Abstract
To determine if Chlamydia muridarum, or other chlamydiae, are enzootic in rodents, we probed a serum bank of wild Peromyscus spp. mice for immunoglobulin G-antibody reactivity to ultraviolet light-inactivated C. muridarum elementary bodies (EBs) using an enzyme-linked immunoassay. Applying a cut-off for a positive reaction of OD405 nm = 0.1 at a 1:20 dilution, we found titratable antibody reactivity in 190 of 247 specimens surveyed (77%, mean OD405 = 0.33 ± 0.26, range = 0.11–1.81, median = 0.25). In addition, serum samples were obtained from a colony of specific pathogen-free Peromyscus spp. maintained at the University of South Carolina and six of 12 samples were reactive (50%, mean OD405 = 0.19 +/− 0.08, range = 0.1–0.32, median = 0.18). Lastly, 40 additional wild Peromyscus spp. were captured in a disparate region of Midwestern USA and 22 serum specimens were reactive (55%, mean OD405 = 0.22 +/− 0.11, range = 0.1–0.48, median = 0.2). Specificity of selected reactive sera for chlamydial antigen was confirmed on Western blot using resolved purified EBs as the detecting antigen. From tissues removed from several mice at necropsy, the gene for chlamydial 16S ribosomal ribonucleic acid (rRNA) was amplified by polymerase chain reaction (PCR). Positive samples of 16S rRNA were subjected to additional PCR for the major outer membrane protein gene (ompA). The amplicons of three select ompA positive samples were sequenced with ≥99% homology with C. muridarum. Our findings indicate that chlamydial infection is enzootic for Peromyscus spp., and that C. muridarum, or a closely related species or strain, is likely the agent in the tested rodent species.
Keywords: rodent, mouse, Chlamydia, Peromyscus
Peromyscus species are commonly infected with chlamydiae.
INTRODUCTION
The Chlamydiaceae is a family of obligate intracellular bacteria of the Order Chlamydiales. The family consists of a single genus, Chlamydia, with several species; each of which naturally infects a select host (Kaltenboeck 2006; Batteiger 2012). Chlamydia pneumoniae is a common human respiratory pathogen, and there are many C. pneumoniae isolates in animals that have been identified (Roulis, Polkinghorne and Timms 2013). Many of the other Chlamydia species are important veterinary pathogens, some of which cause rare but potentially fatal zoonotic infections in humans. An example of this is C. psittaci which causes psittacosis (aka, parrot fever) and is a Class B biothreat agent. The range of human diseases caused by biological variants (aka, biovars) of C. trachomatis is broad but includes ocular disease; sexually transmitted infections; and, respiratory infections and conjunctivitis in neonates (Batteiger 2012). Chlamydial agents also cause an even broader range of diseases in wildlife and many chlamydial agents have been identified or detected throughout the world and in various avian, mammalian and even amphibian and reptilian hosts (Kaltenboeck 2006). In summary, human chlamydial infections and animal chlamydioses have a significant and global impact on the health of humans and animals alike.
Because chlamydial agents are ubiquitous in nature, and also, because it was not uncommon for C. muridarum and select C. psittaci strains to be isolated from laboratory mice of 60–70 years ago, we hypothesized that chlamydial infection could be detected in rodents in nature. We began our search by probing banked wild Peromyscus spp. in order to screen samples for antibodies to C. muridarum. When we observed antibody-positive samples, we followed-up with samples from a domesticated colony of Peromyscus spp. and then conducted a smaller serological and nucleic acid detection (polymerase chain reaction, PCR) survey from freshly caught wild Peromyscus spp. Each survey yielded serological positives and several tissues from different freshly caught wild Peromyscus spp. yielded PCR positives. We conclude from these observations that chlamydial infection is likely to be common in Peromyscus spp.
