Abstract
Type III collagen is the major protein in the walls of blood vessels and hollow organs; it is decreased in patients with vascular Ehlers-Danlos syndrome (EDS). A 52-year-old man was admitted for severe back pain, and right hemothorax was suspected by chest computed tomography. Immediately after embolization for bleeding bronchial artery, aortic dissection occurred and was treated conservatively in the intensive-care unit. Vascular EDS with a mutation of COL3A1 cDNA (c.3175G>A) was diagnosed. When vascular EDS is suspected, the patient should be treated prophylactically, and a genetic examination should be performed to confirm the diagnosis.
Keywords: vascular Ehlers-Danlos syndrome, COL3A1, angiography, aortic dissection, hemothorax
Introduction
Ehlers-Danlos syndrome (EDS) is a rare connective tissue disorder (1,2). All 13 types of EDS are classified based on characteristic manifestations (3). Vascular EDS, previously known as EDS type IV, is the most severe type, and its symptoms are attributed to a reduction in type III collagen production (4-6). Since type III collagen is a major protein in the walls of blood vessels and hollow organs, the symptoms of vascular EDS include bruising, arterial and bowel fragility, and uterine fragility during pregnancy (6).
Although vascular EDS is due to an autosomal dominant defect in the COL3A1 gene, half of vascular EDS patients have no family history; initial manifestations like rupture of blood vessels, intestines, or other organs are diagnostic clues (2,5-7). These symptoms can also suggest other ailments: Marfan syndrome, Loeys-Dietz syndrome, familial artery aneurysm, arterial tortuosity syndrome, and dissection syndromes (6). To distinguish vascular EDS from these other diseases, it is necessary to identify the COL3A1 mutation or to demonstrate a reduced production of type III collagen (2).
We herein report a case of vascular EDS presenting initially as hemothorax without any traumatic injury, subsequently manifesting as aortic dissection after embolization of the bronchial artery during angiography and finally being diagnosed by detection of a mutation in the COL3A1 gene. When hemothorax is seen without any traumatic injury, vascular EDS should be a differential diagnosis, and appropriate examinations should be undertaken to make a diagnosis.
Case Report
A 52-year-old man arrived at the emergency room of our hospital on foot complaining of pain under the right scapula and a sudden onset of a cold sweat 7 hours earlier. He had had hyperuricemia since the age of 30 and spondylolisthesis since the age of 40. No abnormal findings had been pointed out by annual health examinations. He was an ex-smoker of 35 pack-years. His father had died from lung cancer, but he had no family history of any connective tissue diseases or sudden death.
He appeared thin with a height of 179.5 cm, weight of 61.0 kg, and body mass index of 18.9 kg/m2. He had a blood pressure of 185/100 mmHg and heart rate of 110 beats per minute, without oxygenation failure or a fever. His first physical examination was unremarkable. The laboratory examination showed no anemia with hemoglobin of 17.0 g/dL and hematocrit of 49.7%, no coagulation disorder, and no antibodies related to collagen diseases (Table 1). The electrocardiogram showed sinus tachycardia.
Table 1.
Laboratory Findings on First Visit.
