Introduction
Optical coherence tomography (OCT) is a reproducible, noninvasive imaging technology that enables the generation of high resolution, cross-sectional images of retinal structures. OCT has been gaining wide popularity among neurologists for the evaluation of neurological disorders1. In this context, neurologists are often confronted with incidental retinal findings on OCT imaging that may or may not be related to the patient’s primary neurological illness. Such morphological macular abnormalities (MMAs) have been described in the context of a wide array of neurological conditions, including multiple sclerosis (MS)2,3, neuromyelitis optica (NMO)4,5, Alzheimer’s disease6, and Parkinson’s disease7. However, most commonly MMAs are seen with primary ophthalmologic disorders or as part of normal aging8,9.
While neurologists are expected to recognize common patterns of MMAs on OCT (especially those requiring prompt ophthalmologic/retinal referral of patients with potentially vision-threatening findings), there is insufficient data to shed light on the prevalence patterns of these OCT findings in general neurology and neuroimmunology practices. In fact, comprehensive, qualitative reviews of practice-based OCT scans assessing the prevalence of MMAs in MS and NMO to-date are limited. Previous studies addressing this topic have either focused on a single pathological entity2–5 or examined a relatively limited number of symptomatic patients10, making the overall prevalence of such retinal findings hard to discern.
In this study, we systematically analyzed macular OCT scans for qualitative retinal abnormalities in all subjects evaluated in our clinic for suspected or known neuroimmunological disorders. The main aim of this study is to describe the prevalence and characteristics of incidental findings on OCT from a practical point of view in a sample reflective of patients typically seen at an academic neuroimmunology practice.
Methods
Study design and participants:
The Johns Hopkins University institutional review board and ethical standards committee approval was obtained for the study protocol permitting the retrospective review of OCT imaging data and clinical records of all subjects scanned with OCT during the designated time period in a de-identified fashion. Informed consent was waived due to the retrospective nature of the study and de-identified analysis of OCT data.
Data for this cross-sectional analysis was collected in a retrospective fashion from the medical and OCT records of all the patients who underwent OCT imaging in our diverse neuroimmunology clinic which included the Johns Hopkins MS and Transverse Myelitis (TM) centers and the neurology ward of Johns Hopkins Hospital between June 1st 2010 and June 1st 2012. OCT testing is performed on a routine basis for patients presenting with known or suspected neuroimmunological disorders at our clinic and, in order to investigate the incidence of unexpected retinal findings from a practical point of view, all subjects scanned with OCT during the predesignated time period were systematically included in the analysis, regardless of diagnosis. Study participants’ demographics, diagnosis and basic clinical characteristics were determined by review of medical records. Due to the retrospective nature of the study, visual acuity or optic neuritis history was not systematically recorded and, therefore, not included in the final analysis.
Optical coherence tomography:
Retinal imaging was performed using spectral-domain OCT (Cirrus HD-OCT, Model 4000, Software version 5.0; Carl Zeiss Meditec, Dublin, CA) as described in detail elsewhere11. Briefly, macular scans were obtained with the Macular Cube 512×128 protocol. Scans with poor quality or signal strength <7/10 were excluded from the study. For eyes that were unable to fixate due to poor visual function, the patient was instructed to fixate with the fellow eye during acquisition.
Macular cube scans for 1450 subjects enrolled in the study were analyzed qualitatively by two raters (A.V., O.A.; certified per institutional and clinical trial criteria [Optical Coherence Tomography Trial in Multiple Sclerosis; ]) who were blinded to the clinical status of each of the subjects. Each scan was graded based on the presence or absence of MMAs as defined in Supplementary Table 2 and described in detail elsewhere12. To assess inter-rater agreement, a random sample of 100 subjects (200 eyes) chosen from the overall cohort was independently evaluated by the two primary raters. Scans demonstrating MMAs were reviewed and verified by two separate investigators (E.S.S., S.S.) and a retinal specialist (H.Y.); all masked to clinical status.
Statistical methods
Statistical analysis was performed using Stata version 13.1 (StataCorp, College Station, TX). Kappa statistic was used to evaluate inter-rater agreement for the detection of retinal abnormalities. The Shapiro-Wilk test was used to evaluate the normality of distributions. Comparisons between groups were performed using the Student t test (age), χ² test (sex), and Fisher’s exact test (race and diagnosis).
Mixed-effects logistic regression was utilized to evaluate the influence of patient- and eye-level covariates on the odds of identifying MMAs (labeled categorically). Two-level, random-intercept, multivariate logistic regression models were used to account for eye clustering within-subjects. Bonferroni correction for multiple comparisons was undertaken as appropriate. In all analyses, statistical significance was defined as p<0.05.
Results
Macular cubes for 1450 subjects (2900 eyes) were reviewed and 1445 subjects (2872 eyes) were eligible for analysis. Eighteen eyes (0.6%) were excluded due to poor macular cube quality and/or low signal strength (single eye of 8 subjects, both eyes of 5 subjects). Ten eyes had missing macular cube data (less than 1% of the entire study sample). Out of the 1445 subjects examined, 1234 (85%) had one OCT assessment during the designated time period, 173 (12%) had two assessments, and 38 (3%) had three or more (abnormalities seen on at least one OCT scan were included; however, in all MMAs identified, these abnormalities were also present on follow-up scans as well). Based on the kappa statistic, excellent agreement was found between the independent raters for MMA identification: 97% agreement, κ = 0.863 (p<0.001; prevalence index = 0.75; bias index = 0.01). Adjudication of cases in which there was disagreement was undertaken in a consensus revision of all relevant scans with a retinal specialist (H.Y.). In 16 eyes of 14 subjects (5% of MMAs cases), the retinal abnormality could not be reliably categorized into a distinct pathologic entity solely based on OCT data.
