Abstract
Systemic anticoagulation may be beneficial in pulmonary arterial hypertension, but there is no randomized clinical trial data to guide therapeutic decision making, and current guidelines do not account for patient preferences or quality of life. Decision analytic models to evaluate the potential risks and benefits of systemic anticoagulation in pulmonary arterial hypertension patients, focusing on the benefit in quality-adjusted life years, may be helpful in clarifying this uncertainty. We constructed a 31-state Markov decision analytic model to explore anticoagulation and no anticoagulation strategies. Modeled patient characteristics included gender, use of central catheter-based pulmonary arterial hypertension therapy, type of pulmonary arterial hypertension (idiopathic, idiopathic pulmonary arterial hypertension, or connective-tissue associated, connective tissue disease-pulmonary arterial hypertension), and use of oral contraceptive medication by females. Modeled events included mortality, thromboembolic complications, atrial fibrillation, stroke, and anticoagulation bleeding. Deterministic and probabilistic sensitivity analyses were performed. Anticoagulation was favored in all idiopathic pulmonary arterial hypertension cases, with a gain of 0.43–0.51 quality-adjusted life years, and detrimental in all connective tissue disease-pulmonary arterial hypertension cases, with a loss of 0.66–1.89 quality-adjusted life years. Anticoagulation would need to demonstrate a hazard ratio for pulmonary arterial hypertension mortality of 0.95 or better to be favored. In our model, idiopathic pulmonary arterial hypertension patients benefit from anticoagulation in terms of quality-adjusted life years, and connective tissue disease-pulmonary arterial hypertension patients were harmed, with a hazard ratio for pulmonary arterial hypertension mortality of 0.95 or better being required to favorably impact quality-adjusted life years. These results suggest that anticoagulation significantly improves quality adjusted life years and should be offered to all idiopathic pulmonary arterial hypertension patients. Shared decision models based on these results may help clarify therapeutic decision-making uncertainty in pulmonary arterial hypertension patients.
Keywords: health outcomes assessment/cost effectiveness, pulmonary arterial hypertension, anticoagulants, connective tissue disease
Introduction
Pulmonary arterial hypertension (PAH) is a progressive, fatal disease of the pulmonary vasculature.1,2 While the cornerstone of PAH treatment involves targeted vasodilator therapy, thrombotic pulmonary vascular lesions are frequently observed post-mortem, and anticoagulation (AC) with vitamin K antagonists (VKA) may be beneficial in some forms of PAH.1,3–6 The two largest studies to date investigating this question, the Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension (COMPERA) and the Registry to Evaluate Early and Long-Term PAH Disease Management (REVEAL), reached conflicting conclusions regarding the benefit to patients with idiopathic PAH (IPAH) and the harms to patients with connective tissue disease-associated PAH (CTD-PAH) patients.3,4 Consequently, the most recent PAH guidelines suggested that AC therapy may be considered in PAH patients with PAH receiving central catheter-based prostacyclin therapy, and should not be offered to most CTD-PAH patients.1,7 No recommendation was given regarding AC in women receiving estrogen-containing oral contraceptives (OCP), who may be at increased risk of venous thromboembolism.8,9 As a result of this uncertainty, expert opinion has been divided on the benefits of systemic AC therapy in PAH patients who do not have another defined indication for systemic AC therapy, and recent registry data indicate that the minority (∼40%) of IPAH patients receive systemic AC therapy as part of their treatment.10–14 Additionally, although it has been increasingly recognized that shared decision-making incorporating patient values and preferences are important considerations when devising therapeutic strategies in PAH, current PAH guidelines regarding AC to affect pulmonary vascular disease progression focus only on mortality benefits, and the impact of systemic AC on PAH patient quality of life is not well defined.15–17 Thus, we explored these issues by developing a decision analytic model to help clarify the potential benefit or risk, and determine the net clinical benefit in quality-adjusted life years (QALY’s), of AC with VKA’s in IPAH and CTD-PAH across a number of commonly encountered clinical scenarios.
Methods
We used registry data to estimate mortality in IPAH patients, mortality estimates are in close agreement, with a one-year mortality of 18% and a three-year mortality of approximately 26%. Mortality estimates in CTD-PAH patients vary widely, and we estimated the three-year mortality in CTD-PAH patients to be approximately 30–48%.3,4,7,18–24 Given this wide range in mortality rates for CTD-PAH patients, we performed sensitivity analyses in our model using both the lower bounds (30%) and the upper bounds (48%) of the three-year mortality estimates obtained from the literature. The base cases in our model reflected the median age (50 years old) of patients in these registries at the time of diagnosis. Mortality estimates in the literature account for all-cause mortality and do not explicitly describe deaths secondary to PAH alone. Thus, we calculated disease-specific excess mortality rates for PAH, by adjusting all-cause mortality for age and gender using life tables from the Centers for Disease Control and Prevention. We based these adjustments on population-based mortality rates for Caucasians, because the majority of patients described in published PAH studies are of Caucasian descent.
We used the COMPERA and REVEAL studies that provided estimates for the hazard ratio (HR) with VKA AC therapy in IPAH and CTD-PAH patients.3,4 We also incorporated gender differences for mortality rates in our model, as it is well documented that males with PAH have a higher mortality rate than females. We estimated, based on the REVEAL risk calculator, that males had an increased relative risk of mortality of 1.6 as compared to females.25,26
The rate of central catheter-associated deep vein thrombosis (DVT) is estimated at approximately 1% per year.27,28 PE occurs in roughly 37.8% of those with DVT.28–31 We assumed, for the purposes of this model, that DVT rates from central catheters were independent and additive with DVT rates from other sources. We also assumed that DVT rates in PAH patients without central catheters were no different than those in similar patients without PAH. Given the significant hemodynamic impairment of the pulmonary circulation and right ventricular function in these patients, we assumed all pulmonary emboli in PAH patients to be intermediate risk events resulting in hemodynamic instability, with an associated mortality of 19%.32 We included in our model the increased risk of DVT from OCP medications, as the majority of PAH patients are female and the vast majority (specifically those treated with endothelin receptor antagonist medication and prostacyclin therapy) are on OCP’s.7,33,34 Based on large meta analyses, we estimated the baseline non-catheter DVT risk to be roughly 1–2 per 1000 people per year, with an increased relative risk in men of 2 and an increased relative risk in women taking OCP’s of 3.5.35–38 The baseline rate of DVT for patients not receiving systemic AC with VKA therapy was based on baseline DVT risk by gender, use of OCP’s in women, and presence of central catheter (Table 1).
Table 1.
