Abstract
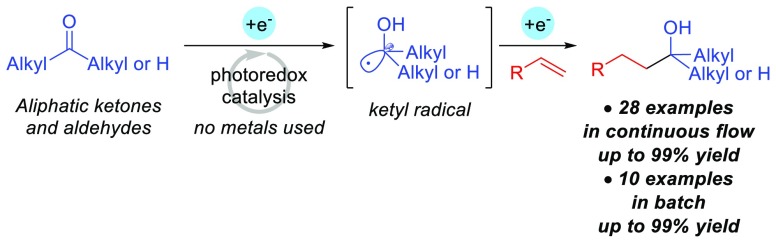
Generation of a ketyl radical from unactivated aliphatic carbonyl compounds is an important strategy in organic synthesis. Herein, catalytic generation and use of a ketyl radical for the reductive coupling of aliphatic carbonyl compounds and styrenes by organic photoredox catalysis is described. The method is applicable to both aliphatic ketones and aldehydes to afford the corresponding tertiary and secondary alcohols in continuous flow and batch. Preliminary mechanistic investigation suggests the catalytic formation of a ketyl radical intermediate.
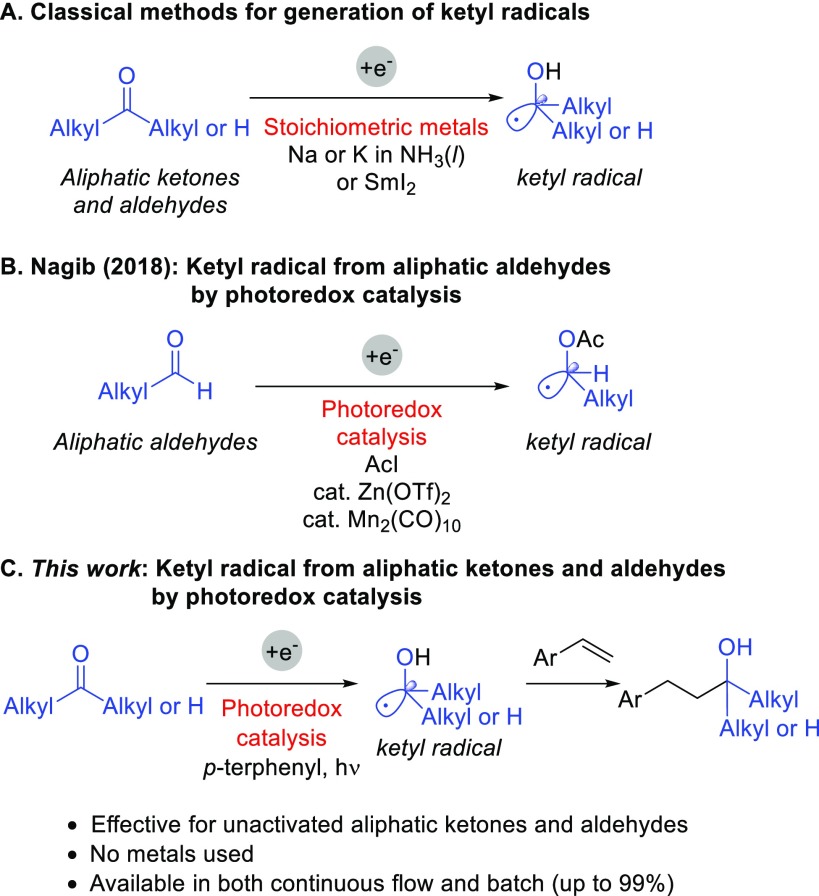
The formation of ketyl radicals from unactivated aliphatic carbonyl compounds is an important strategy to generate carbon–carbon bonds between carbonyl carbons and unsaturated systems.1 Classical protocols using either strongly reducing agents, such as samarium diiodide (SmI2), or dissolving metals, such as sodium and potassium in liquid ammonia, allow access to ketyl radical species and thus enable reductive coupling between unactivated aliphatic carbonyl compounds and unsaturated systems (Scheme 1A).2−4 Although these methodologies have enabled invaluable transformations, the reaction conditions necessitate rigorous laboratory techniques for handling those air- and moisture-sensitive metals. Green chemistry goals require catalytic and inherently safer chemistry for accident prevention. Despite the need for the development of catalytic, inexpensive, and metal-free methods to replace such potentially dangerous or rare earth metal requiring methods, recent methodologies are mostly applicable to aromatic aldehydes5,6 including photoredox catalytic approaches.7−16 The major challenge to reduction of unactivated carbonyl compounds is the standard reduction potentials, which are typically lower than −2 V versus SCE (e.g., cyclohexanone E1/2 = −2.78 V vs SCE and 3-methylbutyraldehyde E1/2 = −2.63 V vs SCE calculated using B3LYP)17 and the discrepancy in reduction potential between aliphatic carbonyl compounds and established visible-light photoredox catalysts (i.e., tris[2-phenylpyridinato-C2,N]iridium(III) [Ir(ppy)3] Ered1/2 = −2.19 V vs SCE in acetonitrile).18−20
Scheme 1. Generation of Ketyl Radical and Reductive Coupling of Unactivated Aliphatic Carbonyl Compounds with Unsaturated Systems.
