Abstract
Background:
Quality of life (QoL) in primary brain tumour (PBT) is often the main outcome measure in an otherwise incurable disease. The impact of psychiatric, cognitive correlates on quality of life in primary brain tumours is less well studied.
Aims and objectives:
The primary objective was to find out the association of psychiatric morbidity, cognitive functions with quality of life in patients with primary brain tumours. The secondary objective was to study whether any association exists with tumour grading, laterality, location and psychiatric morbidity.
Materials and Methods:
100 consecutive patients of PBT were screened in the Neuro-behavioural Clinic. Age, gender matched 52 healthy subjects were taken for comparison. Quality of life (qol) measure (EORTC), Hospital Anxiety Depression Scale (HADS), GHQ (12 item) and Mini Mental State Examination (MMSE) were administered.
Results:
52 PBT cases were included, out of which 17.30% had Organic Anxiety Disorder (F06.4), 23.07% had Organic Mood disorder (F06.3%).Statistically significant association was found in EORTC qol scores and anxiety scores (p 0.001), depressive scores (p 0.029), psychiatric morbidity (p0.000) .Significant association with tumour laterality, depression scores (p0.041) was found. PBT patients had poor quality of life as compared to matched healthy volunteers (p <0.001). Significant negative correlation between EORTC B-20, cognitive scores using Spearman's Rho (p0.005; r - 0.385), implying more symptoms with poor cognitive function scores. Psychiatric morbidity, cognitive dysfunction, poor qol were noted, though no association with tumour grading, location.
Conclusion:
Regular assessments, early intervention will help in improving quality of life in PBT.
Keywords: Anxiety, brain tumors, depression, Mini-Mental State Examination, quality of life, tumor laterality
Brain tumors have an overall incidence of 10.82/100,000 person-years.[1] Primary brain tumors (PBTs) account for 2% of all cancers. Malignant PBTs pose a therapeutic challenge to clinicians with a 5-year survival rate of about 35%. Gliomas constitute about 75% of primary malignant brain tumors, whereas meningiomas are the most common nonmalignant brain tumors.[2] Usually, the detection of brain tumor occurs in advanced stages when its presence has caused unexplained symptoms.
The generalized symptoms in the form of headache, anorexia, nausea, vomiting, seizures, longer sleeps at night, and drowsiness with napping during the day are the common presentations of brain tumors.[3] Clinical presentation depends on the size of tumor and its location, with focal neurological deficits appearing when the lesion presses on the vital brain areas. The behavioral and cognitive defects are noticed The mood symptoms are reported in tumours involving frontal areas, parietal association areas, para-limbic areas and cognitive deficits noticed in frontal and temporal lobe involvement. A recent study carried out by Wang et al. detected the presence of depressive symptoms in postoperative glioma patients using a rating scale.[4] Anxiety symptoms were found to be associated with tumors in the right hemisphere in 101 patients of PBTs.[5] A recent meta-analysis of 37 studies pooled over a period of 35 years by Huang et al. reported the prevalence of depressive symptoms to be 21.7%.[6]
The reported studies on psychiatric morbidity in PBT were based on parameters such as type of tumor, localization, laterality, and demographic details. Studies found the presence of depression and anxiety symptoms in neurofibromatosis[7] and in anteriorly located meningiomas.[8] A recent meta-analysis using six articles found high-grade gliomas to be associated with poor survival.[9] Temporal lobe gliomas were found to be closely related to seizures and psychiatric disorders (72%), more commonly in left-handed individuals.[10] A study by Arnold et al. found that female gender was more likely to develop anxiety, depression, or combined presentation as compared to men in 362 patients visiting a neuro-oncology clinic. The low educational level and low tumor grade emerged as the predictors of these clinical symptoms.[11]
Cognitive deficits had been documented in both high- and low-grade gliomas,[12] frontal meningiomas preoperatively,[13] though a wide variation in the improvement of different cognitive domains was noted postoperatively. Assessment of cognitive functions is now being considered as an important prognostic marker in brain tumor therapy. Mini-Mental State Examination (MMSE) is a useful, short tool for screening neuro-cognitive functions in PBTs. Other associated factors such as tumor location, grading, age, educational status, and treatment interventions also play a role.[14]