Base case values for rates, probabilities, and quality of life.
| Parameters | Base case values | Clinical range or 95% CI | Distribution |
|---|---|---|---|
| Annual mortality rate IPAH | 0.0985 | 0.0970–0.0985 | Log Normal |
| Annual mortality rate CTD-PAH patients (low) | 0.11 | 0.10–0.12 | Log Normal |
| Annual mortality rate CTD-PAH patients (high) | 0.205 | 0.195–0.220 | Log Normal |
| Hazard ratio of AC on IPAH mortality | 0.79 | 0.66–0.94 | Log Normal |
| Hazard ratio of AC on CTD-PAH mortality | 2.03 | 1.09–3.79 | Log Normal |
| Hazard ratio of male gender on PAH mortality | 1.6 | 1.47–2.93 | Log Normal |
| Hazard ratio of AC in preventing DVT | 0.37 | 0.26–0.52 | Log Normal |
| Annual rate of DVT in males no Cath no AC | 0.002 | 0.001–0.004 | Log Normal |
| Annual rate of DVT in males Cath no AC | 0.012 | 0.002–0.022 | Log Normal |
| Annual rate of DVT females no OCP/Cath no AC | 0.001 | 0.0005–0.002 | Log Normal |
| Annual rate of DVT females OCP Cath No AC | 0.011 | 0.005–0.015 | Log Normal |
| Probability of DVT converting to PE | 0.378 | 0.355–0.401 | Beta |
| Probability of death from PE | 0.19 | 0.178–0.198 | Beta |
| Annual rate of bleeding on AC | 0.0253 | 0.02–0.03 | Log Normal |
| Probability of death from bleeding event on AC | 0.1 | 0.05–0.15 | Logit |
| Annual rate of atrial fibrillation | 0.04 | 0.027–0.05 | Log Normal |
| Hazard ratio of AC in prevention stroke from atrial fibrillation in females | 0.26 | 0.23–0.3 | Log Normal |
| Hazard ratio of AC in prevention stroke from atrial fibrillation in males | 0.49 | 0.42–0.58 | Log Normal |
| Annual rate of stroke in females no AC with atrial fibrillation | 0.022 | 0.021–0.023 | Beta |
| Annual rate of stroke in males no AC with atrial fibrillation | 0.014 | 0.012–0.015 | Beta |
| Probability of death from ischemic stroke | 0.2 | 0.16–0.23 | Logit |
| Well (without cardiopulmonary disease) | 1 | N/A | N/A |
| Receiving anticoagulation | 0.99 | 0.9–1.0 | Logit |
| Following major bleeding event | 0.9 | 0.8–1.0 | Logit |
| Following DVT | 0.9 | 0.8–1.0 | Logit |
| Atrial fibrillation | 0.9 | 0.8–1.0 | Logit |
| PE or stroke (after first month) | 0.7 | 0.5–0.9 | Logit |
| IPAH or CTD-PAH | 0.62 | 0.43–0.82 | Logit |
| IPAH or CTD-PAH with Cath | 0.52 | 0.43–0.82 | Logit |
| First month following PE or stroke | 0.11 | 0.05–0.15 | Logit |
| Death | 0 | N/A | N/A |
IPAH: idiopathic pulmonary arterial hypertension; CTD-PAH: connective-tissue-disease associated pulmonary arterial hypertension; AC: anticoagulation with vitamin-K antagonist therapy (Warfarin); Cath: presence of central catheter; OCP: use of oral contraceptive medications; DVT: deep vein thrombosis; PE: pulmonary embolism; CI: confidence interval: N/A: not applicable; Low: low estimate; High: high estimate.
Although the efficacy of AC in PAH patients in preventing DVT’s is not well defined, we estimated that it was at least as good as that observed in other groups of patients, with a relative risk of DVT while on AC of at least 0.37.30,31,39 We made the simplifying assumption that systemic AC protected equally from both central catheter-related DVT as well as DVT from other causes, and was equally protective across gender and etiology of PAH (CTD-PAH versus IPAH), and the rates of DVT across gender, use of OCP’s in women, and presence of central catheters were based on this modeled effectiveness of VKA therapy in these populations (Table 1).
We estimated that PAH patients, who by definition do not have significant additional cardiopulmonary comorbidities and are typically middle-aged at diagnosis, would have the same incidence of atrial fibrillation as the general population in this age group, roughly 4% annually. Additionally, we estimated that the rates of untreated atrial fibrillation-related stroke would approximate a heart failure population with the lowest CHA2DS2VASc scores. We estimated women with PAH would have a CHA2DS2VASc score of two, corresponding to an annual stroke rate of 2.2%, and men would have a score of one and an annual stroke rate of 1.3%.7,38–47 The effectiveness of AC on the prevention of stroke was also based on the CHA2DS2VASc scores, with a HR of 0.26 for stroke in women and 0.49 for stroke in men44–47 (Table 1).
Bleeding was assumed to be similar across all indications for VKA therapy, including atrial fibrillation, previous stroke, PAH, or DVT and pulmonary embolism (PE) therapy. Bleeding rates were estimated from systematic reviews of AC studies utilizing VKA therapy, at approximately 2–3 per 100 patient years.48–50 Only major life-threatening bleeding was considered, with an associated mortality rate of 10%. We assumed patients would start AC therapy if they developed a condition (atrial fibrillation or DVT) that put them at risk of an embolic event, or survived an embolic event (PE or stroke). Similarly, survivors of major bleeding events were no longer treated with AC therapy for the rest of their lives, and there would be no additional risk of bleeding in patients not on systemic AC therapy.
Utilities
We used quality-adjustment factors (QAFs) for less than optimal states of health in order to account for quality of life as well as duration of survival. These were anchored at one for life in a disease-free state of health, and zero for death. QAF’s for survivors of ischemic stroke and major bleeds from AC were obtained from previously published decision analysis studies51–56 (Table 1). Patients with PE and stroke were modeled as having a significantly lower quality of life during the first month after the event, as compared to the quality of life for their remaining lifetime. Quality of life with PAH varied significantly based on functional status, and thus we calculated the QAF for PAH as a weighted average based on the estimated proportion of patients in each of the four functional classes at the time of diagnosis, using data from the REVEAL.19,25 Quality of life estimates for health states were subjected to extensive sensitivity analyses. We used a multiplicative model for calculating QAFs when patients were in health states that incorporated more than one quality of life impacting condition (e.g. PAH and DVT).
Decision analytic model
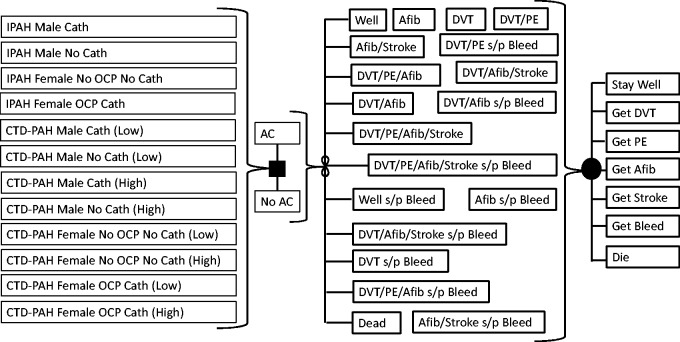
We developed a 31-state Markov state transition decision analytic model to explore outcomes of the two strategies: (1) Anticoagulate with VKA therapy; (2) Do Not Anticoagulate with VKA therapy. We used a standard computer program (Decision Maker, Boston, MA) to build the model, analyze results, and perform sensitivity analyses. Patients were able to enter the Markov simulation from 1 of 12 different disease states based on their gender, mode of PAH therapy (catheter-based or not), type of PAH (IPAH or CTD-PAH), for CTD-PAH patients either a low or high estimate of PAH mortality, and, for females, the use of oral contraceptive medication (OCP) or not. These 12 “base cases” ranged from low risk (no catheter-based therapy or OCP use) to high risk (catheter-based therapy and, for females, concurrent OCP use) clinical scenarios where AC might be considered. This model did not require institutional review board approval. Our primary outcome was the QALY’s for each of the two treatment strategies noted above (AC or no AC).
