Abstract
A 72-year-old man presented with a 6-month history of systemic edema. Hyperpigmentation, hemangioma, pleural effusion, IgG-kappa-type monoclonal protein, high vascular endothelial growth factor values, renal failure, and nerve conduction study abnormalities were also present. Multiparameter flow cytometry (MFC) showed 0.2% neoplastic plasma cells (CD38-, CD56-, and kappa-positive; CD19-, CD27-, and lambda-negative) in the bone marrow leading to POEMS syndrome. Cases involving kappa-type POEMS syndrome are extremely rare. A kidney biopsy revealed membranous proliferative glomerulonephritis-like changes in our case. Lenalidomide-dexamethasone therapy improved the renal function. Detection of neoplastic plasma cells by MFC was useful for the accurate diagnosis and treatment evaluation.
Keywords: POEMS syndrome, IgG kappa monoclonal protein, multiparameter flow cytometry, lenalidomide, membranoproliferative glomerulonephritis (MPGN)
Introduction
POEMS syndrome (Crow-Fukase syndrome, Takatsuki syndrome) is a rare plasma cell dyscrasia characterized by polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, and skin changes (1-3). The various symptoms of this syndrome are thought to result from the high cytokinemia involving cytokines, such as vascular endothelial growth factor (VEGF), but the molecular pathology of the syndrome has not been sufficiently elucidated. In recent years, it has been reported that immunomodulatory drugs (IMiDs) targeting VEGF, such as thalidomide and lenalidomide, have good therapeutic effects for this condition (4-6).
The usefulness of detecting minimal residual disease (MRD) by multiparameter flow cytometry (MFC) has been reported in determining the therapeutic effect after treatment of multiple myeloma (MM) (7). However, the fraction of monoclonal plasma cells in POEMS syndrome is often small. Therefore, it is difficult to identify neoplastic plasma cells by conventional flow cytometry in patients with this syndrome (8).
All cases of POEMS syndrome reported so far have had the lambda-type monoclonal protein, except for four cases previously reported in the literature. We herein report a rare case of POEMS syndrome with IgG-kappa-type monoclonal protein, wherein a small number of neoplastic plasma cells were identified by MFC, and a good therapeutic effect was achieved by lenalidomide-dexamethasone (Ld) therapy.
Case Report
A 72-year-old man was admitted to our hospital because of a 6-month history of systemic edema, pain in the legs, and anorexia. He had a surgical history of prostate cancer eight years ago. A physical examination showed the presence of pitting edema in both legs, hyperpigmentation, white nails, multiple small hemangiomas, gynecomastia, and papillary tenderness, but absence of hypertrichosis (Fig. 1). A hematological examination showed a white blood cell count of 5.8×109/L with a normal differential count, a hemoglobin concentration of 10.1 g/dL, and a platelet count of 56×109/L. The patient was in renal failure, with a serum creatinine level of 1.30 mg/dL. Serum VEGF, plasma VEGF, and serum interleukin-6 (IL-6) concentrations were elevated to 1,790 pg/mL, 1,120 pg/mL, and 9.1 pg/mL, respectively. In addition, endocrine abnormalities, such as elevation of luteinizing hormone, follicle-stimulating hormone, and prolactin, were observed (Table 1). IgG-kappa-type monoclonal protein was observed by serum protein immunofixation, but serum IgG levels were normal. A urinalysis revealed albuminuria, but Bence-Jones protein was not observed.
Figure 1.
Skin changes. (A) Glomeruloid hemangioma and gynecomastia. (B) Hyperpigmentation and peripheral edema of the foot. (C) White nails.
Table 1.
Laboratory Findings on Admission.
