Highlights
-
•
A novel Go/No-go task points to a critical role for the IPS in response inhibition.
-
•
IPS engagement in response inhibition is scaled back with ADHD symptom severity.
-
•
Fronto-parietal connectivity increases when response inhibition is challenging.
-
•
Connectivity modulation is also scaled back with ADHD symptom severity.
Keywords: ADHD, Adults, FMRI, Response inhibition, Connectivity, Parietal cortex
Abstract
Background
Impaired response inhibition is one of the most consistent findings in attention deficit hyperactivity disorder (ADHD). However, the underlying brain mechanisms are not clear. This study aimed to underpin atypical inhibition-related brain activation and connectivity patterns in ADHD using a novel Go/No-go task design, and to determine its association with clinical symptoms of the disorder.
Methods
Forty-eight adults with ADHD performed a Go/No-go task in which target frequency was manipulated during functional MRI. Specific inhibition-related brain activation was correlated with ADHD symptom severity, to assess the relationship of individual differences in engagement of inhibition-related brain circuits with the magnitude of every-day functioning impairments. Finally, generalized psychophysical interaction analyses were carried out to examine whether not only engagement but also functional connectivity between regions implicated in response inhibition is related to symptom severity.
Results
We found no evidence for the expected parietal modulation by increased demand for inhibition at the group-level results. However, this lack of modulation was mediated by individual differences in ADHD symptom severity – increased engagement of the intraparietal sulcus (IPS) in inhibition-demanding events was evident in individuals with less severe symptoms but dissipated with increase in symptomatology. Similarly, functional connectivity between the IPS and the right inferior frontal gyrus (rIFG) was elevated under high inhibitory demand conditions, but this effect diminished with increased symptom severity.
Conclusions
The results highlight the importance of IPS engagement in response inhibition and suggest that IPS modulation may be driven by top-down control from the IFG. Moreover, the current findings force the point of treating ADHD as a continuum whereby brain correlates are scaled with severity of the disorder, and point to the potential use of individual differences in the modulation of IPS activation and connectivity as a neuromarker of ADHD.
1. Introduction
Attention deficit hyperactivity disorder (ADHD) is defined by behavioral symptoms of inattention, hyperactivity and impulsivity; troubling approximately 5% of children and 2.5% of adults (American Psychiatric Association, 2013; Polanczyk et al., 2007; Simon et al., 2009). While ADHD tends to manifest in a range of symptoms, it is thought that deficient response inhibition - reduced ability to suppress inadequate but prepotent response tendencies - is one of the central dysfunctions characterizing the disorder (Barkley, 1997; Nigg, 2001; Wright et al., 2014). The most common paradigms used to study response inhibition are Go/No-go and Stop-Signal tasks (Polner et al., 2015) where poor inhibitory control is behaviorally reflected in high rate of commission errors (i.e. false alarms) in Go/No-go tasks and in long stop-signal reaction time (SSRT) in Stop-Signal tasks (i.e. it takes longer to cancel an ongoing response). It has been consistently demonstrated that response inhibition, as assessed with these tasks, is deficient both in children and in adults with ADHD (Hervey et al., 2004; Lijffijt et al., 2005; Willcutt et al., 2005; Wright et al., 2014).
In contrast with the consistent behavioral effects, previous attempts to uncover the underlying brain mechanism that contributes to impaired response inhibition in ADHD patients have produced mixed results. Findings from functional magnetic resonance imaging (fMRI) studies of ADHD participants (predominantly children) using Go/No-go and Stop-Signal tasks suggest disrupted activation of a right-lateralized fronto-striatal network implicated in response inhibition (see meta-analyses in Cortese et al., 2012; Hart, Radua, Nakao, Mataix-Cols, & Rubia, 2013; McCarthy, Skokauskas, & Frodl, 2014). However, in studies of adults with ADHD, results are much more inconsistent, and the nature of neural disruption is unclear. As summarized by Congdon and colleagues (Congdon et al., 2014), some studies report hypoactivation in ADHD relative to neurotypicals (Cubillo et al., 2010; Montojo et al., 2015; Mulligan et al., 2011; Sebastian et al., 2012), while other report hyperactivation in ADHD (Dillo et al., 2010; Karch et al., 2010), or no difference between patients and neurotypicals (Carmona et al., 2012; Congdon et al., 2014). The problem is exacerbated by the tendency of studies to report an extensive (and sometimes varied) network of regions associated with inhibition, including inferior frontal cortex, the insula, pre-supplementary motor area, medial-superior frontal gyrus, cingulate cortex, the striatum and thalamus. It is therefore possible that a coherent picture will emerge if a more specific set of brain regions can be identified in respect to response inhibition.
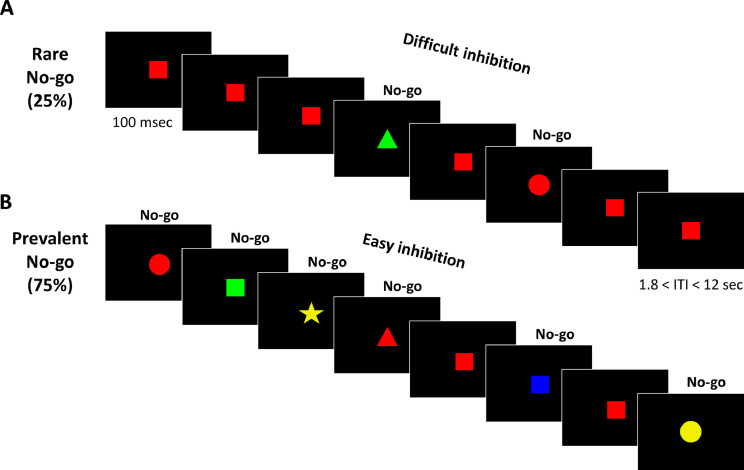
In a recent study with neurotypical adults (Kolodny et al., 2017), we utilized a unique experimental design of a Go/No-go task to increase the specificity of inhibition-related activation. We manipulated the ratio of Go and No-go stimuli, to create two variants of the task: rare-No-go and prevalent-No-go (Fig. 1). While inhibition is required in all No-go trials, a rare-No-go version (only 25% of trials are No-go) yields a tendency to respond in every trial and hence a strong demand for inhibition is posed when a No-go stimulus appears, whereas a prevalent-No-go version (75% of all trials are No-go) requires very little inhibition effort. Thus, contrasting No-go trials from these different contexts pinpoints inhibition-related brain activation. This design is different from common Go/No-go and Stop-signal task designs that compare No-go/Stop trials with Go trials, which therefore capture various differences between the trial types in addition to response inhibition – visual properties, stimulus processing, motor planning and execution. Conversely, directly contrasting between No-go trials (rare vs. prevalent), enables isolating inhibition-related activation while eliminating contaminating motor and visual factors. In our previous study including a sample of neurotypical adults, this approach has been successful in identifying specific inhibition-related activation in the intraparietal sulcus (IPS) and in the left temporo-parietal junction (TPJ) (Kolodny et al., 2017). Crucially, activity in these regions did not show any differences between rare- and prevalent-Go trials, indicating that their activation is not attributable to general processes of saliency detection, attentional capture or expectancy, but is specifically related to inhibition associated with the No-go trials.
Fig. 1.
Experimental design. Illustration of the Go/No-go task, in which participants were shown a series of stimuli. Participants were instructed to respond quickly when a Go stimulus - a red square - was presented in the center of a screen, and to withhold response to all other stimuli. Trials occurred in a randomized order within two types of runs: A) Rare-No-go (25% No-go stimuli and 75% Go stimuli) and B) Prevalent-No-go (75% No-go stimuli and 25% Go stimuli). See MethodsSection 2.3 and Supplementary Materials Section S for a full description of the task.
The specificity achieved by such a task design in identifying response inhibition processes in the brain is particularly relevant in the context of ADHD, which has been also associated with a variety of other (non response-inhibition) impairments, including sustained attention (Hervey et al., 2004; Tsal et al., 2005), response selection (Mullane et al., 2009; Segal et al., 2015), and temporal processing (Dankner et al., 2017; Sonuga-Barke et al., 2010). These, among others, may play a role in Go/No-go tasks, and are likely to alter the processing and performance of both trial types (Go and No-go). Thus, when investigating response inhibition in ADHD, isolating inhibition-related activation from other cognitive processes occurring throughout the task, may prove critical.