Base case values, model parameters, and a cartoon representation of the decision tree figure are summarized in Table 1 and Fig. 1. In all base cases, patients were 50 years old at the start of the model. During each one-month cycle in the Markov simulation, patients could develop atrial fibrillation, or suffer a stroke, DVT, or bleed, based on the annual rates of these events, and modified as appropriate by treatment with AC. AC benefited patients by preventing DVT’s and conversion of DVT to PE, preventing stroke in patients with atrial fibrillation, and augmenting the mortality rate of PAH. During each monthly cycle, patients face a risk of dying from PAH or other non-explicitly modeled causes, developing DVT and PE (and possibly dying), or developing atrial fibrillation and stroke (and possibly dying). Patients receiving AC therapy faced an additional monthly risk of suffering a (potentially fatal) bleed while on AC. The simulation was run for the entire life expectancy of the hypothetical cohort of similar patients.
Fig. 1.
Decision analytic model schematic.
IPAH: idiopathic pulmonary arterial hypertension; CTD-PAH: connective tissue disease-associated pulmonary arterial hypertension; AC: anticoagulation; DVT: deep vein thrombosis; PE: pulmonary embolism; Afib: atrial fibrillation; OCP: oral contraceptive pills; Cath: central catheter-based prostacyclin therapy.
Assumptions
We made a number of simplifying assumptions when constructing our decision analytic model. A major assumption was that the impact of AC, noted in the REVEAL and COMPERA registries, on mortality in IPAH and CTD-PAH patients only accounted for the effects of systemic AC on PAH-specific mortality, and did not include the effects of AC on the mortality from PE complicating DVT, stroke complicating atrial fibrillation, and major bleeding. As noted explicitly by Olsson et al. in the COMPERA study, and implied by Preston et al. in REVEAL, these registries were not designed to capture major bleeding events or the effects of AC on thromboembolic complications, and data regarding the specific effect of AC on bleeding and thromboembolic mortality events are lacking.3,4 Given the importance of these potential complications in the treatment guidelines concerning AC in PAH patients, we chose to explicitly model these events in our decision analytic model.
We assumed fatal outcomes of PE and stroke occurred immediately. Survivors of these events experienced a short-term decrement in quality of life for one month, followed by a somewhat less severe decrement long-term. We considered all PE in PAH patients to be intermediate risk or higher, given pre-existing right ventricular impairment is a consequence of PAH. However, once PE occurred, they had a similar mortality risk as other patients with intermediate or high-risk PE. We assumed the incidence of atrial fibrillation, and the incidence and mortality of atrial fibrillation-associated stroke, to be the same as that of the general population. We considered baseline risk of stroke in patients without atrial fibrillation to be negligible in our population, and that atrial fibrillation remained a persistent risk once it developed. We did not model increased risk of recurrent stroke following an initial stroke, or an increased risk of recurrent DVT following initial DVT.
We assumed the risk of DVT/PE, atrial fibrillation/stroke, death from bleeding on AC, and death from PAH to all be independent and constant over time. We assumed that oral contraceptive therapy did not increase the risk of atrial fibrillation-related stroke. As the prevalence of patent foramen ovale is no higher in PAH patients than in the general population (∼25%), we assumed patients with a central catheter did not have an increased risk of stroke from paradoxical embolism.57–59 We only modeled AC with a VKA, as no studies to date explored the use of any of the direct oral anticoagulant medications. For those receiving catheter therapy, we considered the risk of DVT from the central catheter to be additive to the incidence of DVT in the more general PAH population.
We only considered life-threatening moderate/severe bleeding in this model. Given the limited life expectancy of PAH patients, non-fatal bleeding events were modeled as resulting in a permanent decrement to quality of life (to account for the increased risk of mortality and diminished functional capacity that persists in the six months following hospitalization in PAH patients). Finally, all survivors of DVT or PE, and those who developed atrial fibrillation, crossed over to the AC strategy, with the associated risks and benefits of anticoagulant therapy for these validated indications, and survivors of a major bleed crossed over to the Do Not Anticoagulate strategy and were exposed to the risks and benefits of being without AC in regards to risks of venous thromboembolism, atrial fibrillation, and stroke.
To explore the impact of parameter value uncertainty, we performed deterministic (one-way, two-way, and three-way) sensitivity analyses as well as probabilistic (second-order Monte Carlo) sensitivity analyses. For deterministic sensitivity analyses, parameters (rates, probabilities, and utilities) were varied across wide ranges that at least encompassed either 95% confidence intervals or clinically plausible ranges. For our probabilistic sensitivity analyses, we performed 10,000 iterations of a second-order Monte Carlo simulation using parameter distributions rather than point values (see Table 1).60
Results
We performed a separate analysis for each base case, including high and low mortality estimates for CTD-PAH patients. For all base cases with IPAH, AC with VKA therapy was the favored strategy, with gains ranging between 0.43 and 0.51 QALY’s as compared to no AC (Table 2).
Table 2.
Base case analyses.
| Disease state entering Markov model | Anticoagulate (QALYs) | Do Not Anticoagulate (QALYs) |
|---|---|---|
| IPAH male Cath | 3.53 | 3.10 |
| IPAH male no Cath | 4.27 | 3.78 |
| IPAH female no OCP no Cath | 6.40 | 5.89 |
| IPAH female OCP Cath | 5.23 | 4.73 |
| CTD-PAH male Cath (high mortality) | 0.78 | 1.44 |
| CTD-PAH male no Cath (high mortality) | 0.93 | 1.74 |
| CTD-PAH female no OCP No Cath (high mortality) | 1.50 | 2.71 |
| CTD-PAH female OCP Cath (high mortality) | 1.25 | 2.20 |
| CTD-PAH male Cath (low mortality) | 1.44 | 2.50 |
| CTD-PAH male no Cath (low mortality) | 1.73 | 3.07 |
| CTD-PAH female no OCP no Cath (low mortality) | 2.79 | 4.68 |
| CTD-PAH female OCP Cath (low mortality) | 2.31 | 3.71 |
IPAH: idiopathic pulmonary arterial hypertension; CTD-PAH: connective-tissue disease-associated pulmonary arterial hypertension; AC: anticoagulation with vitamin-K antagonist therapy (Warfarin); QALY: quality-adjusted life years; Cath: presence of central catheter; OCP: oral contraceptive pills; high and low mortality: annual survival estimates in CTD-PAH patients of 55% and 85%, respectively. Values in bold indicate the favored strategy based on higher utility.
