Abstract
Many researches were conducted to assess the association of vitamin E intake on the risk of ovarian cancer, with conflict results. The current meta-analysis of published observational studies aimed to investigate the effect of vitamin E intake on ovarian cancer risk. The summary relative risks (RRs) with corresponding 95% confidence intervals (CIs) were calculated to measure the effectiveness of vitamin E intake on ovarian cancer risk using a random-effects model. As a result, 14 studies including 4597 patients were identified. Eleven studies reported about total vitamin E intake, eight studies about vitamin E intake from food only and five studies about vitamin E intake from supplement only on the risk of ovarian cancer. Overall, the summary RRs on ovarian cancer risk was 0.95 (95%CIs = 0.78–1.16) in total vitamin E intake, 0.99 (95%CIs = 0.77–1.27) in vitamin E intake from food only and 0.82 (95%CIs = 0.54–1.25) in vitamin E intake from supplement only. Results in subgroup analyses by study design and geographic location were consistent with overall result. In conclusions, the findings of this meta-analysis suggested that high intake of vitamin E from food or vitamin E supplement had no significant effect on the risk of ovarian cancer.
Keywords: food, meta-analysis, ovarian cancer, supplement, vitamin E
Introduction
Ovarian cancer is one of the most common malignant tumors in women, accounting for 2.5% of female cancers [1]. About 13,850 women die each year from ovarian cancer; this may be the main causes of cancer death in United States among females [2,3]. Furthermore, the 5-year survival rate with all types of ovarian cancer is about 47% [2,3]. Health education and diet prevention are important ways of preventing ovarian cancer. Vitamin E, also named as tocopherol, contains putative anti-cancer and anti-mutant substances that have long been considered to prevent cancer. It is also a well-known powerful antioxidant, which could protect cells against from oxidative DNA damage and mutagenesis, thereby preventing the onset of certain tumors [4,5]. Previous meta-analyses had been published to explore vitamin E intake on many tumors, such as lung cancer [6], esophageal cancer [7], uterine cervical neoplasm [8] and so on. To our attention, a lot of studies have investigated vitamin E intake from food or from supplement on the risk of ovarian cancer, resulting in inconsistent findings. The reasons for these differences included small sample size, low statistical capacity and/or clinical heterogeneity. To overcome the limitations of individual research and address these inconsistencies, we conducted this meta-analysis to provide a comprehensive overview.
Materials and methods
Search strategy
To collect studies that met the criteria for this meta-analysis, we reviewed all literature on the relationship between vitamin E and ovarian cancer risk. Literature searches were performed using Web of Science, EMBASE and PubMed databases (up to June 1, 2019). The following key words and subject terms were used in the search: “vitamin E” OR “vitamin*” OR “tocopherol” combined with “ovarian cancer” OR “ovarian tumor”. We also manually screened the bibliography of original research and review articles. The initial qualification is determined independently by the two reviewers. Disagreements between the reviewers are resolved by a third-party reviewer at a consensus meeting.
Inclusion criteria and exclusion criteria
To be included in the meta-analysis, these studies must be: (1) case–control, cross-sectional or cohort studies; (2) patients were diagnosed as ovarian cancer; (3) report relative risks (RRs) and 95% confidence intervals (CIs) about vitamin E and ovarian cancer. When two or more articles report the same data, the most recently updated data will be considered. The two reviewers screened the titles and abstracts of the records retrieved from the literature. The full text of the potential related articles is independently searched and evaluated by two reviewers, and the differences in research qualifications are resolved by a third author.
The exclusion criteria were as follows: (1) case reports, conference abstracts, letters, editorials, reviews; (2) overlapping or duplicate studies; (3) irrelevant studies; (4) no available data of RRs and 95%CIs and (5) animal studies.
Data extraction
Two investigators independently extracted these information from all eligible studies selected according to predefined criteria. Data were extracted about the last name of first author, age, study design, country, cases and participants, RR and 95%CI, sources of vitamin E (total vitamin E, vitamin E from food or vitamin E from supplement) and category of vitamin E on ovarian cancer risk.
