Abstract
Traumatic brain injury (TBI) is the leading cause of death in children and adolescents in developed countries, but there are no blood-based biomarkers to support the diagnosis or prognosis of pediatric TBI to-date. Here we report that the plasma levels of osteopontin (OPN), a phosphoprotein chiefly secreted by macrophages and/or activated microglia, may contribute to this goal. In animal models of TBI, while OPN, fibrillary acidic protein (GFAP), and matrix metalloproteinase 9 (MMP-9) were all readily induced by controlled cortical impact in the brains of one-month-old mice, only OPN and GFAP ascended in the blood in correlation with high neurological severity scores (NSS). In children with TBI (three to nine years of age, n = 66), the plasma levels of OPN, but not GFAP, correlated with severe TBI (Glasgow Coma Score ≤ 8) and intracranial lesions at emergency department. In addition, the plasma OPN levels in severe pediatric TBI patients continued to ascend for 72 h and correlated with mortality and the days requiring ventilator or intensive care unit support, whereas the plasma GFAP levels lacked these properties. Together, these results suggest that plasma OPN outperforms GFAP and may be a neuroinflammation-based diagnostic and prognostic biomarker in pediatric TBI.
Keywords: Diagnostic biomarker, glial fibrillary acidic protein, microglia, outcomes, predictive biomarker
Introduction
Head trauma is the leading cause of death and disability in children. In 2013, there were nearly 2.8 million TBI-related emergency department visits, hospitalization, and deaths in the United States.1 Among them, 23.6% fell into the 0–14 years of age group. Because the adult and developing brains have different physiological and metabolic properties, unique guidelines are recommended for the clinical management of pediatric TBI.2,3 For example, although many studies of adult TBI indicated a tight correlation between elevated plasma GFAP levels and computer tomography (CT)-evidenced intracranial lesions at emergency department, this association is missing in children with TBI.4,5 Accordingly, there are no blood-based biomarkers to-date to assist the diagnosis and prognosis in pediatric TBI.
The current search for TBI blood biomarkers emphasizes neuron or astrocyte-specific proteins released to the blood after brain damage.6 In spite of a sound rationale, the brain-to-blood passage of neuron/astrocyte-specific markers partially depends on the glymphatic transport, which could be impaired by intracranial pressure or clinical treatments of TBI.7 Moreover, the stability of these neuron or astrocyte-specific proteins in blood is uncertain. These factors may explain why the plasma GFAP levels typically peaks within 24 h of TBI-onset, making it less useful to monitor the progression of brain damage.6 Given these considerations, we decided to investigate the utility of osteopontin (OPN) as a novel plasma biomarker in pediatric TBI.
OPN, also called secreted phosphoprotein 1 (SPP1), belongs to the small integrin-binding ligand N-link glycoprotein (SIBLINGs) family and has a high stability in the blood and saliva.8–10 OPN exists in a negligible quantity in healthy brains, but it is quickly up-regulated in macrophages and/or activated microglia in a multitude of brain pathologies, including neonatal hypoxia-ischemia, stroke, electrolytic lesion, TBI, and the Alzheimer’s disease models.11–17 We hypothesized that OPN may be a good blood biomarker in pediatric TBI, given the important role of neuroinflammation in TBI, generally healthy conditions in pre-TBI children (hence a low background of plasma OPN), integrin-mediated brain-to-blood transport of OPN, and high stability of OPN in biofluids (Figure 1(a)).18 Moreover, a recent conference abstract reported more than 10-fold increase of OPN in the cerebrospinal fluid in adults TBI patients.19 This report also suggested the utility of OPN as a blood biomarker in TBI.
