Abstract
Colonic varices are usually associated with portal hypertension. Idiopathic colonic varices are extremely rare. A 68-year-old man with a positive fecal occult blood test result underwent colonoscopy. We detected idiopathic ileocolonic varices and a coexisting ascending colon polyp. While reviewing the literature, we found cases of biopsies and polypectomies resulting in significant bleeding. We herein report a case of idiopathic ileocolonic varices coexisting with a colon polyp treated successfully by endoscopy. The coexistence of colonic varices and a colorectal lesion that requires endoscopic treatment may lead to significant bleeding. During management, the development of a treatment strategy and obtaining informed consent are necessary.
Keywords: colon polyp, colonic varices, endoscopic mucosal resection, idiopathic, ileocolonic varices
Introduction
Colonic varices are rare and usually associated with portal hypertension. Idiopathic colonic varices are very rare. In most cases, the major complement is hematochezia. Colon polyps are typically treated by endoscopy, and bleeding is the most common serious complication occurring after endoscopic mucosal resection (1). The coexistence of colonic varices and colorectal lesions that require endoscopic procedures may cause significant bleeding. When reviewing case reports of previous idiopathic colonic varices cases (2-30), we found cases wherein significant bleeding was caused by a biopsy or polypectomy (20,31).
We herein report the first case of idiopathic ileocolonic varices coexisting with a colon polyp treated successfully by endoscopy.
Case Report
A 68-year-old man was referred to our hospital because he had positive fecal occult blood screening test results. He had a history of duodenal ulcer and operation for sinusitis in his 30s. During the examination of the duodenal ulcer, no esophageal varices were detected. He also had a history of hematochezia twice in his 50s. He had never been found to have any liver function abnormality or liver disease, portal hypertension, colon malignancy, pancreatitis, or congestive heart failure. He had no history of abdominal operation, blood transfusion or a family predisposition. He was a social drinker and never drank more than 60 g alcohol per day.
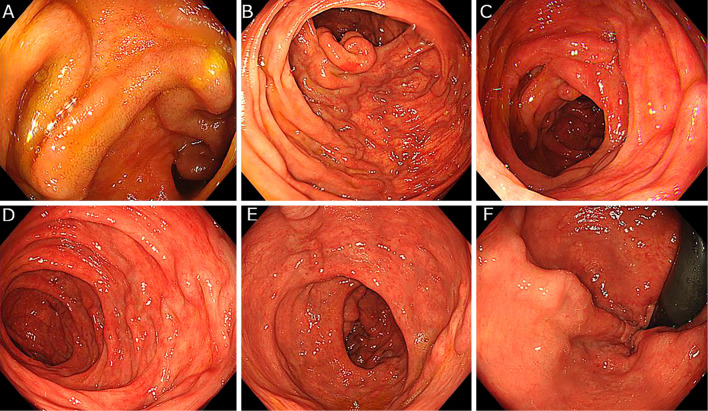
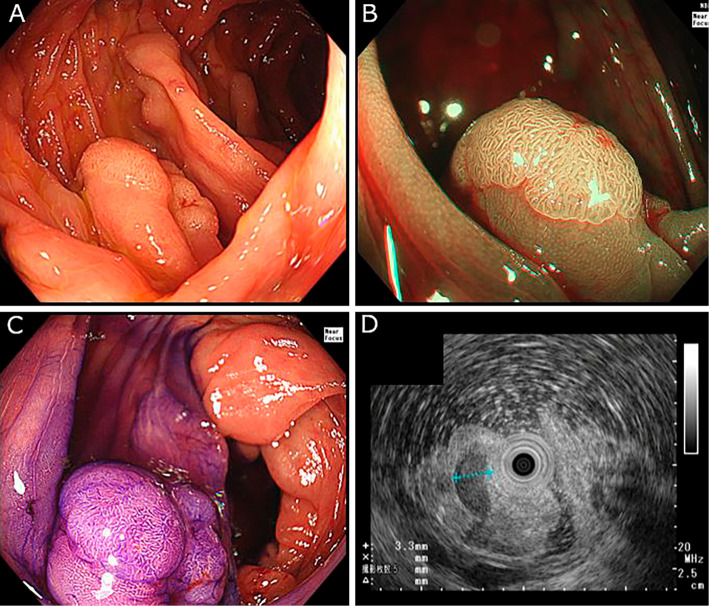
Total colonoscopy revealed the presence of ileocolonic varices in the terminal ileum and throughout the colon, extending from the ascending colon to the rectum (Fig. 1). In addition to ileocolonic varices, the patient had a 12-mm sessile polyp with a clear margin, slightly uneven surface, and faded color without reddish area in the ascending colon on the colonic varices. The Japan Narrow-band imaging Expert Team (JNET) classification (32) was type 2A (Fig. 2A-C).