Statistical analysis
All statistical analyses were conducted using the STATA software V.12.0 (STATA Corp, College Station, Texas, U.S.A.). We conducted a meta-analysis of the relationship about vitamin E on ovarian cancer risk. Pooled results were expressed as RRs and 95%CIs with a random-effect model [9]. We used Cochran Q statistic and I² to assess variation and heterogeneity within and between studies [10]. The heterogeneity test was used to evaluate the null hypothesis that all studies evaluated the same effect. When the Q test P < 0.05 or I² > 50%, it is suggested that the study may be heterogeneous [11]. Sensitivity analysis was used to explore whether one single study had the essential effect on the overall RRs. To assess publication bias, visual observations using the Egger test [12] and the funnel plot [13] were used. When P < 0.05, the difference was statistically significant.
Results
Studies included in the meta-analysis
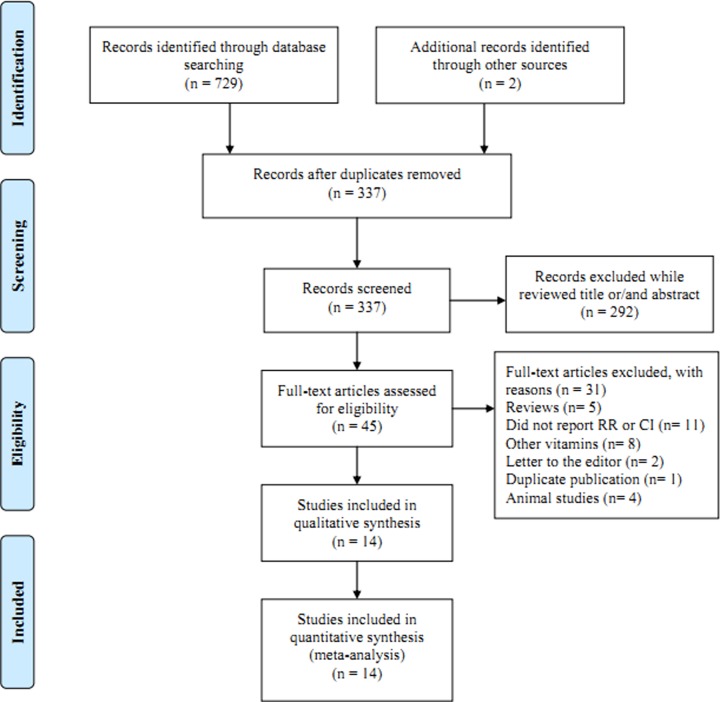
We identified 731 studies using electronic and manual retrieval methods, 394 of which were excluded because they were copies of other reports. Of the remaining 337 articles, 292 articles were excluded after review based on the title and abstract, and 45 were reviewed in full-text. Meanwhile, 31 articles were excluded due to reasons present in Figure 1. Finally, a total of 14 articles [14–27] involving 4597 cases met the inclusion criteria of this meta-analysis. Five articles were cohort design and the remaining nine articles were case–control design. All the studies come from North America except one from Asia. Table 1 summarizes some of the basic features of all included studies.
Figure 1. Flow chart of meta-analysis for exclusion/inclusion of studies.
Table 1. Characteristics of each individual study included in our analysis.