Quality of life (QOL) as an important outcome measure has been studied recently with psychiatric correlates and cognitive functions, though research required for generating evidence-based clinical practice still remains inconclusive.[15,16] Osoba et al. studied the effect of burden of the disease due to recurrence on QOL and found that the patients with glioblastoma multiforme or anaplastic astrocytoma had comparable QOL, though recurrent high-grade glioma had lower QOL scores than other localized ovarian and lung cancer. QOL scores of these recurrent brain tumors were quite similar to metastatic heterogeneous cancers.[17]
The main objective of clinical management of brain cancer is to restore neurologic functioning and the amelioration of psychiatric symptoms. The current treatment modalities such as surgery, radiotherapy, or chemotherapy have not been very promising in this regard. Improvement in QOL is an important indicator for assessing patients' response to treatment. Hence, early assessment of such morbidities and subsequent treatment shall greatly help in improving the QOL. The present study had included PBTs irrespective of their location or grading or laterality. The authors carried out this work with the aim of studying the association between psychiatric and cognitive correlates with QOL in patients with PBTs. The secondary objective was to study whether any association exists with tumor grading, laterality, location (supratentorial and infratentorial), and psychiatric morbidity. We hypothesized that the QOL in patients of PBTs will be lower as compared to the age-gender matched healthy controls.
METHODS
The study was approved by the Institutional Ethical Committee of University College of Medical Sciences, New Delhi, India.
Subjects
All consecutively diagnosed cases of brain tumors visiting the Neurobehavioral Outpatient Clinic during the study period from 2012 to 2015 were screened. The study participants diagnosed as brain tumors (both primary as well as secondary) were referred from the Department of Neurosurgery and Adjacent Oncology Center located in the premises of Guru Teg Bahadur Hospital and University College of Medical Sciences, New Delhi, India. A total of 100 patients in the age range of 18–65 years were screened in the Neurobehavioral Clinic during the study period. The following patients were excluded: 10 patients were found to be suffering from unstable medical condition, 8 patients had delirium, 10 patients refused to take part in the study because of long waiting hours and had to travel long distance, 5 patients had organic psychosis, 10 had cognitive impairment defined as MMSE score <24, and 13 had secondary brain tumors. Finally, 52 patients of PBTs who could read, write, and sign the written informed consent were included. Age- and gender-matched apparently healthy volunteers were recruited through the advertisements from the Neurobehavioral Clinic.
The clinical information and sociodemographic details were noted on the case record pro forma specially designed for the Neurobehavioral Clinic. Data regarding history of patient, examination, investigation, and treatment were collected from the case sheets of patients provided by the Neurosurgery, Neuroradiology, and Oncology Departments. Data also included details such as location and type of tumor, treatment details (chemotherapy/radiotherapy/surgery), duration of treatment, neuroimaging investigations, tumor grading by pathology reports, and neuroimaging. Tumors were graded according to the World Health Organization grading.[18]
A psychiatric evaluation of all the patients was carried out as per the case record pro forma of the Neurobehavioral Clinic. The structured clinical interview was carried out by experienced psychiatrists. The team comprising neuroradiologists, neurosurgeons, and psychiatrists carried out their respective assessments. Tumor location (whether supratentorial, infratentorial, or both) and laterality (whether left, right, midline, or bilateral) were carried out by a neuroradiologist. A psychiatric diagnosis was made according to the International Classification of Diseases, Tenth Revision (ICD-10), and the appropriate intervention was initiated. The following illustrations show different types of primary brain tumours which are as follows: Figure 1 (Frontal lobe biopsy proven Diffuse Astrocytoma, WHO Grade II), Figure 2 (Frontal lobe Butterfly Glioma, WHO Grade IV), Figure 3 (Frontotemporal lobe Glioblastoma Multiforme, WHO GradeIV).
Figure 1.