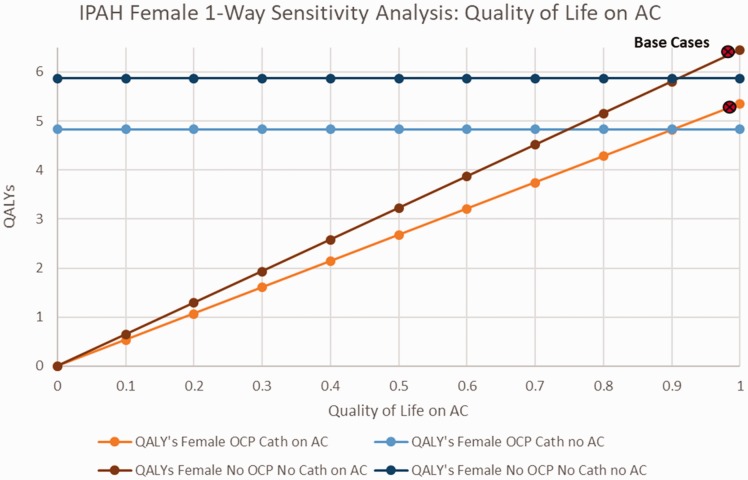
Fig. 2 explores the impact of quality of life while receiving AC therapy, for scenarios of women with IPAH receiving OCPs and catheter-delivered PAH therapy, and women not receiving OCP’s or catheter-based therapy. In both scenarios, as well as in men with IPAH, AC therapy was preferred unless the quality of life while receiving AC dropped below a threshold of 0.9 (online Fig. 1).
Fig. 2.
Sensitivity analysis on IPAH females quality of life with AC.
AC: anticoagulation; QALY: quality-adjusted life years; IPAH: idiopathic pulmonary arterial hypertension; OCP: oral contraceptive pills.
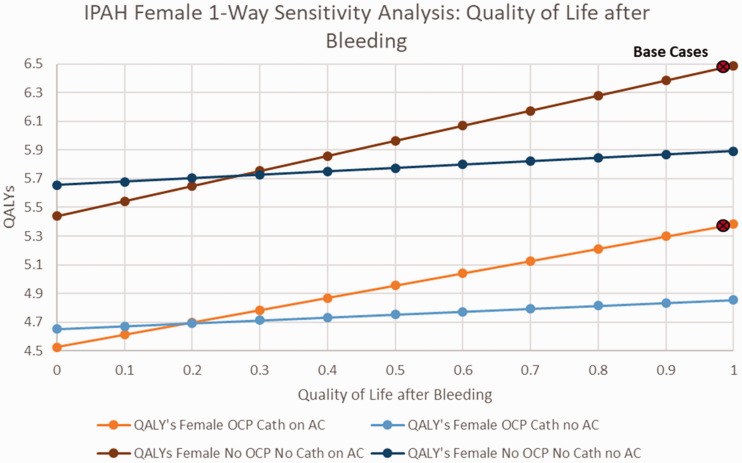
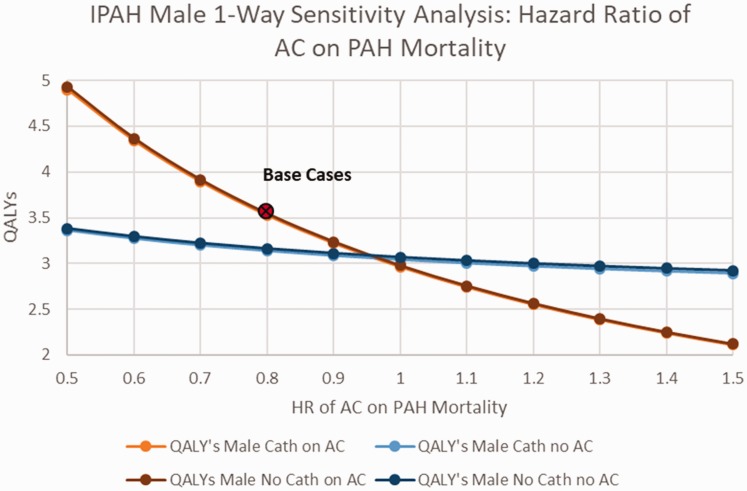
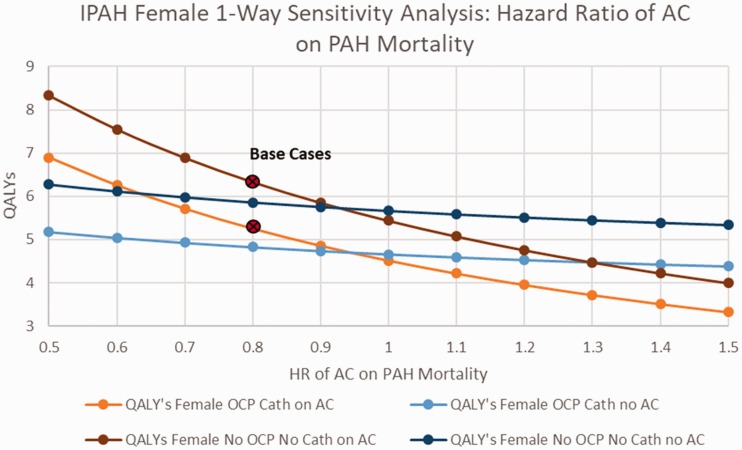
When examining the impact of changes in the rate of major bleeding among patients with IPAH receiving AC therapy, among women, with or without OCP’s and catheter-delivered therapy, AC was preferred unless the rate of bleeding exceeded 0.1/year (base case 0.025) (online Fig. 2). Among IPAH males, AC was preferred unless the rate of major bleeding while receiving AC exceeded 0.15/year (online Fig. 3). Among IPAH females receiving catheter-delivered therapy, the quality of life after a bleeding event would have to drop to below 0.2 before AC would no longer be favored, and below 0.25 for IPAH females not receiving catheter-delivered therapy (Fig. 3). Assessing the benefit of AC on overall mortality from IPAH (base case HR 0.8), AC would need to demonstrate a HR of 0.95 or better on PAH disease mortality to be the favored strategy, regardless of gender (Figs. 4 and 5).
Fig. 3.
Sensitivity analysis on IPAH females quality of life after bleeding.
AC: anticoagulation; QALY: quality-adjusted life years; IPAH: idiopathic pulmonary arterial hypertension; OCP: oral contraceptive pills.
Fig. 4.
Sensitivity analysis on IPAH males hazard ratio of AC on PAH mortality.
AC: anticoagulation; QALY: quality-adjusted life years; IPAH: idiopathic pulmonary arterial hypertension; HR: hazard ratio.
Fig. 5.
Sensitivity analysis on IPAH females hazard ratio of AC on PAH mortality.
AC: anticoagulation; QALY: quality-adjusted life years; IPAH: idiopathic pulmonary arterial hypertension; OCP: oral contraceptive pills; HR: hazard ratio.
The opposite effect of AC was observed for all CTD-PAH patients, with AC resulting in a loss of between 0.66 and 1.89 QALY’s compared to a no-AC strategy, and needing to demonstrate a HR of at least 0.95 or better for all types of CTD-PAH to be the favored strategy (Base case 2.03) (Table 2, online Figs. 4–6). This result was only sensitive to the rate of DVT in CTD-PAH female patients with a low PAH mortality rate, having to exceed 0.6/year (60% annually) among women receiving catheter-based therapy and OCPs, and 0.7/year (70% annually) in women without catheters or OCP’s, in order to make AC the favored strategy (online Fig. 7).
