Abstract
Introduction:
Baclofen and topiramate are GABAergic drugs, and both have been recommended for the treatment of alcohol dependence as anticraving agent. Several studies have demonstrated the effect of baclofen and topiramate as anticraving, but none has compared them. The main aim of the current study was to assess the baclofen and topiramate as anticraving agent in alcohol dependence during 1 month follow-up.
Methodology:
After 1-week detoxification protocol, 94 patients were randomly assigned to either baclofen (n = 49) or topiramate (n = 45) for 1-month follow-up. Patients were assessed with clinical institute withdrawal assessment at baseline, and at 1 week, the Addiction Severity Index, ready to change questionnaire at baseline and weekly assessed with Obsessive and Compulsive drinking scale (OCDS) for craving. At every follow-up, adverse effects were also assessed to check tolerability.
Results:
A marked improvement was observed with baclofen in OCDS in each assessment as compared to topiramate. With baclofen, 61.22% of patients became complete abstinence, as compared to 37.78% in topiramate group. Baclofen was better tolerated as 24.49% patients were dropped out in baclofen group as compared to 33.33% in topiramate group.
Conclusion:
Baclofen has better efficacy and tolerability as compared to topiramate.
Keywords: Alcohol dependence, baclofen, topiramate
Alcohol abuse attributes approximately 4% of global burden of disease.[1] Alcohol dependence is most serious form of alcohol use disorder, and it has been affecting nearly 14% of general population.[2] In addition to alcohol dependence, other form of unhealthy alcohol consumption include such as alcohol abuse and binge drinking.[3] The prevalence rate of alcohol consumption has been increasing, and it raises the importance of this issue for public health and socioeconomic impact.[1] Alcohol dependence is a major problem in India. An estimated 34%–42% of adult Indian population have exposure of alcohol in their lifetime; 5%–7% has been estimated to be abuser of alcohol and 10–20 million persons have been estimated to be in need of treatment for alcohol consumption every year.[4] A national-wide epidemiology study in India has reported prevalence of 21.4% for the current use of alcohol and those at least 17% may be dependent users.[5]
Alcohol dependence is a multifactorial disorder which is influenced by interacting genetic, biological, psychological, and environmental factors. All these factors also involve in relapse after successful detoxification in case of alcohol dependence.[6] One feature noted that before relapse, some abstinent patients have craving or urge for alcohol. This urge may contribute to risk of relapse. Pharmacologically, alcohol is considered to be a potent central nervous system depressant, and its action is mediated through multi-neurotransmitter systems, including the GABAergic, glutamatergic, dopaminergic, serotoninergic, and opiatergic system. The various pharmacological agents are in market, which help in controlling this complex neurobiological network, which is involved in the regulation of alcohol preference, intake, rewarding and craving components of alcohol dependence.[7] Although psychological approach and counseling are essential components in reducing craving and achieving alcohol abstinence,[8] pharmacotherapy may also be necessary in treating individual who are not helped by psychological therapies alone. Several pharmacological therapy which has been available in market and that act in the previously mentioned neurotransmitter systems include naltrexone,[9] acamprosate,[10] fluoxetine,[11] topiramate,[12] and gamma-hydroxybutyric acid.[13] These drugs have been tested in both preclinical and clinical to evaluate their effect on craving, ethanol intake, and alcohol abstinence, but till date no effective therapy has been found in case of alcohol dependence.
With regard to the possible mechanism of action by which baclofen acts and helps in reduce the alcohol consumption and motivation, a recent preliminary microdialysis experiment verified that baclofen decreased dopamine release in the shell of the nucleus accumbes.[14] Different experiments study evidence have been suggested that mesolimbic dopamine neurons are involved in mediation of alcohol intake and reinforcement.[15] Interestingly, gamma-aminobutyric acid (GABA)B receptors are located in ventral tegmental area, and these receptors are present both on the cell body of dopamine neurons and the terminals of glutamatergic afferent neurons.[16] Their activation by GABAB receptor agonist may bring to bear an inhibitory action on dopamine neurons.[17] Baclofen reduces dopamine release and in turn, decrease craving for alcohol.