MATERIALS AND METHODS
Sources and sampling
A serum bank of (N = 247) Peromyscus spp. samples was made available for our initial serological survey. These samples were collected in the 2004–07 timeframe in central Iowa (USA) and genus determined by an experienced veterinarian at Iowa State University (K.B.P.). A second, smaller sample of wild Peromyscus spp. was collected in Illinois in the summers of 2010 and 2011, and the genus and species was determined by a small mammal biologist (K.E.T.) who viewed digital images of the captured mice. A third set of sera was obtained by purchase from the domesticated Peromyscus spp. colony at the University of South Carolina Peromyscus Genetic Stock Center (http://stkctr.biol.sc.edu/). In the studies involving live capture of wild mice (see below), captured mice were anesthetized, blood collected by retro-orbital venous plexus puncture, and plasma separated by centrifugation. Aliquots of plasma were frozen at −30°C until assays were run.
Serological assays
Collected plasma was subjected to a modification of an enzyme-linked immunosorbency assay (ELISA) that we have designed and used frequently to detect and quantify chlamydia -specific Mus musculus immunoglobulin G (IgG) antibody post-infection with C. muridarum (Ramsey, Newhall and Rank 1989; Sigar et al. 2014). The modification consisted of substituting a commercially available affinity-purified phosphatase-conjugated secondary antibody to detect Peromyscus spp. IgG (KPL, Inc. Gaithersburg, MD). Gradient-purified ultraviolet light inactivated C. muridarum elementary bodies (EBs) were used as the capture antigen (Ramsey, Newhall and Rank 1989). When this assay is applied with appropriate secondary antibody to sera from commercially provided inbred specific pathogen-free (SPF) laboratory Mus musculus mice (BALB/c, C3H/HeN, C57BL/6), we routinely set a cut-off for a positive reaction at a O.D.405 reading of 0.1 or higher at 1:10 dilution (approximately two standard deviations above average normal background in uninfected mice). It should be noted that to date, and despite thousands of pre-infection screenings on commercial inbred lab mice, we have never found background reactivity above this number, although an occasional outbred mouse (e.g. Swiss Webster) will provide low background readings mildly elevated above this cutoff. It should also be noted that some of the samples from the serum bank at Iowa State University had been thawed and refrozen prior to our assays due to use in other serological surveys (e.g. West Nile virus). Based on our prior experiences, we do not feel that one or two freeze–thaw cycles altered antibody reactivity in the assays used although if it had, antibody reactivity would be reduced, and thus, our assays may have lost some sensitivity.
In order to determine that the antibodies detected in our screening ELISA reacted specifically against chlamydial antigens and not some putative contaminant in our assay, we applied a modification of an immunoblot assay previously used by us to detect antibodies to chlamydial antigens post-infection with C. muridarum (Ramsey, Newhall and Rank 1989). Briefly, we used centrifugation gradient-purified MoPn EBs resolved on a 10% SDS-polyacrylamide gel before transfer to nitrocellulose membranes. Following standard blocking protocol, we cut nitrocellulose membranes into strips (∼5 mm) and froze the strips individually at −30°C until used. Blot strips were thawed and probed with a 1:100 dilution of selected mid-high titer Peromyscus spp. serum (or BALB/c MoPn immune serum as a positive control). Following thorough wash steps, the strips were then probed with peroxidase-conjugated Peromyscus spp. IgG secondary antibody used in the ELISA assay described above and developed using the TMB substrate development system according to manufacturer's specifications (KPL Inc, Gaithersburg, MD).