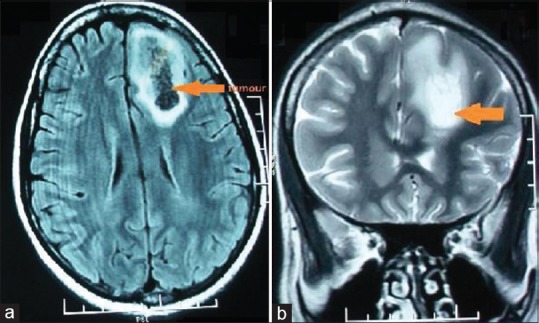
(a) Axial FLAIR and (b) coronal T2-weighted magnetic resonance images reveal an intra-axial heterogeneous signal intensity supratentorial mass with central necrosis and peritumoral edema in the left frontal lobe biopsy-proven diffuse astrocytoma (World Health Organization Grade II)
Figure 2.

(a) Sagittal T2-weighted and (b) axial T1-weighted magnetic resonance images reveal a large ill-defined infiltrative intra-axial midline supratentorial mass involving the corpus callosum with butterfly-shaped extension into both frontal lobe butterfly glioma (World Health Organization Grade IV)
Figure 3.
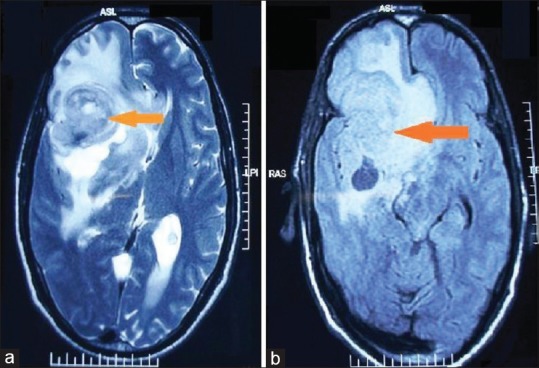
(a) Axial T2-weighted and (b) FLAIR magnetic resonance images reveal an intra-axial poorly marginated heterogeneous signal intensity supratentorial mass with necrosis, hemorrhage, and peritumoral edema in the right frontotemporal lobe glioblastoma multiforme (World Health Organization Grade IV)
Assessments
Patients were evaluated for cognitive function, QOL, anxiety, depression, and psychiatric morbidity using the following tools.
QOL was assessed using the brain module of the European Organization for Research and Treatment of Cancer (EORTC) questionnaire which contains twenty questions. The self-administered questionnaire available in Hindi language[19] was given to all the study participants. The questionnaire comprises four domains such as future uncertainty, visual disorder, motor dysfunction, and communication deficit. There are seven single items. The questions are rated on a scale of 1–4. The items on EORTC QLQ-BN20 (European Organization for Research and Treatment of Cancer Quality of life) measures are scaled, scored, and transformed to a linear scale ranging from 0 to 100. Differences of ≥10 points are classified as meaningful changes in QOL parameter.
The 12-item General Health Questionnaire (GHQ-12) was used to assess psychiatric morbidity.[20] It is a screening tool available in Hindi language. GHQ score of more than 2 is considered to be significant.
Hospital Anxiety and Depression Scale (HADS) questionnaire was used for the assessment of anxiety and depressive symptoms.[21] HADS questionnaire consists of 14 questions. The HADS contains 14 items and consists of two subscales: anxiety and depression. Each item is rated on a four-point scale of 0–3, giving maximum scores of 21 for anxiety and depression, respectively. Scores of 11 or more on either subscale are considered to be a significant “case” of psychological morbidity, while scores of 8–10 represent a “mood disorder.” A score of 7 or below is considered as normal.
Cognitive functions were assessed using MMSE.[22] It contains questions regarding orientation, registration, attention and calculation, recall, and language.
Data analysis
Data were analyzed using Statistical Package (SPSS) version 20 (SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.2011). Association between QOL and psychiatric morbidity, depression, anxiety scores, and cognitive scores was carried out using the Mann–Whitney test. Nonparametric correlations were found using Spearman's rho correlation between QOL parameters, anxiety, depressive symptom scores, cognitive functions, and psychiatric morbidity.
