] [Google Scholar]
- Yalcin I., Bohren Y., Waltisperger E., Sage-Ciocca D., Yin J.C., Freund-Mercier M.-J., Barrot M. A time-dependent history of mood disorders in a murine model of neuropathic pain. Biol. Psychiatry. 2011;70:946–953. doi: 10.1016/j.biopsych.2011.07.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.