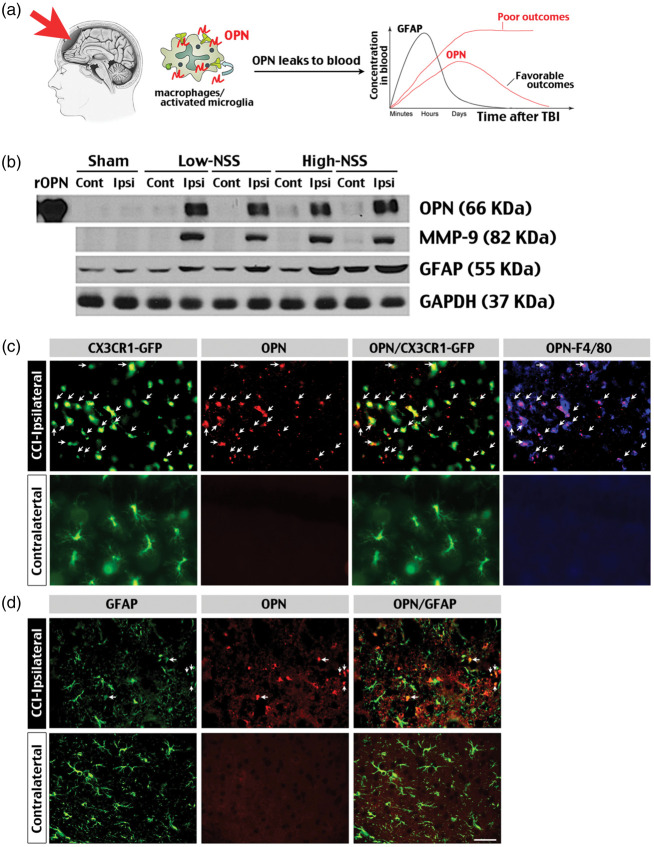
Figure 1.
Induction of brain injury markers after controlled cortical impact (CCI) in juvenile mice. (a) Schematic diagram of using plasma OPN levels to predict the severity and outcomes of TBI. We hypothesized that plasma OPN levels may ascend faster/higher and last longer in more severe pediatric TBI. (b) Immunoblot analysis demonstrated sharp induction of OPN, MMP-9 and GFAP in the ipsilateral hemisphere of one-month-old mice with either a low (≤2) or high (3–4) neurologic severity score (NSS)21 at 48 h after CCI. Shown are the representative results for 8 low-NSS and 4 high-NSS mice. Shams were age-matched mice subjected to craniotomy without CCI. rOPN: mouse recombinant OPN used as positive controls. (c) Immunostaining showed induction of OPN in GFP- and F4/80-positive, activated microglia/macrophages (arrows) in the ipsilateral, but not contralateral hemisphere of CX3CR1GFP/+ mice at 48 h post-CCI. (d) In contrast, few anti-OPN immunosignals were detected in GFAP-positive astrocytes in the ipsilateral or contralateral hemisphere after CCI. Shown are the representative images in n = 8 mice. Scale bar: 20 µm.
Materials and methods
Controlled cortical impact model in mice
The controlled cortical impact (CCI) model was established in juvenile (four-week-old) male C57BL/6 or CX3CR1 mice using an electromagnetic device (Impact One, Leica Biosystems), as previously described.17 Briefly, the mouse was anesthetized by 2% isoflurane, and the body temperature was maintained at 37℃ during surgery. A central incision was made to expose the skull. A 4 mm-diameter circular craniotomy at 0.5 mm from the left side of midline in the center between the Lambda and Bregma was made to expose the dura. The integrity of dura was examined and the dura-damaged animals were excluded; 24 mice entered the experiment and 23 mice completed the surgery with an intact dura. All CCI-injured mice were included for immunoblot and immunostaining analysis. The CCI parameters were velocity at 3 m/s, compression time at 500 ms, and deformation depth at 2 mm. The blood following impact was cleaned and the wound was closed with tissue glue. For shams, craniotomy was made with an intact dura. The body temperature of mice was maintained at 37℃ after surgery until recovery from anesthesia. After CCI, mice were examined daily and assessed for the neurologic severity score (NSS), as previously described.18 Briefly, one point each was given to inability to exit from a circle of 50 cm in diameter when placed in the center for 30 min, loss of righting reflex when left on its back for 30 min, loss of seeking behavior, and hemiplegia or hemiparesis. NSS ≤ 2 at 48 h post-CCI was classified as low-NSS, and NSS of 3–4 was classified as high-NSS. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) and conducted according to the National Institutes of Health Guide for Care and Use of Laboratory Animals. Experiments are performed and reported in accordance with the ARRIVE (Animal Research: Reporting in vivo Experiments) guidelines (https://www.nc3rs.org.uk/arrive-guidelines).