Prevalence and general characteristics of MMAs
The demographic characteristics of study participants are presented in Table 1. Overall, MMAs were detected in 338 eyes of 232 subjects, giving rise to crude prevalence estimates of 11.7% (95% CI 10.6%–13.0%) and 16.1% (95% CI 14.2%–18.0%) respectively. Amongst the subjects in the MMAs group, bilateral abnormalities were noted in 106 subjects (45.7%) and, in the majority of these cases, the abnormality was congruous for both eyes (n=78). Multiple simultaneous retinal pathologies were recorded in 27 eyes of 23 subjects (8.0% of the MMAs eyes).
Table 1:
Summary of demographics and clinical characteristics of the study population
| Demographics | Overall (n=1,445) | MNM group (n=1,213) | MMAs group (n=232) | MNM vs MMAs, p-value |
|---|---|---|---|---|
| Age, yr, mean ±SD | 44.0 ±13 | 42.8 ±12 | 50.3 ±12 | <0.01a |
| Female, n (%) | 1,047 (72) | 896 (74) | 151 (65) | <0.01b |
| Race, n (%): | ||||
| - Caucasian | 1,051 (73) | 887 (73) | 164 (71) | |
| - African-American | 267 (18) | 221 (18) | 46 (20) | |
| - Hispanic | 21 (1) | 20 (2) | 1 (<1) | 0.24c |
| - Asian | 18 (1) | 14 (1) | 4 (2) | |
| - Other | 57 (4) | 43 (4) | 14 (6) | |
| - Unknown | 31 (2) | 28 (2) | 3 (1) | |
| Diagnosis, n (%): | ||||
| - RRMS | 724 (50) | 622 (51) | 102 (44) | |
| - SPMS | 50 (3) | 37 (3) | 13 (6) | |
| - PPMS | 39 (3) | 30 (2) | 9 (4) | |
| - CIS | 60 (4) | 56 (5) | 4 (2) | |
| - NMOSD | 32 (2) | 28 (2) | 4 (2) | |
| - Sarcoidosisd | 55 (4) | 38 (3) | 17(7) | |
| - Idiopathic TM | 97 (7) | 81 (7) | 16 (7) | |
| - Othere | 388 (27) | 321 (26) | 67 (29) | |
Student’s t-test.
χ² test.
Fisher’s exact test.
A diagnosis of neurosarcoidosis was made in 35 individuals, whereas 20 patients were referred for suspected neurosarcoidosis, but diagnosed with sarcoidosis only.
This group constitutes patients seen at the clinic during the predesignated time period who did not have any of the more common neuroimmunological disorders. The details of these disorders are listed in Supplementary Table 1.
Abbreviations: CIS = clinically isolated syndrome; MMAs = morphologic macular abnormalities; MNM = morphologically normal macula; NMOSD = neuromyelitis optica spectrum disorders; PPMS = primary progressive multiple sclerosis; RRMS = relapsing-remitting multiple sclerosis; SD = standard deviation; SPMS = secondary progressive multiple sclerosis; TM = transverse myelitis.
Collectively, patients with MMAs were, on average, older (OR 1.79 per 5-year increase in age; 95% CI 1.5–2.1; p=5×10−5) and more likely to be males (OR 2.45; 95% CI 1.2–5.0; p=0.014) as opposed to subjects with a morphologically normal macula after adjusting for race and diagnosis at presentation. African-Americans tended to be at a higher risk of MMAs as opposed to Caucasians, but this was not statistically significant (OR 2.27; 95% CI 0.96–5.4; p = 0.063). The macular central B-scan report captured the observed macular pathology in only 161 eyes (48%). In 162 eyes (48%), the macular pathology was peripheral and not seen on the macular central B-scan report, whereas 15 cases (4%) were equivocal. Amongst the 338 eyes with MMAs, visual symptomatology (either at the time of the OCT or transiently in the past) were reported in 154 eyes (45.6%). Supplementary Table 3 provides a breakdown of the presence versus absence of visual symptomatology by MMAs category. Data on visual symptoms was missing or unknown for 8 eyes (2.4%). The most common complaint was visual blurring/vision loss. Patients with MMP were statistically more likely to report visual symptomatology in the affected eyes compared to other categories (Fisher’s exact test; p = 0.002).
Characteristics of specific MMAs
The most common MMA observed in this study was drusen, which was noted in 87 subjects (6.0%; Table 2; Figure 1, Panel A), and was present bilaterally in 38 subjects. The occurrence of drusen appeared closely related to age with a 1.80-fold increase in risk per each 5-year increment of age (95% CI 1.4–2.4; p=2×10−5). Patients with sarcoidosis appeared at higher risk of drusen as opposed to patients with other neuroinflammatory disorders (OR 11.36 when compared to relapsing-remitting MS; 95% CI 1.1–119; unadjusted p = 0.042). However, this finding did not retain significance after adjustment for multiple comparisons. No statistically significant differences were observed for other demographic characteristics.