Rodent capture and processing
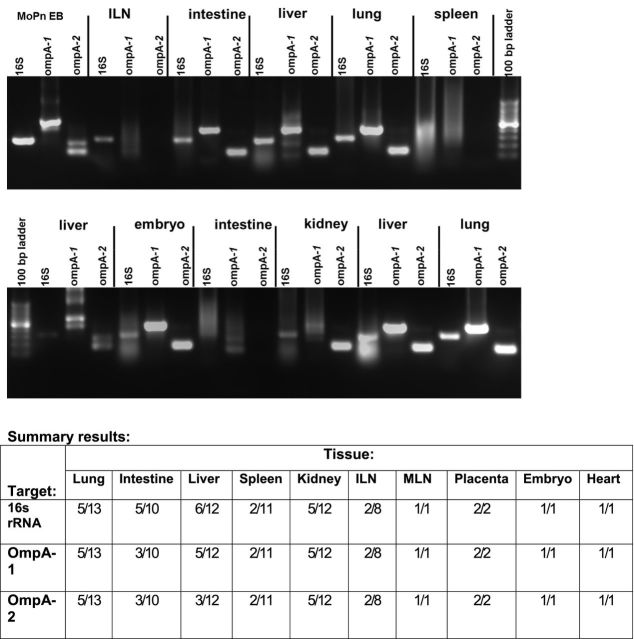
Baited small mammal galvanized folding live catch traps were used to capture the mice in this study (H.B. Sherman Traps, Tallahassee, Florida, USA) following a Centers Disease Control guide for the trapping and survey of wild rodents (Mills et al. 1995). In the field, traps were set and checked daily. Captured mice were anesthetized by isoflurane anesthesia and photographed for later speciation. A log was kept as to date, gender and maturity (e.g. juvenile versus adult) with other annotations as needed. While anesthetized, blood was collected by retro-orbital puncture, and the mouse was immediately euthanized by cervical dislocation. Mice were then necropsied, observations noted and relevant organs, see Fig. 3, were aseptically removed and separated. Five to 15 mg of tissue (depending on organ) was set aside for DNA isolation for PCR. All samples collected in the field were transported on ice to a laboratory at Midwestern University (I.M.S. and K.H.R.) for further processing. All processes and procedures involving the trapping of rodents were approved by the Midwestern University Institutional Animal Care and Use Committee or by the IACUC of Iowa State University.
Figure 3.
PCR detection of Chlamydiae in Peromyscus spp. Panels are representative results of PCR from selected tissues targeting amplification of Chlamydia 16s rRNA, Chlamydia genus-specific regions of ompA (ompA-1) and C. muridarum allele-specific ompA (ompA-2) genes. Diluted Chlamydia ompA-1 PCR product was used as template in the C. muridarum specific ompA-2 PCRs. Products shown were resolved on 1.5% agarose gels with ethidium bromide staining. PCR products of the same targets for C. muridarum genomic DNA (MoPn EB) were used for positive controls. Top panel is from a single male mouse. Bottom panel is from a single pregnant female mouse. Summary results are shown in the table below the panels. ILN: iliac lymph nodes. MLN: mesenteric lymph nodes.
Nucleic acid detection
PCR was conducted on DNA isolated from various tissues (∼5–15 mg of tissue) using DNAeasy Blood & Tissue Kit (Qiagen, Valencia, CA). All PCR reactions utilized Taq PCR Master Mix Kit (Qiagen). PCR conditions and primers for the 16S rRNA gene of Chlamydia were as described previously (Wooters et al. 2009). PCR condition and primers for the genus-specific nested Chlamydia ompA and MoPn-specific ompA were described in (Kaltenboeck, Kousoulas and Storz 1993; Kaltenbock, Schmeer and Schneider 1997).
DNA sequencing of amplification products
Three PCR products of 16S and ompA were purified either by QIAquick gel extraction kit (Qiagen) or QIAquick PCR purification kit (Qiagen). Sequencing was accomplished using Applied Biosystems 3730XL 96 capillary sequencer at the University of Chicago CRC-DNA sequencing facility (Chicago, IL) using cycle sequencing reactions with fluorescent dye terminators. Results were nBLAST'ed and optimized for highly similar sequences (Megablast, NCBI). Sequence alignment of amplification products are provided in Fig. S1(Supporting Information).