Immuno-florescence staining and immunoblotting analysis
All animals were perfused and sacrificed at 48 h post-surgery for tissue collection. Saline perfusion was used for immunoblotting, and 4% paraformaldehyde was perfused for immunohistochemistry. Immunohistochemistry and immunoblotting were performed, as previously published.14 Semi-quantification of immunoblotting was performed using the ImageJ software (NIH, Bethesda, MD). The following antibodies and working dilution were used in immunofluorescence staining: goat anti-OPN (1:100, R&D), anti-goat 588 (1:500, Biolegend), rat anti-F4/80 (1:100, Bio-Rad), biotinylated rat IgG (1:250, Biolegend), Alexa Fluor 647 streptavidin (1:500 Biolegend). The following antibodies and working dilution were used in immunoblotting: goat anti-OPN (1:1000, R&D), goat anti-MMP9 (1:1000, Sigma), rabbit anti-GFAP (1:10,000, Abcam), rabbit anti-actin (1:10,000, Sigma), rabbit anti-transferrin (1:10,000, Sigma); 0.2 µg of mouse recombinant OPN (R&D) was used as positive control in immunoblots.
Luminex assay in mice
Mouse plasma was collected using heparin as an anticoagulant, and centrifuged for 20 min at 1000 × g at 2–8℃ within 30 min. The plasma was diluted 50 times with the buffer in luminex kit (R&D). The luminex bead-based ELISA was assayed and analyzed using a Bio-Plex system (Bio-Rad).
Statistical analysis in animal studies
All data were analyzed using the GraphPad prism 7 analytical software. The OPN and GFAP levels between two groups were compared using the Mann–Whitney test, and p < 0.05 was considered statistically significant. The prognostic value of OPN or GFAP was analyzed using the receiver operating characteristic curve (ROC), as previously described.19 A p-value <0.05 and AUC of 0.7–0.8 was valued as adequate accuracy in diagnosis.
Human study population
The institutional ethnic committee and Institutional Review Board (IRB) of Children’s Healthcare of Atlanta (CHOA) approved this study under the guidelines of the Helsinki Declaration of 1975/1983. Written informed consent was obtained from parents of patients. Subjects were patients between 0 and 18 years of age and brought to the Emergency Department (ED) at the CHOA Scottish Rite and Egleston hospitals with a diagnosis of TBI made by a medical professional. The blood samples TBI patients were collected within 1 h upon arrival, and immediately processed to separate and freeze the plasma fraction. All levels of Glasgow Coma Score (GCS) were eligible, and patients were classified as mild TBI (GCS 13–15), moderate TBI (9–12), or severe TBI (GCS 3–8). The exclusion criteria were children outside the age parameters or had a non-traumatic head injury or medical illness.
ELISA assay of plasma OPN and GFAP in patients
According to the manufacturer’s protocol, the human plasma samples were diluted 25 times with the dilution buffer provided in the kit. If the ELISA readouts of diluted samples were still higher than the assay range, these samples were further diluted until covered by the calibration range. Human plasma samples were analyzed in duplicate using commercial ELISA kits (R&D).
Statistical analysis of clinical data
All data were analyzed using the GraphPad prism 7 analytical software. Levels of OPN or GFAP between two groups were compared with the Mann–Whitney test, and p-value <0.05 was considered statistically significant. Correlation of OPN or GFAP with short-term outcomes (the in-ICU or on-ventilator days) was analyzed using Spearman’s rank correlation test.