| Hematology | Biochemistry | Serology | ||||||||||||
| WBCs | 9,800 | /μL | AST | 29 | U/L | C-reactive protein | 0.1 | mg/dL | ||||||
| Neutrophils | 83.3 | % | ALT | 16 | U/L | |||||||||
| Lymphocytes | 11.6 | % | γ-GTP | 39 | U/L | Anti-nuclear antibody | ×80 | |||||||
| Eosinophils | 0.9 | % | ALP | 252 | U/L | Rheumatoid factor | 11 | U/mL | ||||||
| Monocytes | 3.9 | % | T-Bil | 1.2 | mg/dL | Anti-cardiolipin antibody | 8 | U/mL | ||||||
| Basophils | 0.3 | % | BUN | 15 | mg/dL | MPO-ANCA | 1 | U/mL | ||||||
| Red blood cells | 509 | ×104/μL | Cre | 0.64 | mg/dL | PR3-ANCA | 1 | U/mL | ||||||
| Hb | 17.0 | g/dL | LDH | 216 | U/L | |||||||||
| Hematocrit | 49.7 | % | CK | 172 | U/L | Activated protein C | 94 | % | ||||||
| Platelet | 16.0 | ×104/μL | Na | 140 | mEq/L | Cofactor protein S | 86 | % | ||||||
| Coagulation | K | 4.1 | mEq/L | Lupus anticoagulant (dRVVT) | 1.1 | |||||||||
| APTT | 31.2 | s | Cl | 104 | mEq/L | Prior to neutralization | 43.6 | s | ||||||
| PT/INR | 0.99 | Ca | 9.4 | mg/dL | After neutralization | 39.4 | s | |||||||
| D-Dimer | 0.66 | μg/mL | Alb | 4.5 | g/dL | von Willebrand factor | 292 | % | ||||||
WBCs: white blood cells, Hb: hemoglobin, APTT: activated partial thromboplastin time, PT: prothrombin time, INR: international normalized ratio, AST: aspartate transaminase, ALT: alanine aminotransferase, γ-GTP: γ-glutamyl transpeptidase, ALP: alkaline phosphatase, T-Bil: total bilirubin, BUN: blood urea nitrogen, Cre: creatinine, LDH: lactate dehydrogenase, CK: creatine kinase, Na: sodium, K: potassium, Cl: chlorine, Ca: calcium, Alb: albumin, MPO-ANCA: myeloperoxidase-antineutrophil cytoplasmic antibody, PR3-ANCA: proteinase3-antineutrophil cytoplasmic antibody, dRVVT: dilute Russell’s viper venom time
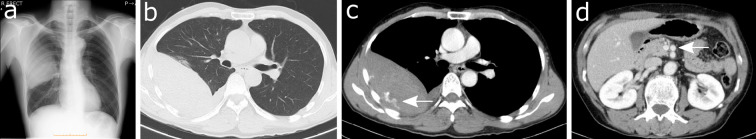
Chest computed tomography (CT) showed a mixed-density soft tissue mass in the right thoracic cavity and leakage of contrast medium from an intercostal artery into the mass, implying hemothorax in the right thoracic cavity (Fig. 1). After admission to the emergency room, a chest tube was inserted, and blood continued to ooze.
Figure 1.
Chest X-ray film and computed tomography (CT) on the first visit. (a) The chest X-ray film shows fan-shaped infiltration spreading from the hilum of the right lung. (b, c) CT reveals a soft tissue mass with uneven density in the right thoracic cavity and contrast medium leak from the intercostal artery into the mass (arrow). (d) There is a 10-mm aneurysm (arrow) in front of the superior mesenteric artery.
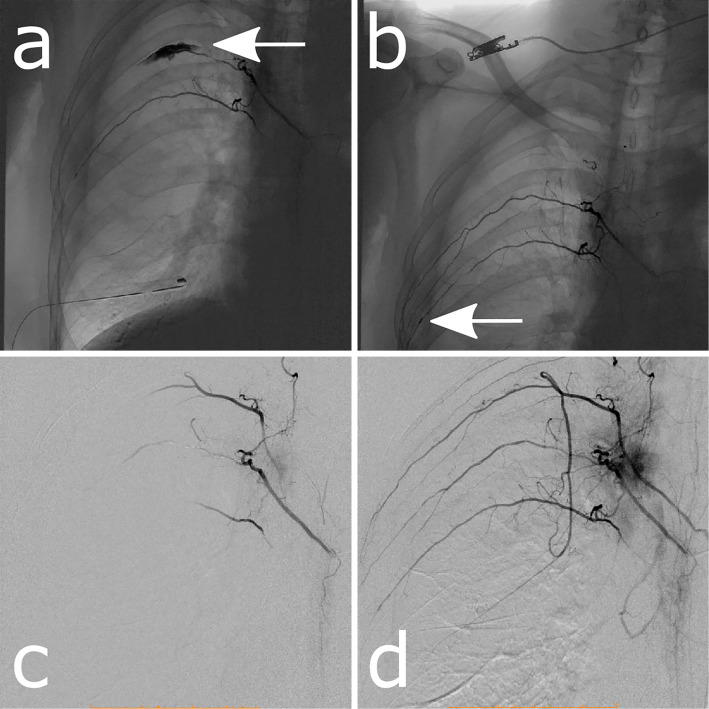
To identify the source of the bleeding, we performed angiography, which revealed irregular leaking of contrast medium to the distal site of the right fifth intercostal artery (Fig. 2a) and an aneurysm at the right sixth intercostal artery (Fig. 2b). The right fourth and sixth intercostal arteries were simultaneously drawn when the right fifth intercostal artery image was constructed, implying that these arteries have a common trunk (Fig. 2c, d). A gelatin sponge was placed at the right fifth intercostal artery for hemostasis.