| Hematology | Biochemistry | Immunological test | Cytokines | Normal range | ||||||||||||
| WBC | 5.8 | ×109/L | AST | 19 | U/L | CRP | 2.81 | mg/dL | plasma VEGF | 1,120 | pg/mL | <38.3 | ||||
| Neut | 70.8 | % | ALT | 11 | U/L | sIL-2R | 1,390 | U/mL | serum VEGF | 1,790 | pg/mL | NA | ||||
| Lym | 15.5 | % | ALP | 217 | U/L | β2-MG | 6.7 | µg/mL | serum IL-6 | 9.1 | pg/mL | <4.0 | ||||
| Mono | 8.4 | % | LDH | 215 | U/L | ACE | 12.0 | U/mL | serum TNF-α | 3.87 | pg/mL | 0.75-1.66 | ||||
| Eo | 4.3 | % | γ-GTP | 130 | U/L | Lysozyme | 13.1 | µg/mL | ||||||||
| Baso | 1.0 | % | TP | 6.6 | g/dL | C3 | 115 | mg/dL | Endocrinology | Normal range | ||||||
| RBC | 3.51 | ×1012/L | Alb | 3.6 | g/dL | C4 | 32 | mg/dL | LH | 30.5 | mIU/mL | 0.79-5.72 | ||||
| Hb | 10.1 | g/dL | T-Bil | 0.4 | mg/dL | CH50 | >60 | U/mL | FSH | 56.9 | mIU/mL | 2.00-8.30 | ||||
| Ht | 29.6 | % | T-Cho | 132 | mg/dL | IgG | 1,199 | mg/dL | ACTH | 54.5 | pg/mL | 7.2-63.3 | ||||
| Plt | 56 | ×109/L | TG | 97 | mg/dL | IgA | 155 | mg/dL | Cortisol | 9.6 | µg/dL | 6.2-18 | ||||
| MCV | 84.3 | fl | LDL-C | 74 | mg/dL | IgM | 101 | mg/dL | Prolactin | 80.40 | ng/mL | 4.29-13.69 | ||||
| MCHC | 34.1 | % | BUN | 21.1 | mg/dL | FLCκ | 155 | mg/L | intact PTH | 33 | pg/mL | 10-65 | ||||
| Ret | 19.1 | ‰ | UA | 8.9 | mg/dL | FLCλ | 44.4 | mg/L | FT3 | 3.0 | pg/mL | 2.2-3.3 | ||||
| Cre | 1.30 | mg/dL | κ/λ | 3.49 | FT4 | 1.8 | ng/dL | 0.8-1.6 | ||||||||
| Coagulation | CK | 63 | IU/L | IgG4 | 26.0 | mg/dL | TSH | 3.41 | µIU/mL | 0.38-4.31 | ||||||
| APTT | 30.7 | s | AMY | 64 | U/L | ANA | 40 | × | ||||||||
| PT | 11.6 | s | ChE | 156 | U/L | SP+HO | Infection | |||||||||
| PT-INR | 1.00 | Ca | 8.5 | mg/dL | RNP | 2.3 | U/mL | IGRA | (-) | |||||||
| FDP | 7.00 | µg/mL | iP | 4.8 | mg/dL | SS-A | 0.7 | U/mL | HBsAg | (-) | ||||||
| Fibrinogen | 423 | mg/dL | Na | 141 | mEq/L | Scl-70 | 1.8 | U/mL | HCV | (-) | ||||||
| ATIII | 92.9 | % | K | 4.1 | mEq/L | Jo-1 | 9.3 | U/mL | TPHA | (-) | ||||||
| D-dimer | 1.75 | µg/mL | Cl | 107 | mEq/L | PR3-ANCA | <1.0 | U/mL | RPR | (-) | ||||||
| ProteinC | 76.1 | % | Fe | 35 | µg/dL | MPO-ANCA | <1.0 | U/mL | ||||||||
| ESR 1h | 41 | mm | TIBC | 204 | µg/dL | RF | 4 | IU/mL | ||||||||
| ESR 2h | 59 | mm | UIBC | 169 | µg/dL | |||||||||||
| Ferritin | 207 | ng/mL | Bone marrow aspiration | |||||||||||||
| Urinalysis | Transferrin | 154 | mg/dL | NCC | 161,300 | /µL | ||||||||||
| UP | (+) | Cys-C | 2.45 | mg/L | MegK | 109.4 | /µL | |||||||||
| 0.276 | g/gCre | Glu | 92 | mg/dL | Plasma cell | 1.2 | % | |||||||||
| OB | (-) | HbA1c | 4.9 | % | G-BAND | 46,XY | ||||||||||
| WBC | (-) | BNP | 83.1 | pg/mL | FISH | |||||||||||
| β2-MG | 28.0 | µg/L | VB12 | 1,163 | pg/mL | del17p | (-) | |||||||||
| NAG | 14.5 | IU/L | IgH/FGFR3 | (-) | ||||||||||||
| IgH/MAF | (-) | |||||||||||||||