Cossy21 and Hasegawa22 independently reported light-mediated intramolecular cyclizations of unactivated ketones and unsaturated systems. However, these methods are limited to specific examples such as the fastest 5-exo intramolecular cyclizations. In 2018, Nagib and co-workers reported the photoredox catalyzed generation of ketyl radicals from aliphatic aldehydes and their coupling with unsaturated systems via in situ activation using acetyl iodide in the presence of catalytic amounts of Zn(OTf)2 and Mn2(CO)10 as a photoredox catalyst (Scheme 1B).23 They demonstrated further application of their method to aliphatic ketones and showed two coupling examples using trifluoroacetone.
In the ketyl radical based strategies illustrated in Scheme 1A and 1B, catalytic intermolecular coupling of aliphatic ketones and unsaturated systems remained a considerable challenge despite the similar reduction potentials of aliphatic aldehydes and ketones.17 Herein, we overcome this challenge by using strongly reducing organic photoredox catalysis under protic conditions. In particular, we demonstrate the metal-free reductive coupling of unactivated aliphatic ketones and aldehydes with styrenes under continuous flow and batch conditions (Scheme 1C).
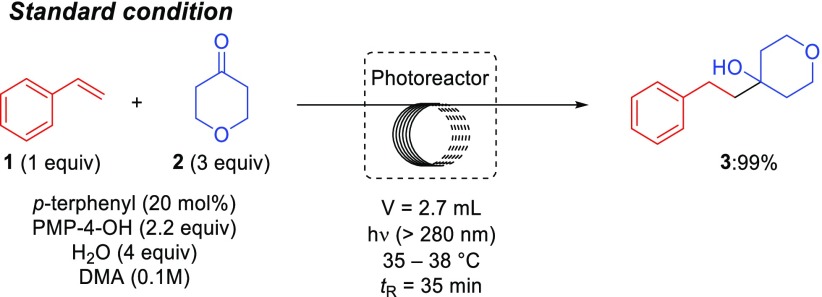
As shown in Table 1, we began our studies by examining the coupling of styrene (1) and tetrahydro-4H-pyran-4-one (2) in the presence of p-terphenyl (20 mol %) as an organic photoredox catalyst and 1,2,2,6,6-pentamethyl-4-piperidinol (PMP-4-OH) in dimethylacetamide (DMA). The reaction was performed in a continuous flow photochemistry system (Figure S1) with a 2.7 mL reactor volume using 0.04 i.d. perfluoroalkoxy (PFA) tubing equipped with a UV lamp and a long-pass filter (λ > 280 nm). After a 35 min residence time, GC analysis revealed quantitative formation of the desired coupled product 3 (99%, Table 1, entry 1). Previously, we reported β-selective hydrocarboxylation using carbon dioxide via p-terphenyl photoredox catalysis in the presence of 1,2,2,6,6-pentamethylpiperidine (PMP) as a reductant in dimethylformamide (DMF) and hexanes.24,25 Application of those conditions to the coupling of 1 and 2 only provided 68% of 3 (entry 2). Notably, use of conditions in entry 1 enables the transformation to be carried out in a continuous flow system without clogging in the absence of a nonpolar cosolvent (i.e., hexanes) that was included to dissolve nonpolar byproducts (e.g., dimer of PMP) under our previously reported PMP/DMF condition.25 As shown in entries 3 and 4, we also conducted the coupling of 1 and 2 in batch (Figure S2) but under otherwise identical conditions to entry 1 and obtained a comparable yield of 3 after 35 min (94%, entry 3) and quantitative yield after 2 h (entry 4).
Table 1. Reductive Coupling of Aliphatic Carbonyl Compounds and Styrenes in Batch and Continuous Flowa.
| entry | conditions | yield of 3 (%)b |
|---|---|---|
| 1 | Standard conditions in flow | 99 |
| 2 | PMP in DMF/hexanes (2:1) instead of PMP-4-OH in DMA | 68 |
| 3 | Standard conditions in batch, 35 min | 94 |
| 4 | Standard conditions in batch, 2 h | 99 |
Reactions were carried out using a continuous-flow photochemical system developed by Beeler.26
Calculated by gas chromatography (GC) analysis using methyl benzoate as an internal standard.