Fig 6.
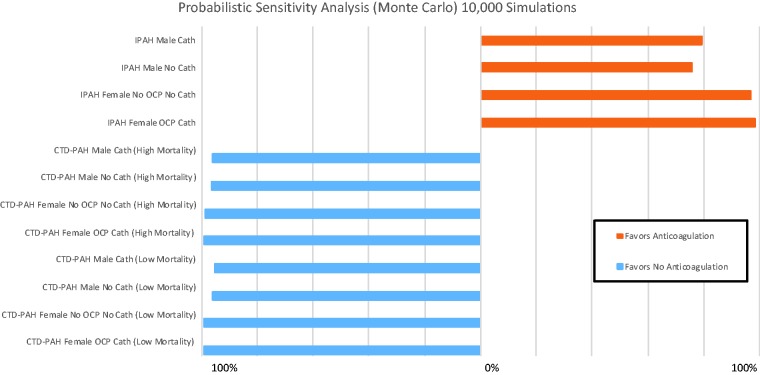
Probabilistic sensitivity analyses results.
IPAH: idiopathic pulmonary arterial hypertension; CTD-PAH: connective-tissue-disease associated pulmonary arterial hypertension; OCP: oral contraceptive pills.
Results were robust to changes in quality of life for atrial fibrillation, DVT, PE, stroke, major bleed, or PAH with or without catheter-delivered therapy. This result also did not depend on the rate of DVT, the effectiveness of AC in preventing DVT, the rate of PE from DVT, the mortality rate of PE, the rate of stroke from atrial fibrillation, the rate of death from stroke, or the rate of death from bleeding events.
In probabilistic sensitivity analyses of CTD-PAH women and men, not anticoagulating was favored in 95.5–99.0% of 10,000 second-order Monte Carlo iterations (Fig. 6). In IPAH, AC was favored in 75.7–79.5% of simulations for men, and in 97.0–98.3% of simulations for women.
Discussion
We found that AC resulted in a gain of up to 0.51 QALY’s in IPAH patients, and a loss of up to 1.89 QALY’s in CTD-PAH patients. Additionally, we observed that AC would need to demonstrate a HR of 0.95 or better on PAH disease mortality to be beneficial. These results were highly robust to variations in model parameters in IPAH females and CTD-PAH patients, and only exhibited minor uncertainty in IPAH males.
Current guidelines suggest that AC should be considered on a case-by-case basis, particularly in IPAH patients receiving therapy through a centrally placed catheter, and should be avoided in CTD-PAH patients.1,7 As a result, considerable uncertainty exists on the benefits of offering systemic AC to all IPAH patients without a secondary indication for AC, and recent registry data indicate that only the minority of IPAH patients are placed on systemic AC.10–14 Additionally, the benefits of systemic AC on PAH quality of life are not well defined, and recommendations for PAH therapy do not currently account for patient values and preferences as part of a shared decision-making model.15–17
Our model indicated that IPAH patients stand to benefit substantially from systemic AC, experiencing an increase of up to 0.51 QALY’s, regardless of gender or the presence of a centrally-placed catheter, and with a high degree of certainty based on the results of our probabilistic sensitivity analyses. These findings suggest that AC should be part of the medical management of pulmonary vascular disease in all IPAH patients, enhances quality-adjusted survival, and has a favorable effect on their disease that exceeds any decreased quality of life or survival from bleeding complications and the difficulties of managing daily VKA therapy. Additionally, when taken in the context of the recent guidelines for diagnosis and management of PAH patients, and PAH registry data, these results indicate that a stronger recommendation for systemic AC in IPAH patients (without a secondary indication for AC) should be considered, and there exists a sizeable proportion of IPAH patients that are not receiving potentially beneficial therapy.
Our results confirm that AC with VKA therapy may harm CTD-PAH patients, worsening their quality of life and survival through exacerbation of their PAH disease, an increase in bleeding complications, and the increased burden of taking systemic AC therapy. This corresponds with the most recent guidelines for diagnosis and treatment of PAH, which indicate that AC is harmful in CTD-PAH patients and should not be offered unless additional compelling medical indications exist for systemic AC therapy.
Although there was greater uncertainty about the net clinical benefit of AC as beneficial in men with IPAH, it was still favored in over 75% of all Monte Carlo simulations. This difference in results between men and women in our probabilistic analyses is likely due to wider confidence intervals in PAH mortality rates for male IPAH patients. Despite this increased uncertainty, the majority of simulations still favored AC in men with IPAH, and they also experienced a greater relative gain in QALY’s with AC than their female counterparts (13% increased QALY’s versus 9% in female IPAH patients), suggesting that men with IPAH may stand to benefit the most from systemic AC, and the benefits of AC in IPAH QALY’s are not gender-specific.
The major factor driving the benefit of AC in PAH patients without a defined secondary indication for AC was the effect of AC on the mortality rate of PAH itself. Our model estimated that a HR of ∼0.95 or greater would be sufficient to make AC the favored strategy in both CTD-PAH and IPAH patients, regardless of other patient factors such as gender or catheter-based prostacyclin therapy. From the literature on AC in PAH mortality, this effect size is highly plausible for IPAH patients, and was observed in the COMPERA (0.79, 95% CI: 0.66–0.94) registry, and falls securely within the 95% confidence interval of effect sizes seen in the REVEAL registry (1.42, 95% CI: 0.86–2.32). In stark contrast to this, our review of the literature indicates that such an effect would be exceedingly unlikely to be seen in CTD-PAH patients, with the REVEAL (1.42, 95% CI: 1.09–3.79) and COMPERA (1.82, 95% CI: 0.94–3.54) registries suggesting that CTD-PAH patients are likely to experience a worsening of their PAH disease while on AC. Not only does this support our conclusion that VKA therapy is likely to result in an improvement in QALY’s in IPAH patients, and is detrimental to CTD-PAH patients, it also provides a minimum effect size of AC on PAH mortality to incorporate into the design and analysis of any future studies on the role of AC (such as with newer oral agents) in PAH patients.9,15–17,61
Our model considered a number of complications related to systemic AC, including bleeding and thromboembolic events, as well as complications particularly relevant to PAH patients, such as the quality of life with catheter-based PAH therapy and the substantial mortality and morbidity of PE in PAH patients who have existing right heart dysfunction. Although we did not observe any clinically plausible thresholds in our deterministic sensitivity analyses, the incorporation of these parameters, their specific relevance to pulmonary vascular disease patients, and the use of a multidimensional outcome measure (QALYs) as the primary outcome of our model are particular strengths of our model. Given the importance of PAH patient perspectives and shared decision making, our model is also well situated to provide a foundation to develop a shared decision-making tool for AC strategies in PAH patients at the bedside.15–17
Our analysis also has a number of limitations. We only modeled the effects of VKA therapy, and the results of our model should not be extrapolated to assessing the risks or benefits of therapy with other systemic anticoagulants (such as direct oral anticoagulants or parenteral therapy). However, it is understood that direct oral anticoagulant therapy is likely to offer comparable or improved protection from thromboembolic events, as well as a more favorable safety profile regarding morbidity and mortality from major bleeding, as compared to VKA therapy, and may be more effective as systemic AC therapy in PAH patients.62–65 If we assume that direct oral anticoagulant therapy is comparable to VKA therapy in affecting the mortality of PAH disease, less burdensome to use than VKA therapy, and incorporating the estimations for the effectiveness of direct oral anticoagulant therapy as compared to VKA therapy in preventing thrombotic complications and resulting in major bleeding, we are able to estimate the potential effect of direct oral AC therapy on IPAH and CTD-PAH QALY’s. As expected, direct oral AC therapy remains favored in IPAH patients, offering a greater improvement in QALY’s (0.5–0.63), and shows the same detrimental effects in CTD-PAH patients, with a loss of between 0.66 and 1.88 QALY’s (online Table 1). If the same effects on PAH mortality are seen with direct oral AC, this therapy would be expected to offer improved quality of life in IPAH patients as compared to VKA therapy, and would be the favored strategy for oral AC in these patients.