WBC: white blood cell, Neut: neutrophil, Lym: lymphocyte, Mono: monocyte, Eo: eosinophil, Baso: basophil, RBC: red blood cell, Hb: hemoglobin, Ht: hematocrit, Plt: platelet, MCV: mean corpuscular volume, MCHC: mean corpuscular hemoglobin concentration, Ret: reticulocyte, APTT: activated partial thromboplastin time, PT: prothrombin time, PT-INR: prothrombin time-international normalized ratio, FDP: fibrin-fibrinogen degradation products, ATIII: antithrombin III, ESR: erythrocyte sedimentation rate, UP: urine protein, OB: occult blood, β2-MG: β2-microglobulin, NAG: N-acetyl glucosaminidase, AST: aspartate aminotransferase, ALT: alanine aminotransferase, LDH: lactate dehydrogenase, γ-GTP: γ-glutamyl transpeptidase, TP: total protein, Alb: albumin, T-Bil: total bilirubin, T-Cho: total cholesterol, TG: triglyceride, LDL-C: low density lipoprotein-cholesterol, BUN: blood urea nitrogen, UA: uric acid, Cre: creatinine, CK: creatine kinase, AMY: amylase, ChE: cholinesterase, Ca: calcium, iP: inorganic phosphorus, Na: sodium, K: potassium, Cl: chloride, Fe: serum iron, TIBC: total iron binding capacity, UIBC: unsaturated iron binding capacity, Cys-C: cystatin-C, Glu: glucose, BNP: brain natriuretic peptide, VB12: vitamin B12, CRP: C-reactive protein, sIL-2R: soluble interleukin-2 receptor, ACE: angiotensin converting enzyme, CH50: 50% hemolytic unit of complement, IgG: immunoglobulin G, IgA: immunoglobulin A, IgM: immunoglobulin M, FLC: free light chain, ANA: anti-nuclear antibody, SP: speckled pattern, HO: homogeneous pattern, RNP: ribonucleoprotein, PR3-ANCA: proteinase3 anti-neutrophil cytoplasmic antibody, MPO-ANCA: myeroperoxidase anti-neutrophil cytoplasmic antibody, RF: rheumatoid factor, NCC: nucleated cell count, MegK: megakaryocyte, FISH: fluorescence in situ hybridization, VEGF: vascular endothelial growth factor, NA: not available, IL-6: Interleukin-6, TNF-α: tumor necrosis factor-α, LH: luteinizing hormone, FSH: follicle stimulating hormone, ACTH: adrenocorticotropic hormone, PTH: parathyroid hormone, FT3: free triiodothyronine, FT4: free thyroxine, TSH: thyroid stimulating hormone, IGRA: interferon-gamma release assay, HBsAg: hepatitis B surface antigen, HCV: hepatitis C virus, TPHA: treponema pallidum hemagglutination test, RPR: rapid plasma reagin
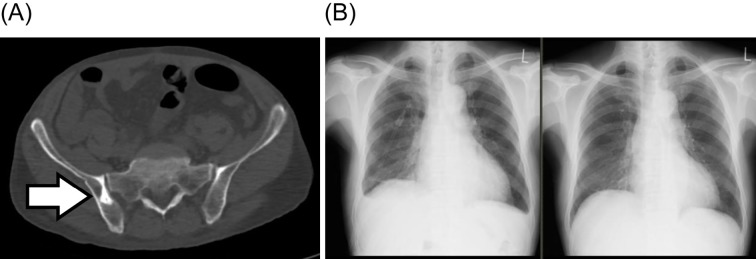
An ultrasound examination showed pericardial effusion of about 400 mL, but the ejection fraction was normal. Whole-body computed tomography showed pleural effusion and pelvic bone sclerosis but no lymphadenopathy, hepatomegaly, or splenomegaly (Fig. 2). Positron emission tomography did not reveal any tumorous accumulation. A nerve conduction study showed polyneuropathy with dominant axonal degeneration accompanied by a decrease in nerve conduction velocity, extension of distant latency, and reduction of compound muscle action potential. Bone marrow aspiration showed 1.2% plasma cells, sometimes associated with atypia (Fig. 3A). An increase was observed in the number of megakaryocytes (109.4 /μL) without atypia. IgG-kappa-type monoclonal protein was also recognized by immunofixation of bone marrow blood (Fig. 3B). MFC (DuraClone, Beckman Coulter, Brea, USA; Table 2) showed 0.2% neoplastic plasma cells with kappa chains (Fig. 3C). A percutaneous kidney biopsy revealed membranoproliferative glomerulonephritis (MPGN)-like changes, but there was no deposition of immunoglobulins or light chains (Fig. 4). Amyloidosis was not observed on a bone marrow biopsy, skin biopsy, renal biopsy, or intestinal biopsy.