| Study, Year | Design | Age | Participants, Cases | Country | Source of vitamin E | Category | RR (95%CI) |
|---|---|---|---|---|---|---|---|
| Chang et al., 2007 | Cohort | <84 | 97,275, 280 |
United States | Total vitamin E intake | >207 versus ≤7 mg/d | 1.46(0.76–2.79) |
| Cramer et al., 2001 | PBCC | >50 | 1065, 549 |
United States | Total vitamin E intake | Q5 versus Q1 | 0.97(0.64–1.49) |
| Fairfield et al., 2001 | Cohort | 30–55 | 80,326, 301 |
United States | Total vitamin E intake From food |
327 IU/day versus 5 IU/day 12 IU/day versus 5 IU/day |
0.88(0.61–1.27) 1.52(1.04–2.21) |
| Fleischauer et al., 2001 | HBCC | ≥18 | 419, 168 |
United States | Total vitamin E intake From food From supplement |
>43.5 versus <11.0 mg/d >10.5 versus <6.5 mg/d >30 mg/d versus none |
0.59(0.30–1.15) 1.82(0.90–3.69) 0.43(0.26–0.72) |
| Gifkins et al., 2012 | PBCC | >21 | 595, 205 |
United States | Total vitamin E intake From food From supplement |
>114.9 versus <21.7 mg/d >11.6 versus <7.4 mg/d Yes versus no |
1.03(0.59–1.78) 0.89(0.45–1.77) 1.63(1.02–2.63) |
| Kushi et al., 1999 | Cohort | 55–69 | 29,083, 139 |
United States | From food | >24.4 versus <6.2 mg/d | 0.91(0.56–1.48) |
| McCann et al., 2001 | HBCC | 20–87 | 1921, 496 |
United States | From food | >9.4 versus ≤4.9 mg/d | 0.58(0.38–0.88) |
| Pan et al. 2004 | PBCC | 20–76 | 2577, 442 |
Canada | From supplement | ≥10 years versus never | 0.49(0.30–0.81) |
| Salazar-Martinez et al., 2002 | HBCC | 20–79 | 713, 84 |
Mexico | Total vitamin E intake | ≥9.4 versus ≤6.3 mg/d | 1.60(0.88–2.95) |
| Silvera et al., 2006 | Cohort | 40–59 | 89,835, 264 |
Canada | Total vitamin E intake From food |
>28 versus <17 mg/d >25 versus <17 mg/d |
1.24(0.85–1.82) 0.87(0.57–1.31) |
| Terry et al. 2017 | PBCC | 20–79 | 1038, 406 |
United States | Total vitamin E intake From food From supplement |
>25.8 versus <6.7 mg/d >9.1 versus <4.1 mg/d ≥13.5 mg/d versus never |
0.91(0.61–1.37) 0.90(0.49–1.67) 0.86(0.61–1.20) |
| Thomson et al., 2008 | Cohort | 50–79 | 133,614, 451 |
United States | Total vitamin E intake From food From supplement |
>403.2 versus <7.4 mg/d >9.4 versus ≤4.9 mg/d >200 mg/d versus never |
1.22(0.89–1.66) 1.05(0.71–1.57) 1.12(0.86–1.45) |
| Tung et al., 2005 | PBCC | 45–75 | 1165, 558 |
United States | Total vitamin E intake | Q4 versus Q1 | 0.80(0.56–1.16) |
| Zhang et al., 2004 | HBCC | 18–75 | 906, 254 |
China | Total vitamin E intake | ≥38.55 versus ≤23.40 mg/d | 0.41(0.24–0.70) |
Abbreviations: CI, confidence intervals; HBCC, hospital-based case–control study; PBCC, population-based case–control study; RR, relative risk.
Total vitamin E intake and ovarian cancer risk
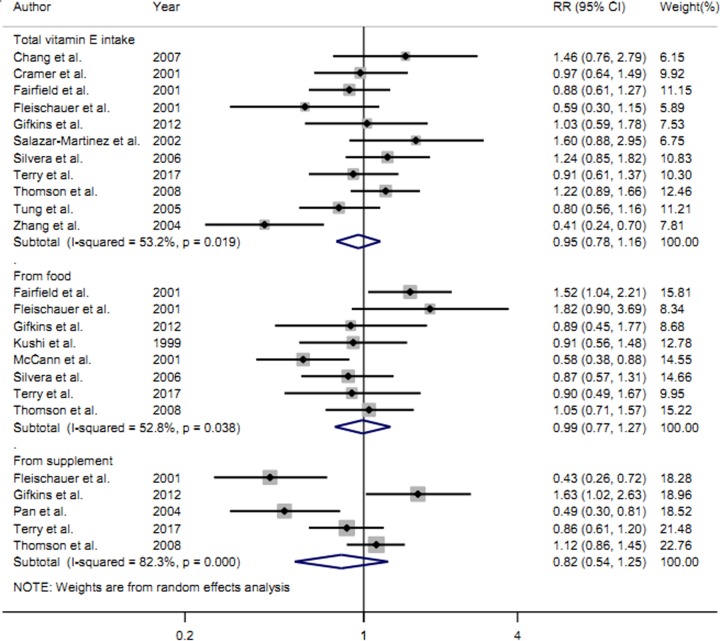
Eleven studies [14–18,22–27] with 3520 cases reported total vitamin E intake on the risk ovarian cancer. Meta-analysis revealed that high category of total vitamin E intake had no significant effect on the risk of ovarian cancer (RRs = 0.95, 95%CIs = 0.78–1.16, I2 = 53.2%, P for heterogeneity = 0.019) (Figure 2). Four studies were cohort design and seven studies were with case–control design. Subgroup analysis by study design showed that the summary RRs on ovarian cancer risk was 1.14 (95%CIs = 0.94–1.38, I2 = 0.0%, P for heterogeneity = 0.417) in cohort studies and 0.84 (95%CIs = 0.64–1.11, I2 = 55.6%, P for heterogeneity = 0.036) in case–control studies. Meanwhile, we further explore the association between ovarian cancer and geographic location. The results suggested that total vitamin E intake is not associated with the risk of ovarian cancer in North America (RRs = 1.02, 95%CIs = 0.88–1.19, I2 = 16.8%, P for heterogeneity = 0.288).