The anticonvulsant topiramate is acted through antagonizing excitatory glutamate receptors at amino-3- hydroxy-5-methylisoxazole-4-propionic acid and kainite receptors and also decreases dopamine release with in mesolimbic system.[18] This dual-action of topiramate helps in dopamine reduction in nucleus accumbens, and it decreases withdrawal symptoms. Thus, targeting both facets of addiction, i.e., craving and withdrawals symptoms, which may be decreased the relapse in alcohol dependence.
On the basis of previous study, the main objective of the present study was to assess the anticraving effect of baclofen and topiramate for alcohol in individuals affected by the current alcoholism. During the study, efficacy and tolerability of baclofen and topiramate were also assessed along with variation on alcohol intake and abstinence from alcohol in treated subjects.
METHODOLOGY
Study design
The study was open-label randomized trial done between January 2019 and April 2019 in de-addiction clinic of the Department of Psychiatry, Shri Guru Gobind Tricentenary Medical College and Hospital, Gurugram, Haryana, India. The inclusion criteria were: (a) Age 18–65 years, (b) availability of reliable informant, (c) fulfilling International Classification of Diseases-10 (ICD-10) Diagnostic Criteria for Research (DCR)[19] criteria for alcohol dependence, (d) patient suitable for out-patient treatment, and (e) patient consenting to participate in study. Exclusion criteria included (a) presence of serious physical illness (as assessed by through physical examination and routine laboratory screening), (b) presence of another pre or co-existing major psychiatric disorder, (c) presence of another drug abuse except nicotine, (d) need for in-patient treatment during detoxification stage of the study, (e) contraindication to either of drugs, (f) clinical instrument for withdrawal assessment score of >10, and (g) patients needing benzodiazepine (lorazepam) during day time. Of the 94 patients who visited the hospital for alcohol-related problems, 67 completed the 1-month follow-up. Participants were assigned either to baclofen group or topiramate group by random allocation table.
The standard detoxification protocol was initiated and completed within 1 week of recruitment in the study. The treatment protocol of the department included use of thiamine and oral administration of lorazepam (4–10 mg in divided doses) which were gradually tapered off within a week. Postdetoxification (baseline) patients were assessed on clinical institute withdrawal assessment (CIWA) for withdrawal symptoms and obsessive and compulsive drinking scale (OCDS) and visual analog scale (VAS) for measure of craving. If CIWA scores were >10, detoxification was continued and reassessed every 2 days. After Baseline assessment patients were allowed to use lorazepam (2–4 mg) at night in case if they had sleep disturbance. Patients and informant were asked not to use lorazepam on days they consumed alcohol. Baclofen sustained-release preparation was prescribed at 20 mg in 1st week while topiramate was prescribed at 50 mg/day in 1st week and 100 mg/day in the 2nd week. Further, escalation of dose for both drugs was determined by clinician based on craving reported by the patient and tolerability of the drug. Baclofen was escalated by 10 mg/day, while topiramate was escalated by 50 mg/day. The assessment of craving (OCDS and VAS), was done at each weekly visit by treating psychiatrist. The alcohol use pattern in the past week, side-effect experienced, and need to increase/reduce or maintain drug dose was determined by treating psychiatrist based on changes in OCDS and VAS scores.
Measures
Participants were diagnosed according to ICD-10 DCR for alcohol dependence. At intake, patients were assessed on a semi-structured pro forma for sociodemographic details, pattern of abuse, life problems related to alcohol consumption, family history of drug and alcohol dependence, prior treatment and occurrence of withdrawal symptoms in the past. Additional measures used at intake were the Addiction Severity Index Scale,[20] readiness to change questionnaire[21] (for the assessment of motivation) and CIWA for withdrawal symptoms.[22]
The objective assessment of craving was estimated with the OCDS[23] at baseline, 1 week, 2nd week, 3rd week, and 4th week. For subjective assessment of craving VAS was used weekly, scores range between 0 and 100. Adverse effects of the treatment in both groups were monitored at every follow-up. At baseline and 4th week, all routine laboratory investigations including liver function were done to rule out any serious medical complication and effect of treatment on liver.
Statistical analysis
A total of 94 patients were recruited in the study, and 27 left the treatment before completing 4 weeks of trial. Chi-square test was used to compare the demographic profile and variable related to alcohol in both groups. Mann–Whitney test was used to assess the variable from the ASI. Mann–Whitney test was used to compare both these group for obsession and compulsion. All tests were two-tailed with statistical significance set at P < 0.5. Data analysis was performed using the SPSS software version 21 (Statistical Package for the Social Sciences, IBM Corp, USA).