Results
Comparison of OPN, GFAP, and MMP-9 in the brain and blood in the CCI model
To examine the possibility of OPN induction after TBI, first we applied CCI to male one-month-old CX3CR1GFP/+ mice, in which microglia and macrophages are tagged by the green fluorescence protein (GFP).20 Immunoblots showed clear induction of OPN and MMP-9, and to a lesser degree GFAP, in the ipsilateral hemisphere of CCI-injured mice that presented either a low (≤2, n = 8) or high (3–4, n = 4) NSS at 48-h recovery (n = 12 total).21 Importantly, no induction of OPN or MMP-9 was detected in the contralateral hemisphere (Figure 1(b)). Similarly, immunostaining showed restricted induction of OPN in the ipsilateral, but not the contralateral hemisphere in CCI-injured CX3CR1GFP/+ mice (Figure 1(c) and (d); n = 4 for each). The anti-OPN immunosignals were mostly detected in GFP- and F4/80-double-positive-activated microglia/macrophages rather than GFAP-positive astrocytes (arrows in Figure 1(c) and (d)). These data suggest that OPN and MMP-9 and GFAP are all robust brain biomarkers in juvenile CCI.
However, immunoblot analysis of the plasma in this CX3CR1GFP/+ mouse cohort indicated selective induction of OPN and GFAP, but not MMP-9, at 48 h post-CCI (Figure 2(a), n = 8 for low-NSS and n = 4 for high-NSS). Quantification confirmed significant increase of plasma OPN (p < 0.01) and GFAP (p < 0.05) in the CCI-injured mice showing high over low NSS (Figure 2(b)). This pattern of high NSS-specific induction of plasma OPN was corroborated by the enzyme-linked immunosorbent assay in post-CCI mice (Figure 2(c), n = 12 total for post-TBI groups). These results raised the possibility that higher plasma OPN levels may indicate more severe brain damage and/or unfavorable outcomes in children with TBI.
Figure 2.

Induction of the plasma OPN and GFAP after CCI in juvenile mice. (a) Immunoblotting showed induction of plasma OPN and GFAP, but not MMP-9, in CCI-injured one-month-old mice with a high- (n = 4), but not low-NSS (n = 8), at 48-h recovery. Shown are the results of three representative low-NSS and high-NSS mice. rOPN: mouse recombinant OPN used as positive controls. (b) Quantification of immunoblot signals revealed a significantly higher level of plasma OPN and GFAP in high-NSS than in low-NSS mice. (c) ELISA (Luminex) corroborated significant induction of plasma OPN levels in high-NSS mice compared with low-NSS mice at 48 h post after CCI (n = 12 total). The p-value was determined by t-test.
Comparison of plasma OPN and GFAP in the acute phase of pediatric TBI
To test this hypothesis, we compared the plasma OPN and GFAP levels in 66 TBI-injured children with or without CT-evidence of intracranial lesions at admission. Our cohort comprises 50 severe TBI (GCS: 3–8; 7.3 ± 0.7 years of age), 5 moderate TBI (GCS: 9–12; 5.9 ± 3.1 years), and 11 mild TBI patients (GCS: 13–15; 7.2 ± 1.4 years) (Table 1). Our analysis showed that the initial plasma OPN levels in children showing intracranial lesions (n = 46, one severe TBI case did not receive CT scan) were significantly higher than those with negative CT findings at admission (n = 19) (Figure 3(a), p = 0.006 by t-test). In contrast, the initial plasma GFAP levels were indistinguishable between those with and without CT evidence of intracranial lesions (p = 0.07), similar to the finding of a recent study.5 In addition, the initial plasma OPN levels were higher in children with severe TBI than those with mild TBI (Figure 3(b), p = 0.02 by t-test), whereas the plasma GFAP levels were similar between the severe- and mild-TBI groups (p = 0.75). The ROC graph analysis confirmed higher accuracy of using the initial plasma OPN level to predict severe TBI (GCS 3–8) than the plasma GFAP level; the area under ROC curve for OPN was 0.7255 (p = 0.02), but only 0.533 for GFAP (p = 0.74, Figure 3(b)). These results suggest that plasma OPN is a better predictor of acute pediatric TBI severity than GFAP.
Table 1.