Table 2:
Characteristics of specific MMAs detected in the study in order of prevalence1
| MMAs | Brief description | n, subjects (eyes)2 | Prevalence, subject-level2* (95% CI) | Prevalence, eye-level2* (95% CI) | Bilateral involvement, n (%) | Remarks |
|---|---|---|---|---|---|---|
| Drusen | Subretinal deposits between the RPE and Bruch’s membrane | 87 (125) | 6.0% (4.9–7.4%) | 4.4% (3.7–5.2%) | 38 (44%) | Increased prevalence with age |
| Epiretinal membrane (ERM) | Fibrocellular tissue growth internal to the ILM; can range from subtle cellophane-like films to thick contractile membranes | 79 (120) | 5.5% (4.4–6.8%) | 4.2% (3.5–5.0%) | 41 (52%) | Increased prevalence with age; macular pucker in 63 eyes (52.5%) |
| Pigment epithelial detachment (PED) | Detachment of the RPE from BM with sub-RPE fluid | 29 (34) | 2.0% (1.4–2.9%) | 1.2% (0.8–1.7%) | 5 (17%) | CSC in 8 eyes (24%) |
| Microcystoid macular pathology (MMP) | Lacunar areas of cystoid degeneration in the INL, OPL, or ONL in a characteristic perifoveal location | 28 (33) | 1.9% (1.3–2.8%) | 1.1% (0.8–1.6%) | 5 (18%) | ON history in 19 eyes (58%); increased risk in African-Americans |
| Foveal cystoid changes | Foveal cystoid changes with or without perifoveal posterior hyaloid detachment | 10 (11) | 0.7% (0.4–1.3%) | 0.4% (0.2–0.7%) | 1 (10%) | FP in 7 eyes (64%); ERM in 5 eyes (45%) |
| Geographic atrophy or other advanced forms of AMD | Focal RPE atrophy, often seen as a complication of AMD | 7 (9) | 0.5% (0.2–1.0%) | 0.3% (0.2–0.6%) | 2 (22%) | ERM in 5 eyes (56%) |
| Central serous chorioretinop athy (CSC) | Subretinal fluid accumulation due to dysfunction of the RPE and/or choroid | 7 (8) | 0.5% (0.2–1.0%) | 0.3% (0.1–0.6%) | 1 (14%) | Associated with PED in 6 eyes (75%) |
| Vitreomacula r traction (VMT) | Posterior vitreous detachment with evidence of retinal traction | 5 (6) | 0.3% (0.1–0.8%) | 0.2% (0.1–0.5%) | 1 (20%) | ERM in 2 eyes (33%) |
| Lamellar macular hole (LMH) | Intraretinal split associated with a thin and irregular foveal floor | 5 (5) | 0.3% (0.1–0.8%) | 0.2% (0.1–0.4%) | 0 (0) | Associated with ERM in all eyes |
| Other, (# of eyes) | Macular pseudohole (2), retinal detachment (2), large FTMH (2), cystoid macular edema (1) | |||||
MMAs could not be categorized solely based on OCT data in 16 eyes of 14 subjects (5% of MMAs cases).
Numbers and percentages are not additive to the total MMAs metrics because of the occurrence of multiple pathologies in 8% of MMAs eyes.
Subject-level prevalence defined as the proportion of subjects identified to have a specific MMAs in at least one eye in the study sample. Eye-level prevalence defined as the proportion of eyes identified to have a specific MMAs amongst all eyes included.
Abbreviations: AMD = age-related macular degeneration; BM = Bruch’s membrane; CI = confidence interval; CSC = central serous chorioretinopathy; ERM = epiretinal membrane; FP = foveal pseudocyst; FTMH = full-thickness macular hole; ILM = inner limiting membrane; INL = inner nuclear layer; MMAs = morphologic macular abnormalities; MMP = microcystoid macular pathology; ON = optic neuritis; ONL = outer nuclear layer; OPL = outer plexiform layer; PED = pigment epithelial detachment; RPE = retinal pigment epithelium.
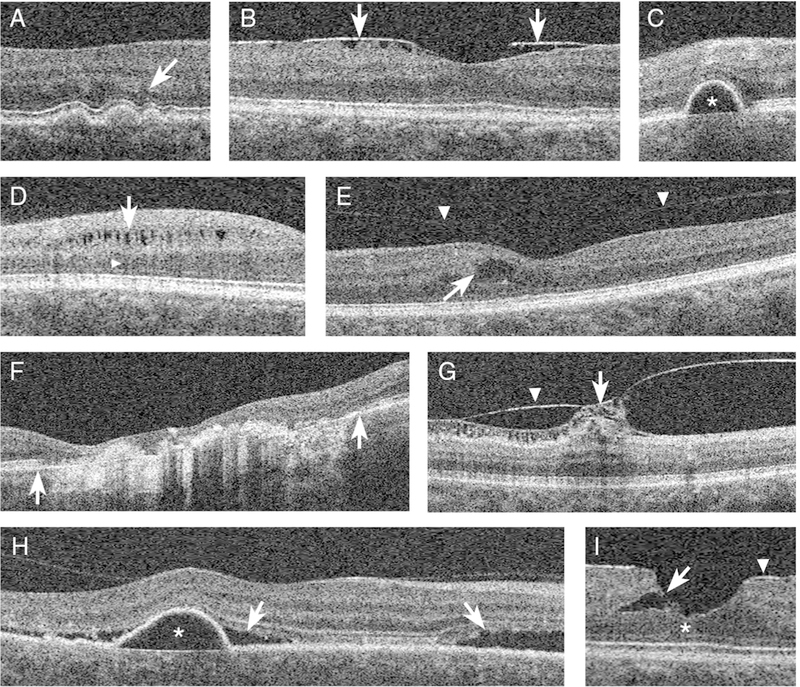
Figure 1: Examples of morphologic macular abnormalities (MMAs) detected in study eyes.