RESULTS
There was no significant difference observed between the baclofen and topiramate groups in term of their socioeconomic characteristic [Table 1], as well as variables related to alcohol abuse history and withdrawals symptoms during the 1st week of abstinence [Tables 2 and 3]. There was no significant difference observed in motivation assessment at baseline in both group [Table 3]. Both groups had similarly high scores on the OCDS and VAS upon admission (baseline), which represent a serious psychosocial impairment [Table 4]. There was no significant difference in drinking pattern in both groups at baseline.
Table 1.
Sociodemographic characteristic of both the groups
| Variables | Baclofen (n=49) | Topiramate (n=45) |
|---|---|---|
| Mean age, years±SD | 41.16±7.898 | 36.40±7.858 |
| Sex (male, female) | 49, 0 | 45, 0 |
| Marital status (S, M, D) | 11, 35, 3 | 9, 32, 4 |
| Socioeconomic status (H, M, L) | 17, 22, 10 | 15, 21, 9 |
| Education (I, UM, IN, G) | 6, 21, 16, 6 | 8, 20, 9, 8 |
| Occupation status (E, UE, R) | 39, 9, 1 | 34, 8, 3 |
| Duration of dependence, years±SD | 8.96±6.541 | 7.60±3.916 |
| Mean age of dependence, years±SD | 31.84±7.493 | 28.28±7.127 |
| REMOVE: Mean CIWA-Ar during 1st week | 5.44±1.557 | 4.88±1.986 |
*All comparison between groups was nonsignificant. S – Single; M – Married; D – Divorced; H – High; M – Middle; L – Low; I – Illitrate; UM – Under-metric; IN – Inter; G – Graduate; E – Employed; UE – Unemployed; R – Retired; SD – Standard deviation; CIWA – Clinical Institute Withdrawal Assessment
Table 2.
Variable related to alcohol consumption in both groups
| Profile | Group | Baclofen, n (%) | Topiramate, n (%) | P | |
|---|---|---|---|---|---|
| 1 | Duration of dependence (year) | ≤5 | 19 (38.77) | 18 (40.0) | 0.751 |
| 6-10 | 12 (24.49) | 12 (26.67) | |||
| 11-15 | 9 (18.37) | 5 (11.11) | |||
| >15 | 9 (18.37) | 10 (22.22) | |||
| 2 | Binge pattern | Present | 29 (59.18) | 24 (53.33) | 0.739 |
| Absent | 20 (40.82) | 21 (46.67) | |||
| 3 | Abstinence | Abstained for at least 1 month in past | 30 (61.22) | 29 (64.44) | 0.081 |
| Never abstinence | 19 (38.78) | 16 (35.56) | |||
| 4 | Nicotine abuser | Present | 40 (81.63) | 41 (91.11) | 0.936 |
| Absent | 9 (18.37) | 4 (8.89) | |||
| 5 | Past history of complication due to alcohol use | Withdrawal seizures | 2 (4.08) | 5 (11.11) | 0.157 |
| Withdrawal delirium | 3 (6.12) | 5 (11.11) | 0.384 | ||
| Alcohol liver disease | 3 (6.12) | 3 (6.67) | 0.552 | ||
| Injury during alcohol intoxication that required medical attention | 5 (10.20) | 5 (11.11) | 0.440 | ||
| 6 | Family history of alcohol intake (specify 1st degree relative) | Present | 35 (71.43) | 34 (75.56) | 0.56 |
| Absent | 14 (28.57) | 11 (24.44) |
Table 3.