Figure 2.
Computed tomography (CT) and chest X-ray. (A) Osteosclerotic lesion on the right side of the pelvis (white arrow). (B) Chest X-ray before lenalidomide-dexamethasone (Ld) treatment showing bilateral pleural effusion (left panel) and on day 13 of Ld therapy showing complete resolution of pleural effusion (right panel).
Figure 3.
Bone marrow aspiration. (A) The smear showing atypical plasma cells. (B) Immunofixation electrophoresis confirmed IgG-kappa monoclonal gammopathy (white arrows). (C) A multiparameter flow cytometry analysis (DuraClone) showing neoplastic plasma cells with kappa light chain. Blue dots: normal plasma cells, purple dots: neoplastic plasma cells.
Table 2.
Antibodies of the Used DuraClone Panels.
| Tube No. | Pacific Blue | Krome Orange | FITC | PE | PC5.5 | PC7 | APC | APC-A750 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | CD38 | CD45 | CD81 | CD27 | CD19 | CD200 | CD138 | CD56 | ||||||||
| 2 | CD45 | CD138 | CD38 | Lambda | CD56 | CD19 | Kappa | - |
Figure 4.

Renal pathological findings. Light microscopy of a hypertrophic glomerulus shows mesangial matrix expansion, narrowing of the glomerular capillary loops, and double contour of the glomerular basement membrane (arrows). Periodic acid-Schiff staining (original magnification ×400).
Since the diagnostic criteria of the Mayo Clinic (9) were satisfied, we finally diagnosed the patient with POEMS syndrome. Because of anemia, thrombocytopenia, and C-reactive protein positivity, overlap with Castleman's disease was considered. However, because there were no enlarged lymph nodes, we excluded Castleman's disease. The patient also met the diagnostic criteria for the recently proposed TAFRO syndrome as he tested positive for fluid retention, thrombocytopenia, high inflammatory response, megakaryocytic hyperplasia, and progressive kidney failure. However, our case showed the presence of monoclonal protein and an abnormality in the nerve conduction test, so TAFRO syndrome was excluded.
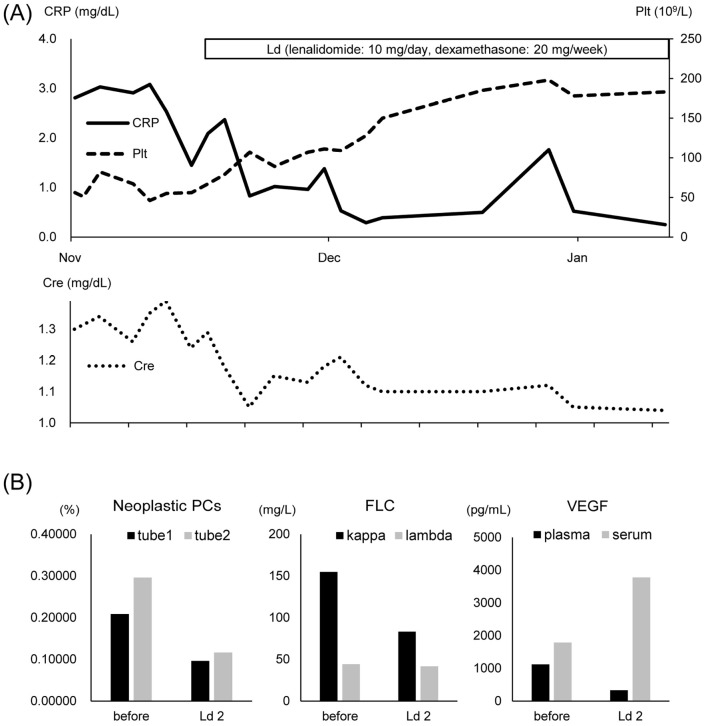
In order to avoid inducing peripheral neuropathy with thalidomide, we chose lenalidomide 10 mg/day and dexamethasone 20 mg/week (Ld) therapy. In accordance with our institutional regulations, all treatments in this case were provided with the approval of the institutional review board and the consent of the patient. Pleural effusion and limb pain quickly improved after treatment was started. In addition, we observed the recovery of the platelet counts, improvement of the renal function, and reduction of the inflammatory response. The treatment efficacy parameters, as evaluated by the Mayo Clinic criteria (10) after two courses of Ld therapy, were as follows: hematologic response, could not be evaluated; VEGF response, partial VEGF response (PRV); clinical response, clinical improvement (IC); and PET response, could not be evaluated. MFC showed a reduction in the fraction of neoplastic plasma cells, confirming the therapeutic effect (Fig. 5). The patient was transferred to a hospital near his residence and continued to receive Ld therapy. The dexamethasone dose was reduced because he developed sleeplessness; however, his symptoms did not relapse after dose reduction. Plasma VEGF levels also did not change.