In the current study, we examine inhibition-related brain activation among adults with ADHD, as measured in a Go/No-go task by contrasting rare-No-go trials against prevalent-No-go trials, using a whole-brain approach as well as ROI analyses in the IPS and TPJ. Furthermore, we investigate whether the extent of inhibition-related activation is related to the severity of ADHD symptoms. Such examination of individual differences in symptoms and in brain function has the benefit of accommodating the considerable heterogeneity in ADHD (Kofler et al., 2016; Nigg et al., 2005), and also provides evidence for the potential clinical significance of abnormal inhibition-related brain activation. Finally, we assess whether altered engagement of the parietal cortex in inhibition is accompanied by altered fronto-parietal connectivity using generalized context-dependent psychophysical interactions (gPPI). Such an analysis may point to possible mechanisms underlying an impaired IPS modulation in response inhibition in ADHD.
2. Methods and Materials
2.1. Participants
Forty-eight adults with ADHD participated in the study. 11 were excluded due to various reasons, described in detail in the Supplementary Materials Section 1 (e.g., abnormal neurological findings, scanner artifacts, excessive motion). Thus, the final sample included 37 participants, 15 men and 22 women, aged between 19 and 34 (mean age = 26.6, SD = 4.0). Participants were recruited through advertisement within university and college campuses. All had a previous diagnosis of ADHD by a qualified clinician, and each participant also completed a full psychiatric evaluation conducted by ST – a certified psychiatrist, which included psychiatric history and mental status examination according to DSM-5 (APA, 2013) criteria. Participants were excluded if they had neurological or psychiatric disorders other than ADHD, including major depression, anxiety, OCD, or psychosis. All participants met criteria for current diagnosis of ADHD and were not using any psychotropic medications other than psychostimulants customary to treat ADHD, and those receiving psychostimulants (11 participants on a regular basis, 20 participants irregularly taking the medicine) had at least 24-hours washout period before each testing session. All had normal or corrected-to-normal vision (glasses were replaced in the scanner with MRI-compatible goggles). Participants had no contraindication to MRI scanning. The study conformed to the Declaration of Helsinki and was approved by the ethics committees of Sheeba medical center and of Tel-Aviv University in Israel. All participants provided written informed consent after receiving a complete description of the study.
2.2. Symptom severity
ADHD current symptom severity was scored using the Hebrew version of the Adult ADHD Self-Report Scale (ASRS) (Kessler et al., 2005; Zohar and Konfortes, 2010). The ASRS is comprised of 18 items corresponding to the DSM diagnostic criteria. For each item, participants indicate how well the symptom describes them currently, on a Likert scale (1=not at all, 5=to a large extent). The total score (overall sum) of the ASRS gives an estimate of a participants’ current symptom severity. The possible range of scores is from 18 to 90.
2.3. Go/No-go task
The Go/No-go task (Kolodny et al., 2017) consisted of a stream of colored shapes, appearing one by one in the center of the screen. Each shape appeared for 100 msec, with varying inter stimulus intervals (Fig. 1). Participants were instructed to respond quickly when a Go stimulus - a red square - was presented, and to withhold response to all other stimuli. We are mostly interested in No-go trials, where participants must withhold their response. The task included two variants: rare-No-go and prevalent-No-go. In the rare-No-go condition, 75% of trials were Go trials and only 25% were No-go trials. In this case, participants are required to respond in the majority of trials, and thus it is difficult to withhold a response in the presence of rare No-go trials. In the prevalent-No-go condition the ratio is inverted – 25% of trials are Go trials and 75% are No-go trials. In this condition, there is no bias to respond; hence the need for inhibition is greatly reduced. A complete description of the task can be found in the Supplementary Materials Section 2.
2.4. Experimental procedure
In order to get familiar with the task prior to the fMRI session, participants attended the lab on a separate day and performed the Go/No-go task in a regular lab setting. During the fMRI scan, participants performed 4 blocks of the task: two of rare-No-go and two of prevalent-No-go, interspersed by an anatomical T1-weighted scan. The order of block types (rare- and prevalent-No-go) was counterbalanced across participants. The stimuli were projected onto a screen and viewed by a mirror mounted on the head coil. Responses were collected via an MRI-compatible response box. Additional scans were acquired after completion of the experimental functional runs, and are not further described in the current paper. The total period of time in the scanner was approximately 60 minutes.
2.5. Behavioral data analysis
Paired t-tests were computed to compare performance in the rare-No-go and prevalent-No-go conditions, in measures of mean reaction time (RT), standard deviation of RT, omission and commission errors. False Discovery Rate (FDR) was applied to correct for multiple comparisons. Effect sizes were estimated by Cohen's d for paired t-tests, adjusted to correct for dependence among means (Morris and DeShon, 2002).
2.6. fMRI analysis
fMRI data processing was carried out using FSL (FMRIB's Software Library, www.fmrib.ox.ac.uk/fsl), version 5.0 (Jenkinson et al., 2012). fMRI acquisition parameters and preprocessing steps are described in detail in the Supplementary Materials Sections 3 and 4. Time-series statistical analysis was carried out using FILM with local autocorrelation correction (Worsley, 2001). Standard GLM fitting was conducted for all subjects. The following events were modeled using a boxcar regressor convolved with a canonical double gamma hemodynamic response function: correct Go, correct No-go, omissions, and commission errors. Null events were not modeled and therefore constitute an implicit baseline. Events were modeled at the time of stimulus onset with duration of 0.1 s. The six motion parameters and temporal derivatives of all regressors were included as covariates of no interest to improve statistical sensitivity. Volumes with framewise displacement (FD) > 0.9 were flagged and regressed out (“motion scrubbing”, Siegel et al., 2014; see more details in the Supplementary Materials Section 5). Results from a contrast of correct No-go trials (i.e. successful inhibitions) versus baseline were fed into the second level analysis. The second level analysis, combining runs within subject, was carried out using a fixed effects model, by forcing the random effects variance to zero in FLAME (FMRIB's Local Analysis of Mixed Effects) (Beckmann et al., 2003; Woolrich, 2008; Woolrich et al., 2004). In order to isolate inhibition-specific activation, a rare-No-go minus prevalent-No-go contrast was computed for each subject. As described above, response inhibition is highly challenging in the rare-No-go, but substantially less so in the prevalent-No-go. Hence the contrast between No-go events in the two conditions reflects the inhibitory process. To control for potential confounds of saliency and expectancy in the abovementioned main contrast, we also computed a rare-Go minus prevalent-Go contrast.
Group analysis was carried out using FLAME (FMRIB's Local Analysis of Mixed Effects) stage 1 (Beckmann et al., 2003; Woolrich, 2008; Woolrich et al., 2004). Z (Gaussianised T/F) statistic images were thresholded using clusters determined by Z>2.3 and a (corrected) cluster significance threshold of P=0.05 (Worsley, 2001), corrected for multiple comparisons using Gaussian random field theory. Activation clusters are reported in MNI coordinates, using Cluster command in FSL. For visualization of results, statistical maps were projected onto an average cortical surface with the use of multifiducial mapping using CARET software (Van Essen, 2005) (http://brainvis.wustl.edu/wiki/index.php/Caret:Download).
ROI analysis was conducted using masks of inhibition-related activation in neurotypical adults (masks are available online linked to (Kolodny et al., 2017)). Percent signal change for each condition (rare-No-go and prevalent-No-go) was computed in reference to an implicit baseline and averaged across voxels within each cluster. Comparison of the resulting values between conditions was conducted using paired t-tests, and further complemented with Bayesian statistics to quantify the support for the null hypothesis (see details in the Supplementary Materials Section 6).
In order to test the effect of symptom severity, a whole-brain regression analysis was conducted at the group level, including demeaned symptom severity scores as a covariate of interest. This allowed for investigation of the relationship between inhibition-related activation, as reflected in the difference between rare-No-go and prevalent-No-go activation, and current symptom severity.
In order to visualize the latter, we extracted percent signal change values for each condition (rare-No-go and prevalent-No-go). Percent signal change was computed in reference to an implicit baseline in clusters from group level analyses, and in intersection masks of current group level analysis with ROI masks of parietal inhibition-related activation in neurotypicals. Percent signal change is plotted against symptom severity scores for visualization only, with no inferential statistics, to avoid circular analysis and inflated correlation values (Poldrack, 2007; Vul et al., 2009).
Three separate Follow-up analyses investigated effects of age, sex and usage of psychostimulant medication. We compared inhibition-related activation between male and female participants; and between participants who are treated regularly with psychostimulants vs. participants using the medication sporadically (only 6 participants were not using any psychostimulants, and these were excluded from this analysis). Finally, we investigated the effect of age on inhibition-related activation by including age as a covariate in the model.