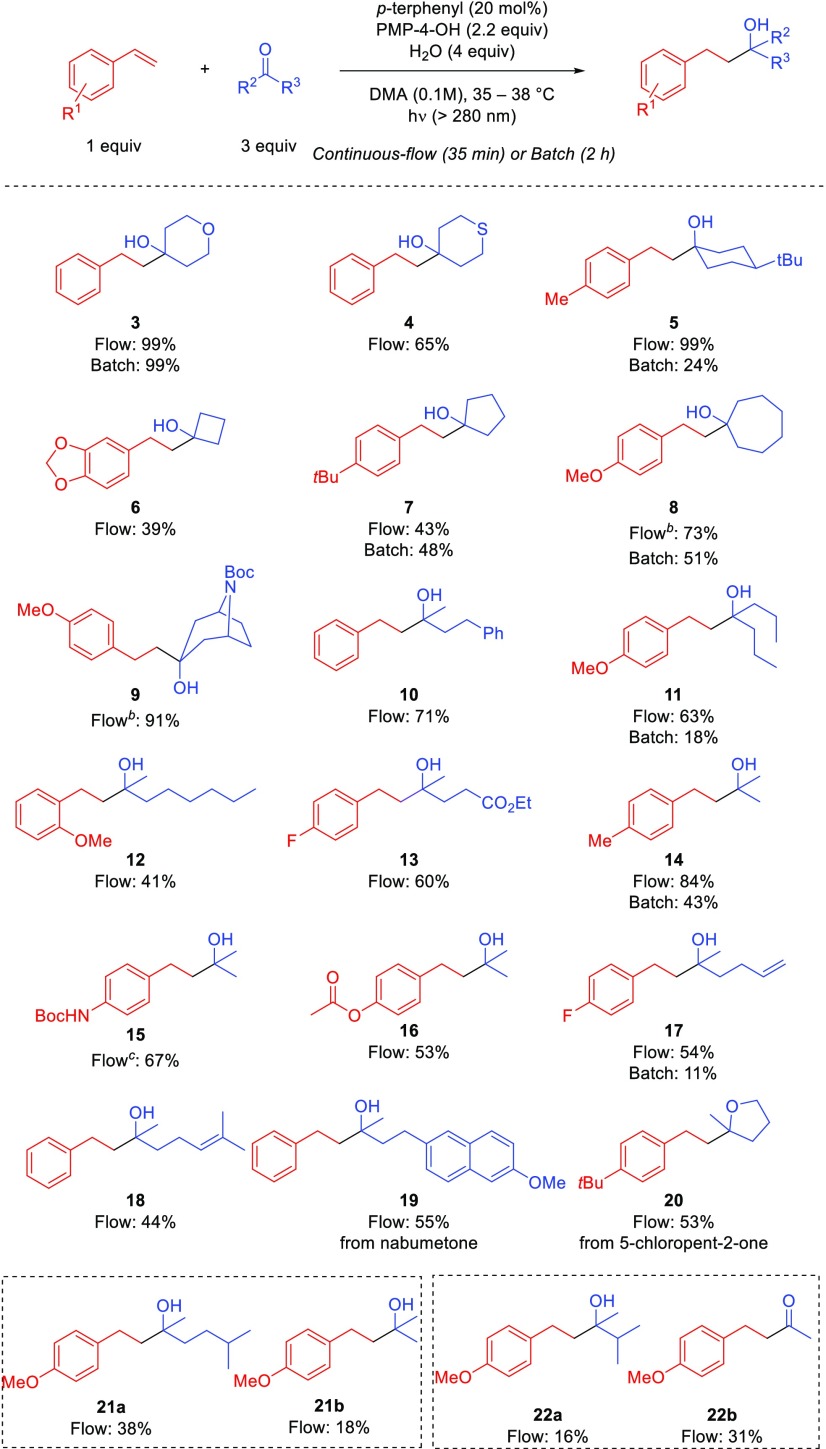
We subsequently examined the scope of the coupling reaction between aliphatic ketones and styrenes under batch and continuous flow conditions as illustrated in Scheme 2. The reaction provided the desired tertiary alcohol products with complete linear selectivity in both batch (2 h) and continuous flow (35 min) in moderate to excellent yields. Cyclic ketones that were six-membered (3–5), four-membered (6), five-membered (7), and seven-membered (8), as well as bicyclic ring structures (9), were tolerated in moderate to excellent yields. Notably, trans-product 5 from 4-tert-butyl cyclohexanone and exo-product 9 from N-Boc-nortropinone were obtained. Linear ketones provided coupled products in moderate yields (10–13). Acetone could be coupled with styrene derivatives in moderate to good yields (14–16). Ketones containing γ,δ-unsaturation provided the desired coupled products in moderate yield (17 and 18) without observations of rearranged byproducts. Nabumetone, a marketed nonsteroidal anti-inflammatory drug (NSAID), was derivatized to provide 19 in 55% yield. We hypothesized that subsequent ring closing would occur with 5-chloropent-2-one. Accordingly, the substituted tetrahydrofuran 20 was obtained as a single product in 53% yield. Although higher frequencies of light (λ > 280 nm) were removed by an optical filter, Norrish type fragmentations27,28 were unavoidable with certain substrates. As exemplified in compound 21, a ketone containing a tertiary γ-hydrogen underwent the coupling to provide the desired product 21a in 38% yield with α,β-cleaved product 21b (18% yield) that was produced by Norrish type II fragmentation. Norrish type I fragmentation was also observed when using an α-tertiary carbonyl. Utilizing 3-methyl-2-butanone, the desired coupled product 22a was