RESULTS
Demographic variables
PBT cases versus age- and gender-matched healthy controls (HCs) were compared for QOL as assessed on EORTC (20-item questionnaire), psychiatric morbidity (as on GHQ-12), anxiety and depressive symptoms on HADS, and cognitive functions on MMSE [Table 1]. No statistically significant difference was found between PBT cases and HCs on religion (P = 0.066) and marital status (P = 0.819). PBT cases were predominantly Hindus (35, 67.30%), whereas 17 (32.69%) were Muslims. Thirty (57.69%) of the study participants were from the middle socioeconomic status, 10 (19.23%) from the upper socioeconomic status, and 12 (23.07%) from the lower socioeconomic status.
Table 1.
Demographic and clinical variables in study sample
| PBT | Control group | Significance (P) | |
|---|---|---|---|
| Gender, n (%) | |||
| Male | 32 (61.53) | 30 (57.69) | 0.689 |
| Female | 20 (38.46) | 22 (42.30) | |
| Characteristic, mean (SD) | |||
| Age | 38.17 (14.06) | 39.54 (14.36) | 0.592 |
| EORTC QOL | 28.02 (5.267) | 21.88 (2.533) | <0.001* |
| GHQ-12 | 2.04 (2.737) | 0.50 (1.820) | 0.000 |
| HADS | 7.56 (7.627) | 3.96 (4.794) | 0.024* |
| MMSE | 25.62 (2.198) | 28.92 (2.936) | <0.001* |
*P<0.05 was considered statistically significant. Chi-square test was used for gender, Mann–Whitney test for HADS and 12-item GHQ. PBT – Primary brain tumor; EORTC QOL – European Organization for Research and Treatment of Cancer quality of life scores; GHQ-12 – 12-item General Health Questionnaire scores; HADS – Hospital Anxiety and Depression Scale scores; MMSE – Mini-Mental State Examination scores; SD – Standard deviation
Psychiatric morbidity and quality of life in primary brain tumor
The Mann–Whitney test showed a statistically significant association between Quality of life scores(EORTC QOL )and anxiety, depression and psychiatric morbidity scores which is shown in Graphs 1, 2, Table 2. Nine (17.30%) study participants had anxiety scores more than or equal to 8 (mean score was 41.28), corresponding with the description of generalized anxiety disorder, classified as an organic anxiety disorder (F = 06.4) using ICD-10. Twelve study participants (23.07%) had more than or equal to 8 score of depressive symptoms (mean score – 34.88), corresponding with the clinical description of depressive disorder, classified under organic mood disorder (F = 06.3) on ICD-10. A statistically significant correlation using Spearman's rho was obtained for EORTC versus anxiety scores (P = 0.001; r = 0.453) and EORTC versus depression scores (P = 0.001; r = 0.449).
Graph 1.
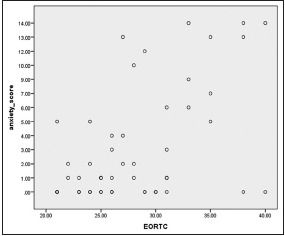
Spearman’s rho correlation between the European Organization for Research and Treatment of Cancer quality of life versus anxiety scores in primary brain tumors (P = 0.001*, r = 0.453), *P < 0.05 considered significant
Graph 2.

Spearman’s rho correlation between the European Organization for Research and Treatment of Cancer quality of life versus depression scores in primary brain tumors (P = 0.001*, r = 0.449), *P < 0.05 considered significant
Table 2.