We did not model the possibility that patients could suffer both DVT and develop atrial fibrillation in the same month. While this is highly unlikely, it could happen in a small proportion of patients. Given the limited life expectancy of PAH patients, we did not consider the possibility that patients could suffer a second stroke or PE, although this limitation likely under-estimated the benefit of systemic AC. We estimated the disease-specific PAH mortality rates from registry data, calibrating our model to the reported total all-cause mortality in IPAH and CTD-PAH patients; however, it is possible our estimates are not reflective of the true mortality rates. Additionally, our model does not account for the increasing rates of atrial fibrillation, stroke, and venous thromboembolism with advancing age, and although the majority of patients in our model did not survive beyond 10 years, we did not explicitly model the changing risks and benefits of systemic AC across the lifespan, and our models conclusions may not be applicable to particularly long-lived patients.
Our model assumes that death following PE or stroke occurs within the first month, and that survivors will recover to a new baseline quality of life after one month. This may not be the case in some patients who experience stroke and PE. We modeled the increased risk of OCP medication on thromboembolic disease as independent and additive to that from catheter-based therapy, but this is likely an oversimplification. Survivors of a major bleeding event were modeled as having a permanent decrease in quality of life. Although this may not be true for all patients, our sensitivity analyses indicated that major bleeding would need to decrease quality of life to an unrealistic 0.25 or less in order to affect the AC decision, and is unlikely to affect the overall conclusions of our model. Although it is well known that race affects PAH mortality, our model is constructed from data on Caucasian patients, which limits its generalizability to other racial groups.66
We assumed PAH patients had the lowest CHA2DS2VASc scores when modeling stroke risk, which may not hold true for all PAH patients, particularly those who are older, and have more cardiovascular risk factors like diabetes and valvular heart disease. It is well established that PAH patients with multiple medical comorbidities such as hypertension, diabetes, and chronic obstructive pulmonary disease have a higher risk of thromboembolic disease, a higher rate of stroke from atrial fibrillation, and a higher risk of death as compared to PAH patients without these conditions.67–70 Additionally, due to their comorbid conditions, many of these patients are also on antiplatelet therapy, which increases the risk of major bleeding when taken concomitantly with AC therapy.71–72 Extending our model to PAH patients with multiple medical comorbidities, we find that the benefits of systemic AC with VKA therapy in IPAH patients are attenuated (only resulting in a gain of 0.28–0.36 QALY’s), and the risks in CTD-PAH patients are diminished (resulting in a loss of only 0.66–1.17 QALY’s) (online Table 2). These changes to the risk-benefit profile of systemic AC in IPAH and CTD-PAH patient, when accounting for multiple medical comorbidities, reflects the complex interplay between benefits (protection from thrombotic events) and risks (increased bleeding risks) that our decision analytic model is designed to study, and further highlights the need for a shared decision-making tool for AC therapy that can be tailored to individual risks, benefits, and patient preferences.
Finally, although we performed one-way and probabilistic sensitivity analyses to assess the effects of uncertainty on our model conclusions, and our model results demonstrated a high degree of certainty, it is possible that in the future newer, more precise epidemiologic data may emerge that is significantly different from the data used to construct our model, which may significantly alter our model’s conclusions.
Conclusion
Systemic AC appears effective at improving outcomes and quality of life and should be offered as disease-modifying therapy to all IPAH patients, worsens quality-adjusted survival and should be avoided in all CTD-PAH patients, and must demonstrate a HR of 0.95 or better on PAH disease mortality to result in a net gain in QALY’s. Results of this analysis support a stronger recommendation for AC with VKA therapy in all IPAH patients without a secondary indication for AC, clarifies effect sizes that future studies would have to achieve in order to definitively demonstrate AC benefit in PAH patients, suggest that a sizeable proportion of patients currently living with IPAH patients may realize improved quality of life if treated with systemic AC, and provide the basis of future shared decision-making tools for clinical use in determining PAH therapeutic strategies at the bedside.
Supplemental Material
Supplemental material, PUL895451 Supplemental Material for Anticoagulation in pulmonary arterial hypertension: a decision analysis by Arun Jose, Mark H. Eckman and Jean M. Elwing in Pulmonary Circulation
Conflict of interest
The author(s) declare that there is no conflict of interest..
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Supplemental material
Supplemental material for this article is available online.
