Abstract
Background
HIV-infected individuals undergoing effective antiretroviral therapy (ART) present an increased risk of atherosclerotic cardiovascular disease. We identified serum metabolites associated with carotid intima-media thickness (c-IMT) and its evolution.
Methods
One hundred forty-three hydrophilic serum metabolites were measured by ultraperformance liquid chromatography coupled with high-resolution mass spectrometry in 49 HIV+ ART+, 48 HIV+ ART-naïve and 50 HIV-negative, age-matched, never-smoking male triads. Metabolites differentially altered between groups (“features”) were defined as having a Benjamini-Hochberg-adjusted P value <.05 from a t test and >0.25 log2 absolute mean fold change in metabolite levels. c-IMT was measured across 12 sites at inclusion in all individuals and at the carotid artery (cca) after a median of 5.1 years in 32 HIV+ ART+ individuals. The difference in c-IMT (cross-sectional analysis) and slope of cca-IMT regression/progression per year (longitudinal analysis) for each log10 (area) increase in metabolite level were estimated with linear regression.
Results
Compared with HIV-, metabolite features of HIV+ ART+ were increased N6,N6,N6-trimethyl-L-lysine and decreased ferulate and 5-hydroxy-L-tryptophan, whereas features of HIV+ ART-naïve were increased malate, kynurenine, 2-oxoglutarate, and indole-3-acetate and decreased succinate and 5-hydroxy-L-tryptophan. In HIV+ ART+ individuals, quinolinate and/or indole-3-acetate were positively associated with c-IMT (P < .03), cca-IMT (P < .03), and cca-IMT progression (P < .008). These associations were not observed in HIV+ ART-naïve or HIV-negative individuals. In HIV+ ART+ individuals, the metabolites xanthosine and uridine, from nucleotide metabolism, and g-butyrobetaine, from lysine/dietary choline degradation, were also positively or negatively associated with c-IMT and/or cca-IMT (all P < .01), but not its evolution.
Conclusions
In these highly selected HIV-positive ART-controlled males, 2 novel metabolites derived from tryptophan catabolism, indole-3-acetate and quinolinate, were associated with c-IMT and its progression.
Keywords: antiretroviral, cardiovascular disease, carotid intima-media thickness, HIV, metabolomics, tryptophan metabolism
Cardiovascular disease (CVD) has become a major concern in HIV-positive individuals, even for those with well-controlled HIV replication during antiretroviral therapy (ART) [1, 2]. Increases in atherosclerotic cardiovascular risk have been reported for HIV-positive individuals, whether based on cardiovascular outcomes or carotid intima-medial thickness (c-IMT), a validated surrogate marker of atherosclerotic vascular disease [3]. Prior studies have linked host factors, such as smoking and illicit drug use, and HIV-related factors, such as known infection duration [4], immune activation [5], and treatment with protease inhibitors [6, 7], to this increased risk. Nevertheless, the precise pathophysiological mechanisms remain uncertain to date.
Metabolomics, able to identify numerous circulating metabolites, could provide important information on the processes involved in atherosclerotic cardiovascular risk. Such analyses have been used in past research, in which metabolite levels were associated with HIV serostatus, severity of HIV infection, low-grade inflammation in HIV-positive individuals with virological suppression, and long-term non-AIDS-related events [8]. Most previous research has focused on the tryptophan (Trp) catabolic pathway and kynurenine/tryptophan ratio (KTR), resulting from indoleamine-pyrrole 2,3-dioxygenase (IDO) activation [9–12], and trimethylamine (TMA) and trimethylamineoxide (TMAO) of the lysine/dietary choline degradation pathway [13, 14].
Notwithstanding the insights gained from these previous studies, their major limitation is that only specific metabolites of distinct pathways were analyzed. To understand the broader scope of potentially involved metabolites, we analyzed 143 hydrophilic metabolites, including all amino acids and metabolic derivatives, and their association with HIV infection and c-IMT. Using data from a previous study in closely matched, never-smoking male adults who were HIV-positive ART-controlled, HIV-positive ART-naïve, or HIV-negative, we took advantage of the minimal confounding bias offered from these triads to evaluate the effect of HIV infection and treatment on metabolite levels. We then focused on HIV-positive ART-treated patients to establish the relationship between metabolite levels and c-IMT. Their correlation with markers of inflammation and HIV infection severity was also assessed.
METHODS
Study Participants and Samples
Participants were selected from the Collaboration on HIV, Inflammation and Cardiovascular disease (ANRS-CHIC) study [4]. Briefly, 150 never-smoker men were enrolled in 3 groups: 50 HIV-1-infected patients >35 years old, taking ART for ≥4 years, and with HIV-1 RNA <400 copies/mL (HIV+ ART+); 50 individually age-matched (±5 years) patients, HIV-positive for ≥2 years, who were naïve to ART (HIV+ ART-); and 50 HIV-negative patients individually age-matched (±5 years) to the index HIV-positive, treated patient (HIV-). Noninclusion criteria were current/former smokers; history of coronary heart disease, stroke, angina, or myocardial infarction; active/chronic viral hepatitis infection; undergoing systemic chemotherapy or steroids. All participants gave their written informed consent, and the protocol was approved by the Hotel-Dieu Ethics Committee.