Quality of life scores versus anxiety, depression, and psychiatric morbidity scores in primary brain tumor
| EORTC QOL | Anxiety scores | n (%) | Mean rank | Sum of ranks | P | |
|---|---|---|---|---|---|---|
| Mean | 28.02 | 7 or < | 43 (82.7) | 23.41 | 1006.50 | 0.001* |
| SD | 5.26 | 8 or > | 9 (17.3) | 41.28 | 371.50 | |
| EORTC QOL | Depression scores | n (%) | Mean rank | Sum of ranks | P | |
| Mean | 28.02 | 7 or < | 40 (76.9) | 23.99 | 959.50 | 0.029* |
| SD | 5.26 | 8 or > | 12 (23.1) | 34.88 | 418.50 | |
| EORTC QOL | GHQ-12 score | n (%) | Mean rank | Sum of ranks | P | |
| Mean | 28.02 | <2 | 35 (67.3) | 20.06 | 702.00 | 0.000* |
| SD | 5.26 | >2 | 17 (32.7) | 39.76 | 676.00 | |
For depression, anxiety, (12-item GHQ) scores versus EORTC scores, nonparametric test, Mann–Whitney test was carried out. *P<0.05 is considered to be statistically significant. EORTC QOL – European Organization for Research and Treatment of Cancer quality of life scores; GHQ-12 – 12-item General Health Questionnaire scores; SD – Standard deviation
Cognitive functions, tumor grade, laterality, and location
A statistically significant correlation was found between EORTC QOL and MMSE scores using Spearman's rho (P = 0.005; r = 0.385).
Twenty-nine patients (55.76%) belonged to Grade 1 and Grade II which were grouped together as low-grade tumors. Twenty-three (44.23%) belonged to high-grade tumors (Grade III and Grade IV). Nonparametric Chi-square test was used between anxiety scores <8, ≥8; depression scores <8, ≥8; GHQ scores <2, ≥2; and low, high tumor grading. No significant association was found between tumor grading and anxiety scores (P = 0.348), depression scores (P = 0.963), and psychological morbidity (P = 0.671).
Tumour location in three categories, namely supratentorial, infratentorial, both supra-infra tentorial is as follows:Supratentorial tumors Category I [36 cases (69.23%)], infratentorial tumors Category II[ 13cases (25%)] and both supratentorial and infratentorial tumors Category III [3cases(5.76%)]. Nonparametric Chi-square test was used between anxiety scores <8, ≥8; depression scores <8, ≥8; GHQ scores <2, ≥2; and tumor location (supratentorial, infratentorial, and both supratentorial and infratentorial tumors). No significant association was found between tumor grading and anxiety scores (P = 0.764), depression scores (P = 0.184), or psychological morbidity (P = 0.638).
Right-sided tumors had close to significant association with anxiety symptoms (P = 0.088). Left-sided tumors had significantly higher (P = 0.041) depression scores (mean rank: 34.38). Tumor laterality of 52 PBT cases was as follows: left side 12 cases (23.07%), right side 22 cases (42.30%), bilateral 10 cases (19.23%), and midline 8 cases (15.38%) distribution. No significant association was found between HADS scores (P = 0.087) and anxiety scores (P = 0.164). A significant association was found between psychological morbidity and tumor laterality (P = 0.054).
No significant association was found between EORTC and tumor location (P = 0.527) or tumor grade (P = 0.337) or laterality (P = 0.617). There was no significant association between cognitive scores on MMSE and tumor grade (P = 0.385) and laterality (P = 0.302).