Figure 2. The forest plot about total vitamin E intake, vitamin E intake from food and vitamin E intake from supplement on ovarian cancer risk.
Vitamin E intake from food only and ovarian cancer risk
Eight studies [16–20,23–25] with 2430 cases about vitamin E intake from food only on ovarian cancer risk were included. Overall, the summary RRs on ovarian cancer risk was 0.99 (95%CIs = 0.77–1.27, I2 = 52.8%, P for heterogeneity = 0.038) in vitamin E intake from food only (Figure 2). Four studies were cohort design and the remaining four studies were with case–control design. Subgroup analysis by study design showed high category of vitamin E intake from food only had no significant association on ovarian cancer risk either in cohort studies (RRs = 1.08, 95%CIs = 0.83–1.40, I2 = 35.9%, P for heterogeneity = 0.197) or in case–control studies (RRs = 0.91, 95%CIs = 0.57–1.46, I2 = 60.8%, P for heterogeneity = 0.054).
Vitamin E intake from supplement only and ovarian cancer risk
Five studies [17,18,21,24,25] with 1672 cases reported vitamin E intake from supplement only on the risk of ovarian cancer. Overall, the summary RRs of vitamin E intake from supplement only on ovarian cancer risk was 0.82 (95%CIs = 0.54–1.25, I2 = 82.3%, P for heterogeneity < 0.001).
Publication bias and sensitivity analysis
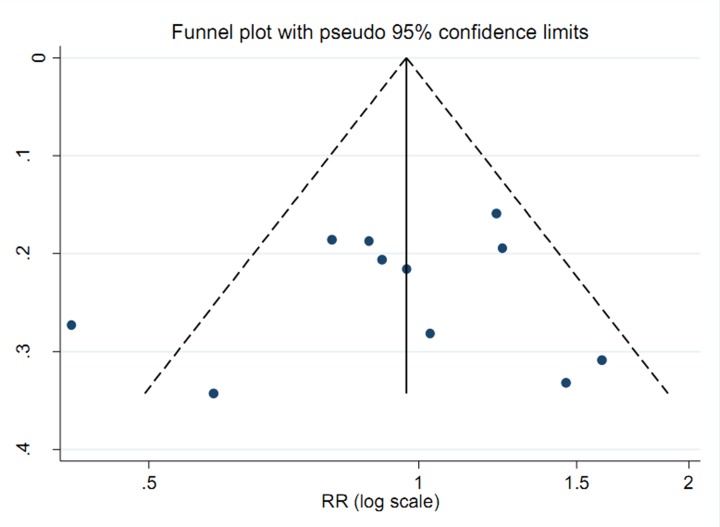
To assess publication bias, Begg’s test was conducted. After the analysis is complete, we did not detect any significant publication bias in total vitamin E intake (P = 0.620), in vitamin E intake from food only (P = 0.876) and in vitamin E intake from supplement only (P = 0.365). Figure 3 shows the funnel plot about total vitamin E intake and ovarian cancer risk. Sensitivity analyses indicated that no singly study had essential effect on the overall results.