Figure 1.
Total colonoscopy revealed the presence of ileocolonic varices at the terminal ileum and throughout the colon, extending from the ascending colon to the rectum, A: Terminal ileum, B: Ascending colon, C: Transverse colon, D: Descending colon, E: Sigmoid colon, F: Rectum.
Figure 2.
A: Conventional endoscopy in the ascending colon shows a 12-mm polyp, existing on the colonic varices, B: Narrow-band imaging, C: Chromoendoscopy, D: Endoscopic ultrasound with a small-caliber ultrasonic probe showed 3- to 4-mm colonic varices in the submucosa.
Endoscopic ultrasonography (EUS) using a small-caliber ultrasonic probe showed 3- to 4-mm colonic varices in the submucosa under the polyp (Fig. 2D). Considering the risk of bleeding after resection, we decided to further continue with the investigation of the portal hypertension and hemodynamics of the ileocolonic varices. The laboratory test results revealed a history of HBV infection, but no other data supported the suspicion of a present viral hepatitis, autoimmune disease, endocrine disease, or other liver disease. Mac-2 binding protein glycan isomer (M2BPGi) was within normal levels (Table 1).
Table 1.
Laboratory Data on Admission.
| <Peripheral blood> | ALT | 13 | U/L | <Serology> | |||||||||
| WBC | 5,580 | /μL | LDH | 173 | U/L | IgG | 1,197 | mg/dL | |||||
| RBC | 466×104 | /μL | ALP | 151 | U/L | IgA | 226 | mg/dL | |||||
| Hb | 14.6 | g/dL | γ-GTP | 19 | U/L | IgM | 54 | mg/dL | |||||
| Ht | 42.8 | % | ChE | 310 | U/L | CRP | 0.03 | μg/dL | |||||
| Plt | 21.6×104 | /μL | T-chol | 248 | mg/dL | Ferritin | 49.8 | μg/dL | |||||
| <Coagulation> | TG | 100 | mg/dL | M2BPGi | 0.39 | COI | |||||||
| PT | 111 | % | UA | 5.4 | mg/dL | <Viral markers> | |||||||
| PT-INR | 0.98 | BUN | 13.6 | mg/dL | HBs-Ag | (-) | |||||||
| APTT | 23 | sec | Cr | 1.1 | mg/dL | HBs-Ab | (-) | ||||||
| FIB | 258 | mg/dL | NH3 | 42 | μg/dL | HBc-Ab | (+) | ||||||
| FDP | <2.0 | μg/mL | Na | 139 | mEq/L | HBe-Ag | (-) | ||||||
| D dimer | 0.8 | μg/mL | K | 4.2 | mEq/L | HBe-Ab | (+) | ||||||
| AT III | 94 | % | Cl | 106 | mEq/L | HBV-DNA (PCR) | (-) | ||||||
| <Biochemistry> | Fe | 139 | μg/dL | HCV-Ab | (-) | ||||||||
| TP | 6.5 | g/dL | UIBC | 179 | μg/dL | CMV pp65 (C10/C11) | (-) | ||||||
| Alb | 4.2 | g/dL | Cu | 89 | μg/dL | EBV VCA-IgM Ab | <10 | × | |||||
| T-Bill | 1.9 | mg/dL | Zu | 86 | μg/dL | EBV VCA-IgG Ab | <10 | × | |||||
| D-Bill | 0.2 | mg/dL | BTR | 8.46 | EBNA | 40 | × | ||||||
| AST | 19 | U/L | AFP | 2.3 | ng/mL | ||||||||