RESULTS
Serological reactivity in samples collected in central Iowa
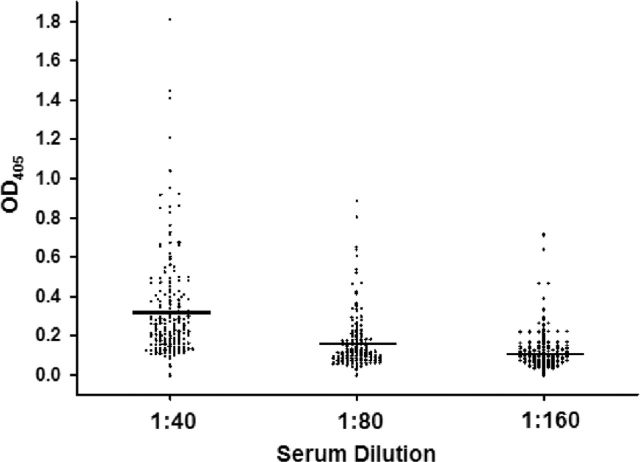
We first addressed the possibility of mouse-adapted chlamydiae being present in a wild-rodent population by conducting serological surveys of banked Peromyscus spp. sera. The genus Peromyscus contains rodent species commonly called ‘deer mice’ and is related to the common house mouse Mus domesticus, and laboratory mouse, Mus musculus (all are in Order Rodentia, suborder Myomorpha, superfamily Muroidea). Because this genera and P. leucopus, in particular, are common carriers of agents of human diseases (Borrelia spp., Ehrlichia spp., Babesia, spp., Hantavirus, etc) and because of the aforementioned ubiquity of chlamydial infections, we hypothesized they could also harbor putative rodent-adaptive chlamydiae. A serum bank was kindly made available to us (K.B.P). The serum bank contained 247 specimens that were collected over a 2 year period in the central and western region of the United States as part of a West Nile virus study. When applied to a modified ELISA to detect IgG antibody directed against C. muridarum EBs, all but 9 of the 247 samples were positive at a 1:10 dilution (OD405 = 0.1). When a more stringent positive threshold was applied (1:20), 190 samples remained positive. Control wells (no serum or with normal Mus musculus serum but with the Peromyscus spp. secondary conjugated antibody) were well below the typical cutoff for a positive reaction (OD405 > 0.1 at 1:10 dilution), indicating these reactions were not due to non-specific binding of the secondary antibody. Still, we thought it possible that false positives could occur due to the modification of the assay. Thus, we again ran the assay at dilutions of 1:40, 1:80 and 1:160. The results of this assay are depicted in Fig. 1. Each point represents the results from a single sample, and the horizontal bars are at the mean for each dilution. At a 1:40 dilution, 190 of 247 samples remained positive with readings above the OD405 0.1 cutoff. In addition, 171 of 247 were positive at 1:80 and 90 of 247 at a dilution of 1:160; indicating that the reactivity was titratable with reference to Peromyscus spp. serum and no other reactants in the assay. This contention was supported by the absence of positive reactions in the serum-free controls.
Figure 1.
Detection of IgG antibody directed against C. muridarum antigens. A bank of 247 Peromyscus spp. serum specimens was queried for IgG antibodies against UV light-inactivated C. muridarum elementary bodies. Serum was initially screened at a 1:10 dilution and positives (≥0.1 0D405) were subject to further two-fold serial dilution.
These results suggested that the reactions were likely specific. However, we still felt a control of serum from known uninfected Peromyscus spp. was lacking. Hence, we purchased 12 samples of serum from the University of South Carolina Peromyscus Genetic Stock Center (USCPGSC http://stkctr.biol.sc.edu/), which domesticates and raises these mice under SPF conditions and provides them to the research community. Interestingly, of the 12 that were tested, six samples yielded positive reactions at a dilution of 1:20 and two remained positive at a dilution of >1:40. One sample gave high OD405 readings at a 1:160 dilution (data not shown). These results were encouraging in that the rate of positive reactions was lower in laboratory-raised and domesticated Peromyscus spp., but also surprised us since we were still observing mostly low-level positives in half of Peromyscus spp. raised under SPF conditions. It should be noted that the testing for SPF pathogens by this supplier does not include any form of chlamydial serology.