Figure 5.
Clinical course. (A) The illustration shows changes in C-reactive protein (CRP), platelets (Plt) and creatinine (Cre) with lenalidomide-dexamethasone (Ld) therapy. (B) The values of neoplastic plasma cells (PCs), free light chain (FLC), and vascular endothelial growth factor (VEGF) before treatment and after two courses of Ld therapy are shown. Antigens in tube 1 and tube 2 are shown in Fig. 3C and Table 2.
Discussion
The detection of MRD has become very important in the treatment of hematological malignancies (11). Generally, MFC is a method for detecting MRD by identifying tumor-specific surface antigens using antibodies labeled with four or more color fluorochromes. In the present case, MFC identified small amounts of neoplastic plasma cells, which are characteristic of POEMS syndrome. As shown in Table 3, it was observed that the surface antigen profile of neoplastic plasma cells in this case (CD27 negative, CD19 negative, CD56 positive, and CD81 diminished) was more similar to that of myeloma cells than to that of monoclonal gammopathy of undetermined significance (MGUS) (12-14). This suggests that neoplastic plasma cells of POEMS syndrome (even in small numbers) are malignant.
Table 3.
Surface Antigen Profiles of Plasma Cell Neoplasms.
| Antigen | Normal PC | MGUS | MM | Present case (POEMS) | ||||
|---|---|---|---|---|---|---|---|---|
| CD138 | + | + | + | + | ||||
| CD38 | + | + | + | + | ||||
| CD56 | - | - or + | + | + | ||||
| CD19 | + | dim+ | - or dim+ | - | ||||
| CD45 | + | - or + | - or dim+ | dim+ | ||||
| CD200 | - | - or + | + | + | ||||
| CD81 | + | - or + | - or dim+ | dim+ | ||||
| CD27 | + | + | - or dim+ | - |
PC: plasma cell, MGUS: monoclonal gammopathy of undetermined significance, MM: multiple myeloma
It has been reported that CD27, which was decreased in the present case, does not decrease in cases of MGUS but does decrease in cases of MM (12,15). Negative CD27 is considered to indicate a poor prognosis in MM (15,16). However, the relationship between the surface antigen profile of neoplastic plasma cells and the therapeutic reactivity/prognosis in POEMS syndrome has not been clarified, so further research is necessary. In addition, in most cases of POEMS syndrome, the proportion of neoplastic plasma cells is lower than that in MM. Therefore, MRD detection by MFC seems to be very useful for the diagnosis and follow-up of POEMS syndrome, in addition to assessments of the clinical symptoms and VEGF values.
Since serum VEGF is strongly influenced by the level of platelets, it is recommended that plasma VEGF levels be used to evaluate the therapeutic effect (10,17). Indeed, serum VEGF levels have been reported to be over 10 times higher than plasma VEGF levels because of the release of VEGF from activated or aggregated platelets (18). Therefore, we used plasma VEGF levels to evaluate the therapeutic effect in our patient with thrombocytopenia.