Demographic and clinical characteristics of the study population.
| Characteristics | Severe TBI | Moderate TBI | Mild TBI |
|---|---|---|---|
| (GCS 3–8) | (GCS 9–12) | (GCS 13–15) | |
| Patients (n) | 50 | 5 | 11 |
| Deceased (n) | 5 | 0 | 0 |
| Gender, n (%) | |||
| Male | 31 (62%) | 4 (80%) | 6 (55%) |
| Female | 19 (38%) | 1 (20%) | 5 (45%) |
| Race, n (%) | |||
| African American | 23 (46%) | 4 (80%) | 4 (36%) |
| Caucasian | 20 (40%) | 0 | 7 (64%) |
| White | 2 (4%) | 0 | 0 |
| Asian | 2 (4%) | 1 (20%) | 0 |
| Unknown | 3 (6%) | 0 | 0 |
| Cause of injury, n (%) | |||
| Traffic incidences | 21 (42%) | 0 | 5 (46%) |
| Incidental fall | 15 (30%) | 1 (20%) | 2 (18%) |
| Violence/assault | 3 (6%) | 0 | 0 |
| Gunshot | 3 (6%) | 1 (20%) | 0 |
| Sport injury | 1 (2%) | 2 (40%) | 1 (9%) |
| Other nonintentional | 3 (6%) | 0 | 2 (18%) |
| Other/unknown | 4 (8%) | 1 (20%) | 1 (9%) |
| CT findings, n (%) | |||
| Intracranial lesions | 40 (80%) | 4 (80%) | 2 (18%) |
| Skull fracture only | 3 (6%) | 0 | 2 (18%) |
| Negative CT | 6 (12%) | 1 (20%) | 7 (64%) |
GCS: Glasgow coma score; TBI: traumatic brain injury
Figure 3.
Prediction of pediatric severe TBI using the plasma OPN and GFAP levels at admission. (a) Scatter plot of the plasma OPN and GFAP levels at admission in pediatric TBI patients with and without intracranial lesions on CT. The p-value was determined using Mann–Whitney test between 19 CT-negative and 46 CT-positive subjects. (b) Scatter plot of the plasma OPN and GFAP levels in children suffered from mild (GCS 13–15, n = 11), moderate (GCS 9–12, n = 5) or severe TBI (GCS 3–8, n = 50) at admission. The p-value was determined using Mann–Whitney test. The receiver operating characteristic (ROC) graph of using the plasma OPN or GFAP levels at admission to predict severe TBI.22 The area under ROC curve is 0.7255 for OPN (p = 0.02) compared with 0.533 for GFAP (p = 0.7435).
Correlation of clinical course with the peak plasma OPN and GFAP levels within 72 h of TBI
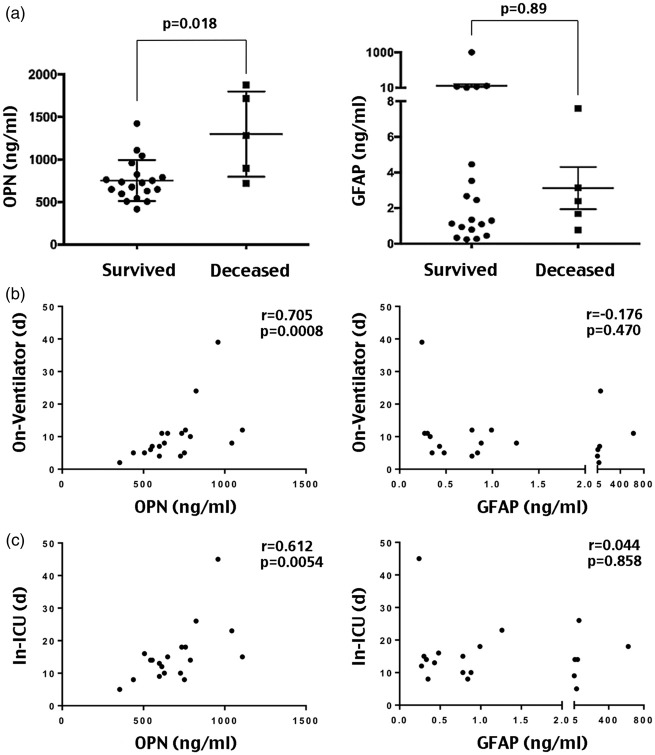
We also collected serial blood samples (at admission, 24, 48, and 72 h of hospitalization) from the severe TBI patients in our cohort (n = 24), from which five children deceased after 72 h. We found that the plasma OPN levels of those later deceased were significantly higher than those survived at 72 h of hospitalization (Figure 4(a), p = 0.018 by t-test), although they were similar at admission and at 24 and 48 h. In contrast, the plasma GFAP levels in children that later deceased and the survivals were inseparable from admission to 72 h of hospitalization (Figure 4(a), p = 0.89 by t-test). These results suggest that the trajectory of plasma OPN levels may predict the outcomes in pediatric TBI.