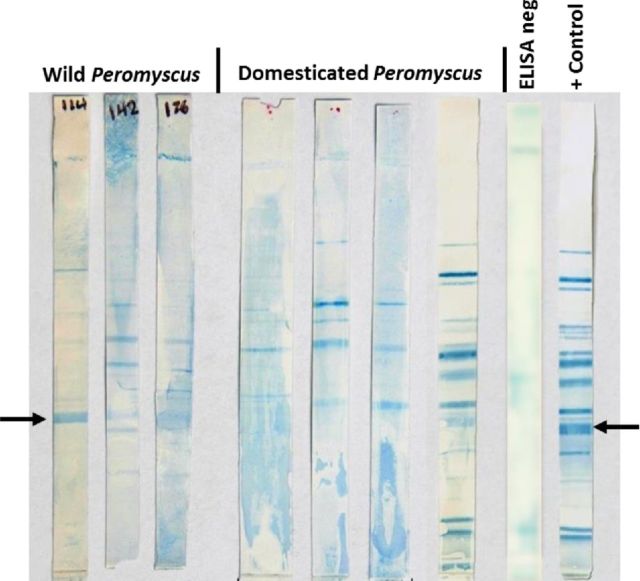
The question remained as to whether we could verify the reactivity as specific to chlamydial antigens. For this purpose, we used a modification of an immunoblot assay that we have previously used to detect antigen specificity of antibodies post-chlamydial infection in our laboratory (Ramsey, Newhall and Rank 1989; Ramsey et al. 1999). The results depicted in Fig. 2 are from both wild and domesticated Peromyscus spp. sources and indicated multiple antigen reactivity on several resolved chlamydial antigens. One of the clearer results came from a domesticated Peromyscus spp. sample which had near identical reactivity as the positive control (closest to positive control) and had given high titer reactivity on ELISA. At least four of the samples showed what appeared to be reactivity with a band consistent with MOMP (arrow), but several others showed reactivity against numerous resolved chlamydial antigens. It was interesting that some novel bands appeared in immunoblots probed with ELISA-reactive Peromyscus spp. sera. This could be a result of different antigen recognition patterns by immunogenetically disparate mice, but this has yet to be proved. It should be noted that when ELISA-negative sera from Peromyscus spp. was used to probe the blots, no reactivity was observed. From these results, we concluded that the seropositive mice had likely been infected with a chlamydial agent of some type. In addition, at least one immunoblot indicated a likely infection with C. muridarum. Even though a hallmark of chlamydial infections is chronic/persistent infections, it is possible that any chlamydial infection had resolved and was not ongoing in these mice at the time sera were collected. Thus, we next sought to determine if there was evidence that Peromyscus spp. may continue to harbor chlamydial pathogens.
Figure 2.
Specificity of ELISA-reactive Peromyscus spp. serum samples on Western blot. Western blotted C. muridarum elementary antigens were probed with a 1:100 dilution of ELISA-reactive Peromyscus spp. serum followed by detection of IgG binding. From the left-right: the first three blots are representative of ELISA-reactive samples obtained from wild-caught Peromyscus spp. mice; the next four blots were from domesticated Peromyscus spp. mice; ELISA neg, the blot was probed with sera that was not reactive on ELISA; the last blot was from a female BALB/c mouse at day 56 post-intravaginal inoculation with C. muridarum (+ control). The arrows on the right and left of the image indicate typical migration and reactivity of the major outer membrane protein (∼43 kDa). Of note, several other bands consistent with specific antibody binding of chlamydial antigens in the positive control blot are observed in the blots probed with Peromyscus spp. serum.