Representative OCT B-scan images demonstrating eight examples of the more commonly detected MMAs in the study. The OCT morphology terms are defined in Supplementary Table 2. A, moderately reflective drusen with overlying photoreceptor layer thinning (arrow). B, focally adherent ERM (arrows) with underlying macular pucker. C, PED characterized by homogenously hyporeflective sub-RPE fluid (star). D, microcystoid macular pathology characterized by lacunar areas of hyporeflectivity within the INL (arrow) and underlying ONL shadowing (arrowhead). E, foveal pseudocyst marked by perifoveal intraretinal cystoid changes (arrow) with signs of overlying incomplete posterior vitreous detachment (arrowheads). F, advanced atrophic age-related macular degeneration (enclosed in the area between the two arrows) with marked thinning of the RPE, increased choroid reflectivity, and overlying retinal tissue loss. G, vitreomacular traction (arrow) caused by an overarching ERM (arrowhead). H, PED (star) with overlying sub-retinal fluid accumulation characteristic of central serous chorioretinopathy (arrows). I, lamellar macular hole characterized by an intraretinal split between the OPL and ONL (arrow) and the lack of a full thickness defect in the photoreceptor layers (star) associated with an overlying ERM (arrowhead).
Abbreviations: ERM = epiretinal membrane; INL = inner nuclear layer; MMAs = morphologic macular abnormalities; OCT = optical coherence tomography; ONL = outer nuclear layer; OPL = outer plexiform layer; PED = pigment epithelial detachment; RPE = retinal pigment epithelium.
Epiretinal membrane (ERM), the second most common finding, was observed in 79 subjects (5.5%), of whom 41 had bilateral pathology (Figure 1, Panel B). Of the 79 patients identified to have an ERM, 20 cases had previously identified ocular conditions associated with secondary ERM formation, namely uveitis (n=7), intraocular surgery (n=6), and retinal detachment (n=4) amongst others. Similar to drusen, older age appeared to be a strong risk factor for the development of ERM with a 4.26-fold increase in risk with each 5 years of increasing age (95% CI 2.3–7.8; p=7×10−3). Globally adherent ERMs were noted in 57 eyes (47.5% of ERM eyes), whereas in 63 eyes (52.5% of ERM eyes) the ERM was focally attached and/or associated with apparent macular traction. In 28 eyes (23% of ERM eyes), ERMs were associated with other retinal pathology, most commonly drusen (n=10), followed by lamellar hole (n=5) and geographic atrophy (n=5).
Pigment epithelial detachment (PED) was observed in 29 subjects (2.0%). This consisted of discrete areas of sub-retinal pigment epithelium (RPE) fluid accumulation in 76% of cases (Figure 1, Panel C). In 24% of PED eyes, evidence of concomitant central serous chorioretinopathy (CSC) was also detected (Figure 1, Panel H). Conversely, among the eight eyes with CSC (0.3% prevalence), a PED or a bump in the RPE was apparent in 6 eyes (75%).
Microcystoid macular pathology (MMP) was prevalent in 28 subjects (1.9%; Figure 1, Panel D). In contrast with the prevalence patterns of MMAs overall, MMP was distinctly associated with a younger age of presentation after adjustment for sex, race, and diagnosis at presentation (OR 0.73 per each 5 years of advancing age; 95% CI 0.6–0.9; p=0.015). Interestingly, African-Americans had a 15.0-fold higher risk of harboring MMP as opposed to Caucasians (95% CI, 4–56; p=5×10−5). This finding remained statistically significant after restricting the analysis to the subgroup of patients with MS or RRMS (p=5×10−4 and 9×10−4 respectively).
In the subset of patients with MS, 24 subjects (47 eyes) were on fingolimod therapy at the time OCT was obtained. Three eyes among this group had evidence of drusen but no cases of fingolimod-associated macular edema or MMP were observed in this cohort. Within the subgroup of patients with MS, increasing age remained an independent predictor of overall MMAs, drusen and ERM prevalence after adjusting for sex and race (Supplementary Table 4). No statistically significant relationships were observed for other demographic variables. Prevalence estimates of the less commonly observed MMAs are listed in Table 2 and illustrated in Figure 1, Panels E-I. A comparison of the prevalence of MMAs amongst specific disease categories is detailed in Table 3. There were no statistically significant differences in the odds of MMAs in pairwise comparisons amongst the different disease categories after adjusting for age, sex, race, and performing Bonferroni correction for multiple comparisons.
Table 3:
| MMAs | RRMS | SPMS | PPMS | CIS | NMOSD | Sarcoidosis | Idiopathic TM |
|---|---|---|---|---|---|---|---|
| MMAs, overall | 141 (9.8) | 19 (19.4) | 16 (20.1) | 4 (3.4) | 7 (11.5) | 24 (22.6) | 26 (13.4) |
| Drusen | 43 (3.0) | 7 (7.1) | 9 (11.7) | 2 (1.7) | 3 (4.9) | 13 (12.3) | 6 (3.1) |
| Epiretinal membrane | 46 (3.2) | 9 (9.2) | 5 (6.5) | - | 1 (1.6) | 5 (4.7) | 15 (7.7) |
| Pigment epithelial detachment | 17 (1.2) | 1 (1.02) | - | 1 (0.9) | 1 (1.6) | 2 (1.9) | 3 (1.6) |
| Microcystoid macular pathology | 23 (1.6) | 2 (2.0) | 3 (3.9) | - | - | 2 (1.9) | - |
| Foveal cystoid changes | 3 (0.2) | 1 (1.0) | - | - | - | - | 3 (1.6) |
| Geographic atrophy or other advanced forms of AMD | 5 (0.4) | - | - | - | - | - | 2 (1.0) |
| Central serous chorioretinopathy | - | - | - | - | - | 1 (0.9) | - |
| Vitreomacular traction | 3 (0.2) | - | - | - | - | 2 (1.9) | 1 (0.5) |
| Lamellar macular hole | 1 (0.1) | 1 (1.0) | - | - | - | - | - |
Data is presented as n (%), where n is the number of eyes and the percentage refers to the proportion of abnormal eyes compared to the total number of eyes for that disease category (i.e. prevalence).