Addiction variable at intake and baseline in both groups
| Baclofen | Topiramate | P | |
|---|---|---|---|
| CIWA (average) | |||
| At intake (values cannot be <10) | 5.44±1.545 | 4.88±1.976 | 0.226 |
| Baseline (values are too low, should be around 7-8) | 1.21±0.512 | 1.08±0.473 | |
| Motivation (%) | |||
| Contemplation | 19 (38.78) | 22 (48.89) | 0.638 |
| Precontemplation | 10 (20.41) | 11 (24.44) | |
| Action | 20 (40.81) | 12 (26.67) | |
| ASI | |||
| Regular alcohol use (years) | 7.44±4.114 | 7.24±4.064 | 0.371 |
| Intoxication (years) | 5.04±2.726 | 4.92±2.628 | 0.673 |
| Money spent per month (RS.) | 3040±1040.051 | 2825±1430.347 | 0.281 |
| Alcohol problem in last month (days) | 29.00±4.734 | 26.36±5.601 | 0.892 |
| Alcohol problem felt by patient | 3.00±0.576 | 2.96±0.841 | 0.783 |
| Need treatment for problem | 2.96±0.543 | 2.92±0.812 | 0.673 |
| Dose of lorazepam (average) | |||
| At first contact | 8.45±0.321 | 7.8±0.619 | |
| At baseline (postdetoxification) | 9.16±0.128 | 8.1±0.284 |
CIWA – Clinical Institute Withdrawal Assessment, ASI – Addiction Severity Index
Table 4.
Score of baclofen and topiramate group in obsessive and compulsive drinking scale during each assessment
| Variables | Group | Baseline | 1st week | 2nd week | 3rd week | 4th week |
|---|---|---|---|---|---|---|
| Dose (mg) (mention values as mean±SD) | Baclofen | - | 20±0.0 | 23.04±6.641 | 23.91±6.593 | 23.81±6.890 |
| Topiramate | - | 25±0.0 | 50±0.0 | 120±25.162 | 125±35.355 | |
| OCD (O) | Baclofen | 13.24±2.654 | 9.96±3.994@ | 6.64±4.156∞ | 3.52±4.155€ | 1.19±3.501© |
| Topiramate | 14.0±3.628 | 13.04±3.702* | 10.08±4.319*,∞ | 8.27±4.783*,€ | 4.05±4.892*,© | |
| OCD (C) | Baclofen | 13.28±2.913 | 7.76±5.789@ | 2.56±5.007∞ | 1.95±4.324€ | 0.81±3.709© |
| Topiramate | 14.36±3.321 | 13.04±3.948* | 9.36±5.724**,∞ | 6.52±6.816**,€ | 2.77±5.275*,© | |
| OCD (T) | Baclofen | 26.52±5.581 | 16.64±10.123@ | 9.21±7.942∞ | 5.48±7.983€ | 2.0±7.036© |
| Topiramate | 28.36±6.769 | 26.08±7.410* | 19.44±9.747**,∞ | 14.5±11.359**,€ | 6.83±9.855*,© | |
| VAS | Baclofen | 81.2±8.817 | 63.2±17.072@ | 39.56±14.924∞ | 24.78±23.906€ | 6.19±18.568© |
| Topiramate | 82.4±10.116 | 78±11.547* | 65.2±17.823*,∞ | 48.63±26.603*,€ | 25.56±28.122*,© | |
| Average drinking days/past week | Baclofen | 6.72±0.791 | 2.36±2.721@ | 0.782±1.730∞ | 0.434±1.502€ | 0.428±1.535© |
| Topiramate | 6.44±1.044 | 5.12±1.833* | 3.32±2.495∞ | 2.22±2.810€ | 1.00±2.143© | |
| Average number of drinks | Baclofen | 10.16±0.862 | 4.10±2.030@ | 2.356±1.466∞ | 1.356±1.160€ | 0.871±1.434© |
| Topiramate | 11.92±0.959 | 6.40±0.931* | 4.96±1.262∞ | 3.045±1.565€ | 1.471±1.395© |
*Significant difference between baclofen and topiramate (0.05), **Significant difference between baclofen and topiramate (0.001), @Significant difference between baseline and 1st week assessment, ∞Significant difference between baseline and 2nd week assessment, €Significant difference between baseline and 3rd week assessment, ©Significant difference between baseline and 4th week assessment. OCD – Obsessive and compulsive drinking; SD – Standard deviation; VAS – Visual Analog Scale
A marked significant reduction in craving was reported at the end of week one in baclofen group as compared to topiramate group [Table 4]. In baclofen group, significant reduction was seen on OCDS and VAS at 1st week as compared to topiramate who showed similar improvement at 2nd week. On comparing on average number of drinks per day and number of days of drinking, baclofen showed significant reduction in numbers of drinks and drinking days at 1st week as compared to topiramate which showed improvement at 2nd week [Table 4]. Outcome in terms of abstinence from alcohol was better with baclofen group. With baclofen, 24 (48.98%) patients showed complete abstinence within 2 weeks of treatment as compared to 8 (17.78%) in topiramate group [Table 5]. Although the patient showed improvement in the form of abstinence with topiramate, it took more than 2 weeks to show results with topiramate as compared to baclofen group [Table 5].