WBC: white blood cell, RBC: red blood cell, Ht: hematocrit, Plt: platelet, PT: protorombin time, INR: international normalized ratio, APTT: active partial thromboplastin time, FIB: fibrinogen, FDP: fibrinogen degradation products, AT III: antithrombin III, TP: total protein, Alb: albumin, T-Bill: total bilirubin, D-bill: direct bilirubin, AST: aspartate aminotransferase, ALT: alanine aminotransferase, LDH: lactate dehydrogenase, ALP: alkaline phosphatase, GTP: glutamyltransferase, ChE: cholinesterase, T-chol: total cholesterol, TG: triglyceride, UA: uric acid, BUN: blood urea nitrogen, Cr: creatinine, NH3: ammonia, Na: natrium, K: kalium, Cl: chloride, Fe: ferrum, UIBC: unsaturated iron binding capacity, Cu: copper, Zu: zinc, BTR: ratio of branched-chain amino acid to tyrosine, AFP: alfa-fetoprotein, M2BPGi: mac-2 binding protein glycan isomer, HBs-Ag: hepatitis B surface antigen, HBs-Ab: hepatitis B surface antibody, HBc-Ab: hepatitis B core antibody, HBe-Ag: hepatitis B e antigen, HBe-Ab: hepatitis B e antibody, HBV-DNA: hepatitis B virus deoxyribonucleic acid, PCR: polymerase chain reaction, HCV-Ab: hepatitis C virus antibody, CMV pp65: cytomegalovirus pp65 antigenemia, EBV VCA-IgM Ab: Epstein-Barr virus-viral capsid antigen immunoglobulin M antibody, EBV VCA-IgG Ab: Epstein-Barr virus viral capsid antigen immunoglobulin G antibody, EBNA: epstein-barr virus nuclear antigen
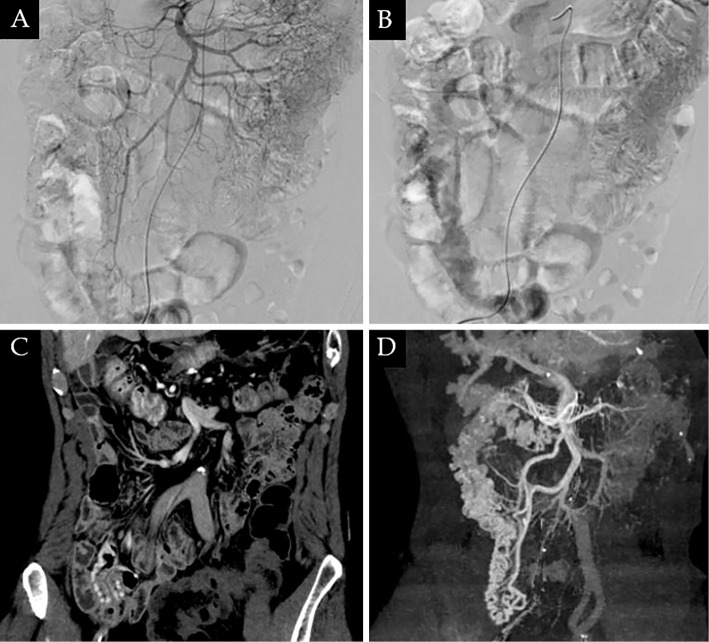
Liver ultrasonography with Shear wave measurement (Hitachi, Tokyo, Japan), an accurate ultrasonographic modality to evaluate liver fibrosis, showed no evidence of liver cirrhosis, according to Vs 1.39 m/s (33). Contrast computed tomography (CT) of the abdomen and pelvis revealed rectal varices, but ileocolonic varices were not detected. Digital subtraction angiography of the superior mesenteric artery showed delayed venous pooling in the ascending colon and terminal ileum. The blood flow was from the superior mesenteric artery to the superior mesenteric vein (Fig. 3A, B). CT during superior mesenteric angiography revealed the development of colonic varices in the ascending colon (Fig. 3C, D). There was no portal vein thrombosis detected on angiography. Considering the risk of bleeding after endoscopic treatment, we thoroughly informed the patient by explaining the possibility of bleeding and potential need for additional treatment, including endoscopic and operative management.
Figure 3.
A, B: Digital subtraction angiography of the superior mesenteric artery showed delayed venous pooling in the ascending colon. C, D: Computed tomography during superior mesenteric angiography showed the development of colonic varices in the ascending colon.