Detection of chlamydial nucleic acids
In order to determine, if there was an endemic presence of chlamydial pathogens in Peromyscus spp., we trapped an additional 40 mice, 20 each in the summers of 2010 and 2011. These mice were trapped in eastern Illinois as opposed to the sera from the serum bank that was collected in central Iowa in the 2005–07 time frame. While the set of Peromyscus spp. from the Iowa serum bank were largely not assigned to species, one set of 30 were classified as P. maniculatus (K.B.P.). Of the set of mice captured in Illinois, the majority (24) of the eastern Illinois trapped mice as P. maniculatus. Eight specimens from Illinois were not photographed, and eight were not sufficiently differentiated in photographs to determine species and could have been P. leucopus (Hoffmeister 2002). Of the sera collected from the 40 mice, 22 reacted positively at a 1:20 dilution in the same ELISA assay used previously (55%, mean OD405 = 0.22 +/− 0.11, range = 0.1–0.48, median = 0.2, data not shown). Selected tissues from seropositive mice were subjected to PCR for chlamydial 16S rRNA or genus-specific or allele-specific regions of ompA: ompA-1 and ompA-2, respectively (Kaltenboeck, Kousoulas and Storz 1993; Kaltenbock, Schmeer and Schneider 1997; Wooters et al. 2009). The results of these assays are shown in Fig. 3 and the accompanying summary table. We were able to detect chlamydial 16s rRNA amplicons in 30 of 71 tissues derived from 13 of the 22 seropositive mice. Chlamydia genus-specific ompA-1 amplicons were detected in 27 of 71 samples and 25 of the 27 Chlamydia genus-specific ompA-1 amplicons also amplified C. muridarum ompA when appropriate species-specific ompA-2 primers were used. In order to rule-out non-specific amplification, three of the PCR+ samples were sequenced: the one 16s rRNA and two ompA-1 amplicons. The 16s rRNA amplicons aligned with 100% bp match and the ompA-1 amplicons each aligned with a 99% match to the published sequence.
DISCUSSION
Despite the ubiquitous nature of chlamydial pathogens and diseases, only two rodent chlamydial pathogens are known. One of these, C. muridarum, is widely used by researchers who apply it as a model for various human chlamydial diseases (Rank 2007). The other, the meningopneumonitis strain of C. psittaci, causes systemic disease that for various reasons has not been used extensively in modeling human diseases in mice (Rank 2007, 2012). Interestingly, neither of these rodent pathogens have been detected or isolated from any source other than laboratory mice (Mus musculus). Both were found by serendipity when mice were being used as ‘incubators’ to culture viruses and diagnose viral diseases in humans prior to the advent and routine use of cell culture for these purposes (Rank 2007). Initially, presumed to be a human virus or other pathogen, C. muridarum was also isolated from several normal control laboratory mice during serial passage of uninoculated mouse tissues—implying a process endemic in the mouse colonies of the mid-twentieth century (Gordon, Freeman and Clampit 1938; Gonnert 1941a,b; Nigg 1942; Nigg and Eaton 1944). Indeed, it was actually quite common during this time frame that researchers would isolate C. muridarum from laboratory mice (the late Dr James Moulder, pers. comm.). This is not surprising if one considers that lab mice of the 1930s and ‘40s were only a step above wild mice with regard to endemic rodent diseases they carried (Fox et al. 2007).