2.7. Functional connectivity analysis: psychophysical interactions
Three generalized psychophysical interaction analyses (gPPI; McLaren et al., 2012), were conducted to examine differential task-based functional connectivity between seed regions derived from the main analysis and other regions of the brain. The three selected seed regions were located in the IPS (two clusters in the left IPS and one cluster in the right IPS), defined from the intersection of the whole-brain analysis result of symptom severity effects with the pre-defined ROIs described above (depicted in Fig. 2B). For each seed region, the seed mask was projected back to the native space of each participant, and an averaged timeseries was extracted from the preprocessed fMRI data. A standard GLM was ran for each run as described for the main analysis, with the addition of gPPI interaction terms, created by multiplying the task regressors (Go and No-go events) with the seed ROI timecourse, and creating a contrast of rare-No-go trials with prevalent-No-go trials. In the group level analysis, demeaned ASRS scores were entered as a covariate of interest, to allow investigation of how functional connectivity patterns of the IPS are modulated by the task demands as well as by individual differences in ADHD symptom severity.
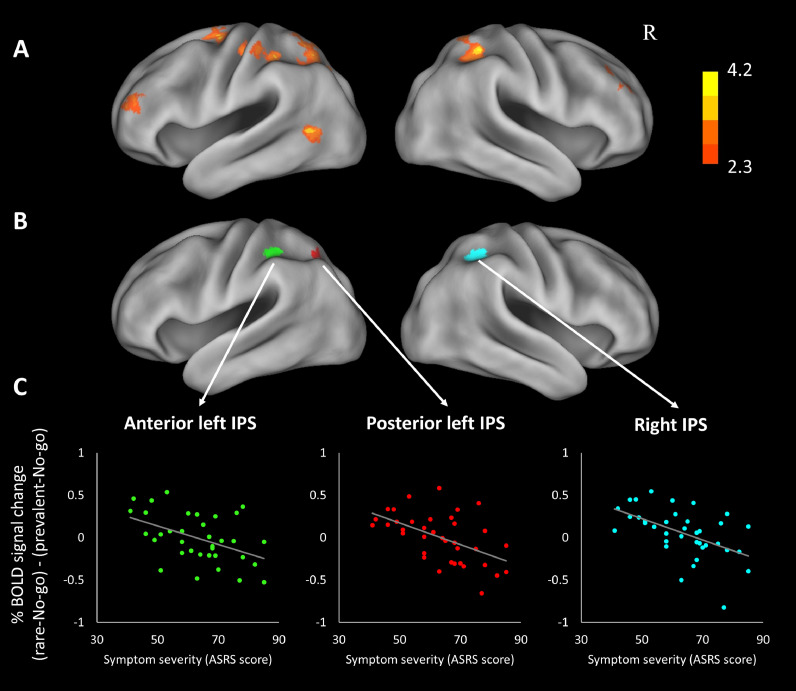
Fig. 2.
(A) Regions where inhibition-related activation is negatively correlated with ADHD symptom severity. See Table 2 for detailed cluster information. The statistical map is corrected for whole-brain multiple comparisons and projected onto an average cortical surface using CARET (R = Right). The color bar represents the z-score. (B) Regions of overlap between pre-defined regions of interest and Fig. 2A; i.e. regions identified in neurotypicals as inhibition-related that also demonstrate correlation with symptom severity in adults with ADHD. (C) Scatter plots visualizing the correlation in these regions among ADHD participants: inhibition-related activation as a function of ADHD symptom severity. Inhibition-related activation quantified as difference in % signal change between rare- and prevalent-No-go, averaged across voxels in the clusters presented in B.
3. Results
3.1. Behavioral results
Behavioral data were examined using paired sampled t-tests comparing the rare-No-Go condition to the prevalent-No-Go condition, in order to assure the experimental manipulation. As expected, and as was previously demonstrated with neurotypicals, RTs for Go trials were significantly faster in the rare-No-go condition (M = 524 msec, SD = 77) than in the prevalent-No-go condition (M = 586 msec, SD = 79; t(36) = 13.0, p<.001, Cohen's d = 2.13), reflecting the tendency to respond quickly that was created in the rare-No-go condition. Critically, commission errors occurred more frequently in the rare-No-go condition (M = 7.6%, SD = 5.7) than in the prevalent-No-go condition (M = 1.0%, SD = 1.2; t(36)=7.8, p<.001, Cohen's d = 2.04). This demonstrates that the inhibition was indeed more demanding in the rare-No-go condition. Standard deviation of RT and rate of omission errors did not differ between conditions (SDRT: M = 82.3 (SD = 34.2) vs. M = 81.9 (SD = 34.3) msec; omissions: M = 3.3% (SD = 4.4) vs M = 4.3% (SD = 6.4), in the rare-No-go and the prevalent-No-go, respectively; t's < 2, n.s.), indicating similar levels of sustained attention (Johnson et al., 2007; Shalev et al., 2011).
3.2. fMRI results
3.2.1. Inhibition-related activation
In order to isolate brain activation which is uniquely associated with inhibition we used the contrast of No-go trials from the two different occurrence rates: the activity in prevalent-No-go trials was subtracted from the activity in rare-No-go trials. This contrast yielded no clusters of activation, i.e. neural activity across the brain in response to No-go stimuli was comparable regardless of the inhibitory demand.
To further examine this we employed regions of interest (ROIs) approach, specifically looking into activation in the right and left IPS and in the left TPJ, previously reported to be modulated by inhibitory demand in this task (Kolodny et al., 2017). Paired t-tests comparing percent signal change calculated within those ROIs, for rare-No-go and for prevalent-No-go, revealed no significant differences in the extent of activation between conditions, in any of the ROIs. Furthermore, Bayesian analysis provided conclusive evidence in favor of the null hypothesis, i.e., no difference between conditions (Table 1). This emphasizes the null results of the whole-brain analysis, showing that for adults with ADHD there is no difference between activation in rare-No-go trials and activation in prevalent-No-go trials.
Table 1.
Lack of parietal modulation in response inhibition among ADHD participants.
| Brain region | % signal change Rare-No-go Mean (SD) | % signal change Prevalent-No-go Mean (SD) | Paired-t statistic df = 37 | BFnull |
|---|---|---|---|---|
| Right IPS | 0.09 (0.20) | 0.04 (0.14) | 1.24 n.s. | 2.83 |
| Posterior Left IPS | 0.07 (0.27) | 0.11 (0.19) | -0.74 n.s. | 4.44 |
| Left TPJ and anterior IPS | 0.07 (0.16) | 0.06 (0.13) | 0.22 n.s. | 5.59 |
Percent signal change for each condition was computed in reference to an implicit baseline and averaged across voxels within each ROI. BFnull = Bayes Factor in favor of the null hypothesis, i.e. no difference between conditions. IPS = intraparietal sulcus; TPJ = temporoparietal junction.
To examine the possibility that the data is noisy, hence rendering any effect difficult to detect, we re-analyzed the data in the traditional approach, contrasting No-go trials with Go-trials in the rare-No-go condition. The activation map revealed occipital and parietal regions, in line with the broad literature on Go/No-go tasks (Criaud and Boulinguez, 2012; Swick et al., 2011) and matching closely with the pattern of activation reported in neurotypicals using the same task design (Kolodny et al., 2017). Frontal regions showed only modest responses, which is also in line with existing ADHD literature (e.g. Congdon et al., 2014; Morein-Zamir et al., 2014; see detailed results in the Supplementary Materials Section 7). These findings rule out the possibility that the absence of specific inhibition-related activation is the result of noisy data. Furthermore, we performed another control analysis contrasting rare-Go trials with prevalent-Go trials. This analysis yielded large distributed activation throughout the brain (see detailed results in the Supplementary Materials Section 8), including in parietal cortex, demonstrating sensitivity to stimulus frequency. This indicates that the absence of response in the main contrast of rare vs. prevalent-No-go trials is specific to No-go stimuli, hence does not reflect overall insensitivity to statistical regularities, and is presumably reflecting insensitivity to the associated inhibitory demand.
3.2.2. Effect of symptom severity
In order to examine the relationship between inhibition-related activation and severity of ADHD symptoms, we added individuals’ ASRS questionnaire scores as a covariate to the GLM, testing for interaction with the difference in activation between rare-No-go and prevalent-No-go, which quantifies inhibition-related neural activity. Interestingly, symptom severity was negatively correlated with such difference in activation in multiple fronto-parietal regions (Fig. 2A and Table 2). Importantly, these include left and right IPS, identified in neurotypicals as involved in response inhibition. This means that participants with ADHD experiencing relatively mild symptom severity demonstrate a pattern of parietal inhibition-related activation that is similar to that found in neurotypicals: higher activation in the IPS towards rare-No-go stimuli than to prevalent-No-go stimuli, i.e., higher activation when inhibition is difficult and demanding. On the other hand, participants who report more severe behavioral manifestation of ADHD show no difference in parietal activation between conditions with different inhibitory demand, or even show a reverse pattern of activation, where IPS is less activated when the demand for inhibition is high. For visualization of this relation between brain activation and behavioral symptoms, we show % signal change differences as a function of symptom severity, in regions of overlap between the current results and the pre-defined ROIs in the IPS (Fig. 2B and C). Additional scatter plots for all clusters of activation, as well as for the left TPJ ROI, are included in the Supplementary Materials section 91.