After obtaining his informed consent, we decided to perform endoscopic mucosal resection (EMR) because the JNET classification was type 2A, suggesting a low-grade intramucosal neoplasia (32). In addition, the tumor size was less than 20 mm, and we considered complete resection to be possible by EMR. During EMR, we shortened the time of exposure to electricity as much as possible in order to reduce the risk of electrocautery damage. After resection, we performed clip closure to help prevent post-EMR bleeding, and no immediate or delayed post-EMR bleeding was noted. A histopathological analysis revealed high-grade tubular adenoma of the colon. A colonoscopy six months after EMR revealed no change in the ileocolonic varices and no other lesions requiring excision.
Discussion
Varices are frequently associated with portal hypertension resulting from liver cirrhosis and other less-common causes. Colonic varices are rare, and it is reported that the incidence of colonic varices is 0.07% (2 of 2,912 cases) (34), whereas that of rectal varices is 3-7% in cirrhotic patients on endoscopy (35,36). Idiopathic ileocolonic varices with no underlying causes of portal hypertension are extremely uncommon. The most common cause of portal hypertension is liver cirrhosis. Other causes of portal hypertension, such as pre-hepatic abnormalities (e.g., portal or splenic vein thrombosis, malignancy, pancreatitis, postsurgical state), post-hepatic abnormalities (e.g., Budd-Chiari syndrome, congestive heart disease), or intrahepatic non-cirrhotic abnormalities (37,38), can induce colonic varices. Varices develop as venous collaterals when the portal pressure remains higher than the hepatic venous pressure, a condition called portal hypertension. Reopening of collapsed embryonic channels and reversal of the flow of existing adult veins is caused by portal hypertension, which can lead to the development of varices (39). The diagnosis of idiopathic colonic varices is made by detecting varices and excluding portal hypertension as a cause. In our case, laboratory testing revealed a history of hepatitis B virus (HBV) infection, but no other data suggested viral hepatitis, autoimmune disease, endocrine disease, or any other liver disease. Mac2 Binding Protein Glucosylation Isomer (M2BPGi), an accurate, reliable, and reproducible marker for the assessment of liver fibrosis (40), was within normal levels (Table 1). Shear wave measurement (Hitachi), an ultrasonographic modality for assessing liver cirrhosis (33), showed no evidence of liver cirrhosis. There was no portal or splenic vein thrombosis, according to contrast-enhanced CT and angiography of the abdomen and pelvis.
To our knowledge, there have been 29 reports on 33 patients with idiopathic colonic varices published since 1986 (Table 2) (2-30). On reviewing these cases, the average age was 44.8 years old, and 17 (77.3%) of the 22 patients were men (4,6-10,12,13,18,19,21,23,24,27,29). A family tendency was evaluated in 16 cases, and 6 cases had familial tendency (9,20,25). Occurrence site of varices was mentioned in 23 cases, and 6 cases (26.1%) were ileocolonic varices (6,16,21,22,27,28) and 12 (52.2%) were total colonic varices (2,3,5,17,19,20,23,24,29,30). The entire colon was involved in almost 80% of cases. A major complement was hematochezia in most cases (3,4,6-8,10,12,13,16-24,28,29), and some cases presented with diarrhea (20,24,26). Among the 19 cases of hematochezia, 7 (36.8%) required an operation as treatment (3,7,9,12,18,21,22). Endoscopic treatment of active bleeding from idiopathic ileocolonic varices has not been reported yet. There were only two asymptomatic cases (27,30), and both were detected by screening colonoscopy.
Table 2.
Characteristics of 33 Cases of Idiopathic Colonic Varices.