References
- 1.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 2.Montani D, Gunther S, Dorfmuller P, et al. Pulmonary arterial hypertension. Orphanet J Rare Dis 2013; 8: 1750–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olsson KM, Delcroix M, Ghofrani HA, et al. Anticoagulation and survival in pulmonary arterial hypertension: results from the Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension (COMPERA). Circulation 2014; 129: 57–65. [DOI] [PubMed] [Google Scholar]
- 4.Preston IR, Roberts KE, Miller DP, et al. Effect of warfarin treatment on survival of patients with pulmonary arterial hypertension (PAH) in the registry to evaluate early and long-term PAH disease management (REVEAL). Circulation 2015; 132: 2403–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wagenvoort CA, Wagenvoort N. Primary pulmonary hypertension. A pathologic study of the lung vessels in 156 clinically diagnosed cases. Circulation 1970; 42: 1163–1184. [Google Scholar]
- 6.Pietra GG, Edwards WD, Kay JM, et al. Histopathology of primary pulmonary hypertension. A qualitative and quantitative study of pulmonary blood vessels from 58 patients in the National Heart, Lung, and Blood institute primary pulmonary hypertension registry. Circulation 1989; 80: 1198–1206. [DOI] [PubMed] [Google Scholar]
- 7.Galie N, Channick RN, Frantz RP, et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J 2019; 53: pii: 1801889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sidney S, Petitti DB, Soff GA, et al. Venous thromboembolic disease in users of low-estrogen combined estrogen-progestin oral contraceptives. Contraception 2004; 70: 3–10. [DOI] [PubMed] [Google Scholar]
- 9.Oedingen C, Scholz S, Razum O. Systemic review and meta-analysis of the association of combined oral contraceptives on the risk of venous thromboembolism: the role of progestogen type and estrogen dose. Thromb Res 2018; 165: 68–78. [DOI] [PubMed] [Google Scholar]
- 10.Roldan T, Rios JJ, Villamanan E, et al. Complications associated with the use of oral anticoagulation in patients with pulmonary arterial hypertension from two referral centers. Pulm Circ 2017; 7: 692–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan MS, Usman MS, Siddiqui TJ, et al. Is anticoagulation beneficial in pulmonary arterial hypertension? Circ Cardiovasc Qual Outcomes 2018; 11: e004757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arvanitaki A, Boutsikou M, Anthi A, et al. Epidemiology and initial management of pulmonary arterial hypertension: real-world data from the Hellenic pulmonary hypertension registry (HOPE). Pulm Circ 2019; 9: 2045894019877157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gabriel L, Delavenne X, Bedouch P, et al. Risk of direct oral anticoagulant bioaccumulation in patients with pulmonary hypertension. Respiration 2016; 91: 307–315. [DOI] [PubMed] [Google Scholar]
- 14.Roldan T, Landzberg MJ, Deicicchi DJ, et al. Anticoagulation in patients with pulmonary arterial hypertension: an update on current knowledge. J Heart Lung Transplant 2016; 35: 151–164. [DOI] [PubMed] [Google Scholar]
- 15.Parikh KS, Gray MP, Rubin LJ, et al. Overdue to understand anticoagulation in pulmonary arterial hypertension. Pulm Circ 2018; 8: 2045893217743184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galie N, McLaughlin VV, Rubin LJ, et al. An overview of the 6th world symposium on Pulmonary Hypertension. Eur Respir J 2019; 53: pii: 1802148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mcgoon MD, Ferrari P, Armstrong I, et al. The importance of patient perspectives in pulmonary hypertension. Eur Respir J 2019; 53: 1801919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benza RL, Gomberg-Maitland M, Miller DP, et al. The REVEAL registry risk score calculator in patients newly diagnosed with pulmonary arterial hypertension. Chest 2012; 141: 354–362. [DOI] [PubMed] [Google Scholar]
- 19.McLaughlin VV, Shillington A, Rich S. Survival in primary pulmonary hypertension: the impact of epoprostenol therapy. Circulation 2002; 106: 1477–1482. [DOI] [PubMed] [Google Scholar]
- 20.Gall H, Felix JF, Schneck FK, et al. The Giessen pulmonary hypertension registry: survival in pulmonary hypertension subgroups. J Heart Lung Transplant 2017; 36: 957–967. [DOI] [PubMed] [Google Scholar]
- 21.Ruiz-Cano MJ, Escribano P, Alonso R, et al. Comparison of baseline characteristics and survival between patients with idiopathic and connective tissue disease-related pulmonary arterial hypertension. J Heart Lung Transplant 2009; 28: 621–627. [DOI] [PubMed] [Google Scholar]
- 22.Condliffe R, Howard LS. Connective tissue disease-associated pulmonary arterial hypertension. F1000Prime Rep 2015; 7: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawut S, Tacihman DB, Archer-Chicko C, et al. Hemodynamics and survival in patients with pulmonary arterial hypertension related to systemic sclerosis. Chest 2003; 123: 344–350. [DOI] [PubMed] [Google Scholar]
- 24.Lefevre G, Dauchet L, Hachulla E, et al. Survival and prognostic factors in systemic sclerosis-associated pulmonary hypertension: a systematic review and meta-analysis. Arthritis Rheum 2013; 65: 2413–2423. [DOI] [PubMed] [Google Scholar]
- 25.Benza RL, Miler DP, Gomberg-Maitland M, et al. Predicting survival in pulmonary arterial hypertension: insights from the registry to evaluate early and long-term pulmonary arterial hypertension disease management (REVEAL). Circulation 2010; 122: 164–172. [DOI] [PubMed] [Google Scholar]
- 26.Mair KM, Johansen AKZ, Wright AF, et al. Pulmonary arterial hypertension: basis of sex differences in incidence and treatment response. Br J Pharmacol 2014; 171: 567–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chopra V, Anand S, Hickner A, et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta-analysis. Lancet 2013; 382: 311–325. [DOI] [PubMed] [Google Scholar]
- 28.Kirkpatrick A, Rathbun S, Whitsett T, et al. Prevention of central venous catheter-associated thrombosis: a meta-analysis. Am J Med 2007; 120: 901.e1–e13. [DOI] [PubMed] [Google Scholar]
- 29.Prandoni P, Polistena P, Bernadi E, et al. Upper-extremity deep vein thrombosis: risk factors, diagnosis, and complications. Arch Intern Med 1997; 157: 57–62. [PubMed] [Google Scholar]
- 30.Monreal M, Ruiz J, Olazabal A, et al. Deep venous thrombosis and the risk of pulmonary embolism. A systematic study. Chest 1992; 102: 677–681. [DOI] [PubMed] [Google Scholar]
- 31.Ikhlague N, Seshadri V, Kathula S, et al. Efficacy of prophylactic warfarin for prevention of thalidomide-related deep vein thrombosis. Am J Hematol 2006; 81: 420–422. [DOI] [PubMed] [Google Scholar]
- 32.Quezada CA, Bikdeli B, Barrios D, et al. Meta-analysis of prevalence and short-term prognosis of hemodynamically unstable patients with symptomatic acute pulmonary embolism. Am J Cardiol 2019; 123: 684–689. [DOI] [PubMed] [Google Scholar]
- 33.Olsson KM, Jais X. Birth control and pregnancy management in pulmonary hypertension. Semin Respir Crit Care Med 2013; 34: 681–688. [DOI] [PubMed] [Google Scholar]
- 34.Lai YC, Potoka KC, Champion HC, et al. Pulmonary arterial hypertension: the clinical syndrome. Circ Res 2014; 115: 115–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Segeman BH, de Bastos M, Rosendaal FR, et al. Different combined oral contraceptives and the risk of venous thrombosis: systematic review and network meta-analysis. BMJ 2013; 347: f5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roach REJ, Cannegieter SC, Lijfering WM. Differential risks in men and women for first and recurrent venous thrombosis: the role of genes and environment. J Thromb Haemost 2014; 13: 886–887. [DOI] [PubMed] [Google Scholar]