DISCUSSION
QOL is an important treatment outcome measure that needs an emphasis in patients with brain tumor. A review by King et al. emphasized the need for QOL measures in brain tumor patients as a routine clinical practice.[23] The literature lacks robust research evidence of the use of health-related QOL measures in gliomas.[24] Both high- and low-grade gliomas did not find any difference in QOL measures at baseline preoperatively, though subjective individual factors vary.[25]
In a study by Bunevicious et al., moderate-to-severe levels of depression and anxiety scores on HADS were found in both gliomas (28%) and meningiomas (36%).[26] Meningiomas with short survival rates were associated with severe depressive symptoms. Our study found a statistically significant association between EORTC QOL measure and anxiety and depression scores. Boele et al. emphasized the need for assessment of psychiatric symptoms including mood disturbances for improving the overall functioning of the brain tumor patients.[27] A study by D'Angelo et al. found that the prevalence of state and trait anxiety symptoms remained unchanged in pre- and postoperative brain tumor patients, though depressive symptoms significantly increase in a 1-year longitudinal follow-up period.[28]
Tumor laterality, mainly right-sided tumors, was found to be associated with anxiety symptoms.[5] However, in our study, this association was quite close to significance (P = 0.088) between anxiety symptoms and tumor laterality. Depression was found to be significantly associated (P = 0.041) with tumor laterality, mainly left-sided tumors. In the present study sample, left-sided tumors mainly were located in the frontal and temporal areas. In a recent review by Madhusoodanan et al., left-sided tumors were associated with frontal lobe and akinesia.[29] The authors clinically diagnosed 23.07% of the patients as organic mood disorder (F = 06.3) using the ICD-10 criteria of depressive episode, a finding quite similar to a study by Rooney et al. which also found 20.6% (±6.4%) of study participants to be suffering from major depressive disorder using a structured interview schedule.[30]
There was a significant negative correlation between EORTC QOL measures and cognitive scores as noted on MMSE, implying higher symptom load with poor cognitive scores in our PBT patients. A review by Liu et al. highlighted the importance of MMSE as a useful screening tool for cognitive functions in brain tumor patients. Assessment of cognitive functions is gaining importance as a prognostic marker for brain tumors.[31] A study by Bunevicius et al. found an association between MMSE scores and QOL measures (except social functioning).[32]
No association was found between tumor grading with QOL EORTC measures in our research, which is in line with another study by Mahalakshmi and Vanisree which showed that both high- and low-grade tumors were associated with poor QOL measures.[33] A review by Taphoorn et al. reported inconsistent findings of tumor grading (high/low) with QOL measures in different studies.[34] The present study had utilized the standard EORTC B20 QOL measure, which in a meta-analysis by Fountain et al. was used in 11 studies on low-grade gliomas.[24] The current study had an advantage of this measure being available in the local language for administration specifically for brain tumor patients. PBT patients had poor QOL (P < 0.001) with higher mean scores on EORTC B20 indicating higher symptom load and poor cognitive scores (P < 0.001) in PBT patients as compared to matched healthy volunteers.
Our study, however, had the limitation of a small sample size which is a drawback in other PBT studies also due to high mortality and critical condition of this group of patients.
CONCLUSIONS
This research improves our understanding of the relationship between brain tumors and psychiatric morbidity and hence suggests the importance of psychiatric evaluation in a patient of brain tumor. Psychiatric morbidity, cognitive dysfunction, poor qol were noted as significant findings, though in this study no association with tumour grading or location was found. QOL in patients with brain tumor can be drastically improved if depression, anxiety, and cognitive functioning of the PBT patients are assessed routinely and intervened early.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors are thankful to Dr. Reema Kumari, Ex Associate Professor, Department of Neuroradiology, IHBAS, Dilshad Garden, New Delhi - 110 095, India, for assistance.