Figure 2.
Angiography of bronchial arteries. (a) Contrast medium leaks out irregularly into the distal site of the right fifth intercostal artery (arrow). (b) An aneurysm is apparent at the right sixth intercostal artery (arrow). (c, d) The right fourth and sixth intercostal arteries are revealed by enhancement of the right fifth intercostal artery. These right intercostal arteries are supplied from a common trunk.
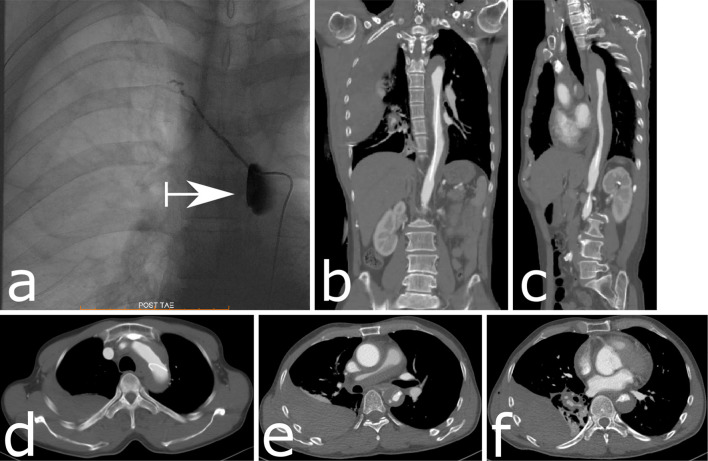
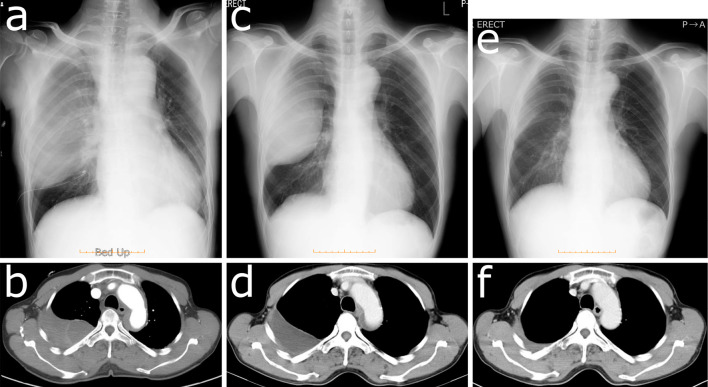
Immediately afterward, severe back pain developed, and fentanyl to relieve pain was started in the angiography room. Contrast medium was stagnant in a false lumen of the descending aorta at the origin of the right fifth intercostal artery (Fig. 3a). CT showed Stanford type A aortic dissection from the thoracic descending aorta to the ascending aorta (Fig. 3b-f). We started to administer nicardipine and continued to observe the patient without surgery in the intensive-care unit (ICU). Five days later, the false lumen had shrunk, and he left the ICU (Fig. 4a-d). He was discharged without recurrence of symptoms 22 days after the aortic dissection was noted and returned to his occupation. The hematoma had also shrunk after his discharge (Fig. 4e, f).
Figure 3.