obtained in 16% yield along with α-carbon cleavage product 22b in 31% yield. Unfortunately, α-quaternary carbonyl and α-phenyl carbonyl compounds did not provide any product under the current optimized conditions. Selected batch reactions were also performed to compare yields. Batch yields were lower (5, 8, 11, 14, and 17) or comparable (3 and 7) to those in flow.
Scheme 2. Scope of the Coupling Reaction Between Aliphatic Ketones and Styrenes.
Isolated yield of the coupling product.
tR = 17 min.
tR = 30 min.
A range of electron-rich and electron-neutral substituents on styrene were tolerated including protected amines (15), protected alcohols (6, 8, 12, and 16), fluorine (17), and alkyl chains (5 and 7) (Scheme 2). Styrenes with extended conjugation (e.g., 4-vinyl biphenyl) or electron-withdrawing substituents did not provide the desired coupled products. We observed reduced styrene as the major byproducts in these reactions which suggests that the reduction of styrene derivatives under photoredox catalysis may not induce the desired coupling (vide infra).
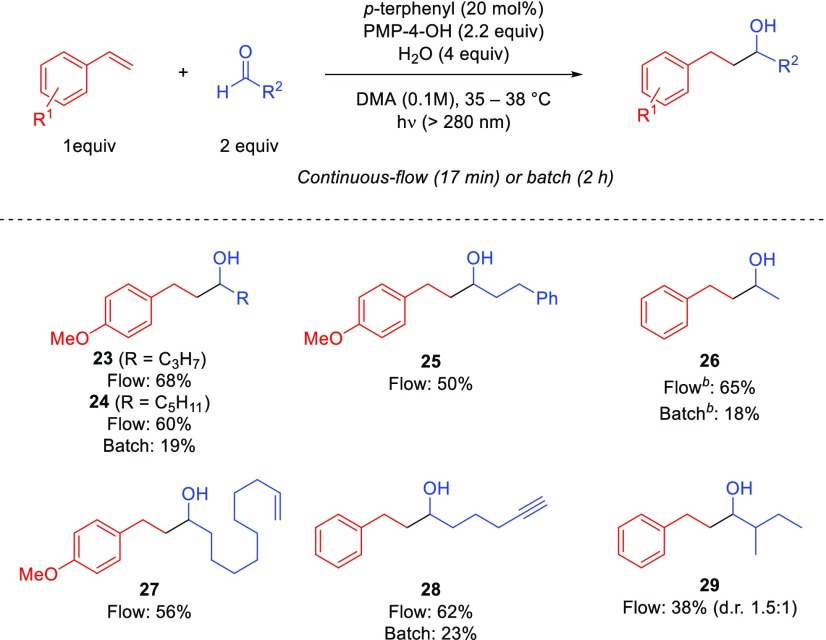
We next evaluated unactivated aliphatic aldehydes to afford secondary alcohol products (Scheme 3). The corresponding linear coupled products 23–25 with 4-vinylanisole were produced in 17 min of residence time. Acetaldehyde also underwent the desired coupling to provide 26 in 65% yield. 10-Undecenal containing a distal double bond was tolerated to provide 27 in 56% yield without detection of rearranged products under radical conditions. Hex-5-ynal provided a moderate yield of the desired product 28. 2-Methylbutanal also underwent the desired transformation to give 29 in 38% yield. Selected batch reactions to afford 24, 26, and 28 provided lower yields (18–23%) compared to the corresponding continuous flow reactions.