REFERENCES
- 1.de Robles P, Fiest KM, Frolkis AD, Pringsheim T, Atta C, St Germaine-Smith C, et al. The worldwide incidence and prevalence of primary brain tumors: A systematic review and meta-analysis. Neuro Oncol. 2015;17:776–83. doi: 10.1093/neuonc/nou283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lapointe S, Perry A, Butowski NA. Primary brain tumours in adults. Lancet. 2018;392:432–46. doi: 10.1016/S0140-6736(18)30990-5. [DOI] [PubMed] [Google Scholar]
- 3.Levin VA. Neuro-oncology: An overview. Arch Neurol. 1999;56:401–4. doi: 10.1001/archneur.56.4.401. [DOI] [PubMed] [Google Scholar]
- 4.Wang Q, Qi F, Song X, Di J, Zhang L, Zhou Y, et al. A prospective longitudinal evaluation of cognition and depression in postoperative patients with high-grade glioma following radiotherapy and chemotherapy. J Cancer Res Ther. 2018;14:S1048–51. doi: 10.4103/0973-1482.199431. [DOI] [PubMed] [Google Scholar]
- 5.Mainio A, Hakko H, Niemelä A, Tuurinkoski T, Koivukangas J, Räsänen P. The effect of brain tumour laterality on anxiety levels among neurosurgical patients. J Neurol Neurosurg Psychiatry. 2003;74:1278–82. doi: 10.1136/jnnp.74.9.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang J, Zeng C, Xiao J, Zhao D, Tang H, Wu H, et al. Association between depression and brain tumor: A systematic review and meta-analysis. Oncotarget. 2017;8:94932–43. doi: 10.18632/oncotarget.19843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Talaei-Khoei M, Riklin E, Merker VL, Sheridan MR, Jordan JT, Plotkin SR, et al. First use of patient reported outcomes measurement information system (PROMIS) measures in adults with neurofibromatosis. J Neurooncol. 2017;131:413–9. doi: 10.1007/s11060-016-2314-7. [DOI] [PubMed] [Google Scholar]
- 8.Kessler RA, Loewenstern J, Kohli K, Shrivastava RK. Is psychiatric depression a presenting neurologic sign of meningioma? A critical review of the literature with causative etiology. World Neurosurg. 2018;112:64–72. doi: 10.1016/j.wneu.2018.01.074. [DOI] [PubMed] [Google Scholar]
- 9.Shi C, Lamba N, Zheng LJ, Cote D, Regestein QR, Liu CM, et al. Depression and survival of glioma patients: A systematic review and meta-analysis. Clin Neurol Neurosurg. 2018;172:8–19. doi: 10.1016/j.clineuro.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 10.Lukshina AA, Urakov SV, Loshakov VA. Psychiatric disorders in temporal lobe gliomas. Zh Vopr Neirokhir Im N N Burdenko. 2010;3:25–31. [PubMed] [Google Scholar]
- 11.Arnold SD, Forman LM, Brigidi BD, Carter KE, Schweitzer HA, Quinn HE, et al. Evaluation and characterization of generalized anxiety and depression in patients with primary brain tumors. Neuro Oncol. 2008;10:171–81. doi: 10.1215/15228517-2007-057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raysi Dehcordi S, Mariano M, Mazza M, Galzio RJ. Cognitive deficits in patients with low and high grade gliomas. J Neurosurg Sci. 2013;57:259–66. [PubMed] [Google Scholar]
- 13.Tucha O, Smely C, Preier M, Becker G, Paul GM, Lange KW. Preoperative and postoperative cognitive functioning in patients with frontal meningiomas. J Neurosurg. 2003;98:21–31. doi: 10.3171/jns.2003.98.1.0021. [DOI] [PubMed] [Google Scholar]
- 14.Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 15.Pan-Weisz TM, Kryza-Lacombe M, Burkeen J, Hattangadi-Gluth J, Malcarne VL, McDonald CR. Patient-reported health-related quality of life outcomes in supportive-care interventions for adults with brain tumors: A systematic review. Psychooncology. 2019;28:11–21. doi: 10.1002/pon.4906. [DOI] [PubMed] [Google Scholar]
- 16.Wagner A, Shiban Y, Lange N, Joerger AK, Hoffmann U, Meyer B, et al. The relevant psychological burden of having a benign brain tumor: A prospective study of patients undergoing surgical treatment of cranial meningiomas. J Neurosurg. 2019;11:1–8. doi: 10.3171/2018.8.JNS181343. doi: 10.3171/2018.8.JNS181343. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.Osoba D, Brada M, Prados MD, Yung WK. Effect of disease burden on health-related quality of life in patients with malignant gliomas. Neuro Oncol. 2000;2:221–8. doi: 10.1093/neuonc/2.4.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016;131:803–20. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 19.Budrukkar A, Jalali R, Kamble R, Parab S. Translation and pilot validation of Hindi translation of assessing quality of life in patients with primary brain tumours using EORTC brain module (BN-20) J Cancer Res Ther. 2006;2:166–70. doi: 10.4103/0973-1482.29826. [DOI] [PubMed] [Google Scholar]