For this study, we included participants with available serum samples. Individuals with severe renal dysfunction (estimated creatinine clearance <30 mL/min) were excluded due to the strong effect of kidney function on metabolite concentrations [15].
Carotid Intima-Media Thickness Measurements
c-IMT was calculated as a composite measure (12-site mean) of the maximal common carotid artery (cca)–IMT, bifurcation IMT, and internal carotid artery IMT bilaterally, outside of plaque, and are reported in mm [4]. c-IMT measurements were performed offline with quality IMT automatic measurement software. A subgroup of patients in the HIV+ ART+ group also had a second measurement of cca-IMT a median (interquartile range) of 5.1 (4.8–5.3) years from participating in the cross-sectional study.
Laboratory Measurements
Individuals had been fasting (12 hours) before their visit. Blood samples were retrieved and processed at a single center and stored at –80°C until use. Interleukin (IL)-6, IL-10 (Bender Medsystems, Burlingame, CA, USA), resistin, and soluble(s)CD14 (R&D Systems, Minneapolis, MN, USA) levels were analyzed using an enzyme-linked immunosorbent assay. Serum ultrasensitive C-reactive protein (us-CRP) was measured by immononephelometry on an IMMAGE analyzer (Beckman-Coulter, Miami, FL, USA). Plasma D-dimer was measured by enzyme-linked fluorescent assay on a VIDAS analyzer (Biomérieux, Marcy-l’Etoile, France). Inducible protein (IP)–10, fractalkine, Monokine induced by gamma interferon (MIG), monocyte chemoattractant protein (MCP)–1, E-selectin, and tumor necrosis factor (TNF)–α were quantified from plasma using the BD Cytometric Bead Array system (BD, Franklin Lakes, NJ, USA).
Immunological Function
Cell samples were available on a randomly selected subset of 30 HIV+ ART+ patients [16]. Activated CD8+ and CD4+ memory T cells (CCR7-CD27-CD45RA+/-, defined by expression of the CD38 and Ki67 markers) and immunosenescence (defined by expression of CD57 on memory T cells) were analyzed on a LSR2 flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA) with appropriate isotype controls and color compensation.
Ultraperformance Liquid Chromatography Coupled With High-Resolution Mass Spectrometry Metabolomics
Reference compounds and a 13C and 15N stable isotope-labeled mix of amino acids were purchased from Sigma-Aldrich (Saint Quentin Fallavier, France), along with liquid chromatography–mass spectrometry (LC-MS)–grade solvents, acetonitrile, and formic acid. Deionized water was obtained from a Milli-Q Elix system fitted with a LC-PaK and a MilliPak filter at 0.22 μm (Merck Millipore, Guyancourt, France).
Sample preparation and liquid chromatography coupled with high-resolution mass spectrometer experiments were carried out as reported [17, 18]. Briefly, 8 volumes of frozen acetonitrile (–20°C) containing internal standard (labeled mixture of amino acids at 12.5 µg/mL) were added to 100 µL of serum samples and vortexed. The resulting samples were then sonicated and incubated at 4°C during 1 hour for slow protein precipitation. Samples were centrifuged for 20 minutes at 20.000×g at 4°C. Supernatants were transferred to another series of tubes and then dried and stored at –80°C before LC-MS analyses. Samples were reconstituted in a 3-fold dilution of H2O/ACN (95/05).
Ultraperformance liquid chromatography coupled with high-resolution mass spectrometry experiments were performed using a chromatographic column Discovery HS F5-PFPP, 5 µm, 2.1×150 mm (Sigma, Saint Quentin Fallavier, France) at 35°C in a UPLC Waters Acquity (Waters Corp, Saint-Quentin-en-Yvelines, France) and Q-Exactive mass spectrometer (Thermo Fisher Scientific, Illkirch, France).
Statistical Analysis
To perform our analysis, we used pathways of the major mapped metabolites from the Kyoto Encyclopedia of Genes and Genomes (KEGG) [19] that have been involved in previous studies on HIV/ART or c-IMT. These metabolic pathways were purine and pyrimidine metabolism, lysine and dietary choline catabolism, tryptophan catabolism, and citrate cycle. All metabolite levels are reported in log10-transformed area values (log10 (area)).