Scrutiny of the original reports of isolation of a rodent chlamydial pathogen indicates that C. muridarum was likely a gastrointestinal pathogen or commensal that had incidentally infected the respiratory tract (as reviewed in Rank 2007; Yeruva et al. 2013). Investigators found that the organism was highly infectious and pathogenic for hamsters and produced pneumonia similar to that seen in mice inoculated with human influenza virus—but, it was less infectious for ferrets and not infectious at all for rabbits (Nigg 1942). Since the time of the original isolation, there have been scant reports of chlamydiae isolated from laboratory mice and it is likely that that one of these was a C. psittaci strain (Ata, Stephenson and Storz 1971) and another was C. muridarum or closely related to it (Fox et al. 1993; Zhang et al. 1993). Unfortunately, the latter isolate was lost during freezer failure (Fox, pers. comm.). Each of these reports related to laboratory-reared rodents. To our knowledge, only one other attempt to detect or isolate chlamydiae from non-domesticated small mammal sources has been reported. Stephan et al. (2014) reported using nucleic acid detection methodologies to detect chlamydial infection in European shrews and reported only rare positives, indicating very low endemicity of chlamydial infection (Stephan et al. 2014).
In the present report, we present convincing evidence that Peromyscus spp. are infected or colonized with a chlamydial agent of some sort. Based on initial sequencing results of PCR products, it would appear that this agent is either a strain of C. muridarum or some closely related species. Our results indicate that this agent is enzootic in Peromyscus spp. of the upper Midwestern USA because of the high rate of seropositive mice in two regions over two periods 5–7 years apart. It is interesting that laboratory—reared and ‘specific pathogen-free’ Peromyscus spp. also had serological evidence of chlamydial infection. We interpret these findings that chlamydial infection is endemic in Peromyscus spp. of the upper Midwestern area of the United States and that the chlamydial etiology is either C. muridarum or a very closely related strain or species. Our initial attempts to culture from the tissues collected in this study were unsuccessful.
Nonetheless, we are in hopes of isolating and identifying either new species of rodent-adapted chlamydiae or new strains of C. muridarum which are adapted to Peromyscus spp. and thus broaden the repertoire of pathogens available to model human chlamydial infection and disease. Additionally, comparative genomics, when applied to novel chlamydial isolates, can be used to better understand the natural history of adaptation and evolution of chlamydial pathogens and thereby provide better insights and understanding of chlamydial biology.
Supplementary Material
SUPPLEMENTARY DATA
Conflict of interest. None declared.
REFERENCES
- Ata FA, Stephenson EH, Storz J. Inapparent respiratory infection of inbred Swiss mice with sulfadiazine-resistant, iodine-negative Chlamydiae. Infect Immun. 1971;4:506–7. doi: 10.1128/iai.4.4.506-507.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batteiger BE. Chlamydia infection and epidemiology. In: Tan M, Bavoil PM, editors. Intracellular Pathogens I: Chlamydiales. Washington, DC, USA: ASM Press; 2012. pp. 1–26. [Google Scholar]
- Fox JG, Barthold S, Newcomer C, et al. The Mouse in Biomedical Research. Vol. 1. New York, NY: Academic Press; 2007. Ch. 1–3. [Google Scholar]
- Fox JG, Stills HF, Paster BJ, et al. Antigenic specificity and morphologic characteristics of Chlamydia trachomatis, strain SFPD, isolated from hamsters with proliferative ileitis. Lab Anim Sci. 1993;43:405–10. [PubMed] [Google Scholar]
- Gonnert R. Die Bronchopneumoniae, eine neue Viruskrankheit der Maus. Zbl Bakt Parasitenkunde und Infektions krankheiten. 1941a;147:161–73. [Google Scholar]
- Gonnert R. Ueber ein neues, dem Erreger des Lymphogranuloma inquinale aenliches Maeusevirus. Klin Wochenschr. 1941b;20:76–8. [Google Scholar]