Stanford type A aortic dissection from the thoracic descending aorta to the ascending aorta after embolization of the right fifth intercostal artery. (a) Contrast medium is stagnant at the origin of the right fifth intercostal artery in the descending aorta. (b) A coronal section of the vessels on CT shows dissection in the thoracic descending aorta with a narrow true lumen of the aorta and catheter existing in the true lumen. An uneven density in the right thoracic cavity is revealed. (c) A sagittal section of the vessels on CT also shows dissection in the descending aorta with a narrow true lumen. (d-f) An axial section of the vessels on CT shows dissection at the arch of the aorta, with a catheter and a narrowed true lumen. The right pulmonary trunk and arteries are compressed and narrowed by a mediastinal hematoma. An area of uneven density on the dorsal side in the right thoracic cavity is revealed (d). The dissection extends to the ascending aorta, and there is an area of uneven density on the dorsal side of the right thoracic cavity (e). The dissection with the mediastinal hematoma extends from the descending aorta to the ascending aorta. (f) Contrast-enhanced CT reveals a feature that is similar in appearance to an ulcer-like projection that protrudes into the false lumen at the descending aorta. An area of uneven density stretches to the caudal side in the right thoracic cavity.
Figure 4.
The follow-up chest X-ray film and CT scan. The false lumen of the aorta shrank, and the hemothorax did not expand 1 day (a, b), 15 days (c) and 18 days (d) after the aortic dissection had occurred. The hematoma was almost diminished three months after his discharge (e, f).
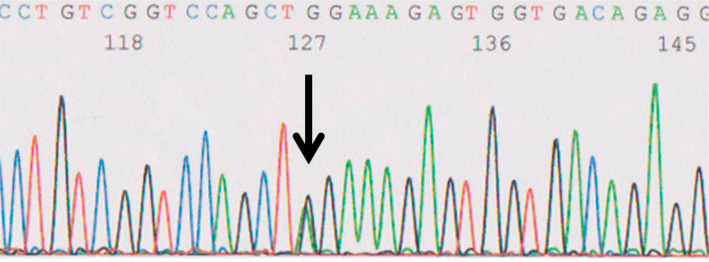
Since connective tissue disorders including vascular EDS were suspected, we referred him to the division of personalized genetic medicine at Nippon Medical School Hospital. To obtain his consent to the examination, a medical geneticist explained about genetic testing based on the following points: maintenance of the confidentiality of the patient's individual information and examination results; possibility of incidental pathogenic findings and the right to refuse to be informed of the results; possibility of familial disease; reconfirmation of his intention regarding genetic testing for his family members, after knowing results of his examination, if a responsible mutation is detected; genetic counseling; and mental supports by psychiatrists, clinical psychologists, medical geneticists and genetic counselors. After obtaining his written consent, total RNA was extracted from his peripheral blood cells, and a missense mutation (c.3175G>A) of COL3A1 cDNA (i.e., a glycine-to-arginine substitution at codon 1059) was found (Fig. 5). This result confirmed the diagnosis of vascular Ehlers-Danlos syndrome. To confirm the diagnosis, we tried to check the COL3A1 gene of his family. Unfortunately, his three children did not consent to this assessment. Celiprolol was therefore started at 50 mg/day and gradually increased to 400 mg/day, while an angiotensin II receptor blocker was added to a calcium channel blocker for stabilization of blood pressure. No vascular complications have recurred thus far.
Figure 5.
The sequence of the COL3A1 gene located on 2q31. The cDNA was from the peripheral blood cells. Each nucleotide is indicated by a different color: adenine (A), green; cytosine (C), blue; guanine (G), black; and thymine (T), red. A heterozygous missense mutation (c.3175G>A) was found (arrow).
Discussion
In the present case, vascular EDS was suspected and diagnosed based on the manifestations of vascular vulnerability, including hemothorax without any traumatic injury, aortic dissection immediately after angiography and embolization of the bronchial artery. At the time, it was difficult to diagnose the patient with vascular EDS because he had no family history of any connective tissue diseases or sudden death and had had abnormalities of the joint or skin that were unremarkable. Hyperextensibility of the skin or hypermobility of large joints are often clues to the presence of EDS, but these manifestations are unusual for vascular EDS (1). Symptoms of vascular EDS are related to decreased production of type III collagen, and the diagnostic criteria were revised in 2017 (Table 2) (3). However, these findings are not necessary for the diagnosis of vascular EDS, and there are cases where the disease has had little or no effect on the appearance of the face or skin (8).