- 20.Goldberg D. Manual of the General Health Questionnaire. Windsor, England: NFER Publishing; 1978. [Google Scholar]
- 21.Snaith RP. The hospital anxiety and depression scale. Health Qual Life Outcomes. 2003;1:29. doi: 10.1186/1477-7525-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murray KJ, Scott C, Zachariah B, Michalski JM, Demas W, Vora NL, et al. Importance of the mini-mental status examination in the treatment of patients with brain metastases: A report from the radiation therapy oncology group protocol 91-04. Int J Radiat Oncol Biol Phys. 2000;48:59–64. doi: 10.1016/s0360-3016(00)00600-3. [DOI] [PubMed] [Google Scholar]
- 23.King S, Exley J, Parks S, Ball S, Bienkowska-Gibbs T, MacLure C, et al. The use and impact of quality of life assessment tools in clinical care settings for cancer patients, with a particular emphasis on brain cancer: Insights from a systematic review and stakeholder consultations. Qual Life Res. 2016;25:2245–56. doi: 10.1007/s11136-016-1278-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fountain DM, Allen D, Joannides AJ, Nandi D, Santarius T, Chari A. Reporting of patient-reported health-related quality of life in adults with diffuse low-grade glioma: A systematic review. Neuro Oncol. 2016;18:1475–86. doi: 10.1093/neuonc/now107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drewes C, Sagberg LM, Jakola AS, Solheim O. Perioperative and postoperative quality of life in patients with glioma-A longitudinal cohort study. World Neurosurg. 2018;117:e465–74. doi: 10.1016/j.wneu.2018.06.052. [DOI] [PubMed] [Google Scholar]
- 26.Bunevicius A, Deltuva VP, Tamasauskas A. Association of pre-operative depressive and anxiety symptoms with five-year survival of glioma and meningioma patients: A prospective cohort study. Oncotarget. 2017;8:57543–51. doi: 10.18632/oncotarget.15743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boele FW, Rooney AG, Grant R, Klein M. Psychiatric symptoms in glioma patients: From diagnosis to management. Neuropsychiatr Dis Treat. 2015;11:1413–20. doi: 10.2147/NDT.S65874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.D'Angelo C, Mirijello A, Leggio L, Ferrulli A, Carotenuto V, Icolaro N, et al. State and trait anxiety and depression in patients with primary brain tumors before and after surgery: 1-year longitudinal study. J Neurosurg. 2008;108:281–6. doi: 10.3171/JNS/2008/108/2/0281. [DOI] [PubMed] [Google Scholar]
- 29.Madhusoodanan S, Ting MB, Farah T, Ugur U. Psychiatric aspects of brain tumors: A review. World J Psychiatry. 2015;5:273–85. doi: 10.5498/wjp.v5.i3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rooney AG, McNamara S, Mackinnon M, Fraser M, Rampling R, Carson A, et al. Screening for major depressive disorder in adults with cerebral glioma: An initial validation of 3 self-report instruments. Neuro Oncol. 2013;15:122–9. doi: 10.1093/neuonc/nos282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu R, Page M, Solheim K, Fox S, Chang SM. Quality of life in adults with brain tumors: Current knowledge and future directions. Neuro Oncol. 2009;11:330–9. doi: 10.1215/15228517-2008-093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bunevicius A, Tamasauskas S, Deltuva V, Tamasauskas A, Radziunas A, Bunevicius R. Predictors of health-related quality of life in neurosurgical brain tumor patients: Focus on patient-centered perspective. Acta Neurochir (Wien) 2014;156:367–74. doi: 10.1007/s00701-013-1930-7. [DOI] [PubMed] [Google Scholar]
- 33.Mahalakshmi P, Vanisree AJ. Quality of life measures in glioma patients with different grades: A preliminary study. Indian J Cancer. 2015;52:580–5. doi: 10.4103/0019-509X.178395. [DOI] [PubMed] [Google Scholar]
- 34.Taphoorn MJ, Sizoo EM, Bottomley A. Review on quality of life issues in patients with primary brain tumors. Oncologist. 2010;15:618–26. doi: 10.1634/theoncologist.2009-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]