Table 5.
Alcohol use pattern in both groups
| Variables | Group | 1st week (%) | 2nd week (%) | 3rd week (%) | 4th week (%) |
|---|---|---|---|---|---|
| Total number of patients on follow up | Baclofen | 49 (100) | 40 (81.63) | 38 (77.55) | 37 (75.51) |
| Topiramate | 44 (97.78) | 38 (84.44) | 31 (68.69) | 30 (66.67) | |
| Abstinence | Baclofen | 21 (42.86.0) | 24 (48.98) | 26 (53.06) | 30 (61.22) |
| Topiramate | 2 (4.44) | 8 (17.78) | 14 (31.11) | 17 (37.78) | |
| Reduce alcohol quantity | Baclofen | 15 (30.61) | 11 (22.45) | 7 (14.29) | 4 (8.16) |
| Topiramate | 17 (37.78) | 20 (44.44) | 12 (26.67) | 9 (20.0) | |
| No improvement | Baclofen | 13 (26.53) | 5 (10.20) | 5 (10.20) | 3 (6.13) |
| Topiramate | 25 (55.56) | 10 (22.22) | 5 (11.11) | 4 (8.89) | |
| Drop out | Baclofen | 0 | 9 (18.37) | 11 (22.45) | 12 (24.49) |
| Topiramate | 1 (2.22) | 7 (15.56) | 14 (31.11) | 15 (33.33) |
Reported adverse effects were presented in Table 6. A considerable proportion of topiramate group had some adverse effects, but no significant difference was recorded compared with baclofen group. All adverse effects were tolerable, and no treatment was required for this. In baclofen group, 75.71% patients completed the trial as compared to topiramate group (66.67%). This drop out may be because of higher adverse effects due to topiramate [Table 5]. Drop out with topiramate was reported at higher dose around 150–200 mg/day.
Table 6.
Adverse effects reported by study groups
| Variable | Baclofen (n=49), n (%) | Topiramate (n=45), n (%) |
|---|---|---|
| Headache | 3 (6.12) | 5 (11.11) |
| Tremor | 0 (0.0) | 4 (8.89) |
| Decrease appetite | 2 (4.08.0) | 4 (8.89) |
| Sedation | 2 (4.08) | 3 (6.67) |
| Nausea | 3 (6.12) | 3 (6.67) |
| Constipation | 0 (0.0) | 2 (4.44) |
| Abdominal pain | 0 (0.0) | 2 (4.44) |
*All results were nonsignificant
DISCUSSION
GABA modulation drugs, such as baclofen and topiramate, have been shown to have anticraving effects in several studies. To the best our knowledge, neither of them has been in direct comparison with other anticraving agents for their efficacy and tolerability although both medications have not been currently approved for the treatment of alcohol dependence preliminary studies have suggested anticraving effects in those with alcohol dependence. Randomized double-blind placebo-controlled trials involving topiramate and (drug/placebo) have demonstrated its efficacy in improving drinking behavior and maintaining abstinence.[12] Topiramate has been found to be inferior to disulfiram in terms of days to relapse[24] and superior to naltrexone in term of reducing craving.[25] In case of baclofen, mostly comparison was done with placebo[26] or benzodiazepine.[27]
Baseline comparisons: Both groups were comparable at sociodemographic characteristics. Mean age of patients was 41.16 years in baclofen group and 36.40 years in topiramate group. All were male, and most were married.