Table 2.
| Brain region | Hemisphere | N voxels | Max Z-stat | x | y | z |
|---|---|---|---|---|---|---|
| [rare-No-go minus prevalent-No-go] activation, negatively correlated with symptom severity | ||||||
| Precuneus, superior parietal cortex, IPS | L | 16,318 | 4.2 | -9 | -68 | 58 |
| Superior frontal gyrus | L | 8313 | 3.6 | -26 | 0 | 72 |
| IPS | R | 5473 | 4.0 | 35 | -41 | 51 |
| Occipito-temporal lobe | L | 3086 | 3.6 | -52 | -60 | 6 |
| Lateral prefrontal cortex | L | 3052 | 3.7 | -35 | 45 | 15 |
| Middle frontal gyrus | R | 2223 | 3.6 | 38 | 54 | 31 |
N Voxels: number of activated voxels per cluster; Max Z-stat: maximum z-statistic for each cluster; x, y, and z are MNI coordinates for peak of each cluster. R = right; L = left. IPS = intraparietal sulcus.
Admittedly, the contrast of rare-No-go with prevalent-No-go overcomes many shortcomings common in the response inhibition literature as discussed earlier, but still contains some possible confounds that are inherent to the design. Specifically, rare-No-go trials are more unexpected than prevalent-No-go trials, and might be eliciting neural responses that are related to expectancy or saliency detection. To distinguish such effects from inhibition-related activations, we conducted an analogous analysis based on Go trials, i.e. examining the interaction of ASRS questionnaire scores with the difference in activation between rare-Go and prevalent-Go. The analysis revealed clusters in bilateral superior frontal gyrus and left inferior frontal gyrus (see detailed results in the Supplementary Materials Section 8) where difference in activation between rare-Go and prevalent-Go were negatively associated with ASRS scores. However, no such activation was seen in the parietal cortex nor in any other regions identified in the No-go trials analysis. These results confirm that the activation observed in the IPS is indeed inhibition-related and not associated with general frequency effects.
3.2.3. Functional connectivity: generalized context-dependent psychophysiological interactions
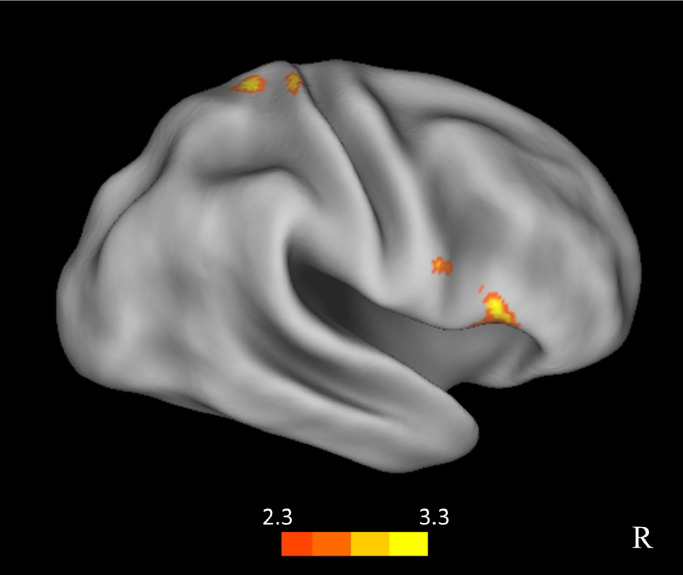
To further explore the possible underlying mechanisms of response-inhibition we used the right and left IPS activation clusters depicted in Fig. 2B, i.e. the intersection of the current whole-brain results with the pre-defined ROIs, as seed-regions for whole-brain gPPI analyses. We were particularly interested in the relationship between the IPS and frontal regions previously suggested to be central to inhibition, including the inferior frontal gyrus and the pre-supplementary motor area (Aron et al., 2014, 2004; Chambers et al., 2009; Meffert et al., 2016; Sharp et al., 2010), hypothesizing that top-down control from frontal regions may be driving the IPS response to increased inhibitory demand. A whole-brain exploratory analysis with the anterior left IPS seed, including the ASRS symptom scores as a group-level covariate, yielded a cluster of activation in the right inferior frontal gyrus (rIFG), and a cluster in the right primary somatosensory cortex (postcentral gyrus; Fig. 3, Table 3). When relaxing the statistical threshold, the same cluster in the rIFG emerged also when using the other two seed regions: the right IPS and the posterior left IPS. This finding indicates that the modulation of connectivity between the IPS and the rIFG by the inhibitory demand correlates with the extent of ADHD severity: fronto-parietal connectivity is elevated when response inhibition is challenging (larger for rare-No-go trials than prevalent-No-go trials), but less so for participants reporting high occurrence of ADHD symptoms.
Fig. 3.
gPPI results for a seed region in the left anterior IPS (seed region shown in Fig. 2B). Regions in the right IFG and in the right parietal cortex where the modulation of correlation with the IPS seed time course by the inhibitory demand negatively correlated with symptom severity of ADHD.
Table 3.
| Brain region | Hemisphere | N voxels | Max Z-stat | x | y | z |
|---|---|---|---|---|---|---|
| [rare-No-go minus prevalent-No-go] interaction with left IPS seed region in gPPI analysis, negatively correlated with symptom severity | ||||||
| IFG | R | 1822 | 3.4 | 59 | 22 | -4 |
| Postcentral gyrus, superior parietal lobule | R | 1794 | 3.32 | 28 | -34 | 70 |
N Voxels: number of activated voxels per cluster; Max Z-stat: maximum z-statistic for each cluster; x, y, and z are MNI coordinates for peak of each cluster. R = right; L = left. IFG = inferior frontal gyrus.
3.2.4. Effect of sex, medication status and age
Follow-up analyses compared inhibition-related activation in male vs. female participants, participants medicated regularly vs. those using psychostimulants sporadically, and investigated the effect of age on inhibition-related activation. None of these analyses yielded significant results.
4. Discussion
In the present study we aimed to characterize abnormal patterns of brain activation underlying impaired response inhibition in adult participants with ADHD. The novelty of the current study, compared to previous similar attempts, is in applying a unique task design that enables isolation of inhibition-related activation. Contrasting rare-No-go trials with prevalent-No-go trials avoided contamination of the results by visual and motor components that are present in the Go trials. Behaviorally, it was established that adults with ADHD had more difficulty inhibiting responses in the rare-No-go condition than in the prevalent-No-go condition (with more commission errors expressing inhibition failures), confirming a substantial increase in inhibitory demand in this condition. While we have not compared performance between ADHD and controls directly in this study, the rate of commission errors of participants in the rare-No-go condition was 7.6% - higher than that reported for neurotypical adults in the same task (4%, Kolodny et al., 2017), which fits with previous reports on deficits in inhibition of a prepotent response in participants with ADHD (Nigg, 2000; Willcutt et al., 2005; Wright et al., 2014). Interestingly, although the behavioral effect of No-go trial prevalence was substantial, imaging results indicated no change in parietal responses between rare- and prevalent-No-go conditions. This is in contrast with the pattern of inhibition-related activation in parietal regions that was recently reported in neurotypicals (Kolodny et al., 2017).
Importantly, the lack of parietal cortex recruitment is specifically tied to the inhibitory demand: contrasting Go trials from different prevalence yielded extensive brain-wide activations, including in the parietal cortex, but not in the regions of interest in the IPS, demonstrating intact sensitivity to stimulus frequencies. This analysis is also an indication that the lack of IPS recruitment is not a result of increased noise or variability in the ADHD cohort, which would undermine this control analysis.
While we found no differentiated parietal engagement for response inhibition on the group level, using an individual differences approach it was revealed that inhibition-related activation in the parietal cortex (including bilateral IPS) was, in fact, modulated by ADHD symptom severity. Participants who reported moderate ADHD symptoms did show the expected inhibition-related activation (similar to neurotypicals) but this was eliminated in participants who evaluated themselves as experiencing relatively high severity of symptoms. The link between ADHD symptom severity and brain dynamics was further corroborated using a gPPI analysis assessing functional connectivity between the IPS and other brain regions in the context of response inhibition. We found that the similarity of activation time courses between IPS and rIFG, hypothesized to reflect functional connectivity between the two regions, depended on the inhibitory demand and increased with inhibition difficulty, but crucially this effect was compromised with elevated symptom severity of ADHD.