Figure 4.
Correlation of the peak plasma OPN and GFAP levels within 72 h of hospitalization with mortality and the length of on-ventilator or in-intensive care unit (ICU) in children with severe TBI. (a) Comparison of the highest plasma OPN or GFAP levels within 72 h of hospitalization between children with severe TBI that survived (n = 19) or later deceased (n = 5). The p-value was determined by Mann–Whitney test. (b) Correlation analysis of the highest plasma OPN or GFAP levels within 72 h of hospitalization with the eventual days that required ventilator support during hospitalization in children with severe TBI (n = 19). The Spearman’s rank correlation coefficient (r) is 0.705 for OPN (p = 0.0008), and −0.176 for GFAP (p = 0.470). (c) In the same cohort of pediatric severe TBI, the Spearman’s rank correlation coefficient (r) between the highest plasma OPN levels within 72 h of TBI-onset and the in-ICU days is 0.612 for OPN (p = 0.0054) versus 0.0440 for GFAP (p = 0.858).
Therefore, we plotted the highest plasma OPN and GFAP levels within 72 h of admission against the days requiring on-ventilator or in-intensive care unit (ICU) support, as the measurements for short-term outcomes, in 19 severe TBI-injured children (excluding the five patients that deceased after 72 h). This analysis showed significant correlation between the peak plasma OPN levels and the duration requiring ventilator or ICU support. The Spearman’s rank coefficient (r) between the peak plasma OPN levels and the on-ventilator days was 0.705 (Figure 4(b), p = 0.0008), and 0.6112 for in-ICU days (Figure 4(c), p = 0.0054). In contrast, the peak plasma GFAP levels within 72 h of hospitalization were poorly correlated with the days requiring ventilator or ICU support (p = 0.470 and p = 0.858, respectively). Thus, the plasma OPN levels also outperformed GFAP levels to predict short-term outcomes of severe pediatric TBI in our cohort.
Discussion
Current management of TBI uses radiographic imaging and neurological examinations to predict and monitor the severity of brain injury, without the assistance by simple laboratory tests. Blood-based biomarkers that correlate with the clinical severity and progression of TBI would assist acute triage, early intervention of complications, and rehabilitation planning.3,6 The need for TBI blood biomarkers is particularly pressing in pediatrics, because children in the 0–4, 5–14, or 15–24 years groups have the highest rates of TBI-related emergency department visits (2–4 times higher than those in the 25–44 years group).1 In addition, many blood biomarkers with demonstrated utility in adult TBI, such as GFAP, have been shown to be less useful in the pediatric population.4,5
Given the critical role of neuroinflammation in TBI, we reasoned that the proteins secreted by active microglia/macrophages, efficiently passing the blood–brain barrier, and possessing a high stability in body fluids may serve as plasma biomarkers in pediatric TBI. In particular, OPN is a good candidate with several unique properties. First, the brain OPN level is negligible in healthy subjects, but readily up-regulated by activated microglia and macrophages in multiple neurological conditions, including neonatal hypoxia-ischemia, stroke, electrolytic lesion, TBI, and Alzheimer’s disease models, although the functions of OPN in brain injury remain uncertain.11–17 Second, OPN exhibits efficient brain-to-blood transport, presumably due to its integrin-binding property, and a high stability in the blood and saliva.8,9,10,14 Third, perhaps except for the shaken baby syndrome, most pediatric TBI patients have healthy brains prior to the incident, which may provide a greater signal-to-noise ratio for plasma OPN after TBI. Finally, it has been shown that the elevated plasma OPN derives from microglia and correlates with the severity of brain damage in neonatal hypoxic-ischemic injury models.14 Together, these unique attributes suggest that OPN may be a good plasma biomarker in pediatric TBI.