Table 2.
Diagnostic Criteria of Vascular Ehlers-Danlos Syndrome (3).
| Inheritance | |
| Autosomal dominant | |
| Major criteria | |
| Family history of vascular Ehlers-Danlos Syndrome with documented causative variant in COL3A1 | |
| Arterial rupture at a young age | |
| Spontaneous sigmoid colon perforation in the absence of known diverticular disease or other bowel pathology | |
| Uterine rupture during the third trimester in the absence of previous C-section and/or severe peripartum perineum tears | |
| Carotid-cavernous sinus fistula formation in the absence of trauma | |
| Minor criteria | |
| Bruising unrelated to identified trauma and/or in unusual sites such as cheeks and back | |
| Thin, translucent skin with increased venous visibility | |
| Characteristic facial appearance | |
| Spontaneous pneumothorax | |
| Acrogeria | |
| Talipes equinovarus | |
| Congenital hip dislocation | |
| Hypermobility of small joints | |
| Tendon and muscle rupture | |
| Keratoconus | |
| Gingival recession and gingival fragility | |
| Early onset varicose veins (under age 30 and nulliparous if female) |
Since vascular EDS is likely to be suspected based on the clinical features and family history, and given its rarity (1 in 50,000-200,000 of the population), its diagnosis before the occurrence of serious initial symptoms is difficult and sometimes not confirmed (1,6). To confirm the diagnosis, a mutational analysis of the COL3A1 gene or biochemical examination of collagen production is recommended. A biochemical examination to confirm a reduction in type III collagen was not conducted in the present case because recurrent bleeding events due to tissue vulnerability and the detection of a COL3A1 mutation made us hesitate to conduct a tissue biopsy. Tissues for a biochemical examination may be able to be collected if he suffers from another event, such as intestinal rupture, that requires unavoidable surgical intervention.
Hemothorax was the initial symptom of vascular EDS in this case, and aortic dissection developed immediately after intervention for bronchial arterial bleeding. An aneurysm or dissection or rupture of vessels in the patients of vascular EDS typically involves the renal, iliac, femoral, mesenteric, hepatic, carotid, subclavian, ulnar, popliteal and tibial arteries, and coronary rupture is rare (8). Intercostal aneurysm rupture was recently reported in only one case from Japan, and intercostal aneurysm rapture as the onset manifestation is rare, as described in the paper (9). Although pathological evidence of thoracic complications in vascular EDS has already been reported in a few post-mortem cases (10), the occurrence of aortic dissection after intervention has hardly been reported. The complications of angiography in vascular EDS patients are reported to be hemorrhaging from the puncture site, intracranial hemorrhaging, heart rupture, aortic dissection, intraabdominal hemorrhaging, intrathoracic hemorrhaging and others. Such hemorrhaging events occur repeatedly after the first treatment of the complications, and when or where the next complications will occur is unpredictable (11). We should therefore pay close attention when performing guidewire manipulation in order to avoid these complications.
In the present case, hemothorax of uncertain origin was noted and led to the suspicion of blood vessel rupture in the chest. Therefore, angiography had to be performed in order to identify the source of the bleeding. It has been reported that 37% of patients with vascular EDS die of complications from blood vessel injury in the chest, and in all cases of carotid cavernous sinus fistula, coil embolization for the carotid vessels was safely performed (12). When contrast medium was leaking from the right fifth intercostal artery, we realized that the bleeding was continuous and decided to conduct embolization of this artery. Since aortic dissection had occurred after embolization in this case of uncertain pathogenesis, conservative observation might have been an option as long as the blood pressure was stabilized. Aortic dissection can occur in any case; therefore, angiography should be performed cautiously, and alternative candidate causes should continue to be considered. Even though the cause of aortic dissection in our case was uncertain, an elevated blood pressure during angiography or vascular injury from some type of insult was suspected; the guidewire did not stray into the false lumen. Although aortic dissection can be treated surgically, we chose to treat it conservatively in the ICU to avoid the risk of further serious complications, as these sequential events suggested the presence of a co-morbidity, such as a connective tissue disorder.