- 37.Prencipe M, Culasso F, Rasura M, et al. Long-term prognosis after a minor stroke: 10-year mortality and major stroke recurrence rates in a hospital-based cohort. Stroke 1998; 29: 126–132. [DOI] [PubMed] [Google Scholar]
- 38.Feigin VL, Lawes CM, Bennett DA, et al. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol 2003; 2: 43–53. [DOI] [PubMed] [Google Scholar]
- 39.Ridker PM, Goldhaber SZ, Danielson E, et al. Long-term, low-intensity warfarin therapy for the prevention of recurrent venous thromboembolism. N Engl J Med 2003; 348: 1425–1434. [DOI] [PubMed] [Google Scholar]
- 40.Agarwal S, Hachamovitch R, Menon V. Current trial-associated outcomes with warfarin in prevention of stroke in patients with nonvalvular atrial fibrillation: a meta-analysis. Arch Intern Med 2012; 172: 623–631. [DOI] [PubMed] [Google Scholar]
- 41.Wanamaker B, Cascino T, McLaughlin V, et al. Atrial arrhythmias in pulmonary hypertension: pathogenesis, prognosis, and management. Arrhythm Electrophysiol Rev 2018; 7: 43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gundland A, Xian Y, Peterson ED. Prestroke and poststroke antithrombotic therapy in patients with atrial fibrillation: results from a nationwide cohort. JAMA Netw Open 2018; 1: e180171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lip GYH, Skjoth F, Rasmussen LH, et al. Oral anticoagulation, aspirin, or no therapy in patients with nonvalvular AF with 0 or 1 stroke risk factor based on the CHA2DS2-VASc Score. J Am Coll Cardiol 2015; 65: 1385–1394. [DOI] [PubMed] [Google Scholar]
- 44.Jackson LR, 2nd, Kim S, Fonarow GC, et al. Stroke risk and treatment in patients with atrial fibrillation and low CHA2DS2-VASc Scores: findings from the ORBIT-AF 1 and II Registries. J Am Heart Assoc 2018; 7: e008764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Friberg L, Rosenqvist M, Lip GY. Net clinical benefit of warfarin in patients with atrial fibrillation: a report from the Swedish atrial fibrillation cohort study. Circulation 2012; 125: 2298–2307. [DOI] [PubMed] [Google Scholar]
- 46.Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010; 137: 263–272. [DOI] [PubMed] [Google Scholar]
- 47.Friberg L, Rosenqvist M, Lip GY. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J 2012; 33: 1500–1510. [DOI] [PubMed] [Google Scholar]
- 48.Gomez-Outes A, Lagunar-Ruiz J, Terleira-Fernandez A, et al. Causes of death in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol 2016; 68: 2508–2521. [DOI] [PubMed] [Google Scholar]
- 49.Roskell NS, Samuel M, Noack H, et al. Major bleeding in patients with atrial fibrillation receiving vitamin K antagonists: a systematic review of randomized and observational studies. Europace 2013; 15: 787–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carrier M, Le Gal G, Wells PS, et al. Systematic review: case-fatality rates of recurrent venous thromboembolism and major bleeding events among patients treated for venous thromboembolism. Ann Intern Med 2010; 152: 578–589. [DOI] [PubMed] [Google Scholar]
- 51.Eckman MH, Singer DE, Rosand J, et al. Moving the tipping point: the decision to anticoagulate patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes 2012; 4: 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gage BF, Cardinalli AB, Owens DK. The effect of stroke and stroke prophylaxis with aspirin or warfarin on quality of life. Arch Intern Med 1996; 156: 1829–1836. [PubMed] [Google Scholar]
- 53.Tavoly M, Utne KK, Jelsness-Jorgenson L, et al. Health-related quality of life after pulmonary embolism: a cross-sectional study. BMJ Open 2016; 6: e013086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klok FA, van Kralingren KW, van Djik AP, et al. Quality of life in long-term survivors of acute pulmonary embolism. Chest 2010; 138: 1432–1440. [DOI] [PubMed] [Google Scholar]
- 55.Garin MC, Clark L, Chumney EC, et al. Cost-utility of treatments for pulmonary arterial hypertension: a Markov state-transition decision analysis model. Clin Drug Investig 2009; 29: 635–646. [DOI] [PubMed] [Google Scholar]
- 56.Coyle K, Coyle D, Blouin J, et al. Cost effectiveness of first-line oral therapies for pulmonary arterial hypertension: a modeling study. Pharmacoeconomics 2016; 34: 509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Homma S, Sacco RL. Patent foramen ovale and stroke. Circulation 2005; 112: 1063–1072. [DOI] [PubMed] [Google Scholar]
- 58.Mojadidi MK, Zaman MO, Elgendy IY, et al. Cryptogenic stroke and patent foramen ovale. J Am Coll Cardiol 2018; 71: 1035–1043. [DOI] [PubMed] [Google Scholar]
- 59.Gallo de Morales G, Vakil A, Moua T. Patent foramen ovale in idiopathic pulmonary arterial hypertension: long-term risk and morbidity. Respir Med 2016; 118: 53–57. [DOI] [PubMed] [Google Scholar]
- 60.Doubilet P, Begg CB, Wienstein MC, et al. Probabilistic sensitivity analysis using Monte Carlo simulation. A practical approach. Med Decis Making 1985; 5: 157–177. [DOI] [PubMed] [Google Scholar]
- 61.Olschewski H, Rich S. Are anticoagulants still indicated in pulmonary arterial hypertension? Pulm Circ 2018; 8: 2045894018807681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Granger CB, Alexander JH, McMurray JJV, et al. Apixiban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365: 981–992. [DOI] [PubMed] [Google Scholar]
- 63.Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomized trials. Lancet 2014; 383: 15–21. [DOI] [PubMed] [Google Scholar]
- 64.Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009; 361: 2342–2352. [DOI] [PubMed] [Google Scholar]
- 65.Margelidon-Cozzolino V, Delavenne X, Catella-Chatron J, et al. Indications and potential pitfalls of anticoagulants in pulmonary hypertension: would DOACs become a better option than VKAs? Blood Rev Elsevier 2019; 37: 100579. [DOI] [PubMed] [Google Scholar]
- 66.Al-Naamani N, Paulus JK, Roberts KE. Racial and ethnic differences in pulmonary arterial hypertension. Pulm Circ 2017; 7: 793–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Poms AD, Turner M, Farber HW, et al. Comorbid conditions and outcomes in patients with pulmonary arterial hypertension: a REVEAL registry analysis. Chest 2013; 144: 169–176. [DOI] [PubMed] [Google Scholar]
- 68.McGoon MD, Benza RL, Escribano-Subias P, et al. Pulmonary arterial hypertension: epidemiology and registries. J Am Coll Cardiol 2013; 62: D51–D59. [DOI] [PubMed] [Google Scholar]
- 69.Chung WS, Lin CL, Kao CH. Diabetes increases the risk of deep-vein thrombosis and pulmonary embolism: a population-based cohort study. Thromb Haemost 2015; 114: 812–818. [DOI] [PubMed] [Google Scholar]
- 70.Ambrosetti M, Ageno W, Spanevello A, et al. Prevalence and prevention of venous thromboembolism in patients with acute exacerbations of COPD. Thromb Res 2003; 112: 203–207. [DOI] [PubMed] [Google Scholar]
- 71.Paikin JS, Wright DS, Eikelbloom JW. Effectiveness and safety of combined antiplatelet and anticoagulant therapy: a critical review of the evidence from randomized controlled trials. Blood Rev 2011; 25: 123–129. [DOI] [PubMed] [Google Scholar]
- 72.Melkonian M, Jarzebowski W, Pautas E, et al. Bleeding risk of antiplatelet drugs compared with oral anticoagulants in older patients with atrial fibrillation: a systematic review and meta-analysis. J Thromb Haemost 2017; 15: 1500–1510. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, PUL895451 Supplemental Material for Anticoagulation in pulmonary arterial hypertension: a decision analysis by Arun Jose, Mark H. Eckman and Jean M. Elwing in Pulmonary Circulation