No statistically significant differences were found in the odds of MMAs amongst the different disease categories (using the presence/absence of MMAs as dependent variable and the diagnosis as a categorical co-variate), using multilevel logistic regression models with pairwise comparisons adjusting for age, sex, race, and performing Bonferroni correction for multiple comparisons.
Abbreviations: AMD = age-related macular degeneration; CIS = clinically isolated syndrome; MMAs = morphologic macular abnormalities; NMOSD = neuromyelitis optica spectrum disorders; PPMS = primary progressive multiple sclerosis; RRMS = relapsing-remitting multiple sclerosis; SPMS = secondary progressive multiple sclerosis; TM = transverse myelitis.
The prevalence data for drusen and ERM (the two most common abnormalities observed in the study) were compared to age-matched, population-based estimates obtained from the Beaver Dam Eye study13,14 (Table 4). Drusen prevalence rates for the overall cohort were similar to population-based estimates between the ages of 43 to 54 years and significantly lower between the ages of 55 to 64, and 65 to 74 years (p=0.003 and 0.013 respectively). Similar trends were seen in the MS subgroup, although they did not reach statistical significance. Interestingly, ERM prevalence rates for the overall cohort were consistently higher than those seen in the general population amongst all ages included in the comparison, but reached statistical significance only in the age groups 43 to 54, and 65 to 74 years (p=0.001 and 1×10−4 respectively). This appeared to be driven mainly by the subgroup of patients with MS as illustrated in Table 4. No statistically significant differences in drusen or ERM prevalence were noted among the different MS subtypes. As a sensitivity analysis, the ERM prevalence data were also compared to population-based estimates from the Visual Impairment project15, which revealed similar trends, particularly in the 40 to 69 years age epoch (Supplementary Table 5). The prevalence estimates for patients older than 75 years were unreliable due to the small sample size, thereby precluding meaningful comparisons.
Table 4:
Age-matched prevalence estimates of drusen and epiretinal membrane in this study (overall and in the multiple sclerosis subgroup) compared to population-based estimates obtained from the Beaver Dam Eye Study
| Age, yrs | Drusen prevalence, % (No. at Risk; 95% CI)1 | ERM prevalence, % (No. at Risk; 95% CI)2 | ||||
|---|---|---|---|---|---|---|
| Overall cohort | MS subgroup | Population-based estimates | Overall cohort | MS subgroup | Population-based estimates2 | |
| <43 | 3.2 (665; 2.1–4.8) | 2.7 (407; 1.5–4.8) | - (0) | 1.4 (665; 0.7–2.6) | 1.0 (407; 0.4–2.6) | - (0) |
| 43–54 | 7.4 (488; 5.4–10.1) | 6.5 (262; 4.1–10.2) | 7.1 (1504; 5.9–8.5) | 4.3 (488; 2.8–6.5) * | 5.0 (262; 2.9–8.4) * | 1.7 (1419; 1.1–2.5) |
| 55–64 | 8.6 (221; 5.5–13.1) ǂ | 8.1 (124; 4.4–14.5) | 16.3 (1301; 14.3–18.4) | 11.8 (221; 8.1–16.8) | 12.9 (124; 8.0–20.1) | 6.7 (1145; 5.3–8.3) |
| 65–74 | 10.6 (66; 5.0–21.0) ǂ | 15 (20; 4.4–40.4) | 23.9 (1249; 21.6–26.4) | 30.3 (66; 20.2–42.7) * | 35 (20; 16.4–59.6) * | 11.7 (1035; 9.8–13.8) |
| 75+ | 80 (5; 0.1–99.2) | - (0) | 44.3 (713; 40.6–48.1) | 60 (5; 8.1–96.2) | - (0) | 8.5 (526; 6.3–11.3) |
Population-based estimate data extracted from Klein et al15.
Population-based estimate data obtained by weighted-averaging of the ERM prevalence estimates of both genders in each age stratum of the right eyes included in the Beaver Dam Eye study16.
Denotes a significantly higher prevalence rate compared to general population estimates.
Denotes a significantly lower prevalence rate compared to general population estimates.
Abbreviations: CI = confidence interval; ERM = epiretinal membrane; MS = multiple sclerosis.
Discussion
OCT has been gaining momentum in the field of neurology as an important tool to help elucidate discrete patterns of anterior visual pathway injury and aid in the diagnosis and monitoring of neurological conditions. Our investigation characterizes general retinal pathologies seen on OCT in neuroimmunology practice illustrating that in patients undergoing OCT as part of their neurologic evaluation, unexpected or incidental MMAs are not uncommon and occur, on average, in one out of seven patients. The strongest risk factor involved in the detection of MMAs is age, emphasizing the importance of close OCT scan review particularly in older patient populations.
While many of the retinal findings described may exhibit an innocuous appearance, a proportion are associated with significant visual morbidity or have the potential to progress to more deleterious complications, thus warranting further ophthalmologic evaluation and management (Table 5 and Supplementary Figure 1). It is important to note that nearly half of the detected MMAs (48%) were peripherally located and not apparent on central horizontal or vertical B-scans (which are often the only images present on automated OCT reports), and therefore may be easily overlooked. In practice, this underscores the importance of comprehensive OCT scan review instead of relying solely on central B-scan representative images. Next, we will address specific MMAs and their clinical relevance in order of their prevalence.