Figure 3. Funnel plot for the analysis of publication bias between total vitamin E intake and ovarian cancer risk.
Discussion
Numerous of studies about vitamin E intake and ovarian cancer risk have been published, with conflicting results. We therefore conducted the present study to clarify whether high category of total vitamin E intake, vitamin E intake from food and vitamin E intake from supplement had some effective on the development of ovarian cancer. A total of 14 papers involving 4597 ovarian cancer cases were used in the present study. Pooled results suggested that high category of total vitamin E intake, vitamin E intake from food only and vitamin E intake from supplement only are not associated with the risk of ovarian cancer. Subgroup analyses by geographic location and study design in total vitamin E intake and subgroup analysis by study design in vitamin E intake from food only obtained consistent results with overall result. These results of the present study suggested to clinicians and researchers that there is no need to supplement vitamin E or intentionally eat more vitamin E.
Between-study heterogeneity existed in our study, which may affect the conclusions of the meta-analysis, although random effects model have been carried out. We used meta-regression to explore the potential heterogeneity. To our attention, geographic location may be a covariate for this high heterogeneity in total vitamin E intake. When we performed the subgroup analysis by geographic location, the I2 was reduced to 16.8% in North America populations and no analysis for Asia due to only one study from Asia. The result was not changed in North America. We did not detect any other influence factors on this high heterogeneity in total vitamin E intake, vitamin E intake from food and vitamin E intake from supplement.
Previous review by Koushik et al. [28] had explored the association about vitamin E intake on ovarian cancer risk from Pooling Project investigations of ovarian cancer risk. They concluded that consumption of vitamin E did not play a major role in ovarian cancer risk. Another review published by Crane et al. [29] concluded that no association was demonstrated for vitamin E intake on ovarian cancer risk while only included three papers. Results in the current meta-analysis were consistent with the previous reviews. Although our study did not obtain a positive result, we included more studies than the above-mentioned reviews. Furthermore, we also explored the association about total vitamin E intake, vitamin E intake from food and vitamin E intake from supplement on ovarian cancer risk, respectively. Even though, trial sequential analysis should be performed to see if more investigations were needed [30].
Some limitations should be stated in this meta-analysis. First, any literature-based review and meta-analysis are facing a major threat, namely reporting bias (only recruiting published English literature). Second, the sample size of some studies is relatively small. Third, almost all the included studies come from North America, and the results in our study are suitable for North America only. Therefore, to explore the relationship about vitamin E on the risk of ovarian cancer, more studies with other countries are warranted to further confirm this result. There is no doubt that the limitations mentioned above may affect our final conclusions. However, this meta-analysis also has its advantages. To the best of our knowledge, our meta-analysis is the comprehensive evidence to provide vitamin E intake on ovarian cancer risk. Similarly, compared with individual studies, our data on the relationship between vitamin E intake and ovarian cancer risk, due to the results of multiple independent analysis, improved statistical power and resolution, resulting in higher accuracy. However, given the limitations described above, further research is needed to address bias, confusion and opportunity.
Conclusions
In conclusions, the findings of this meta-analysis indicated that high intake of vitamin E from food or vitamin E supplement had no significant effect on the risk of ovarian cancer. More studies are required to further explore these associations due to some limitations existed in our study.
Abbreviations
- CI
confidence interval
- HBCC
hospital-based case–control study
- PBCC
population-based case–control study
- RR
relative risk
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
The authors declare that there are no sources of funding to be acknowledged.
Author Contribution
Y.X.L. and H.R.Z. contributed to the conception and design of this research. Y.X.L., H.R.Z. and F.J.M. contributed to the completion of articles, the data extraction. T.T. calculated the data. Y.X.L. and J.Y.X. write the manuscript. F.J.Y. reviewed and revised the manuscript. The entire author approved the final manuscript.