Indeed, the modulation of IPS-IFG functional connectivity by the inhibitory demand fits with theories that highlight the role of the IFG in response inhibition: the IFG is activated in various tasks requiring inhibition (Cai and Leung, 2011; Chikazoe et al., 2009; Garavan, 2002; Rubia et al., 2003) and patients with damage to this brain region present behavioral inhibitory failures (Aron et al., 2003; Rieger et al., 2003). These findings gave rise to the long-standing view of the IFG as a critical locus of response inhibition (Aron et al., 2014, 2004). In recent years the specificity of the IFG to inhibitory function has been questioned (Chatham et al., 2012; Hampshire et al., 2010; Sharp et al., 2010; Swick and Chatham, 2014), and it has been suggested that the involvement of the IFG in response inhibition tasks results from its role in domain-general fronto-parietal networks that support broader cognitive control functions such as context monitoring (Banich and Depue, 2015; Chatham et al., 2012; Criaud et al., 2017; Hampshire and Sharp, 2015). In light of this view, and given our findings here and in Kolodny et al. (2017), we suggest that while the IFG is not specifically engaged in inhibition per se, its function in top-down control is essential in modulating the parietal cortex according to the inhibitory demand to achieve efficient response inhibition. Interestingly, a recent exploratory analysis of parcellation-based functional connectivity of the IFG during a stop-signal task identified the IPS as essential for response inhibition, supporting our current framework (Osada et al., 2019).
The failure to modulate parietal activation in individuals who report more severe ADHD symptoms may be a consequence of altered responses in the frontal regions, reflecting malfunctioning of cognitive control in general. This is supported by behavioral findings indicating a general deficit in context monitoring, including abnormally effortful task switching deficits (Cepeda et al., 2000; King et al., 2007) and lack of sensitivity to temporal regularities (Dankner et al., 2017), and by neuroimaging evidence for hypoactivation of frontal regions, which is well documented in ADHD in a variety of tasks (Cubillo et al., 2012; Dickstein et al., 2006). Alternatively, lack of parietal modulation in individuals who reported severe ADHD symptoms can stem from abnormal connectivity in fronto-parietal pathways. In this case the identification of the context and the stimuli properties (e.g. novelty, saliency, prevalence) might be intact, but the ability to act upon this information is limited by altered top-down connectivity to other brain regions. This possibility is supported by reports of disrupted functional connectivity in the fronto-parietal cognitive control network in ADHD (Konrad and Eickhoff, 2010; Sripada et al., 2014), as well as in other neural networks including the default-mode network, the ventral attention network, and motor, saliency, and reward-based networks (Castellanos and Aoki, 2016; O'Halloran et al., 2018). However, hypo-activation and hypo-connectivity are bound together, and our current data cannot tease these possibilities apart.
The current results also demonstrate how individual differences analysis can unravel effects that may be concealed when using only group-averaged statistics. This is especially important in research of ADHD, which is a highly heterogeneous disorder, in its symptomatic manifestation as well as in neuropsychological profiles and in psychopathological pathways (Castellanos et al., 2006; Nigg et al., 2005; Sonuga-Barke et al., 2010; Wåhlstedt et al., 2009). Moreover, while current diagnosis is based on a categorical model of ADHD, recent studies support the conceptualization of ADHD as a dimensional phenomenon, that represents a continuum of attentional dysfunction from normal functioning to extreme deficit (Coghill and Sonuga-Barke, 2012; Frazier et al., 2007; Salum et al., 2014). The current results highlight the merit of adopting a dimensional experimental design over the traditional comparison of patients with neurotypicals. While group comparison is unquestionably valuable, it suffers at the same time from fundamental pitfalls when clinical groups are involved, primarily its vulnerability to general group differences in variability and in noise (e.g., motion artifacts, structural abnormalities, etc.; Nakao et al., 2011; Satterthwaite et al., 2012). An ideal approach would thus be adopting a fully dimensional design, with a large number of participants that represents the complete range of attentional functioning. In the current study, however, to balance sample size and statistical power with the dimensional approach, and to align with current clinical standards of ADHD definition, we chose to focus on the extremity of the attention spectrum, including only participants with established and rigorous ADHD diagnosis, and analyzing individual differences within this clinical group. The convergence of the whole brain analysis in the clinical group to the same regions of interest in the parietal cortex that were pre-defined from neurotypicals’ activation maps provides strong evidence for the contribution of the IPS to response inhibition. Inclusion of participants from across the entire range of attention functioning in future large-scale studies would allow further and wider examination of this hypothesis.
A small number of previous studies focusing on inhibition also used a similar dimensional approach, conducting a whole-brain regression analysis between ADHD symptoms and brain function, but resulted in inconsistent findings (Congdon et al., 2014; Cubillo et al., 2010; Schneider et al., 2010). Those studies reported both negative and positive correlations of inhibition-related activation with symptom scores, across a variety of brain regions. However, these studies were based on the classic contrast of No-go or Stop trials versus Go trials. This contrast might include activation that is unrelated to inhibition per se, but is a consequence of motor, visual and/or perceptual processes that distinguish No-go from Go events. As such, it might also depend heavily on the specific stimuli used in a certain study, and on factors other than response inhibition that may be associated with the syndrome, all of which may contribute to the inconsistencies among studies. Consequently, the current results cannot be directly compared to these previous reports, due to the fundamental difference in the contrasts of interest.
One factor that has been previously suggested to impact inhibition-related activation in adults with ADHD is medication status. Current psychostimulants treatment as well as the history of medication usage has been associated with the neural substrates of inhibition (Congdon et al., 2014). While studies in children and youth with ADHD show that acute administration of psychostimulants is linked with increased stopping-related activation and even normalization of the signal to the level of neurotypicals, there is no conclusive evidence regarding long-term effects (Rubia et al., 2014; Schweren et al., 2013), and there is only one report of such effects in adults (Bush et al., 2008; Rubia et al., 2014). In the current study all of the participants had a history of psychostimulants usage, and most of them were currently treated. Thus, we could not directly address the effect of long-term medication. However, when comparing participants reporting regular usage of psychostimulants with participants reporting occasional usage of psychostimulants, no differences were found in inhibition-related activation. Future research is needed to inspect whether medication-naïve participants show similar effects to those reported in the current study.
To conclude, our findings whereby IPS engagement in response inhibition scales with symptom severity, point to this signal's potential usefulness as a neuromarker of ADHD in adults and as a target for intervention. The fact that fronto-parietal connectivity was similarly sensitive to individual differences in symptom severity highlight the two possible sources of this pattern of results – frontal hypoactivation (possibly associated with reduced context sensitivity) or fronto-parietal disconnection (possibly leading to impaired ability to act upon the changing context). Both frontal activation and brain-wide connectivity patterns can be effected by pharmacological intervention (Rubia et al., 2009; Schmidt et al., 2017; Sweitzer et al., 2017), and as recently shown, also by neurofeedback training (Alegria et al., 2017; Rubia et al., 2018). Thus, future research can test whether normalization of IFG responses would extend to normalization in IPS-IFG connectivity and/or can lead to enhanced IPS modulation during response inhibition, and whether such neural effects would be associated with changes in symptom severity and other aspects of everyday functioning. Targeting individual differences in such intervention studies can increase the impact and therapeutic value of such programs.
The current approach and findings could also be relevant to other disorders associated with response inhibition abnormalities, such as psychosis, schizophrenia, anxiety and obsessive-compulsive disorders, where a similar framework can be used to examine the underlying neural mechanisms of impaired response inhibition on a group and individual levels.
Funding
This study was funded by grant no. 3-7331 from the Chief Scientist of the Israeli Ministry of Health.
Disclosures
None of the authors report any conflicts of interest.
CRediT authorship contribution statement
Tamar Kolodny: Conceptualization, Methodology, Software, Formal analysis, Investigation, Writing - original draft, Writing - review & editing, Visualization. Carmel Mevorach: Conceptualization, Methodology, Investigation, Writing - review & editing, Supervision. Pnina Stern: Investigation, Project administration. Natalie Biderman: Investigation, Visualization. Maya Ankaoua: Investigation. Shlomit Tsafrir: Investigation. Lilach Shalev: Conceptualization, Investigation, Writing - review & editing, Supervision, Funding acquisition.
Footnotes
Note that the scatter plots are for visualization only, while statistical inference is relying on the whole-brain analysis. While the reader might be inclined to look for correlation values accompanying this type of scatterplots, computing any inferential correlational statistics in this case is misleading, since the selection of regions was based on the same data rendering the analyses non-independent. As demonstrated and discussed at length by Vul and colleagues, reporting correlation values in this situation of a non-independent analysis results in biased and inflated numbers (Poldrack and Mumford, 2009; Poldrack, 2007; Vul et al., 2009).
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.nicl.2019.102119.