Indeed, the present study provides two sets of evidence (from preclinical and clinical results) to support this hypothesis. In preclinical experiments, we showed that while OPN and MMP-9 and GFAP were all sensitive brain biomarkers in TBI-injured juvenile mice (Figure 1), only OPN and to a lesser degree GFAP rose in blood and correlated with a high neurological severity score at 48 h after TBI (Figure 2). The neurological severity score in the present study is a short form compared with other similar studies.21 The somewhat variable neurological severity following the same CCI parameters may be partially due to the younger age of mice (four-week-old) in our study. Although the dynamics of plasma OPN induction after experimental TBI is yet to be fully characterized (since we only examined the brain and plasma OPN levels at 48 h after CCI), our results nonetheless suggest that OPN may be a better blood biomarker than GFAP in pediatric TBI.
Consistent with this notion, our analysis of the archived samples from 66 post-TBI children showed that the initial plasma OPN level outperforms GFAP for predicting severe TBI (GCS ≤ 8) and CT-evidenced intracranial lesion (Figure 3). In fact, the area under ROC curve between plasma GFAP and severe TBI was 0.533 (p = 0.74), a value that is close to no discrimination, in our cohorts (Figure 3(b)). Close inspection indicated that the plasma GFAP levels are relatively low (<10 ng/ml) and have larger variation among individuals, similar to the findings in a recent study,5 which may have contributed to its poor diagnostic power. Our results also showed that the peak plasma OPN level within 72 h of TBI onset has greater correlation with mortality and the length requiring ventilator or ICU support in hospitalization than the peak plasma GFAP level in the same period (Figure 4). These results suggest that plasma OPN level is a useful biomarker to assist the diagnosis and predict the outcomes in pediatric TBI.
Our study, however, has the following limitations. First, the present study was conducted in one medical center using 66 archived plasma samples. A larger (multi-center) prospective study is required to confirm our findings. For example, the diagnostic utility of plasma GFAP in adult TBI was initially hinted in a small cohort and later confirmed by the Transforming Research and Clinical Knowledge in Traumatic Brain Injury Study (TRACK-TBI) that involved 215 cases.4 Second, our retrospective study only used on-ventilator and in-ICU days as the objective short-term outcomes. In future studies, extended monitoring beyond 72 h of TBI onset and the use of Glasgow Outcome Scale-Extended (GOSE) at six months of recovery will be important to ensure the prognostic value of plasma OPN. Finally, we suggest that the utility of OPN as a blood biomarker in adult TBI, either singularly or in combination with other indicators, should be considered.
In summary, the advantage of OPN as a blood biomarker in pediatric TBI may derive from its strong induction by activated microglia/macrophages, efficient brain-to-blood transport, and high stability in biofluids. Given these unique attributes, the diagnostic and predictive value of plasma OPN in pediatric TBI warrants further investigation.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Institute of Health grants (NS084744, NS095064, NS093446, and HD080429 to C-Y.K. and NS103597 to A.R.) and a Children’s Healthcare of Atlanta (CHOA) Friends Research Grant to A.R.
Acknowledgements
We thank Dr. Ton DeGrauw for encouragement and advice throughout this study, Dr. Beverly Rogers for laboratory support, Ms. Blaire Holbrook for logistical support, and Ms. Beena Desai for administrative coordination.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
AR, C-YK and NG designed the study; NG, X-Z-B, BW and IS performed the experiments; JJC and AR collected clinical samples; LSB collected and analyzed the clinical history of patients; AR, C-YK, NG and LSB analyzed the data; NG, C-YK and AR drafted the manuscript; and all authors reviewed the final draft of the manuscript.