Scheme 3. Scope of the Coupling Reaction Between Aliphatic Aldehydes and Styrenes.
Isolated yield of the coupling product.
4 equiv of acetaldehyde were used.
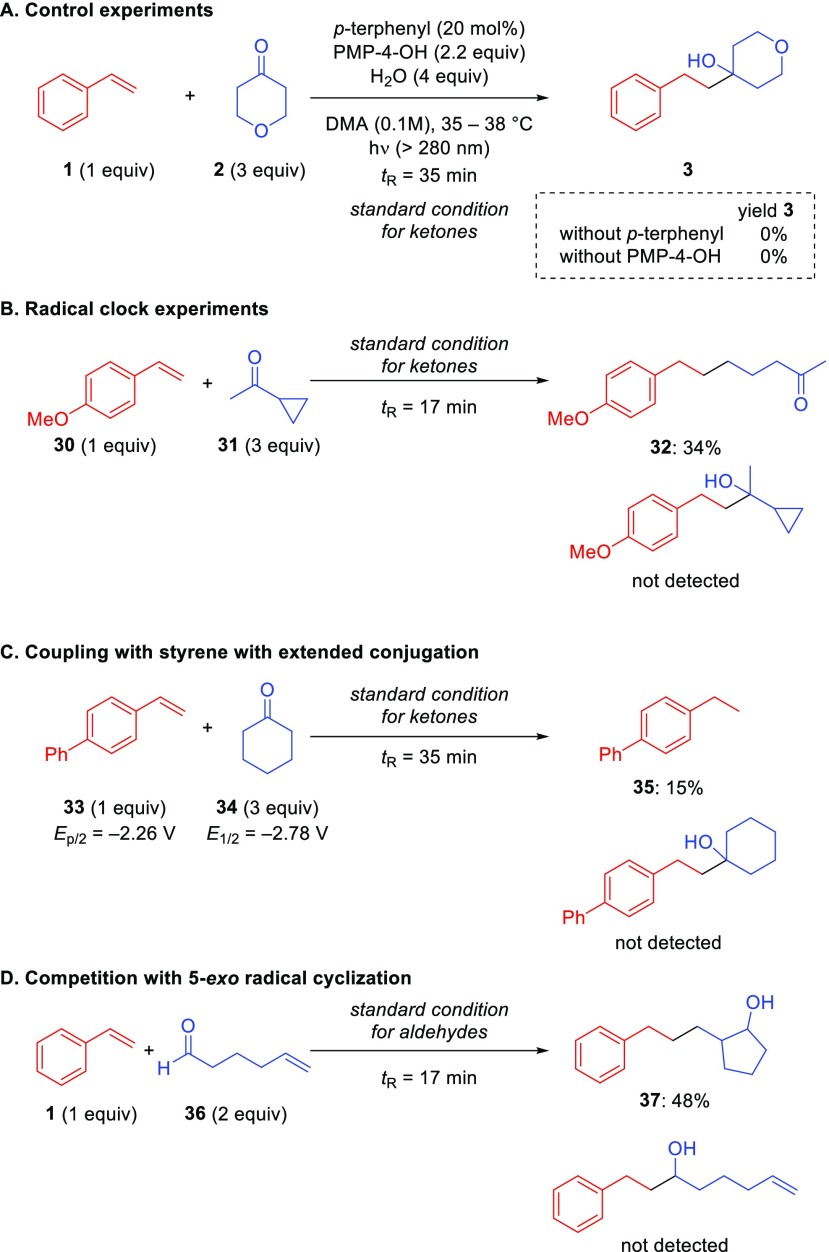
Preliminary investigations have provided some insight into the mechanism of the reaction. First, control experiments conducted in the absence of p-terphenyl did not provide any product, indicating that the direct charge transfer between ketone and amine under UV irradiation is not likely (Scheme 4A). Second, in the absence of PMP-4-OH under otherwise optimized conditions, no product was observed, highlighting its crucial role in the net reductive reaction.
Scheme 4. Preliminary Mechanistic Investigations.