Appendix. Supplementary materials
References
- Alegria A.A., Wulff M., Brinson H., Barker G.J., Norman L.J., Brandeis D., Stahl D., David A.S., Taylor E., Giampietro V., Rubia K. Real-time fMRI neurofeedback in adolescents with attention deficit hyperactivity disorder. Hum. Brain Mapp. 2017;38:3190–3209. doi: 10.1002/hbm.23584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . American Psychiatric Pub.; 2013. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®) [Google Scholar]
- Aron A.R., Fletcher P.C., Bullmore E.T., Sahakian B.J., Robbins T.W. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat. Neurosci. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Aron A.R., Robbins T.W., Poldrack R.A. Inhibition and the right inferior frontal cortex: One decade on. Trends Cogn. Sci. 2014;18:177–185. doi: 10.1016/j.tics.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Aron A.R., Robbins T.W., Poldrack R.A. Inhibition and the right inferior frontal cortex. Trends. Cogn. Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Banich M.T., Depue B.E. Recent advances in understanding neural systems that support inhibitory control. Curr. Opin. Behav. Sci. 2015;1:17–22. [Google Scholar]
- Barkley R.A. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol. Bull. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Beckmann C.F., Jenkinson M., Smith S.M. General multilevel linear modeling for group analysis in FMRI. Neuroimage. 2003;20:1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Bush G., Spencer T.J., Holmes J., Shin L.M., Valera E.M., Seidman L.J., Makris N., Surman C., Aleardi M., Mick E., Biederman J. Functional magnetic resonance imaging of methylphenidate and placebo in attention-deficit/hyperactivity disorder during the multi-source interference task. Arch. Gen. Psych. 2008;65:102–114. doi: 10.1001/archgenpsychiatry.2007.16. [DOI] [PubMed] [Google Scholar]
- Cai W., Leung H.-C. Rule-guided executive control of response inhibition: functional topography of the inferior frontal cortex. PLoS One. 2011;6:e20840. doi: 10.1371/journal.pone.0020840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona S., Hoekzema E., Ramos-Quiroga J.A., Richarte V., Canals C., Bosch R., Rovira M., Carlos Soliva J., Bulbena A., Tobeña A., Casas M., Vilarroya O. Response inhibition and reward anticipation in medication-naïve adults with attention-deficit/hyperactivity disorder: A within-subject case-control neuroimaging study. Hum. Brain Mapp. 2012;33:2350–2361. doi: 10.1002/hbm.21368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos F.X., Aoki Y. Intrinsic Functional Connectivity in Attention-Deficit/Hyperactivity Disorder: A Science in Development. Biol. Psych. Cogn. Neurosci. Neuroimag. 2016;1:253–261. doi: 10.1016/j.bpsc.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos F.X., Sonuga-Barke E.J.S., Milham M.P., Tannock R. Characterizing cognition in ADHD: Beyond executive dysfunction. Trends Cogn. Sci. 2006;10:117–124. doi: 10.1016/j.tics.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Cepeda N.J., Cepeda M.L., Kramer a F. Task switching and attention deficit hyperactivity disorder. J. Abnorm. Child Psychol. 2000;28:213–226. doi: 10.1023/a:1005143419092. [DOI] [PubMed] [Google Scholar]
- Chambers C.D., Garavan H., Bellgrove M.A. Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neurosci. Biobehav. Rev. 2009;33:631–646. doi: 10.1016/j.neubiorev.2008.08.016. [DOI] [PubMed] [Google Scholar]
- Chatham C.H., Claus E.D., Kim A., Curran T., Banich M.T., Munakata Y. PLoS One 7; 2012. Cognitive control reflects context monitoring, not motoric stopping, in response inhibition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikazoe J., Jimura K., Asari T., Yamashita K., Morimoto H., Hirose S., Miyashita Y., Konishi S. Functional dissociation in right inferior frontal cortex during performance of go/no-go task. Cereb. Cortex. 2009;19:146–152. doi: 10.1093/cercor/bhn065. [DOI] [PubMed] [Google Scholar]
- Coghill D., Sonuga-Barke E.J.S. Annual research review: Categories versus dimensions in the classification and conceptualisation of child and adolescent mental disorders - Implications of recent empirical study. J. Child Psychol. Psychiatry Allied Discip. 2012;53:469–489. doi: 10.1111/j.1469-7610.2011.02511.x. [DOI] [PubMed] [Google Scholar]
- Congdon E., Altshuler L.L., Mumford J.A., Karlsgodt K.H., Sabb F.W., Ventura J., McGough J.J., London E.D., Cannon T.D., Bilder R.M., Poldrack R.A. Neural activation during response inhibition in adult attention-deficit/hyperactivity disorder: Preliminary findings on the effects of medication and symptom severity. Psychiatry Res. Neuroimag. 2014;222:17–28. doi: 10.1016/j.pscychresns.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese S., Kelly C., Chabernaud C., Proal E., Di Martino A., Milham M.P., Castellanos F.X. Toward Systems Neuroscience of ADHD:A Meta-Analysis of 55 fMRI Studies. Am. J. Psych. 2012;169:1038–1055. doi: 10.1176/appi.ajp.2012.11101521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criaud M., Boulinguez P. Have we been asking the right questions when assessing response inhibition in go/no-go tasks with fMRI? A meta-analysis and critical review. Neurosci. Biobehav. Rev. 2012;37:11–23. doi: 10.1016/j.neubiorev.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Criaud M., Longcamp M., Anton J.-L., Nazarian B., Roth M., Sescousse G., Strafella A.P., Ballanger B., Boulinguez P. Testing the physiological plausibility of conflicting psychological models of response inhibition: A forward inference fMRI study. Behav. Brain Res. 2017;333:192–202. doi: 10.1016/j.bbr.2017.06.030. [DOI] [PubMed] [Google Scholar]
- Cubillo A., Halari R., Ecker C., Giampietro V., Taylor E., Rubia K. Reduced activation and inter-regional functional connectivity of fronto-striatal networks in adults with childhood Attention-Deficit Hyperactivity Disorder (ADHD) and persisting symptoms during tasks of motor inhibition and cognitive switching. J. Psychiatr. Res. 2010;44:629–639. doi: 10.1016/j.jpsychires.2009.11.016. [DOI] [PubMed] [Google Scholar]
- Cubillo A., Halari R., Smith A., Taylor E., Rubia K. A review of fronto-striatal and fronto-cortical brain abnormalities in children and adults with Attention Deficit Hyperactivity Disorder (ADHD) and new evidence for dysfunction in adults with ADHD during motivation and attention. Cortex. 2012;48:194–215. doi: 10.1016/j.cortex.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Dankner Y., Shalev L., Carrasco M., Yuval-Greenberg S. Prestimulus Inhibition of Saccades in Adults With and Without Attention-Deficit / Hyperactivity Disorder as an Index of Temporal Expectations. Psychol. Sci. 2017;28:835–850. doi: 10.1177/0956797617694863. [DOI] [PubMed] [Google Scholar]
- Dickstein S.G., Bannon K., Castellanos F.X., Milham M.P. The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. J. Child Psychol. Psychiatry. 2006;47:1051–1062. doi: 10.1111/j.1469-7610.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- Dillo W., Göke A., Prox-Vagedes V., Szycik G.R., Roy M., Donnerstag F., Emrich H.M., Ohlmeier M.D. Neuronal correlates of ADHD in adults with evidence for compensation strategies - a functional MRI study with a Go/No-Go paradigm. Ger. Med. Sci. 2010;8:1–8. doi: 10.3205/000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier T.W., Youngstrom E.a., Naugle R.I. The latent structure of attention-deficit/hyperactivity disorder in a clinic-referred sample. Neuropsychology. 2007;21:45–64. doi: 10.1037/0894-4105.21.1.45. [DOI] [PubMed] [Google Scholar]
- Garavan H. Dissociable Executive Functions in the Dynamic Control of Behavior: Inhibition, Error Detection, and Correction. Neuroimage. 2002;17:1820–1829. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- Hampshire A., Chamberlain S.R., Monti M.M., Duncan J., Owen A.M. The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage. 2010;50:1313–1319. doi: 10.1016/j.neuroimage.2009.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A., Sharp D.J. Contrasting network and modular perspectives on inhibitory control. Trends Cogn. Sci. 2015;19:445–452. doi: 10.1016/j.tics.2015.06.006. [DOI] [PubMed] [Google Scholar]