References
- 1.Taylor CA, Bell JM, Breiding MJ, et al. Traumatic Brain Injury-Related Emergency Department Visits, Hospitalizations, and Deaths – United States, 2007 and 2013. MMWR Surveill Summ 2017; 66: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giza C, Mink R, Madikians A. Pediatric traumatic brain injury: not just little adults. Curr Opin Crit Care 2007; 13: 143–152. [DOI] [PubMed] [Google Scholar]
- 3.Au AK, Clark RSB. Paediatric traumatic brain injury: prognostic insights and outlooks. Curr Opin Neurol 2017; 30: 565–572. [DOI] [PubMed] [Google Scholar]
- 4.Okonkwo DO, Yue JK, Puccio AM, et al. GFAP-BDP as an acute diagnostic marker in traumatic brain injury: results from the prospective transforming research and clinical knowledge in traumatic brain injury study. J Neurotrauma 2013; 30: 1490–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mondello S, Kobeissy F, Vestri A, et al. Serum concentrations of ubiquitin C-terminal hydrolase-L1 and glial fibrillary acidic protein after pediatric traumatic brain injury. Sci Rep 2016; 6: 28203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adrian H, Marten K, Salla N, et al. Biomarkers of traumatic brain injury: temporal changes in body fluids. eNeuro 2016; 3: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plog BA, Dashnaw ML, Hitomi E, et al. Biomarkers of traumatic injury are transported from brain to blood via the glymphatic system. J Neurosci 2015; 35: 518–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bellahcene A, Castronovo V, Ogbureke KU, et al. Small integrin-binding ligand N-linked glycoproteins (SIBLINGs): multifunctional proteins in cancer. Nat Rev Cancer 2008; 8: 212–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lanteri P, Lombardi G, Colombini A, et al. Stability of osteopontin in plasma and serum. Clin Chem Lab Med 2012; 50: 1979–1984. [DOI] [PubMed] [Google Scholar]
- 10.Gopal N, Rajagambeeram R, Venkatkumar S, et al. Association of salivary osteopontin levels with glycaemic status and microalbuminuria – in patients with type 2 diabetes mellitus. J Clin Diagn Res 2016; 10: BC06–08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellison JA, Velier JJ, Spera P, et al. Osteopontin and its integrin receptor alpha(v)beta3 are upregulated during formation of the glial scar after focal stroke. Stroke 1998; 29: 1698–1706. [DOI] [PubMed] [Google Scholar]
- 12.Chen W, Ma Q, Suzuki H, et al. Osteopontin reduced hypoxia-ischemia neonatal brain injury by suppression of apoptosis in a rat pup model. Stroke 2011; 42: 764–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Velthoven CT, Heijnen CJ, van Bel F, et al. Osteopontin enhances endogenous repair after neonatal hypoxic-ischemic brain injury. Stroke 2011; 42: 2294–2301. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Dammer EB, Zhang-Brotzge X, et al. Osteopontin is a blood biomarker for microglial activation and brain injury in experimental hypoxic-ischemic encephalopathy. eNeuro 2017. 4(1): ENEURO.0253-16.2016. DOI: 10.1523/ENEURO.0253-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan JL, Reeves TM, Phillips LL. Osteopontin expression in acute immune response mediates hippocampal synaptogenesis and adaptive outcome following cortical brain injury. Exp Neurol 2014; 261: 757–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Gertten C, Flores Morales A, Holmin S, et al. Genomic responses in rat cerebral cortex after traumatic brain injury. BMC Neurosci 2005; 6: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rentsendorj A, Sheyn J, Fuchs DT, et al. A novel role for osteopontin in macrophage-mediated amyloid-beta clearance in Alzheimer’s models. Brain Behav Immun 2017; 67: 163–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simon DW, McGeachy MJ, Bayir H, et al. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat Rev Neurol 2017; 13: 171–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antonios A, Anastasia D, Agapi K, et al. Osteopontin as indicator of traumatic brain injury severity and progression. In: 3rd international conference on neurological disorders and brain injury, 17–18 April 2017. London, UK.
- 20.Osier ND, Dixon CE. The controlled cortical impact model: applications, considerations for researchers, and future directions. Front Neurol 2016; 7: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shapira Y, Shohami E, Sidi A, et al. Experimental closed head injury in rats: mechanical, pathophysiologic, and neurologic properties. Crit Care Med 1988; 16: 258–265. [DOI] [PubMed] [Google Scholar]
- 22.Swets JA. Measuring the accuracy of diagnostic systems. Science 1988; 240: 1285–1293. [DOI] [PubMed] [Google Scholar]