| No. | Age | Sex | Location | Family tendency |
Symptom | Treatment | Prognosis | Ref. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 71 | F | colonic | ND | asymptomatic | none | stable | [30] | ||||||||
| 2 | 76 | M | colonic | ND | hematochezia | preserved | stable | [29] | ||||||||
| 3 | 37 | M | ileocolonic | ND | hematochezia | preserved (ARB) | disappeared | [28] | ||||||||
| 4 | 54 | M | ileocolonic | none | asymptomatic | none | stable | [27] | ||||||||
| 5 | 38 | F | colonic | none | diarrhea | none | stable | [26] | ||||||||
| 6 | ND | ND | ND | (+) | ND | ND | ND | [25] | ||||||||
| 7 | ND | ND | ND | (+) | ND | ND | ND | [25] | ||||||||
| 8 | ND | ND | ND | (+) | ND | ND | ND | [25] | ||||||||
| 9 | 30 | M | colonic | none | hematochezia, diarrhea | preserved | stable | [24] | ||||||||
| 10 | 44 | M | colonic | none | hematochezia | preserved | stable | [23] | ||||||||
| 11 | 20 | F | ileocolonic | ND | hematochezia | operation | stable | [22] | ||||||||
| 12 | 21 | M | ileocolonic | none | hematochezia | operation | stable | [21] | ||||||||
| 13 | 61 | F | colonic | (+) | hematochezia, diarrhea | preserved | stable | [20] | ||||||||
| 14 | 43 | M | colonic | none | hematochezia | preserved (propranolol) | decreased | [19] | ||||||||
| 15 | 24 | M | rectum-descending | none | hematochezia | operation | stable | [18] | ||||||||
| 16 | 74 | F | colonic | ND | hematochezia | preserved | stable | [17] | ||||||||
| 17 | 64 | ND | ileocolonic | ND | hematochezia | operation | stable | [16] | ||||||||
| 18 | ND | ND | ND | ND | ND | ND | ND | [15] | ||||||||
| 19 | ND | ND | ND | ND | ND | ND | ND | [14] | ||||||||
| 20 | 30 | M | hepatic flexure | ND | hematochezia | preserved | ND | [13] | ||||||||
| 21 | 37 | M | sigmoid | ND | hematochezia | operation | stable | [12] | ||||||||
| 22 | ND | ND | ND | ND | ND | ND | ND | [11] | ||||||||
| 23 | 27 | M | ND | ND | hematochezia | preserved | stable | [10] | ||||||||
| 24 | 56 | M | ND | (+) | ND | ND | ND | [9] | ||||||||
| 25 | 28 | M | ND | (+) | ND | ND | ND | [9] | ||||||||
| 26 | 81 | M | ND | ND | hematochezia | ND | ND | [8] | ||||||||
| 27 | 32 | M | rectum-sigmoid | none | hematochezia | operation | stable | [7] | ||||||||
| 28 | 25 | M | ileocolonic | ND | hematochezia | preserved | stable | [6] | ||||||||
| 29 | ND | ND | colonic | ND | ND | ND | ND | [5] | ||||||||
| 30 | 58 | M | rectum-sigmoid, caecum | none | hematochezia | preserved | stable | [4] | ||||||||
| 31 | ND | ND | colonic | none | hematochezia | operation | stable | [3] | ||||||||
| 32 | ND | ND | colonic | ND | ND | ND | ND | [2] | ||||||||
| 33 | ND | ND | colonic | ND | ND | ND | ND | [2] |
ND: Not detected, ARB: Angiotensin receptor blocker
Screening colonoscopy is an effective method of reducing the risk of death due to colon cancer (41), and its spread might be the reason for these recent asymptomatic cases. We detected colonic varices by conducting screening colonoscopy and established the diagnosis by EUS, digital subtraction angiography, and CT angiography. CT angiography might be the most reliable method for making a diagnosis, and digital subtraction angiography might be the only method for determining the blood flow in the varices. As we showed in our case, EUS or transrectal Doppler sonography have also been reported to be useful for making a diagnosis (18,19). Capsule endoscope may also be a reliable tool for investigating the location of varices (28). A barium enema can reportedly depict colonic varices as tumor-like serpiginous lesions (20). However, it is difficult to exclude polyps, cancer, or other colorectal lesions using a barium enema.
Colonic varices may be misinterpreted as polyps, cancer, air bubbles, or fecal material (2,20). There are cases of a biopsy or polypectomy resulting in a significant bleeding (20,31). In asymptomatic ileocolonic varices, as in our case, a careful examination using a variety of techniques is required in order to avoid significant complications of endoscopy.