Considering the reduction potentials of styrene derivatives (e.g., styrene E1/2 = −2.58 V vs SCE in DMF)29 and aliphatic ketones (e.g., cyclohexanone E1/2 = −2.78 V vs SCE),17 both species can be reduced under the p-terphenyl catalytic system (E0 = −2.63 V vs SCE in DMF).30 To probe the formation of a ketyl radical intermediate in the reaction, we conducted a radical clock experiment using cyclopropyl methyl ketone (31), although its reduction potential (E0 = −3.38 V vs SCE derived from Marcus theory)31 is out of the range of the p-terphenyl catalytic system (Scheme 4B). Nevertheless, we obtained the rearranged product 32 in 34% yield, while the closed cyclopropyl ring product was not detected by GCMS. This result suggests that the ketyl radical intermediate is involved in the mechanism. The discrepancy between the reduction potentials of certain unactivated carbonyl compounds and the p-terphenyl catalyst can be attributed to the protic conditions of our protocol. Based on literature reports,11,32 the reduction of carbonyl compounds in protic media occurs at less negative potentials than in aprotic solvents due to protonation, leading to the protonation of the primary carbonyl radical anion and a σ-radical located at the carbon atom in slightly protic organic solvents. We hypothesize that the reduction of unactivated carbonyl compounds by p-terphenyl catalysis is modulated by the presence of both PMP-4-OH and water in the reaction medium.
Next, we tested the more conjugated 4-vinyl biphenyl (33, Ep/2 = −2.26 V vs SCE in DMF, Figure S3) which should lead to the reduction of 4-vinyl biphenyl (33) over the cyclohexanone (34, E1/2 = −2.78 V vs SCE calculated using B3LYP)17 under photoredox catalysis (Scheme 4C). Since the product we observed was 4-ethyl biphenyl (35) in 15% yield, the single-electron reduction of styrene followed by nucleophilic attack at the carbonyl appears less likely.
Lastly, we investigated the competition experiment between the intermolecular addition to styrene and 5-exo cyclization using hex-5-enal (36) (Scheme 4D). The exclusive formation of the rearranged product 37 indicates that 5-exo cyclization outcompetes the intermolecular addition to styrene. This rearranged product provides indirect evidence of the formation of a radical intermediate derived from the carbonyl group.
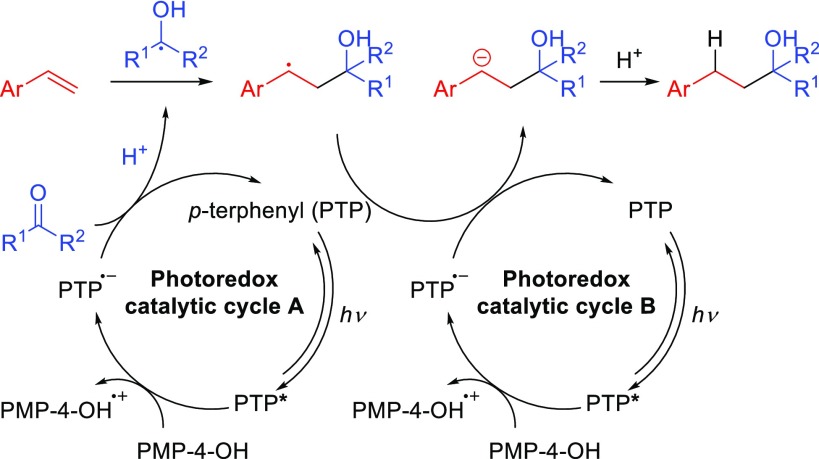
Although further mechanistic studies are warranted, we propose the catalytic mechanism summarized in Scheme 5. Photoexcitation of the organic photoredox catalyst, p-terphenyl (PTP), produces the excited singlet state of p-terphenyl (PTP*),30 which undergoes single-electron transfer (SET) with the reductant, PMP-4-OH, to generate the strong reducing p-terphenyl radical anion (PTP•–) and the PMP-4-OH radical cation (Photoredox catalytic cycle A). The p-terphenyl radical anion (PTP•–) would reduce carbonyl to a ketyl radical in protic media. The ketyl radical then adds to the β-position of styrene and produces a stable benzylic radical intermediate which is further reduced to a benzylic anion by photoredox catalytic cycle B.25 Finally, the styrene-carbonyl coupled product is formed by a protonation of the benzylic anion. The reactive PMP-4-OH radical cation is quenched by dimerization after the deprotonation (compounds S2; see Supporting Information).
Scheme 5. Proposed Mechanism.
In summary, metal-free reductive coupling of aliphatic carbonyl compounds and styrenes was developed using p-terphenyl photoredox catalysis in both batch and continuous flow. This method has been shown to be compatible with a range of ketone and aldehyde derivatives with electron-rich and -neutral styrenes. Preliminary mechanistic investigations suggest the catalytic formation of a ketyl radical to enable the desired coupling.
Acknowledgments
This work was supported by the Bill and Melinda Gates Foundation. H.S. thanks Amgen Graduate Fellowship in Synthetic Chemistry. We thank Rachel L Beingessner (MIT) for her advice and support.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.9b04235.