In the current study, the main aim of the study was to compare the anticraving effect of both drugs in alcohol dependence. Both of these drugs showed significant improvement at the end of study [Table 4], but baclofen has shown improvement earlier as compared to topiramate. In baclofen group, 73.47% patients have shown significant abstinence (42.86%) or reduce dose (30.61%) within 1 week of treatment [Table 5] and were maintained abstinence throughout the treatment period. In case of baclofen, the patient showed significant improvement within 1st week as compared to topiramate which took 2 weeks to show improvement [Table 4]. This increase efficacy of baclofen over topiramate may be related to its suppressants effect on anticraving; indeed, baclofen produced a rapid decrease in compulsive and obsessive components of craving, as indicated by immediate reduction in mean score of both OCDS subscales [Figures 1 and 2]. This reduction in obsession and compulsion with baclofen lead to significant decrease drinking dose and drinking days. At baseline both groups have similar results in term of drinking days, and amount of alcohol drank in a day, but baclofen showed significant reduction in dose within 2 weeks as compared to topiramate [Table 4]. This observation is in agreement with data reported by Ling et al.[28] as anticraving effect of baclofen in cocaine dependence and also with Addolorato et al. 2000,[29] 2002[26] suggest that baclofen has anticraving effect within a week or two in alcohol dependence. As mentioned in most studies[26,27,28,29] that 20–30 mg/day is therapeutic and efficacious dose for baclofen in alcohol dependence. That's why, patients showed effect within 2 weeks of use with baclofen.
Figure 1.
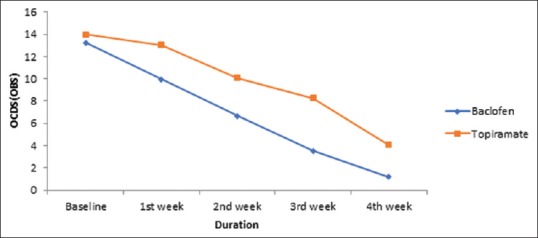
Comparison between obsessive and compulsive drinking scale (obsession) on each assessment
Figure 2.
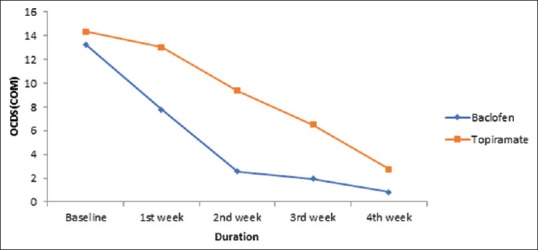
Comparison between obsessive and compulsive drinking scale (compulsion) on each assessment
In case of topiramate, dose was gradually increased, and max dose used in our study was 200 mg/day. In topiramate group, therapeutic effect was evident later than 2 weeks of treatment, and this was maintained till the end of 4th week. At the end of 2 weeks (100 mg/day), patients reported significant reduction in craving with topiramate, as reported by VAS and OCDS. At 2 week, 62.22% patients reported complete abstinence (17.78%) or reduction in alcohol consumption dose of alcohol (44.44%) [Table 5]. It would, therefore, be of interest to determine whether a small dose of topiramate as compared to large dose around more than 200 mg/day would be efficacious over the length of the trial period. Earlier most of the drug trials were in favor of giving high dose of topiramate,[30] but in our study, patients showed that improvement with dose of 100 mg/day could be possible. This observation is in keeping with suggestion pointed out by Paparrigopoulos et al., and demonstrate by another group where-in a dose of 75 mg of topiramate showed anticraving effect.[31] In our study, lorazepam was used for detoxification in 1st week, but dose was decreased up to 1–2 mg/day after 1 week of detoxification. Lorazepam was primarily given for sedation only in night dose after 1 week and discontinued in most of the patients if they had normal sleep. Although lorazepam also has anticraving effect, in our study it is unlikely to influence craving effect it has a short half-life and was given only as bed-time dose.
If we compared the side effect profile, baclofen was better tolerated as compared to topiramate. In topiramate group, 15 (33.33%) patients did not complete the trial as compared to 12 (24.49%) in baclofen group. All patients dropped out after 2nd week in topiramate, when increased above 100 mg/day due to its poor tolerability effect. In baclofen group, two (50%) patients dropped out before 2nd week and two (50%) patients after 2 weeks. None of them dropped out due to side effect.
The main limitations of the present study are: (a) relatively small sample size, which reduces the statistical significance of our finding, (b) long-term follow-up would provide information about the long-term efficacy of topiramate and baclofen, which was not possible due to 1 month follow up in study.