Almost all patients with POEMS syndrome have the lambda-type monoclonal protein (19-21). This is because monoclonal proteins have a specific V lambda subfamily gene that can cause high cytokinemia involving cytokines, such as VEGF via some signals (22). However, the mechanism has not been fully elucidated. In contrast, POEMS syndrome with kappa-type monoclonal protein is very rare. Table 4 shows the details of five POEMS syndrome cases with kappa-type monoclonal protein, including our own (23-26). All patients showed some endocrine abnormality. Interestingly, renal failure was a complication in three cases, including our own. It has been reported that about 20% of POEMS syndrome cases have associated renal dysfunction (27). In our earlier study, we reported the clinicopathological features of 52 patients with POEMS syndrome with renal complications. Over half of the patients had renal dysfunction with serum creatinine >1.5 mg/dL, and 10% of the patients needed renal replacement therapy. The histological findings of the kidney in POEMS syndrome frequently include MPGN-like glomerular changes, such as glomerular swelling, mesangial proliferation, mesangiolysis, and endothelial and mesangial cell enlargement (28). These glomerular changes are thought to be caused by cytokines, such as VEGF and IL-6 (29). Nephropathy in POEMS syndrome is significantly improved by treatment with new agents, including lenalidomide, compared to treatment without these agents (27). In our case, Ld therapy improved the renal function as plasma VEGF levels decreased. For patients with renal dysfunction, it is necessary to reduce the dose of lenalidomide in order to prevent hematologic toxicity and maintain optimal plasma concentrations (30). Lenalidomide can be safely used in patients with POEMS syndrome with renal dysfunction.
Table 4.
Clinical Findings and Therapies in Patients with Kappa-type Monoclonal Protein.
| Characteristic | #1 (23) | #2 (24) | #3 (25) | #4 (26) | #5 (present case) | % Affected (9) |
|---|---|---|---|---|---|---|
| Sex/Age, Years | M/64 | M/57 | F/50 | M/60 | M/72 | |
| Polyneuropathy | + | + | + | + | + | 100 |
| demyelinating or axonal degeneration | mixed | demyelinating | demyelinating | ? | mixed | |
| Organomegaly | - | + | ? | + | - | 45-85 |
| Hepatomegaly | - | + | ? | ? | - | 24-78 |
| Splenomegaly | - | + | ? | + | - | 22-70 |
| Lymphadenopathy | - | ? | ? | - | - | 26-74 |
| Castleman’s disease | ? | ? | ? | ? | - | 11-25 |
| Endocrinopathy | + | + | + | + | + | 67-84 |
| Gonadal axis abnormality | + | ? | ? | + | + | 55-89 |
| Adrenal axis abnormality | ? | ? | ? | ? | - | 16-33 |
| Increased prolactin value | ? | ? | ? | ? | + | 5-20 |
| Gynecomastia or galactorrhea | + | + | ? | ? | + | 12-18 |
| Diabetes mellitus | + | - | + | ? | - | 3-36 |
| Hypothyroidism | - | + | ? | ? | - | 9-67 |
| M protein | + | + | + | + | + | 100 |
| Monoclonal plasma cell dyscrasia | + | + | + | + | + | 100 |
| M protein on serum electrophoresis | + | + | + | + | + | 24-54 |
| M protein type | IgG-kappa | IgG-kappa | IgG-kappa | IgG-kappa | IgG-kappa | |
| Plasma cells in bone marrow | 1.0% | no increase | 7.6% | 20.0% | 1.2% | <5% |
| Bone lesions | + | - | + | - | + | 27-97 |
| Skin changes | + | + | + | - | + | 68-89 |
| Hyperpigmentation | + | ? | + | - | + | 46-93 |
| Acrocyanosis and plethora | + | + | ? | - | + | 19 |
| Hemangioma/telangiectasia | ? | ? | ? | - | + | 9-35 |
| Hypertrichosis | + | ? | + | - | - | 26-74 |
| Thickening | + | ? | ? | - | + | 5-43 |
| Clubbing | + | ? | ? | ? | - | 5-49 |
| Extravascular volume overload | + | + | ? | + | + | 29-87 |
| Peripheral edema | + | + | ? | ? | + | 24-89 |
| Ascites | - | ? | ? | + | - | 7-54 |
| Pleural effusion | - | ? | ? | + | + | 3-43 |
| Pericardial effusion | - | ? | ? | + | + | 1-64 |
| Nephropathy | ? | + | ? | + | + | NA |
| renal failure | ? | + | ? | + | + | NA |
| renal biopsy | ? | MPGN-like | ? | FSGS | MPGN-like | |