The treatment of idiopathic colonic varices remains unclear. While most cases with hematochezia show no active bleeding during colonoscopy, others require emergency operation (16) or extensive colectomy (3,7,21,22). There have been no reports of endoscopic treatment for active bleeding caused by idiopathic ileocolonic varices. The short-term prognosis seems good, but the long-term prognosis is unclear. A family history of death from gastrointestinal hemorrhaging was reported in one case (20). Very few attempts have been made to treat colorectal lesions (e.g., polyps, carcinoma) accompanied by colonic varices. Depending on the bleeding status, an operation seems to be the safest method, but it is highly invasive. As mentioned above, there are cases of significant bleeding arising from a biopsy or a polypectomy (20,31). Such cases show that endoscopic treatment can cause significant complications related to colorectal varices. Generally, active lower gastrointestinal bleeding is first treated by endoscopic hemostasis therapy, including mechanical, thermal, and injection therapy or a combination thereof (42). The majority of bleeding events after a colonoscopy are managed successfully by endoscopy, whereas very few cases require operation (43). When colorectal lesions coexist with colonic varices and require treatment, an endoscopic approach should be considered from the point of invasiveness. In our case, an interventional approach to stop the bleeding was considered difficult because the blood flow of the colorectal varices was from the mesenteric artery to the mesenteric vein. Therefore, we planned endoscopic clipping or endoscopic injection sclerotherapy to manage the bleeding, and informed consent was obtained, including consent regarding the operative method. At the time of EMR, we shortened the electricity time as much as possible. We proceeded with clip closure in order to prevent post-EMR bleeding, and no immediate or delayed bleeding was noted.
Cold snare polypectomy has a lower risk of post-polypectomy bleeding than does hot snare polypectomy (44). This shows that a longer time of exposure to electricity might increase the risk of post-polypectomy bleeding. Prophylactic clipping is reportedly unnecessary for preventing post-polypectomy bleeding with polyps smaller than 2 cm in diameter (45). The polyp in our case was smaller than 2 cm, but we proceeded with clip closure to prevent post-EMR bleeding, as this case had coexistent colonic varices.
To our knowledge, this is the first report of idiopathic ileocolonic varices with a colon polyp treated successfully by endoscopy. The diagnosis of idiopathic colonic varices requires a careful examination using a variety of techniques. During management, the development of a treatment strategy and obtaining informed consent are required.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Metz AJ, Bourke MJ, Moss A, Williams SJ, Swan MP, Byth K. Factors that predict bleeding following endoscopic mucosal resection of large colonic lesions. Endoscopy 43: 506-511, 2011. [DOI] [PubMed] [Google Scholar]
- 2. Vella-Camilleri FC, Friedrich R, Vento AO. Diffuse colonic varices: an uncommon cause of intestinal bleeding. Am J Gastroenterol 81: 492-494, 1986. [PubMed] [Google Scholar]
- 3. Nikolopoulos N, Xynos E, Datsakis K, Kasapidis P, Vassilakis JS. Varicosis coli totalis: report of a case of idiopathic aetiology. Digestion 47: 232-235, 1990. [DOI] [PubMed] [Google Scholar]
- 4. Iredale JP, Ridings P, McGinn FP, Arthur MJ. Familial and idiopathic colonic varices: an unusual cause of lower gastrointestinal haemorrhage. Gut 33: 1285-1288, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shrestha R, Dunkelberg JC, Schaefer JW. Idiopathic colonic varices: an unusual cause of massive lower gastrointestinal hemorrhage. Am J Gastroenterol 90: 496-497, 1995. [PubMed] [Google Scholar]