The lifespan of affected individuals varies widely from 10 to 80 years, and the median age at death is about 51 years (12,13). The lifespan is influenced by a mutation in COL3A1, which encodes the pro-α1 chains of type III collagen; these chains have a (Gly-X-Y)n repeat structure (14). The exon-skipping mutation of COL3A1 occurs less frequently than other mutations of COL3A1, and patients with exon-skipping mutations have the shortest median survival time. Substitution for triplet glycine residues in the triple helical domain results in a better prognosis than exon-skipping mutations, but substitution by bulky residues results in more severe phenotypes than that by smaller residues. Heterozygosity for COL3A1-null alleles delays the onset of complications by 20 years (6,15). The missense mutation c.3175G>A in COL3A1 cDNA, which converts glycine to arginine at codon 1059 in the (Gly-X-Y)n repeat structure in the triple helical domain, was detected in this case and has not been previously reported on the ClinVar website (https://www.ncbi.nlm.nih.gov/clinvar/). However, the pathogenic missense mutation c.3176G>T in COL3A1, which converts glycine to valine at codon 1059, just as in our case, had been already reported on the ClinVar website. Similar pathogenic mutations leading to substitution for glycine in the (Gly-X-Y)n repeat structure at other positions in the triple helical domain are the most common mutation type in the COL3A1 gene. The (Gly-X-Y)n repeat structure is necessary for the triple helix formation of the pro-α1 chains of type III collagen encoded by the COL3A1 gene. In particular, glycine residues are positioned in the center of the triple helix for dense packing along the central axis of chains to form type III collagen and stable collagen fibrils in extracellular spaces. Therefore, substitution of glycine in the (Gly-X-Y)n repeat structure leads to the production of nonfunctional type III collagen and vulnerability of collagen tissue (14). We assume that the mutation in our case inhibits the encoding of glycine at the same codon which was previously reported to be pathogenic, and therefore it could also fail to generate the (Gly-X-Y)n repeat structure.
Blood pressure stabilization is the recommended treatment for patients with vascular EDS. In a randomized trial that was relatively large for a rare disease, celiprolol, a β1-adrenoceptor antagonist with β2-adrenoceptor agonist action, was shown to protect against severe vascular EDS complications, including cardiac and arterial events, by causing vasodilation or by boosting the production of type III collagen (16,17).
It is difficult to consider vascular EDS as a differential diagnosis before treatment or conducting an examination for complications because it is a rare disease, and its complication is often revealed or assessed under urgent circumstances. In a previous report, vascular EDS was diagnosed before vascular surgery in only 26% of patients (18). Our case report suggests that vascular EDS should be suspected in patients who develop bleeding or blood vessel collapse of uncertain origin, and patients with vascular EDS should be treated prophylactically. For the confirmation of the diagnosis, genetic examinations need to be performed at specialized facilities with specialists in clinical genetics who are accustomed to handling genetic information, although they are currently approved by the health insurance program in Japan. Medical practitioners must understand the autosomal dominant nature of EDS and carefully consider the results of genetic examinations.