CONCLUSION
Baclofen has shown better efficacy and tolerability as compared to topiramate. Baclofen has shown his effect within 2 weeks, but topiramate needs more than 2 weeks to show his effect. Low-dose topiramate can be used as anticraving, which is a surprised result in this study. Furthermore studies are required to confirm the finding in the current study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Room R, Babor T, Rehm J. Alcohol and public health. Lancet. 2005;365:519–30. doi: 10.1016/S0140-6736(05)17870-2. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the united states. Results from the national comorbidity survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 3.Saitz R, Horton NJ, Sullivan LM, Moskowitz MA, Samet JH. Addressing alcohol problems in primary care: A cluster randomized, controlled trial of a systems intervention. The screening and intervention in primary care (SIP) study. Ann Intern Med. 2003;138:372–82. doi: 10.7326/0003-4819-138-5-200303040-00006. [DOI] [PubMed] [Google Scholar]
- 4.Ray R, editor. South East Asia Drug Demand Reduction Report. New Delhi: UNDCP Regional Office for South Asia; 1998. UNDCP Regional Office for South Asia. Country profile-India; pp. 259–61. [Google Scholar]
- 5.Ray R. The Extent, Pattern and Trends of Drug Abuse in India. National Survey. 2004 [Google Scholar]
- 6.Hesler RK, Miller WR, editors. Handbook of Alcoholism Treatment Approaches: Effective Alternatives. 2nd ed. Elmsford, NY: Pergamon Press; 1989. Treating alcohol problem: toward an informed eclecticism; pp. 12–4. [Google Scholar]
- 7.Lobo IA, Harris RA. GABAA receptors and alcohol. Pharmacol Biochem Behav. 2008;90:90–4. doi: 10.1016/j.pbb.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dall Aglio C, Caputo F, Baudanza P, Castelli E, Marsigli L, Addolorato G, et al. Short and long term treatment of alcohol dependence. Alcol Eur J Alcohol Stud. 1997;9:61–4. [Google Scholar]
- 9.Volpicelli JR, Alterman AI, Hayashida M, O'Brien CP. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49:876–80. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- 10.Whitworth AB, Fischer F, Lesch OM, Nimmerrichter A, Oberbauer H, Platz T, et al. Comparison of acamprosate and placebo in long-term treatment of alcohol dependence. Lancet. 1996;347:1438–42. doi: 10.1016/s0140-6736(96)91682-7. [DOI] [PubMed] [Google Scholar]
- 11.Naranjo CA, Poulos CX, Bremner KE, Lanctot KL. Fluoxetine attenuates alcohol intake and desire to drink. Int Clin Psychopharmacol. 1994;9:163–72. doi: 10.1097/00004850-199409000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Johnson BA, Rosenthal N, Capece JA, Wiegand F, Mao L, Beyers K, et al. Topiramate for treating alcohol dependence: A randomized controlled trial. JAMA. 2007;298:1641–51. doi: 10.1001/jama.298.14.1641. [DOI] [PubMed] [Google Scholar]
- 13.Addolorato G, Cibin M, Caputo F, Capristo E, Gessa GL, Stefanini GF, et al. Gamma-hydroxybutyric acid in the treatment of alcoholism: Dosage fractioning utility in non-responder alcoholic patients. Drug Alcohol Depend. 1998;53:7–10. doi: 10.1016/s0376-8716(98)00094-5. [DOI] [PubMed] [Google Scholar]
- 14.Colombo G, Addolorato G, Agabio R, Carai MA, Pibiri F, Serra S, et al. Role of GABA (B) receptor in alcohol dependence: Reducing effect of baclofen on alcohol intake and alcohol motivational properties in rats and amelioration of alcohol withdrawal syndrome and alcohol craving in human alcoholics. Neurotox Res. 2004;6:403–14. doi: 10.1007/BF03033315. [DOI] [PubMed] [Google Scholar]
- 15.Weiss F, Porrino LJ. Behavioral neurobiology of alcohol addiction: Recent advances and challenges. J Neurosci. 2002;22:3332–7. doi: 10.1523/JNEUROSCI.22-09-03332.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowery NG, Hudson AL, Price GW. GABAA and GABAB receptor site distribution in the rat central nervous system. Neuroscience. 1987;20:365–83. doi: 10.1016/0306-4522(87)90098-4. [DOI] [PubMed] [Google Scholar]