- 6. Schilling D, Maier M, Kohler B, Wurmel W, Jakob P, Riemann JF. Idiopathic mesenteric varices causing lower gastrointestinal bleeding. Eur J Gastroenterol Hepatol 8: 177-179, 1996. [DOI] [PubMed] [Google Scholar]
- 7. Detry RJ, Kartheuser A, Moisse R, et al. Idiopathic non-familial rectal and colonic varices requiring sigmoidorectal resection and coloanal anastomosis. Eur J Gastroenterol Hepatol 8: 1023-1026, 1996. [DOI] [PubMed] [Google Scholar]
- 8. Loffeld RJ, van Bochove A, de Graaf JC. Colonic varices: an unusual cause of occult blood loss. Ned Tijdschr Geneeskd 140: 2467-2469, 1996(in Dutch, Abstract in English). [PubMed] [Google Scholar]
- 9. Lopez-Cepero Andrada JM, Lopez Silva M, Ferre Alamo A, Salado Fuentes M, Benitez Roldan A. Familial colonic varices: report of two cases. Gastroenterol Hepatol 23: 341-343, 2000(in Spanish, Abstract in English). [PubMed] [Google Scholar]
- 10. Place RJ. Idiopathic colonic varices as a cause of lower gastrointestinal bleeding. South Med J 93: 1112-1114, 2000. [PubMed] [Google Scholar]
- 11. Van Gossum M, Reuss K, Moussaoui M, Bourgeois V. Idiopathic colonic varices: an unusual cause of massive lower gastrointestinal hemorrhage. Acta Gastroenterol Belg 63: 397-399, 2000. [PubMed] [Google Scholar]
- 12. Abraham-Igwe C, Patel R. Idiopathic colonic varices: a case report. Endoscopy 34: 680, 2002. [DOI] [PubMed] [Google Scholar]
- 13. Mehta R, Deepak S, John A, Balakrishnan V. Idiopathic colonic varices. Indian J Gastroenterol 23: 30-31, 2004. [PubMed] [Google Scholar]
- 14. Vuillemin E, Croquet V, Coumeau D, Ouali L. Idiopathic ileocolonic varices: a rare cause of lower gastrointestinal bleeding. Gastroenterol Clin Biol 28: 1183-1184, 2004. (in French). [DOI] [PubMed] [Google Scholar]
- 15. Keren D, Rainis T, Stermer E, Goldstein O, Lavy A. Extensive idiopathic colonic varices in a young patient. Dig Dis Sci 50: 1175-1176, 2005. [DOI] [PubMed] [Google Scholar]
- 16. Lopes LM, Ramada JM, Certo MG, et al. Massive lower gastrointestinal bleeding from idiopathic ileocolonic varix: report of a case. Dis Colon Rectum 49: 524-526, 2006. [DOI] [PubMed] [Google Scholar]
- 17. Simvoulakis E, Viazis N, Pipis P, Stefanidis G, Avgerinos A. Diffuse idiopathic colonic varices presenting with lower gastrointestinal bleeding in an elderly patient: a case report and review of the literature. Acta Gastroenterol Belg 69: 15-19, 2006. [PubMed] [Google Scholar]
- 18. Han JH, Jeon WJ, Chae HB, et al. A case of idiopathic colonic varices: a rare cause of hematochezia misconceived as tumor. World J Gastroenterol 12: 2629-2632, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Francois F, Tadros C, Diehl D. Pan-colonic varices and idiopathic portal hypertension. J Gastrointestin Liver Dis 16: 325-328, 2007. [PubMed] [Google Scholar]
- 20. Zaman L, Bebb JR, Dunlop SP, Jobling JC, Teahon K. Familial colonic varices-a cause of “polyposis” on barium enema. Br J Radiol 81: e17-e19, 2008. [DOI] [PubMed] [Google Scholar]
- 21. Krishna RP, Singh RK, Ghoshal UC. Recurrent lower gastrointestinal bleeding from idiopathic ileocolonic varices: a case report. J Med Case Rep 4: 257, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gentilli S, Aronici M, Portigliotti L, Pretato T, Garavoglia M. Idiopathic ileo-colonic varices in a young patient. Updates Surg 64: 235-238, 2012. [DOI] [PubMed] [Google Scholar]
- 23. Akkucuk S, Aydogan A, Paltaci I, Temiz M. Diffuse idiopathic varices in the colon characterized by lower gastrointestinal bleeding. Ulus Cerrahi Derg 30: 109-111, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Speicher MV, Keegan MT, Kirk KE. A case of idiopathic colonic varices. J Am Osteopath Assoc 114: 56-59, 2014. [DOI] [PubMed] [Google Scholar]
- 25. Boland P, Leonard J, Saunders M, Bursey F. Familial idiopathic small-bowel and colonic varices in three siblings. Endoscopy 46: 893-897, 2014. [DOI] [PubMed] [Google Scholar]