Table 5:
Examples of retinal abnormalities warranting further ophthalmological referral if incidentally detected on OCT imaging
| Type of retinal abnormality | Urgency of ophthalmological referral |
|---|---|
| Multiple or aggregate drusen deposits | Routine |
| Epiretinal membrane | Routine, maybe urgent if there are signs of macular edema/retinal traction or progressive visual compromise |
| Pigment epithelial detachment | Routine |
| Foveal pseudocyst | Urgent |
| Geographic atrophy or other advanced forms of AMD | Urgent |
| Central serous chorioretinopathy | Routine, maybe urgent if treatment with systemic corticosteroids is contemplated |
| Lamellar macular hole | Urgent |
| Retinal detachment | Ocular emergency |
| Macular hole | Urgent |
| Cystoid macular edema | Urgent |
Abbreviations: AMD = age-related macular degeneration
Our results suggest that retinal pathology associated with or indicative of age-related macular degeneration (AMD), such as drusen, PED, and geographic atrophy, appear to be among the most prevalent findings in neuroimmunology practice. When compared to age-matched prevalence estimates from US-based population studies13, the prevalence patterns for drusen appear to be closely similar to those seen in the general population for the epoch of patients aged 43 to 54 years, but appeared lower between the ages of 55 to 74 years (Table 4). While macular drusen may arguably be the first clinically evident sign of AMD, its presence often times denotes age-related changes or could serve as an epiphenomenon of RPE cell dysfunction in the absence of any looming threat of visual compromise16,17. In this context, a fundamental point to be made is that identifying the type of drusen is critical in terms of estimating an individual’s risk of AMD, especially when it is incidentally found without any referable visual complaints17. For instance, hard drusen (appearing as small, distinct, and segregated yellow deposits on fundus imaging) has less than 3% risk of developing into advanced AMD at 15 years, as opposed to soft drusen (appearing as larger, indistinct areas that tend to cluster together) where the incidence can range between 13 to 53% depending on the exact morphology of the deposits18,19. Such distinction between soft and hard drusen is often not readily apparent solely based on OCT imaging and, therefore, further ophthalmologic evaluation is vital for risk stratification.
Similar to prior reports, advanced age was an independent risk factor for ERM prevalence in our analysis15,20. One principal finding, however, was that patients with MS appeared to have a two-to-three-fold higher risk of ERMs compared to general population estimates obtained from the Beaver Dam and Visual Impairment project studies after adjustment for age (Table 4 and Supplementary Table 5 respectively). Previous investigations have highlighted a central role for intra-ocular inflammation, particularly uveitis, in the pathogenesis and formation of ERMs21. Interestingly, uveitis was the most common cause of secondary ERM in our cohort, a factor that is exceedingly relevant in patients with MS and sarcoidosis, who are at increased risk of pars planitis and panuveitis22. Therefore, it can be hypothesized that MS patients appear to have a higher incidence of clinical and subclinical retinal inflammation that might be driving the formation of ERMs or other forms of retinal pathology, such as MMP, occurring subsequently2.
The fourth most common MMAs reported in our investigation was MMP, a retinal abnormality that has gained wide attention as a marker of visual disability in patients with various forms of optic neuropathy. In recent years, practice-based studies have described prevalence data on MMP ranging from 3 to 6% in patients with MS and has been linked to prior history of optic neuritis2,3,23,24. Longitudinal studies examining the timing of MMP development following optic neuritis are lacking, however, cross-sectional studies have shown that this can occur on the order of months following confirmed visual pathway injury25. Furthermore, the presence of this abnormality has been linked to the severity of inner retinal layer thinning and a younger age at presentation26. Indeed, in contrast to other retinal pathologies reported in this investigation, MMP was associated with a higher likelihood of visual symptomatology and had a compelling trend of decreased risk with advancing age. Our study, however, demonstrates that racial factors add another dimension when it comes to explaining some of the heterogeneity behind MMP occurrence, with African-Americans having a 15-fold higher risk of MMP as opposed to Caucasians. This was mostly driven by the subset of patients with MS in the study. A conclusive reasoning behind this predisposition is hard to propose. One of the most appealing explanations, however, is that African-Americans MS patients appear to exhibit a more aggressive phenotype of retinal damage characterized by accelerated retinal nerve fiber and ganglion cell layer thinning compared to Caucasian MS patients27,28. This tendency may be a driving factor for reactive INL changes, including MMP, occurring in tandem. In addition, genetic factors may underlie an increased susceptibility towards blood-retinal barrier disruption or a dysregulated glial inflammatory response induced by ganglion cell death culminating in INL edema. In accordance with these observations, potential relationships between genetic biomarkers and retinal damage in MS and/or optic neuritis warrants further exploration.
A salient point underscored by the findings of this study is the importance of recognizing subtypes of MMAs that may influence clinical decision making. For example, CSC (observed in 0.5% of subjects) develops due to leakage of fluid into the subretinal space, which can ultimately lead to acute visual loss mimicking acute optic neuritis. CSC was observed in 7 subjects in this study: one patient with neurosarcoidosis, three patients with possible MS, one patient with ALS, and two patients in whom the diagnosis was unclear. Corticosteroid use has a long track record of precipitating and/or exacerbating CSC and their use might be avoided in this setting29. Furthermore, susceptible patients with CSC in whom corticosteroid therapy is contemplated for other neurological or systemic disorders should be counseled about the risk of developing acute ocular complications. A similar issue arises with fingolimod therapy whereby macular edema has been reported as an important adverse event occurring in 0.5% of patients30. No cases of fingolimod-associated macular edema were observed in our study. This probably relates to the small size of MS patients on fingolimod therapy in our cohort of patients (n=24), since its US Food and Drug Administration approval in September 2010 occurred shortly after the start of patient recruitment. Therefore, this has likely limited the total number of MS patients on fingolimod as well as the length of therapy prior to OCT acquisition.