| Other signs | ||||||
| Thrombocytosis | ? | ? | ? | - | - | 54-88 |
| Thrombotic diatheses | ? | ? | ? | + | + | NA |
| Polycythemia | - | - | - | - | - | 12-19 |
| Papilledema | ? | ? | ? | ? | - | 29-64 |
| Decreased DLCO | ? | ? | ? | ? | ? | >15 |
| Pulmonary hypertension | ? | ? | ? | ? | ? | 36 |
| Weight loss | ? | + | ? | ? | ? | 37 |
| hyperhidrosis | - | ? | + | + | - | NA |
| Fatigue | ? | + | + | ? | + | 31 |
| Therapy | MP | IVIG/PSL/CY/ mPSLpulse |
Ld | CyA+PSL/ VCD/VTD |
Ld |
In conclusion, our case suggested that identification of neoplastic plasma cells by MFC is important for obtaining a proper diagnosis and enacting treatment of POEMS syndrome. In the future, MFC will be essential for the treatment of this syndrome. Ld therapy might be effective in cases of POEMS syndrome for reducing VEGF levels. It is also important to clarify the cause of kidney dysfunction by a renal biopsy in POEMS syndrome patients with renal failure. Furthermore, since POEMS syndrome with kappa-type monoclonal protein is very rare, it is necessary to increase our knowledge about its clinical presentation and therapeutic reactivity.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Crow RS. Peripheral neuritis in myelomatosis. Br Med J 2: 802-804, 1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bardwick PA, Zvaifler NJ, Gill GN, Newman D, Greenway GD, Resnick DL. Plasma cell dyscrasia with polyneuropathy, organomegaly, endocrinopathy, M protein, and skin changes: the POEMS syndrome. Report on two cases and a review of the literature. Medicine (Baltimore) 59: 311-322, 1980. [DOI] [PubMed] [Google Scholar]
- 3. Takatsuki K, Sanada I. Plasma cell dyscrasia with polyneuropathy and endocrine disorder: clinical and laboratory features of 109 reported cases. Jpn J Clin Oncol 13: 543-555, 1983. [PubMed] [Google Scholar]
- 4. Misawa S, Sato Y, Katayama K, et al. Safety and efficacy of thalidomide in patients with POEMS syndrome: a multicentre, randomised, double-blind, placebo-controlled trial. Lancet Neurol 15: 1129-1137, 2016. [DOI] [PubMed] [Google Scholar]
- 5. Nozza A, Terenghi F, Gallia F, et al. Lenalidomide and dexamethasone in patients with POEMS syndrome: results of a prospective, open-label trial. Br J Haematol 179: 748-755, 2017. [DOI] [PubMed] [Google Scholar]
- 6. Li J, Huang XF, Cai QQ, et al. A prospective phase II study of low dose lenalidomide plus dexamethasone in patients with newly diagnosed polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, and skin changes (POEMS) syndrome. Am J Hematol 93: 803-809, 2018. [DOI] [PubMed] [Google Scholar]
- 7. Jelinek T, Bezdekova R, Zatopkova M, et al. Current applications of multiparameter flow cytometry in plasma cell disorders. Blood Cancer J 7: e617, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dao LN, Hanson CA, Dispenzieri A, Morice WG, Kurtin PJ, Hoyer JD. Bone marrow histopathology in POEMS syndrome: a distinctive combination of plasma cell, lymphoid, and myeloid findings in 87 patients. Blood 117: 6438-6444, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dispenzieri A. POEMS syndrome: 2017 update on diagnosis, risk stratification, and management. Am J Hematol 92: 814-829, 2017. [DOI] [PubMed] [Google Scholar]
- 10. D'Souza A, Lacy M, Gertz M, et al. Long-term outcomes after autologous stem cell transplantation for patients with POEMS syndrome (osteosclerotic myeloma): a single-center experience. Blood 120: 56-62, 2012. [DOI] [PubMed] [Google Scholar]
- 11. Ben Lassoued A, Nivaggioni V, Gabert J. Minimal residual disease testing in hematologic malignancies and solid cancer. Expert Rev Mol Diagn 14: 699-712, 2014. [DOI] [PubMed] [Google Scholar]
- 12. Raja KR, Kovarova L, Hajek R. Review of phenotypic markers used in flow cytometric analysis of MGUS and MM, and applicability of flow cytometry in other plasma cell disorders. Br J Haematol 149: 334-351, 2010. [DOI] [PubMed] [Google Scholar]