- 26. Dina I, Braticevici CF. Idiopathic colonic varices: case report and review of literature. Hepat Mon 14: e18916, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Peixoto A, Silva M, Pereira P, Macedo G. Giant idiopathic pancolonic varices - a rare entity. Clin Res Hepatol Gastroenterol 40: 255-256, 2016. [DOI] [PubMed] [Google Scholar]
- 28. Kawasaki K, Kakisaka K, Matsumoto T. Idiopathic ileocolonic varices depicted by colon capsule endoscopy. Dig Endosc 28: 615, 2016. [DOI] [PubMed] [Google Scholar]
- 29. Kahl R, Patel K, George K, Piper M. Idiopathic colonic varices: a rare cause of recurrent lower gastrointestinal bleeding. ACG Case Rep J 4: e122, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sunkara T, Caughey ME, Culliford A, Gaduputi V. Idiopathic isolated colonic varices: an extremely rare condition. J Clin Med Res 10: 63-65, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vescia FG, Babb RR. Colonic varices: a rare, but important cause of gastrointestinal hemorrhage. J Clin Gastroenterol 7: 63-65, 1985. [DOI] [PubMed] [Google Scholar]
- 32. Sano Y, Tanaka S, Kudo SE, et al. Narrow-band imaging (NBI) magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team. Dig Endosc 28: 526-533, 2016. [DOI] [PubMed] [Google Scholar]
- 33. Yada N, Tamaki N, Koizumi Y, et al. Diagnosis of fibrosis and activity by a combined use of strain and shear wave imaging in patients with liver disease. Dig Dis 35: 515-520, 2017. [DOI] [PubMed] [Google Scholar]
- 34. Feldman M Sr., Smith VM, Warner CG. Varices of the colon. Report of three cases. JAMA 179: 729-730, 1962. [DOI] [PubMed] [Google Scholar]
- 35. Zaman A, Hapke R, Flora K, Rosen H, Benner K. Prevalence of upper and lower gastrointestinal tract findings in liver transplant candidates undergoing screening endoscopic evaluation. Am J Gastroenterol 94: 895-899, 1999. [DOI] [PubMed] [Google Scholar]
- 36. Rabinovitz M, Schade RR, Dindzans VJ, Belle SH, Van Thiel DH, Gavaler JS. Colonic disease in cirrhosis. An endoscopic evaluation in 412 patients. Gastroenterology 99: 195-199, 1990. [DOI] [PubMed] [Google Scholar]
- 37. Biecker E. Portal hypertension and gastrointestinal bleeding: diagnosis, prevention and management. World J Gastroenterol 19: 5035-5050, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Webster GJ, Burroughs AK, Riordan SM. Review article: portal vein thrombosis - new insights into aetiology and management. Aliment Pharmacol Ther 21: 1-9, 2005. [DOI] [PubMed] [Google Scholar]
- 39. Sato T, Akaike J, Toyota J, Karino Y, Ohmura T. Clinicopathological features and treatment of ectopic varices with portal hypertension. Int J Hepatol 2011: 960720, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Toshima T, Shirabe K, Ikegami T, et al. A novel serum marker, glycosylated Wisteria floribunda agglutinin-positive Mac-2 binding protein (WFA (+) -M2BP), for assessing liver fibrosis. J Gastroenterol 50: 76-84, 2015. [DOI] [PubMed] [Google Scholar]
- 41. Doubeni CA, Corley DA, Quinn VP, et al. Effectiveness of screening colonoscopy in reducing the risk of death from right and left colon cancer: a large community-based study. Gut 67: 291-298, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Strate LL, Gralnek IM. ACG clinical guideline: management of patients with acute lower gastrointestinal bleeding. Am J Gastroenterol 111: 459-474, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Paraskeva KD, Paspatis GA. Management of bleeding and perforation after colonoscopy. Expert Rev Gastroenterol Hepatol 8: 963-972, 2014. [DOI] [PubMed] [Google Scholar]
- 44. Kawamura T, Takeuchi Y, Asai S, et al. A comparison of the resection rate for cold and hot snare polypectomy for 4-9 mm colorectal polyps: a multicentre randomised controlled trial (CRESCENT study). Gut 67: 1950-1957, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Matsumoto M, Kato M, Oba K, et al. Multicenter randomized controlled study to assess the effect of prophylactic clipping on post-polypectomy delayed bleeding. Dig Endosc 28: 570-576, 2016. [DOI] [PubMed] [Google Scholar]