One particular strength of this study is the systematic selection of an entire cohort of neurologic patients undergoing OCT, using the same acquisition protocol, during a predesignated time period, thereby minimizing a diversity of potential selection biases. Although, due to the retrospective nature of the study, visual complaints were not systematically captured in a uniform method amongst the study participants, which limited our ability to correlate OCT findings with specific symptomatology. That being said, the goal of this study is to demonstrate that the presence of certain MMAs on OCT merits further ophthalmological evaluation, regardless of visual symptomatology, to establish the ocular diagnosis and for risk stratification purposes. Another point worthy of mentioning is that our population of patients were primarily selected from a neuroimmunology-based practice, which limits the generalizability of our findings to other neurological patient populations.
In summary, as OCT starts to make its way into routine neurologic practice, our study highlights the need for pragmatic review of OCT scan images as many incidental MMAs can masquerade behind neurological complaints. Neurologists involved in the interpretation of OCT images should be equipped with the tools necessary to identify common qualitative retinal findings and, more importantly, recognize the need for appropriate referral to an ophthalmologist or retinal specialist for further evaluation (Table 5). In research applications, many of these morphological findings can impact the performance of automated macular segmentation algorithms and recent quality control criteria have been instituted to minimize this occurrence31. Collectively, employing measures for reliable identification of MMAs in neurologic practice represents an important step towards improving patient outcomes by early recognition of retinal disease before symptoms and irreversible visual loss are underway.
Supplementary Material
Acknowledgments
Funding:
This study was funded by a Race to Erase MS grant to SS and National Institutes of Health grant 5R01NS082347 to PAC.
Dr. Vidal-Jordana: has received speaking honoraria from Sanofi-Aventis, and Novartis, and has received consulting fees from Biogen-Idec and Roche.
Dr. Balcer has received consulting honoraria from Biogen.
Dr. Frohman has received speaker and consulting fees from Novartis, Genzyme, Acorda, and TEVA.
Dr. Calabresi has received personal compensation for consulting and serving on scientific advisory boards from Vertex, Vaccinex, Merck, and Abbvie and has received research funding from Biogen-IDEC, MedImmune, and Novartis.
Dr. Saidha has received consulting fees from Medical Logix for the development of CME programs in neurology, consulting fees from Axon Advisors LLC, Educational Grant Support from Novartis & Teva Neurosciences, speaking honoraria from the National Association of Managed Care Physicians, Family Medicine Foundation of West Virginia, and Advanced Studies in Medicine and served on a scientific advisory board for Biogen-Idec, Genzyme and Novartis. He also receives research funding from the Race to Erase MS and Genentech Corporation.
Dr. Newsome has received consultant fees for scientific advisory boards from Biogen, and Genentech and has received research funding (paid directly to institution) from Biogen, Novartis, Genentech, Department of Defense and the National MS Society.
Footnotes
Conflicts of interest:
The authors report no conflicts of interest pertaining to the work presented in this manuscript.
Disclosures:
Dr. Al-Louzi, Dr. Sotirchos, Dr. Beh, Dr. Ying, and J. Button report no disclosures.
References:
- 1.Calabresi P, Balcer L, Frohman E. Optical Coherence Tomography in Neurologic Diseases. Cambridge University Press; 2015. [Google Scholar]
- 2.Saidha S, Sotirchos ES, Ibrahim M a, et al. Microcystic macular oedema, thickness of the inner nuclear layer of the retina, and disease characteristics in multiple sclerosis: a retrospective study. Lancet Neurol. 2012;11(11):963–972. doi: 10.1016/S1474-4422(12)70213-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gelfand JM, Nolan R, Schwartz DM, Graves J, Green AJ. Microcystic macular oedema in multiple sclerosis is associated with disease severity. Brain. 2012;135(Pt 6):1786–1793. doi: 10.1093/brain/aws098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sotirchos ES, Saidha S, Byraiah G, et al. In vivo identification of morphologic retinal abnormalities in neuromyelitis optica. Neurology. 2013;80(15):1406–1414. doi: 10.1212/WNL.0b013e31828c2f7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gelfand JM, Cree BA, Nolan R, Arnow S, Green AJ. Microcystic inner nuclear layer abnormalities and neuromyelitis optica. JAMA Neurol. 2013;70(5):629–633. doi: 10.1001/jamaneurol.2013.1832. [DOI] [PubMed] [Google Scholar]
- 6.Moschos MM, Markopoulos I, Chatziralli I, et al. Structural and functional impairment of the retina and optic nerve in Alzheimer’s disease. Curr Alzheimer Res. 2012;9(7):782–788. http://www.ncbi.nlm.nih.gov/pubmed/22698074 Accessed February 12, 2014. [DOI] [PubMed] [Google Scholar]
- 7.Bodis-Wollner I. Retinopathy in Parkinson Disease. J Neural Transm. 2009;116(11):1493–1501. doi: 10.1007/s00702-009-0292-z. [DOI] [PubMed] [Google Scholar]