- 17.Yoshida M, Yokoo H, Tanaka T, Emoto H, Tanaka M. Opposite changes in the mesolimbic dopamine metabolism in the nerve terminal and cell body sites induced by locally infused baclofen in the rat. Brain Res. 1994;636:111–4. doi: 10.1016/0006-8993(94)90183-x. [DOI] [PubMed] [Google Scholar]
- 18.Xiao C, Shao XM, Olive MF, Griffin WC, 3rd, Li KY, Krnjević K, et al. Ethanol facilitates glutamatergic transmission to dopamine neurons in the ventral tegmental area. Neuropsychopharmacology. 2009;34:307–18. doi: 10.1038/npp.2008.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization. ICD-10 Classifications of Mental and Behavioural Disorder: Clinical Descriptions and Diagnostic Guidelines. Geneva. World Health Organization. 1992 [Google Scholar]
- 20.Leonhard C, Mulvey K, Gastfriend DR, Shwartz M. The addiction severity index: A field study of internal consistency and validity. J Subst Abuse Treat. 2000;18:129–35. doi: 10.1016/s0740-5472(99)00025-2. [DOI] [PubMed] [Google Scholar]
- 21.Budd R, Rollnick S. The structure of the readiness to change questionnaire: A test of Prochaska and Diclemente's transtheoretical model. Br J Health Psychol. 1996;1:365–76. [Google Scholar]
- 22.Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: The revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar) Br J Addict. 1989;84:1353–7. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- 23.Anton RF, Moak DH, Latham P. The obsessive compulsive drinking scale: A self-rated instrument for the quantification of thoughts about alcohol and drinking behavior. Alcohol Clin Exp Res. 1995;19:92–9. doi: 10.1111/j.1530-0277.1995.tb01475.x. [DOI] [PubMed] [Google Scholar]
- 24.De Sousa AA, De Sousa J, Kapoor H. An open randomized trial comparing disulfiram and topiramate in the treatment of alcohol dependence. J Subst Abuse Treat. 2008;34:460–3. doi: 10.1016/j.jsat.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 25.Flórez G, Saiz PA, García-Portilla P, Alvarez S, Nogueiras L, Bobes J, et al. Topiramate for the treatment of alcohol dependence: Comparison with naltrexone. Eur Addict Res. 2011;17:29–36. doi: 10.1159/000320471. [DOI] [PubMed] [Google Scholar]
- 26.Addolorato G, Caputo F, Capristo E, Domenicali M, Bernardi M, Janiri L, et al. Baclofen efficacy in reducing alcohol craving and intake: A preliminary double-blind randomized controlled study. Alcohol Alcohol. 2002;37:504–8. doi: 10.1093/alcalc/37.5.504. [DOI] [PubMed] [Google Scholar]
- 27.Addolorato G, Leggio L, Agabio R, Colombo G, Gasbarrini G. Baclofen: A new drug for the treatment of alcohol dependence. Int J Clin Pract. 2006;60:1003–8. doi: 10.1111/j.1742-1241.2006.01065.x. [DOI] [PubMed] [Google Scholar]
- 28.Ling W, Shoptaw S, Majewska D. Baclofen as a cocaine anti-craving medication: A preliminary clinical study. Neuropsychopharmacology. 1998;18:403–4. doi: 10.1016/S0893-133X(97)00128-0. [DOI] [PubMed] [Google Scholar]
- 29.Addolorato G, Caputo F, Capristo E, Colombo G, Gessa GL, Gasbarrini G, et al. Ability of baclofen in reducing alcohol craving and intake: II – Preliminary clinical evidence. Alcohol Clin Exp Res. 2000;24:67–71. [PubMed] [Google Scholar]
- 30.Johnson BA, Ait-Daoud N, Bowden CL, DiClemente CC, Roache JD, Lawson K, et al. Oral topiramate for treatment of alcohol dependence: A randomised controlled trial. Lancet. 2003;361:1677–85. doi: 10.1016/S0140-6736(03)13370-3. [DOI] [PubMed] [Google Scholar]
- 31.Paparrigopoulos T, Tzavellas E, Karaiskos D, Kourlaba G, Liappas I. Treatment of alcohol dependence with low-dose topiramate: An open-label controlled study. BMC Psychiatry. 2011;11:41. doi: 10.1186/1471-244X-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]