References
- 1.Jelovac D. and Armstrong D.K. (2011) Recent progress in the diagnosis and treatment of ovarian cancer. CA Cancer J. Clin. 61, 183–203 10.3322/caac.20113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jemal A., Siegel R., Xu J. and Ward E. (2010) Cancer statistics, 2010. CA Cancer J. Clin. 60, 277–300 10.3322/caac.20073 [DOI] [PubMed] [Google Scholar]
- 3.Nasioudis D., Ko E.M., Haggerty A.F., Giuntoli R.L. II, Burger R.A., Morgan M.A. et al. (2019) Isolated distant lymph node metastases in ovarian cancer. Should a new substage be created? Gynecol. Oncol. Rep. 28, 86–90 10.1016/j.gore.2019.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diao Q.X., Zhang J.Z., Zhao T., Xue F., Gao F., Ma S.M. et al. (2016) Vitamin E promotes breast cancer cell proliferation by reducing ROS production and p53 expression. Eur. Rev. Med. Pharmacol. Sci. 20, 2710–2717 [PubMed] [Google Scholar]
- 5.Zheng N., Gao Y., Ji H., Wu L., Qi X., Liu X. et al. (2016) Vitamin E derivative-based multifunctional nanoemulsions for overcoming multidrug resistance in cancer. J. Drug Target. 24, 663–669 10.3109/1061186X.2015.1135335 [DOI] [PubMed] [Google Scholar]
- 6.Zhu Y.J., Bo Y.C., Liu X.X. and Qiu C.G. (2017) Association of dietary vitamin E intake with risk of lung cancer: a dose-response meta-analysis. Asia Pac. J. Clin. Nutr. 26, 271–277 [DOI] [PubMed] [Google Scholar]
- 7.Cui L., Li L., Tian Y., Xu F. and Qiao T. (2018) Association between Dietary Vitamin E Intake and Esophageal Cancer Risk: An Updated Meta-Analysis. Nutrients 10, pii: E801 10.3390/nu10070801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu X., Li S., Zhou L., Zhao M. and Zhu X. (2017) Effect of vitamin E supplementation on uterine cervical neoplasm: A meta-analysis of case-control studies. PLoS One 12, e0183395 10.1371/journal.pone.0183395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DerSimonian R. and Laird N. (1986) Meta-analysis in clinical trials. Control. Clin. Trials 7, 177–188 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 10.Higgins J.P., Thompson S.G., Deeks J.J. and Altman D.G. (2003) Measuring inconsistency in meta-analyses. BMJ 327, 557–560 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins J.P. and Thompson S.G. (2004) Controlling the risk of spurious findings from meta-regression. Stat. Med. 23, 1663–1682 10.1002/sim.1752 [DOI] [PubMed] [Google Scholar]
- 12.Egger M., Davey Smith G., Schneider M. and Minder C. (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Begg C.B. and Mazumdar M. (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- 14.Chang E.T., Lee V.S., Canchola A.J., Clarke C.A., Purdie D.M., Reynolds P. et al. (2007) Diet and risk of ovarian cancer in the California Teachers Study cohort. Am. J. Epidemiol. 165, 802–813 10.1093/aje/kwk065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cramer D.W., Kuper H., Harlow B.L. and Titus-Ernstoff L. (2001) Carotenoids, antioxidants and ovarian cancer risk in pre- and postmenopausal women. Int. J. Cancer 94, 128–134 10.1002/ijc.1435 [DOI] [PubMed] [Google Scholar]
- 16.Fairfield K.M., Hankinson S.E., Rosner B.A., Hunter D.J., Colditz G.A. and Willett W.C. (2001) Risk of ovarian carcinoma and consumption of vitamins A, C, and E and specific carotenoids: a prospective analysis. Cancer 92, 2318–2326 [DOI] [PubMed] [Google Scholar]
- 17.Fleischauer A.T., Olson S.H., Mignone L., Simonsen N., Caputo T.A. and Harlap S. (2001) Dietary antioxidants, supplements, and risk of epithelial ovarian cancer. Nutr. Cancer 40, 92–98 10.1207/S15327914NC402_3 [DOI] [PubMed] [Google Scholar]