- Hart H., Radua J., Nakao T., Mataix-Cols D., Rubia K. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry. 2013;70:185–198. doi: 10.1001/jamapsychiatry.2013.277. [DOI] [PubMed] [Google Scholar]
- Hervey A.S., Epstein J.N., Curry J.F. Neuropsychology of adults with attention-deficit/hyperactivity disorder: a meta-analytic review. Neuropsychology. 2004;18:485–503. doi: 10.1037/0894-4105.18.3.485. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Beckmann C.F., Behrens T.E.J., Woolrich M.W., Smith S.M. Fsl. Neuroimage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Johnson K.A., Kelly S.P., Bellgrove M.A., Barry E., Cox M., Gill M., Robertson I.H. Response variability in Attention Deficit Hyperactivity Disorder: Evidence for neuropsychological heterogeneity. Neuropsychologia. 2007;45:630–638. doi: 10.1016/j.neuropsychologia.2006.03.034. [DOI] [PubMed] [Google Scholar]
- Karch S., Thalmeier T., Lutz J., Cerovecki A., Opgen-Rhein M., Hock B., Leicht G., Hennig-Fast K., Meindl T., Riedel M., Mulert C., Pogarell O. Neural correlates (ERP/fMRI) of voluntary selection in adult ADHD patients. Eur. Arch. Psychiatry Clin. Neurosci. 2010;260:427–440. doi: 10.1007/s00406-009-0089-y. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Adler L., Ames M., Demler O., Faraone S., Hiripi E., Howes M.J., Jin R., Secnik K., Spencer T., Ustun T.B., Walters E.E. The World Health Organization Adult ADHD Self-Report Scale (ASRS): a short screening scale for use in the general population. Psychol. Med. 2005;35:245–256. doi: 10.1017/s0033291704002892. [DOI] [PubMed] [Google Scholar]
- King J.A., Colla M., Brass M., Heuser I., von Cramon D. Inefficient cognitive control in adult ADHD: evidence from trial-by-trial Stroop test and cued task switching performance. Behav. Brain Funct. 2007;3:42. doi: 10.1186/1744-9081-3-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofler M.J., Sarver D.E., Spiegel J.A., Day T.N., Harmon S.L., Wells E.L. Heterogeneity in ADHD: Neurocognitive predictors of peer, family, and academic functioning. Child Neuropsychol. 2016:1–27. doi: 10.1080/09297049.2016.1205010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodny T., Mevorach C., Shalev L. Isolating response inhibition in the brain: Parietal vs. frontal contribution. Cortex. 2017;88:173–185. doi: 10.1016/j.cortex.2016.12.012. [DOI] [PubMed] [Google Scholar]
- Konrad K., Eickhoff S.B. Is the ADHD brain wired differently? A review on structural and functional connectivity in attention deficit hyperactivity disorder. Hum. Brain Mapp. 2010;31:904–916. doi: 10.1002/hbm.21058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lijffijt M., Kenemans J.L., Verbaten M.N., van Engeland H. A meta-analytic review of stopping performance in attention-deficit/hyperactivity disorder: deficient inhibitory motor control? J. Abnorm. Psychol. 2005;114:216–222. doi: 10.1037/0021-843X.114.2.216. [DOI] [PubMed] [Google Scholar]
- McCarthy H., Skokauskas N., Frodl T. Identifying a consistent pattern of neural function in attention deficit hyperactivity disorder: a meta-analysis. Psychol. Med. 2014;44:869–880. doi: 10.1017/S0033291713001037. [DOI] [PubMed] [Google Scholar]
- McLaren D.G., Ries M.L., Xu G., Johnson S.C. A generalized form of context-dependent psychophysiological interactions (gPPI): A comparison to standard approaches. Neuroimage. 2012;61:1277–1286. doi: 10.1016/j.neuroimage.2012.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meffert H., Hwang S., Nolan Z.T., Chen G., Blair J.R. Segregating attention from response control when performing a motor inhibition task: Segregating attention from response control. Neuroimage. 2016;126:27–38. doi: 10.1016/j.neuroimage.2015.11.029. [DOI] [PubMed] [Google Scholar]
- Montojo C.A., Congdon E., Hwang L., Jalbrzikowski M., Kushan L., Vesagas T.K., Jonas R.K., Ventura J., Bilder R.M., Bearden C.E. Neural mechanisms of response inhibition and impulsivity in 22q11.2 deletion carriers and idiopathic attention deficit hyperactivity disorder. NeuroImage Clin. 2015;9:310–321. doi: 10.1016/j.nicl.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morein-Zamir S., Dodds C., van Hartevelt T.J., Schwarzkopf W., Sahakian B., Müller U., Robbins T. Hypoactivation in right inferior frontal cortex is specifically associated with motor response inhibition in adult ADHD. Hum. Brain Mapp. 2014;35:5141–5152. doi: 10.1002/hbm.22539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris S.B., DeShon R.P. Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychol. Methods. 2002;7:105–125. doi: 10.1037/1082-989x.7.1.105. [DOI] [PubMed] [Google Scholar]
- Mullane J.C., Corkum P.V., Klein R.M., McLaughlin E. Interference control in children with and without ADHD: a systematic review of flanker and simon task performance. Child Neuropsychol. 2009;15:321–342. doi: 10.1080/09297040802348028. [DOI] [PubMed] [Google Scholar]
- Mulligan R.C., Knopik V.S., Sweet L.H., Fischer M., Seidenberg M., Rao S.M. Neural correlates of inhibitory control in adult attention deficit/hyperactivity disorder: evidence from the Milwaukee longitudinal sample. Psychiatry Res. 2011;194:119–129. doi: 10.1016/j.pscychresns.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao T., Radua J., Rubia K., Mataix-Cols D. Gray matter volume abnormalities in ADHD: Voxel-based meta-analysis exploring the effects of age and stimulant medication. Am. J. Psychiatry. 2011;168:1154–1163. doi: 10.1176/appi.ajp.2011.11020281. [DOI] [PubMed] [Google Scholar]
- Nigg J.T. Is ADHD a disinhibitory disorder? Psychol. Bull. 2001;127:571–598. doi: 10.1037/0033-2909.127.5.571. [DOI] [PubMed] [Google Scholar]
- Nigg J.T. On inhibition/disinhibition in developmental psychopathology: views from cognitive and personality psychology and a working inhibition taxonomy. Psychol Bull. 2000;126:220–246. doi: 10.1037/0033-2909.126.2.220. [DOI] [PubMed] [Google Scholar]
- Nigg J.T., Willcutt E.G., Doyle A.E., Sonuga-Barke E.J.S. Causal heterogeneity in attention-deficit/hyperactivity disorder: do we need neuropsychologically impaired subtypes? Biol. Psychiatry. 2005;57:1224–1230. doi: 10.1016/j.biopsych.2004.08.025. [DOI] [PubMed] [Google Scholar]
- O'Halloran L., Cao Z., Ruddy K., Jollans L., Albaugh M.D., Aleni A., Potter A.S., Vahey N., Banaschewski T., Hohmann S., Bokde A.L.W., Bromberg U., Büchel C., Quinlan E.B., Desrivières S., Flor H., Frouin V., Gowland P., Heinz A., Ittermann B., Nees F., Orfanos D.P., Paus T., Smolka M.N., Walter H., Schumann G., Garavan H., Kelly C., Whelan R. Neural circuitry underlying sustained attention in healthy adolescents and in ADHD symptomatology. Neuroimage. 2018;169:395–406. doi: 10.1016/j.neuroimage.2017.12.030. [DOI] [PubMed] [Google Scholar]
- Osada T., Ohta S., Ogawa A., Tanaka M., Suda A., Kamagata K., Hori M., Aoki S., Shimo Y., Hattori N., Shimizu T., Enomoto H. An essential role of the intraparietal sulcus in response inhibition predicted by parcellation-based network Title : An essential role of the intraparietal sulcus in response inhibition predicted by parcellation-based network Takahiro Osada, 1 Shinri Oht. J. Neurosci. 2019 doi: 10.1523/JNEUROSCI.2244-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk G., De Lima M.S., Horta B.L., Biederman J., Rohde L.A. The worldwide prevalence of ADHD: A systematic review and metaregression analysis. Am. J. Psychiatry. 2007;164:942–948. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- Poldrack R.a., Mumford J.a. Independence in ROI analysis: Where is the voodoo? Soc. Cogn. Affect. Neurosci. 2009;4:208–213. doi: 10.1093/scan/nsp011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack R.A. Region of interest analysis for fMRI. Soc. Cogn. Affect. Neurosci. 2007;2:67–70. doi: 10.1093/scan/nsm006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polner B., Aichert D., Macare C., Costa A., Ettinger U. Gently restless: association of ADHD-like traits with response inhibition and interference control. Eur. Arch. Psychiatry Clin. Neurosci. 2015;265:689–699. doi: 10.1007/s00406-014-0531-7. [DOI] [PubMed] [Google Scholar]