- Gordon FB, Freeman G, Clampit JM. A pneumonia-producing filterable agent from stock mice. P Soc Exp Biol Med. 1938;39:450–3. [Google Scholar]
- Hoffmeister DF. Mammals of Illinois. Urbanna and Chicago, Illinois, USA: University of Illinois Press; 2002. [Google Scholar]
- Kaltenbock B, Schmeer N, Schneider R. Evidence for numerous omp1 alleles of porcine Chlamydia trachomatis and novel chlamydial species obtained by PCR. J Clin Microbiol. 1997;35:1835–41. doi: 10.1128/jcm.35.7.1835-1841.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltenboeck B. Recent advances in the knowledge of animal Chlamydial infections. In: Chernesky MA, Caldwell HD, Christiansen G, et al., editors. Proceedings of the 11th International Symposium on Human Chlamydial Infections. San Francisco, CA, USA: 2006. pp. 399–408. International Chlamydia Symposium. [Google Scholar]
- Kaltenboeck B, Kousoulas KG, Storz J. Structures of and allelic diversity and relationships among the major outer membrane protein (ompA) genes of the four chlamydial species. J Bacteriol. 1993;175:487–502. doi: 10.1128/jb.175.2.487-502.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills JN, Childs JE, Ksiazek TG, et al. Method for Trapping and Sampling Small Mammals for Virologic Testing. Atlanta, Georgia USA: US Department of Health and Human Services, USPHS, Centers for Disease Control; 1995. [Google Scholar]
- Nigg C. An unidentified virus which produces pneumonia and systemic infection in mice. Science. 1942;95:49–50. doi: 10.1126/science.95.2454.49-a. [DOI] [PubMed] [Google Scholar]
- Nigg C, Eaton MD. Isolation from normal mice of a pneumotropic virus which forms elementary bodies. J Exp Med. 1944;79:497–510. doi: 10.1084/jem.79.5.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey KH, Cotter TW, Salyer RD, et al. Prior genital tract infection with a murine or human biovar of Chlamydiatrachomatis protects mice against heterotypic challenge infection. Infect Immun. 1999;67:3019–25. doi: 10.1128/iai.67.6.3019-3025.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey KH, Newhall WJ, Rank RG. Humoral immune response to chlamydial genital infection of mice with the agent of mouse pneumonitis. Infect Immun. 1989;57:2441–6. doi: 10.1128/iai.57.8.2441-2446.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rank RG. Chlamydial diseases. In: Fox J, Barthold S, Newcomer C, et al., editors. The Mouse in Biomedical Research. New York: Academic Press; 2007. [Google Scholar]
- Rank RG. In vivo Chlamydial infection. In: Tan M, Bavoil PM, editors. Intracellular Pathogens, I: Chlamydiales. Washington, DC, USA: ASM Press; 2012. pp. 285–310. [Google Scholar]
- Roulis E, Polkinghorne A, Timms P. Chlamydia pneumoniae: modern insights into an ancient pathogen. Trends Microbiol. 2013;21:120–8. doi: 10.1016/j.tim.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Sigar IM, Schripsema JH, Wang Y, et al. Plasmid deficiency in urogenital isolates of Chlamydiatrachomatis reduces infectivity and virulence in a mouse model. Pathog Dis. 2014;70:61–9. doi: 10.1111/2049-632X.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan S, Guerra D, Pospischil A, et al. Chlamydiaceae and Chlamydia-like organisms in free-living small mammals in Europe and Afghanistan. J Wildlife Dis. 2014;50:195–204. doi: 10.7589/2013-08-194. [DOI] [PubMed] [Google Scholar]
- Wooters MA, Kaufhold RM, Field JA, et al. A real-time quantitative polymerase chain reaction assay for the detection of Chlamydia in the mouse genital tract model. Diagn Micr Infec Dis. 2009;63:140–7. doi: 10.1016/j.diagmicrobio.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Yeruva L, Spencer N, Bowlin AK, et al. Chlamydial infection of the gastrointestinal tract: a reservoir for persistent infection. Pathog Dis. 2013;68:88–95. doi: 10.1111/2049-632X.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YX, Fox JG, Ho Y, et al. Comparison of the major outer-membrane protein (MOMP) gene of mouse pneumonitis (MoPn) and hamster SFPD strains of Chlamydiatrachomatis with other Chlamydia strains. Mol Biol Evol. 1993;10:1327–42. doi: 10.1093/oxfordjournals.molbev.a040079. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.