- 18.Gifkins D., Olson S.H., Paddock L., King M., Demissie K., Lu S.E. et al. (2012) Total and individual antioxidant intake and risk of epithelial ovarian cancer. BMC Cancer 12, 211–220 10.1186/1471-2407-12-211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kushi L.H., Mink P.J., Folsom A.R., Anderson K.E., Zheng W., Lazovich D. et al. (1999) Prospective study of diet and ovarian cancer. Am. J. Epidemiol. 149, 21–31 10.1093/oxfordjournals.aje.a009723 [DOI] [PubMed] [Google Scholar]
- 20.McCann S.E., Moysich K.B. and Mettlin C. (2001) Intakes of selected nutrients and food groups and risk of ovarian cancer. Nutr. Cancer 39, 19–28 10.1207/S15327914nc391_3 [DOI] [PubMed] [Google Scholar]
- 21.Pan S.Y., Ugnat A.M., Mao Y., Wen S.W., Johnson K.C. and Canadian Cancer Registries Epidemiology Research, G (2004) A case-control study of diet and the risk of ovarian cancer. Cancer Epidemiol. Biomarkers Prev. 13, 1521–1527 [PubMed] [Google Scholar]
- 22.Salazar-Martinez E., Lazcano-Ponce E.C., Gonzalez Lira-Lira G., Escudero-De los Rios P. and Hernandez-Avila M. (2002) Nutritional determinants of epithelial ovarian cancer risk: a case-control study in Mexico. Oncology 63, 151–157 10.1159/000063814 [DOI] [PubMed] [Google Scholar]
- 23.Silvera S.A., Jain M., Howe G.R., Miller A.B. and Rohan T.E. (2006) Carotenoid, vitamin A, vitamin C, and vitamin E intake and risk of ovarian cancer: a prospective cohort study. Cancer Epidemiol. Biomarkers Prev. 15, 395–397 10.1158/1055-9965.EPI-05-0835 [DOI] [PubMed] [Google Scholar]
- 24.Terry P.D., Qin B., Camacho F., Moorman P.G., Alberg A.J., Barnholtz-Sloan J.S. et al. (2017) Supplemental Selenium May Decrease Ovarian Cancer Risk in African-American Women. J. Nutr. 147, 621–627 10.3945/jn.116.243279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomson C.A., Neuhouser M.L., Shikany J.M., Caan B.J., Monk B.J., Mossavar-Rahmani Y. et al. (2008) The role of antioxidants and vitamin A in ovarian cancer: results from the Women’s Health Initiative. Nutr. Cancer 60, 710–719 10.1080/01635580802233983 [DOI] [PubMed] [Google Scholar]
- 26.Tung K.H., Wilkens L.R., Wu A.H., McDuffie K., Hankin J.H., Nomura A.M. et al. (2005) Association of dietary vitamin A, carotenoids, and other antioxidants with the risk of ovarian cancer. Cancer Epidemiol. Biomarkers Prev. 14, 669–676 10.1158/1055-9965.EPI-04-0550 [DOI] [PubMed] [Google Scholar]
- 27.Zhang M., Lee A.H. and Binns C.W. (2004) Reproductive and dietary risk factors for epithelial ovarian cancer in China. Gynecol. Oncol. 92, 320–326 10.1016/j.ygyno.2003.10.025 [DOI] [PubMed] [Google Scholar]
- 28.Koushik A., Wang M., Anderson K.E., van den Brandt P., Clendenen T.V., Eliassen A.H. et al. (2015) Intake of vitamins A, C, and E and folate and the risk of ovarian cancer in a pooled analysis of 10 cohort studies. Cancer Causes Control. 26, 1315–1327 10.1007/s10552-015-0626-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crane T.E., Khulpateea B.R., Alberts D.S., Basen-Engquist K. and Thomson C.A. (2014) Dietary intake and ovarian cancer risk: a systematic review. Cancer Epidemiol. Biomarkers Prev. 23, 255–273 10.1158/1055-9965.EPI-13-0515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fu W., Zhuo Z.J., Chen Y.C., Zhu J., Zhao Z., Jia W. et al. (2017) NFKB1 -94insertion/deletion ATTG polymorphism and cancer risk: Evidence from 50 case-control studies. Oncotarget 8, 9806–9822 [DOI] [PMC free article] [PubMed] [Google Scholar]