Experimental details and compound characterizations (PDF)
Author Present Address
† Department of Chemical Engineering, Massachusetts Institute of Technology, 77 Massachusetts Ave., Cambridge, MA 02139 (USA)
The authors declare no competing financial interest.
Supplementary Material
References
- Hart D. J. Free-Radical Carbon-Carbon Bond Formation in Organic Synthesis. Science 1984, 223, 883–887. 10.1126/science.223.4639.883. [DOI] [PubMed] [Google Scholar]
- Kahn B. E.; Rieke R. D. Carbonyl Coupling Reactions Using Transition Metals, Lanthanides, and Actinides. Chem. Rev. 1988, 88, 733–745. 10.1021/cr00087a002. [DOI] [Google Scholar]
- McMurry J. E. Carbonyl-Coupling Reactions Using Low-Valent Titanium. Chem. Rev. 1989, 89, 1513–1524. 10.1021/cr00097a007. [DOI] [Google Scholar]
- Szostak M.; Fazakerley N. J.; Parmar D.; Procter D. J. Cross-Coupling Reactions Using Samarium(II) Iodide. Chem. Rev. 2014, 114, 5959–6039. 10.1021/cr400685r. [DOI] [PubMed] [Google Scholar]
- Nguyen K. D.; Park B. Y.; Luong T.; Sato H.; Garza V. J.; Krische M. J. Metal-Catalyzed Reductive Coupling of Olefin-Derived Nucleophiles: Reinventing Carbonyl Addition. Science 2016, 354, aah5133. 10.1126/science.aah5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y.-L.; Liu Y.-Y.; Wu Y.-M.; Wang Y.-X.; Lin Y.-T.; Ye M. Iron-Catalyzed Regioselective Transfer Hydrogenative Couplings of Unactivated Aldehydes with Simple Alkenes. Angew. Chem., Int. Ed. 2016, 55, 6315–6318. 10.1002/anie.201602130. [DOI] [PubMed] [Google Scholar]
- Ischay M. A.; Anzovino M. E.; Du J.; Yoon T. P. Efficient Visible Light Photocatalysis of [2 + 2] Enone Cycloadditions. J. Am. Chem. Soc. 2008, 130, 12886–12887. 10.1021/ja805387f. [DOI] [PubMed] [Google Scholar]
- Tarantino K. T.; Liu P.; Knowles R. R. Catalytic Ketyl-Olefin Cyclizations Enabled by Proton-Coupled Electron Transfer. J. Am. Chem. Soc. 2013, 135, 10022–10025. 10.1021/ja404342j. [DOI] [PubMed] [Google Scholar]
- Du J.; Skubi K. L.; Schultz D. M.; Yoon T. P. A Dual-Catalysis Approach to Enantioselective [2 + 2] Photocycloadditions Using Visible Light. Science 2014, 344, 392–396. 10.1126/science.1251511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. N.; Ngai M.-Y. Recent Developments in Transition-Metal Photoredox-Catalysed Reactions of Carbonyl Derivatives. Chem. Commun. 2017, 53, 13093–13112. 10.1039/C7CC06287G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima M.; Fava E.; Loescher S.; Jiang Z.; Rueping M. Photoredox-Catalyzed Reductive Coupling of Aldehydes, Ketones, and Imines with Visible Light. Angew. Chem., Int. Ed. 2015, 54, 8828–8832. 10.1002/anie.201501556. [DOI] [PubMed] [Google Scholar]
- Berger A. L.; Donabauer K.; König B. Photocatalytic Barbier Reaction – Visible-Light Induced Allylation and Benzylation of Aldehydes and Ketones. Chem. Sci. 2018, 9, 7230–7235. 10.1039/C8SC02038H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz J. L.; Schäfers F.; Tlahuext-Aca A.; Lückemeier L.; Glorius F. Diastereoselective Allylation of Aldehydes by Dual Photoredox and Chromium Catalysis. J. Am. Chem. Soc. 2018, 140, 12705–12709. 10.1021/jacs.8b08052. [DOI] [PubMed] [Google Scholar]
- Lee K. N.; Lei Z.; Ngai M.-Y. β-Selective Reductive Coupling of Alkenylpyridines with Aldehydes and Imines via Synergistic Lewis Acid/Photoredox Catalysis. J. Am. Chem. Soc. 2017, 139, 5003–5006. 10.1021/jacs.7b01373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava E.; Nakajima M.; Nguyen A. L. P.; Rueping M. Photoredox-Catalyzed Ketyl–Olefin Coupling for the Synthesis of Substituted Chromanols. J. Org. Chem. 2016, 81, 6959–6964. 10.1021/acs.joc.6b01006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Q.; Dong J.; Song H.; Wang Q. Visible-Light Photocatalysis of the Ketyl Radical Coupling Reaction. Chem. - Eur. J. 2018, 10.1002/chem.201804873. [DOI] [PubMed] [Google Scholar]