- Rieger M., Gauggel S., Burmeister K. Inhibition of ongoing responses following frontal, nonfrontal, and basal ganglia lesions. Neuropsychology. 2003;17:272–282. doi: 10.1037/0894-4105.17.2.272. [DOI] [PubMed] [Google Scholar]
- Rubia K., Alegria A.A., Cubillo A.I., Smith A.B., Brammer M.J., Radua J. Effects of stimulants on brain function in attention-deficit/hyperactivity disorder: A systematic review and meta-analysis. Biol. Psychiatry. 2014;76:616–628. doi: 10.1016/j.biopsych.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K., Criaud M., Wulff M., Alegria A., Brinson H., Barker G., Stahl D., Giampietro V. Functional connectivity changes associated with fMRI neurofeedback of right inferior frontal cortex in adolescents with ADHD. Neuroimage. 2018;188:43–58. doi: 10.1016/j.neuroimage.2018.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K., Halari R., Cubillo A., Mohammad A.-M., Brammer M., Taylor E. Methylphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication-naïve children with ADHD during a rewarded continuous performance task. Neuropharmacology. 2009;57:640–652. doi: 10.1016/j.neuropharm.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Rubia K., Smith A.B., Brammer M.J., Taylor E. Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. Neuroimage. 2003;20:351–358. doi: 10.1016/s1053-8119(03)00275-1. [DOI] [PubMed] [Google Scholar]
- Salum G.a., Sonuga-Barke E., Sergeant J., Vandekerckhove J., Gadelha A., Moriyama T.S., Graeff-Martins a S., Manfro G.G., Polanczyk G., Rohde L.a P. Mechanisms underpinning inattention and hyperactivity: neurocognitive support for ADHD dimensionality. Psychol. Med. 2014;44:1–13. doi: 10.1017/S0033291714000919. [DOI] [PubMed] [Google Scholar]
- Satterthwaite T.D., Wolf D.H., Loughead J., Ruparel K., Elliott M.A., Hakonarson H., Gur R.C., Gur R.E. Impact of in-scanner head motion on multiple measures of functional connectivity: Relevance for studies of neurodevelopment in youth. Neuroimage. 2012;60:623–632. doi: 10.1016/j.neuroimage.2011.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A., Müller F., Dolder P.C., Schmid Y., Zanchi D., Liechti M.E., Borgwardt S. Comparative effects of methylphenidate, modafinil, and mdma on response inhibition neural networks in healthy subjects. Int. J. Neuropsychopharmacol. 2017;20:712–720. doi: 10.1093/ijnp/pyx037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M.F., Krick C.M., Retz W., Hengesch G., Retz-Junginger P., Reith W., Rösler M. Impairment of fronto-striatal and parietal cerebral networks correlates with attention deficit hyperactivity disorder (ADHD) psychopathology in adults - a functional magnetic resonance imaging (fMRI) study. Psychiatry Res. 2010;183:75–84. doi: 10.1016/j.pscychresns.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Schweren L.J.S., de Zeeuw P., Durston S. MR imaging of the effects of methylphenidate on brain structure and function in Attention-Deficit/Hyperactivity Disorder. Eur. Neuropsychopharmacol. 2013;23:1151–1164. doi: 10.1016/j.euroneuro.2012.10.014. [DOI] [PubMed] [Google Scholar]
- Sebastian a, Pohl M.F., Klöppel S., Feige B., Lange T., Stahl C., Voss A., Klauer K.C., Lieb K., Tüscher O. Disentangling common and specific neural subprocesses of response inhibition. Neuroimage. 2012;64:601–615. doi: 10.1016/j.neuroimage.2012.09.020. [DOI] [PubMed] [Google Scholar]
- Segal D., Mashal N., Shalev L. Semantic conflicts are resolved differently by adults with and without ADHD. Res. Dev. Disabil. 2015;47:416–429. doi: 10.1016/j.ridd.2015.09.024. [DOI] [PubMed] [Google Scholar]
- Shalev L., Ben-Simon A., Mevorach C., Cohen Y., Tsal Y. Conjunctive Continuous Performance Task (CCPT)–a pure measure of sustained attention. Neuropsychologia. 2011;49:2584–2591. doi: 10.1016/j.neuropsychologia.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Sharp D.J., Bonnelle V., De Boissezon X., Beckmann C.F., James S.G., Patel M.C., Mehta M.A. Distinct frontal systems for response inhibition, attentional capture, and error processing. Proc. Natl. Acad. Sci. 2010;107:6106–6111. doi: 10.1073/pnas.1000175107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel J.S., Power J.D., Dubis J.W., Vogel A.C., Jessica A., Schlaggar B.L., Petersen S.E. Statistical improvements in functional magnetic resonance imaging analyses produced by censoring high-motion data points. Hum. Brain Mapp. 2014;35:1981–1996. doi: 10.1002/hbm.22307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon V., Czobor P., Balint S., Meszaros A., Bitter I. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br. J. Psychiatry. 2009;194:204–211. doi: 10.1192/bjp.bp.107.048827. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke E., Bitsakou P., Thompson M. Beyond the Dual Pathway Model: Evidence for the Dissociation of Timing, Inhibitory, and Delay-Related Impairments in Attention-Deficit/Hyperactivity Disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2010;49:345–355. doi: 10.1016/j.jaac.2009.12.018. [DOI] [PubMed] [Google Scholar]
- Sripada C., Kessle D., Fang Y., Welsh R.C., Kumar Krishan Prem, Angstadt M. Disrupted network architecture of the resting brain in attention-deficit/hyperactivity disorder. Hum. Brain Mapp. 2014;35:4693–4705. doi: 10.1002/hbm.22504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweitzer M.M., Kollins S.H., Kozink R.V, Hallyburton M., English J., Addicott Merideth A Oliver, Jason A McClernon F.J. ADHD, Smoking Withdrawal, and Inhibitory Control: Results of a Neuroimaging Study with Methylphenidate Challenge. Neuropsychopharmacology. 2017;43:851–858. doi: 10.1038/npp.2017.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick D., Ashley V., Turken U. Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. Neuroimage. 2011;56:1655–1665. doi: 10.1016/j.neuroimage.2011.02.070. [DOI] [PubMed] [Google Scholar]
- Swick D., Chatham C.H. Ten years of inhibition revisited. Front. Hum. Neurosci. 2014;8 doi: 10.3389/fnhum.2014.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsal Y., Shalev L., Mevorach C. The diversity of attention deficits in ADHD: the prevalence of four cognitive factors in ADHD versus controls. J. Learn. Disabil. 2005;38:142–157. doi: 10.1177/00222194050380020401. [DOI] [PubMed] [Google Scholar]
- Van Essen D.C. A population-average, landmark- and surface-based (PALS) atlas of human cerebral cortex. Neuroimage. 2005;28:635–662. doi: 10.1016/j.neuroimage.2005.06.058. [DOI] [PubMed] [Google Scholar]
- Vul E., Harris C., Winkielman P., Pashler H. Puzzingly high correlations in fMRI studies of emotion, personality, and social cognition. Perspect. Psychol. Sci. 2009;4:274–290. doi: 10.1111/j.1745-6924.2009.01125.x. [DOI] [PubMed] [Google Scholar]
- Wåhlstedt C., Thorell L.B., Bohlin G. Heterogeneity in ADHD: Neuropsychological pathways, comorbidity and symptom domains. J. Abnorm. Child Psychol. 2009;37:551–564. doi: 10.1007/s10802-008-9286-9. [DOI] [PubMed] [Google Scholar]
- Willcutt E.G., Doyle A.E., Nigg J.T., Faraone S.V, Pennington B.F. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol. Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Woolrich M. Robust group analysis using outlier inference. Neuroimage. 2008;41:286–301. doi: 10.1016/j.neuroimage.2008.02.042. [DOI] [PubMed] [Google Scholar]
- Woolrich M.W., Behrens T.E.J., Beckmann C.F., Jenkinson M., Smith S.M. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage. 2004;21:1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Worsley K.J. Chapter 14 Statistical Analysis of Activation Images. In: Jezzard P., Matthew P.M., Smith S.M., editors. Functional MRI: An Introduction to Methods. Oxford University Press; Oxford, UK: 2001. pp. 251–270. [Google Scholar]
- Wright L., Lipszyc J., Dupuis A., Thayapararajah S.W., Schachar R. Response inhibition and psychopathology: A meta-analysis of go/no-go task performance. J. Abnorm. Psychol. 2014;123:429–439. doi: 10.1037/a0036295. [DOI] [PubMed] [Google Scholar]
- Zohar A.H., Konfortes H. Diagnosing ADHD in Israeli adults: The psychometric properties of the adult ADHD self report scale (ASRS) in hebrew. Isr. J. Psychiatry Relat. Sci. 2010;47:308–313. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.