- 8.Joshi M, Agrawal S, Christoforidis JB. Inflammatory mechanisms of idiopathic epiretinal membrane formation. Mediators Inflamm. 2013;2013:192582. doi: 10.1155/2013/192582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson MW. Posterior vitreous detachment: evolution and complications of its early stages. Am J Ophthalmol. 2010;149(3):371–82.e1. doi: 10.1016/j.ajo.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 10.Towler HM, Lightman S. Symptomatic intraocular inflammation in multiple sclerosis. Clin Experiment Ophthalmol. 2000;28(2):97–102. http://www.ncbi.nlm.nih.gov/pubmed/10933771 Accessed July 30, 2017. [DOI] [PubMed] [Google Scholar]
- 11.Saidha S, Syc SB, Ibrahim M a, et al. Primary retinal pathology in multiple sclerosis as detected by optical coherence tomography. Brain. 2011;134(Pt 2):518–533. doi: 10.1093/brain/awq346. [DOI] [PubMed] [Google Scholar]
- 12.Newsome SD, Ratchford JN. Optical coherence tomography pathologies to know about in clinical practice In: Optical Coherence Tomography in Neurologic Diseases. Cambridge University Press; 2015:145–155. [Google Scholar]
- 13.Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology. 1992;99(6):933–943. http://www.ncbi.nlm.nih.gov/pubmed/1630784 Accessed June 26, 2016. [DOI] [PubMed] [Google Scholar]
- 14.Klein R, Klein BE, Wang Q, Moss SE. The epidemiology of epiretinal membranes. Trans Am Ophthalmol Soc. 1994;92:403–25-30 http://www.ncbi.nlm.nih.gov/pubmed/7886875 Accessed June 26, 2016. [PMC free article] [PubMed] [Google Scholar]
- 15.McCarty DJ, Mukesh BN, Chikani V, et al. Prevalence and associations of epiretinal membranes in the visual impairment project. Am J Ophthalmol. 2005;140(2):288–294. doi: 10.1016/j.ajo.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 16.Anderson DH, Mullins RF, Hageman GS, Johnson LV. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2002;134(3):411–431. doi: 10.1016/S0002-9394(02)01624-0. [DOI] [PubMed] [Google Scholar]
- 17.Williams MA, Craig D, Passmore P, Silvestri G. Retinal drusen: harbingers of age, safe havens for trouble. Age Ageing. 2009;38(6):648–654. doi: 10.1093/ageing/afp136. [DOI] [PubMed] [Google Scholar]
- 18.Buch H, Nielsen NV, Vinding T, Jensen GB, Prause JU, la Cour M. 14-year incidence, progression, and visual morbidity of age-related maculopathy: the Copenhagen City Eye Study. Ophthalmology. 2005;112(5):787–798. doi: 10.1016/j.ophtha.2004.11.040. [DOI] [PubMed] [Google Scholar]
- 19.Klein R, Klein BEK, Knudtson MD, Meuer SM, Swift M, Gangnon RE. Fifteen-year cumulative incidence of age-related macular degeneration: the Beaver Dam Eye Study. Ophthalmology. 2007;114(2):253–262. doi: 10.1016/j.ophtha.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell P, Smith W, Chey T, Wang JJ, Chang A. Prevalence and associations of epiretinal membranes. The Blue Mountains Eye Study, Australia. Ophthalmology. 1997;104(6):1033–1040. http://www.ncbi.nlm.nih.gov/pubmed/9186446 Accessed January 25, 2016. [DOI] [PubMed] [Google Scholar]
- 21.Nicholson BP, Zhou M, Rostamizadeh M, et al. Epidemiology of epiretinal membrane in a large cohort of patients with uveitis. Ophthalmology. 2014;121(12):2393–2398. doi: 10.1016/j.ophtha.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biousse V, Trichet C, Bloch-Michel E, Roullet E. Multiple sclerosis associated with uveitis in two large clinic-based series. Neurology. 1999;52(1):179–179. doi: 10.1212/WNL.52.1.179. [DOI] [PubMed] [Google Scholar]
- 23.Kaufhold F, Zimmermann H, Schneider E, et al. Optic neuritis is associated with inner nuclear layer thickening and microcystic macular edema independently of multiple sclerosis. PLoS One. 2013;8(8):e71145. doi: 10.1371/journal.pone.0071145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burggraaff MC, Trieu J, de Vries-Knoppert WAEJ, Balk L, Petzold A. The clinical spectrum of microcystic macular edema. Invest Ophthalmol Vis Sci. 2014;55(2):952–961. doi: 10.1167/iovs.13-12912. [DOI] [PubMed] [Google Scholar]
- 25.Al-Louzi O, Button J, Newsome SD, Calabresi PA, Saidha S. Retrograde trans-synaptic visual pathway degeneration in multiple sclerosis: A case series. Mult Scler J. 2017;23(7):1035–1039. doi: 10.1177/1352458516679035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abegg M, Dysli M, Wolf S, Kowal J, Dufour P, Zinkernagel M. Microcystic macular edema: retrograde maculopathy caused by optic neuropathy. Ophthalmology. 2014;121(1):142–149. doi: 10.1016/j.ophtha.2013.08.045. [DOI] [PubMed] [Google Scholar]
- 27.Phillips PH, Newman NJ, Lynn MJ. Optic neuritis in African Americans. Arch Neurol. 1998;55(2):186–192. http://www.ncbi.nlm.nih.gov/pubmed/9482360. [DOI] [PubMed] [Google Scholar]
- 28.Kimbrough DJ, Sotirchos ES, Wilson J a., et al. Retinal damage and vision loss in African American multiple sclerosis patients. Ann Neurol. 2015;77(2):228–236. doi: 10.1002/ana.24308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carvalho-Recchia CA, Yannuzzi LA, Negrão S, et al. Corticosteroids and central serous chorioretinopathy. Ophthalmology. 2002;109(10):1834–1837. http://www.ncbi.nlm.nih.gov/pubmed/12359603 Accessed January 27, 2016. [DOI] [PubMed] [Google Scholar]
- 30.Jain N, Bhatti MT. Fingolimod-associated macular edema: incidence, detection, and management. Neurology. 2012;78(9):672–680. doi: 10.1212/WNL.0b013e318248deea. [DOI] [PubMed] [Google Scholar]
- 31.Tewarie P, Balk L, Costello F, et al. The OSCAR-IB consensus criteria for retinal OCT quality assessment. PLoS One. 2012;7(4):e34823. doi: 10.1371/journal.pone.0034823. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.