- Roth H.; Romero N.; Nicewicz D. Experimental and Calculated Electrochemical Potentials of Common Organic Molecules for Applications to Single-Electron Redox Chemistry. Synlett 2016, 27, 714–723. 10.1055/s-0035-1561297. [DOI] [Google Scholar]
- Prier C. K.; Rankic D. A.; MacMillan D. W. C. Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev. 2013, 113, 5322–5363. 10.1021/cr300503r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero N. A.; Nicewicz D. A. Organic Photoredox Catalysis. Chem. Rev. 2016, 116, 10075–10166. 10.1021/acs.chemrev.6b00057. [DOI] [PubMed] [Google Scholar]
- Shaw M. H.; Twilton J.; MacMillan D. W. C. Photoredox Catalysis in Organic Chemistry. J. Org. Chem. 2016, 81, 6898–6926. 10.1021/acs.joc.6b01449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belotti D.; Cossy J.; Pete J. P.; Portella C. Synthesis of Bicyclic Cyclopentanols by Photoreductive Cyclization of.Delta.,.Epsilon.-Unsaturated Ketones. J. Org. Chem. 1986, 51, 4196–4200. 10.1021/jo00372a018. [DOI] [Google Scholar]
- Hasegawa E.; Takizawa S.; Seida T.; Yamaguchi A.; Yamaguchi N.; Chiba N.; Takahashi T.; Ikeda H.; Akiyama K. Photoinduced Electron-Transfer Systems Consisting of Electron-Donating Pyrenes or Anthracenes and Benzimidazolines for Reductive Transformation of Carbonyl Compounds. Tetrahedron 2006, 62, 6581–6588. 10.1016/j.tet.2006.03.061. [DOI] [Google Scholar]
- Wang L.; Lear J. M.; Rafferty S. M.; Fosu S. C.; Nagib D. A. Ketyl Radical Reactivity via Atom Transfer Catalysis. Science 2018, 362, 225–229. 10.1126/science.aau1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo H.; Katcher M. H.; Jamison T. F. Photoredox Activation of Carbon Dioxide for Amino Acid Synthesis in Continuous Flow. Nat. Chem. 2017, 9, 453–456. 10.1038/nchem.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo H.; Liu A.; Jamison T. F. Direct β-Selective Hydrocarboxylation of Styrenes with CO 2 Enabled by Continuous Flow Photoredox Catalysis. J. Am. Chem. Soc. 2017, 139, 13969–13972. 10.1021/jacs.7b05942. [DOI] [PubMed] [Google Scholar]
- Telmesani R.; Park S. H.; Lynch-Colameta T.; Beeler A. B. [2 + 2] Photocycloaddition of Cinnamates in Flow and Development of a Thiourea Catalyst. Angew. Chem., Int. Ed. 2015, 54, 11521–1125. 10.1002/anie.201504454. [DOI] [PubMed] [Google Scholar]
- Norrish Type I Reaction: (Norrish Type I Process, Norrish Type I Cleavage). Comprehensive Organic Name Reactions and Reagents; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Norrish Type II Reaction: (Norrish Type II Process, Norrish Type II Photoreaction, Yang Cyclization). Comprehensive Organic Name Reactions and Reagents; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Filardo G.; Gambino S.; Silvestri G.; Gennaro A.; Vianello E. Electrocarboxylation of Styrene through Homogeneous Redox Catalysis. J. Electroanal. Chem. Interfacial Electrochem. 1984, 177, 303–309. 10.1016/0022-0728(84)80232-6. [DOI] [Google Scholar]
- Matsuoka S.; Kohzuki T.; Pac C.; Ishida A.; Takamuku S.; Kusaba M.; Nakashima N.; Yanagida S. Photocatalysis of Oligo(p-Phenylenes): Photochemical Reduction of Carbon Dioxide with Triethylamine. J. Phys. Chem. 1992, 96, 4437–4442. 10.1021/j100190a057. [DOI] [Google Scholar]
- Phillips J. P. PhD. Dissertation, Virginia Polytechnic Institute and State University, 1998. [Google Scholar]
- Organic Electrochemistry: Revised and Expanded, 5th ed.; Hammerich O., Speiser B., Eds.; CRC Press: 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.