This case was reported at the 45th Annual Meeting of the Japanese Society of Intensive Care Medicine.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Pepin M, Schwarze U, Superti-Furga A, Byers PH. Clinical and genetic features of Ehlers-Danlos syndrome type IV, the vascular type. N Engl J Med 342: 673-680, 2000. [DOI] [PubMed] [Google Scholar]
- 2. Abel MD, Carrasco LR. Ehlers-Danlos syndrome: classifications, oral manifestations, and dental considerations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 102: 582-590, 2006. [DOI] [PubMed] [Google Scholar]
- 3. Malfait F, Francomano C, Byers P, et al. The 2017 international classification of the Ehlers-Danlos syndromes. Am J Med Genet C Semin Med Genet 175: 8-26, 2017. [DOI] [PubMed] [Google Scholar]
- 4. Beighton P, De Paepe A, Steinmann B, Tsipouras P, Wenstrup RJ. Ehlers-Danlos syndromes: revised nosology, Villefranche, 1997. Ehlers-Danlos National Foundation (USA) and Ehlers-Danlos Support Group (UK). Am J Med Genet 77: 31-37, 1998. [DOI] [PubMed] [Google Scholar]
- 5. Matsushita A, Takayanagi N, Ishiguro T, et al. A case of Ehlers-Danlos syndrome suspected from pulmonary hematoma due to disruption of the lung. Nihon Kokyuki Gakkai Zasshi (J Jpn Respir Soc) 47: 704-710, 2009(in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 6. Byers PH, Belmont J, Black J, et al. Diagnosis, natural history, and management in vascular Ehlers-Danlos syndrome. Am J Med Genet C Semin Med Genet 175: 40-47, 2017. [DOI] [PubMed] [Google Scholar]
- 7. Germain DP. Ehlers-Danlos syndrome type IV. Orphanet J Rare Dis 2: 32, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. De Paepe A, Malfait F. The Ehlers-Danlos syndrome, a disorder with many faces. Clin Genet 82: 1-11, 2012. [DOI] [PubMed] [Google Scholar]
- 9. Fukui T, Sumitomo S, Ishikawa M, et al. Intercostal artery rupture associated with vascular-type Ehlers-Danlos syndrome - Report of a case. Nihon Kokyuki Geka Gakkai Zasshi (J Jpn Assoc Chest Surg) 30: 80-86, 2016(in Japanese, Abstract in English). [Google Scholar]
- 10. Shields LB, Rolf CM, Davis GJ, Hunsaker JC. Sudden and unexpected death in three cases of Ehlers-Danlos syndrome type IV. J Forensic Sci 55: 1641-1645, 2010. [DOI] [PubMed] [Google Scholar]
- 11. Asai K, Toyota S, Hayakawa K, et al. Cartoid cavernous fistula in a patient with Ehlers-Danlos syndrome type IV: a case report. No Kekkan-nai Chiryo (J Neuroendovasc Ther) 7: 94-100, 2013(in Japanese, Abstract in English). [Google Scholar]
- 12. Pepin MG, Schwarze U, Rice KM, Liu M, Leistritz D, Byers PH. Survival is affected by mutation type and molecular mechanism in vascular Ehlers-Danlos syndrome (EDS type IV). Genet Med 16: 881-888, 2014. [DOI] [PubMed] [Google Scholar]
- 13. Frank M, Albuisson J, Ranque B, et al. The type of variants at the COL3A1 gene associates with the phenotype and severity of vascular Ehlers-Danlos syndrome. Eur J Hum Genet 23: 1657-1664, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eagleton MJ. Arterial complications of vascular Ehlers-Danlos syndrome. J Vasc Surg 64: 1869-1880, 2016. [DOI] [PubMed] [Google Scholar]
- 15. Plancke A, Holder-Espinasse M, Rigau V, Manouvrier S, Claustres M, Khau Van Kien P. Homozygosity for a null allele of COL3A1 results in recessive Ehlers-Danlos syndrome. Eur J Hum Genet 17: 1411-1416, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ong KT, Perdu J, De Backer J, et al. Effect of celiprolol on prevention of cardiovascular events in vascular Ehlers-Danlos syndrome: a prospective randomised, open, blinded-endpoints trial. Lancet 376: 1476-1484, 2010. [DOI] [PubMed] [Google Scholar]
- 17. Nawarskas JJ, Cheng-Lai A, Frishman WH. Celiprolol: a unique selective adrenoceptor modulator. Cardiol Rev 25: 247-253, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Oderich GS, Panneton JM, Bower TC, et al. The spectrum, management and clinical outcome of Ehlers-Danlos syndrome type IV: a 30-year experience. J Vasc Surg 42: 98-106, 2005. [DOI] [PubMed] [Google Scholar]