- 13. Olteanu H, Harrington AM, Kroft SH. CD200 expression in plasma cells of nonmyeloma immunoproliferative disorders: clinicopathologic features and comparison with plasma cell myeloma. Am J Clin Pathol 138: 867-876, 2012. [DOI] [PubMed] [Google Scholar]
- 14. Binder M, Bacher U. The role of CD81 for plasma cell dyscrasias. Leuk Res 38: 292-293, 2014. [DOI] [PubMed] [Google Scholar]
- 15. Guikema JE, Hovenga S, Vellenga E, et al. CD27 is heterogeneously expressed in multiple myeloma: low CD27 expression in patients with high-risk disease. Br J Haematol 121: 36-43, 2003. [DOI] [PubMed] [Google Scholar]
- 16. Arana P, Paiva B, Cedena MT, et al. Prognostic value of antigen expression in multiple myeloma: a PETHEMA/GEM study on 1265 patients enrolled in four consecutive clinical trials. Leukemia 32: 971-978, 2018. [DOI] [PubMed] [Google Scholar]
- 17. D'Souza A, Hayman SR, Buadi F, et al. The utility of plasma vascular endothelial growth factor levels in the diagnosis and follow-up of patients with POEMS syndrome. Blood 118: 4663-4665, 2011. [DOI] [PubMed] [Google Scholar]
- 18. Hashiguchi T, Arimura K, Matsumuro K, et al. Highly concentrated vascular endothelial growth factor in platelets in Crow-Fukase syndrome. Muscle Nerve 23: 1051-1056, 2000. [DOI] [PubMed] [Google Scholar]
- 19. Soubrier MJ, Dubost JJ, Sauvezie BJ;. French Study Group on POEMS Syndrome POEMS syndrome: a study of 25 cases and a review of the literature. Am J Med 97: 543-553, 1994. [DOI] [PubMed] [Google Scholar]
- 20. Dispenzieri A, Kyle RA, Lacy MQ, et al. POEMS syndrome: definitions and long-term outcome. Blood 101: 2496-2506, 2003. [DOI] [PubMed] [Google Scholar]
- 21. Abe D, Nakaseko C, Takeuchi M, et al. Restrictive usage of monoclonal immunoglobulin lambda light chain germline in POEMS syndrome. Blood 112: 836-839, 2008. [DOI] [PubMed] [Google Scholar]
- 22. Kawajiri-Manako C, Mimura N, Fukuyo M, et al. Clonal immunoglobulin λ light-chain gene rearrangements detected by next generation sequencing in POEMS syndrome. Am J Hematol 93: 1161-1168, 2018. [DOI] [PubMed] [Google Scholar]
- 23. Romas E, Storey E, Ayers M, Byrne E. Polyneuropathy, organomegaly, endocrinopathy, M-protein and skin change (POEMS) syndrome with IgG kappa paraproteinemia. Pathology 24: 217-220, 1992. [DOI] [PubMed] [Google Scholar]
- 24. Dursun B, Artac M, Varan HI, Akkaya BK, Karpuzoglu G, Suleymanlar G. An atypical case of POEMS syndrome with IgG kappa M protein and end stage renal failure. Int Urol Nephrol 37: 581-585, 2005. [DOI] [PubMed] [Google Scholar]
- 25. Sethi S, Tageja N, Arabi H, Penumetcha R. Lenalidomide therapy in a rare case of POEMS syndrome with kappa restriction. South Med J 102: 1092-1093, 2009. [DOI] [PubMed] [Google Scholar]
- 26. Pulivarthi S, Gurram MK. An atypical presentation of POEMS syndrome with IgG kappa type M protein and normal VEGF level: Case report and review of literature. J Cancer Res Ther 14: 679-681, 2018. [DOI] [PubMed] [Google Scholar]
- 27. Ye W, Wang C, Cai QQ, et al. Renal impairment in patients with polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy and skin changes syndrome: incidence, treatment and outcome. Nephrol Dial Transplant 31: 275-283, 2016. [DOI] [PubMed] [Google Scholar]
- 28. Nakamoto Y, Imai H, Yasuda T, Wakui H, Miura AB. A spectrum of clinicopathological features of nephropathy associated with POEMS syndrome. Nephrol Dial Transplant 14: 2370-2378, 1999. [DOI] [PubMed] [Google Scholar]
- 29. Soubrier M, Sauron C, Souweine B, et al. Growth factors and proinflammatory cytokines in the renal involvement of POEMS syndrome. Am J Kidney Dis 34: 633-638, 1999. [DOI] [PubMed] [Google Scholar]
- 30. Kobayashi T, Miura M, Niioka T, et al. Phase II clinical trial of lenalidomide and dexamethasone therapy in Japanese elderly patients with newly diagnosed multiple myeloma to determine optimal plasma concentration of lenalidomide. Ther Drug Monit 40: 301-309, 2018. [DOI] [PubMed] [Google Scholar]