In an initial analysis, we examined metabolite differences between HIV/ART groups. First, we compared the overall variation of metabolite levels, within the 4 selected pathways, across groups using an F-statistic from Wilks’ lambda in a multivariate analysis of variance (MANOVA). Second, we identified metabolite features that were differentially altered between HIV/ART groups. Mean metabolite levels were compared between (i) HIV+ ART+ vs HIV-, (ii) HIV+ ART- vs HIV-, and (iii) HIV+ ART+ vs HIV+ ART- using an unpaired t test. P values were adjusted using the Benjamini-Hochberg procedure. The log2 mean fold change of the log10 (area) levels between groups was also calculated for each metabolite. We defined metabolite features as those with an adjusted P value <.05 and a >.25 or <–.25 log2 mean fold change in log10 (area). This analysis was carried out using the R package “omu.”
In a subsequent analysis, we focused on HIV+ ART+ participants and studied the relationship between metabolite levels and c-IMT. We first used univariable analyses to identify candidate metabolites, which were tested in subsequent analyses. First, change in c-IMT for each log10 (area) increase in metabolite level was estimated with linear regression in univariable analysis. A multivariable analysis model was constructed in which an a priori selection of covariables based on prior research [4, 15] (age, prior hypertension, diabetes, and creatinine clearance) and metabolites with P < .1 in univariable analysis were included, whereas metabolites with P > .1 were removed in backwards-stepwise fashion. Second, the same metabolites associated with c-IMT in univariable analysis were used to model (1) change in cca-IMT for each log10 (area) increase in metabolite level and (2) change in slope of cca-IMT regression/progression per year for each log10 (area) increase in metabolite concentration among those with 2 cca-IMT measurements. Univariable and multivariable models were constructed as above. Significance for these analyses was determined by a P value <.05, and no P value adjustments were made in order to avoid adverse reduction in type II error [20].
Other analyses were conducted to determine the correlation (Spearman rank) between metabolites of the same pathway, as illustrated with correlation networks using Cytoscape, version 3.6.1 [21], and to determine the correlation between metabolites and markers of inflammation/immunity or HIV severity, as illustrated with heatmaps using the R package “gplots.” Significance for these analyses was determined by a P value <.05.
Statistical analysis was performed using STATA (version 12.1; College Station, TX, USA) and R (version 3.2.0; Vienna, Austria).
RESULTS
Characteristics of the Study Population
Of the 150 included males, 2 without an available serum sample and 1 with severe renal dysfunction in the HIV+ group were not included.
Demographic, HIV-related, and cardiovascular-related characteristics are described in Supplementary Table 1. The large majority had a BMI <30 kg/m2 (97%), and only 6% had ≥1 comorbidity (diabetes, prior hypertension, or moderate renal dysfunction). Any use of cannabis, cocaine, and/or methamphetamine within <12 months was most common in HIV+ ART+ participants (28.6%), followed by HIV+ ART- (12.5%) and HIV- (2.0%) participants (P < .001). In the HIV+ ART+ group, HIV-infection was mostly controlled, with a median ART duration >4 years and 94% under viral suppression (<50 copies/mL).
Differences in Metabolites Between HIV/ART Groups
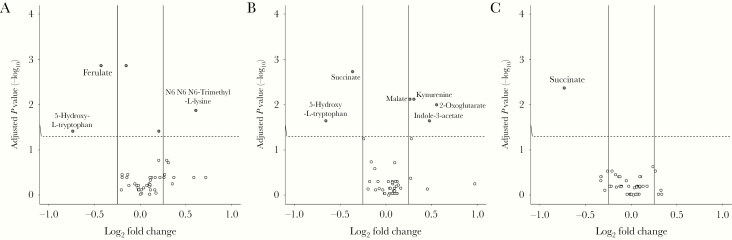
Using MANOVA, significant variations in metabolite levels were observed in the tryptophan metabolism (P < .001) and citrate cycle (P < .001) pathways, but not purine/pyrimidine metabolism (P = .08) or lysine and dietary choline catabolism (P = .14). Metabolite features were as follows: HIV+ ART+ vs HIV-: increased relative levels of N6,N6,N6-trimethyl-L-lysine and decreased ferulate and 5-hydroxy-L-tryptophan (Figure 1A); HIV+ ART- vs HIV-: increased malate, kynurenine, 2-oxoglutarate, and indole-3-acetate and decreased succinate and 5-hydroxy-L-tryptophan (Figure 1B); and HIV+ ART+ vs HIV+ ART-: decreased succinate (Figure 1C).
Figure 1.
Metabolite features of the HIV and antiretroviral therapy (ART) groups. Adjusted P values (-log10) from a t test comparing mean metabolite levels in log10 (area) between groups are plotted against their relative log2 mean fold change. Each dot represents a metabolite; metabolite names are only given for those fulfilling criteria as a metabolite feature: adjusted P value <.05 and log2 mean fold change >0.25 or <–0.25. Comparisons are between: HIV+ ART+ vs HIV- (A), HIV+ ART- vs HIV- (B), and HIV+ ART+ vs HIV+ ART- (C).
Correlation Between Metabolites of the Same Pathway According to HIV/ART Groups
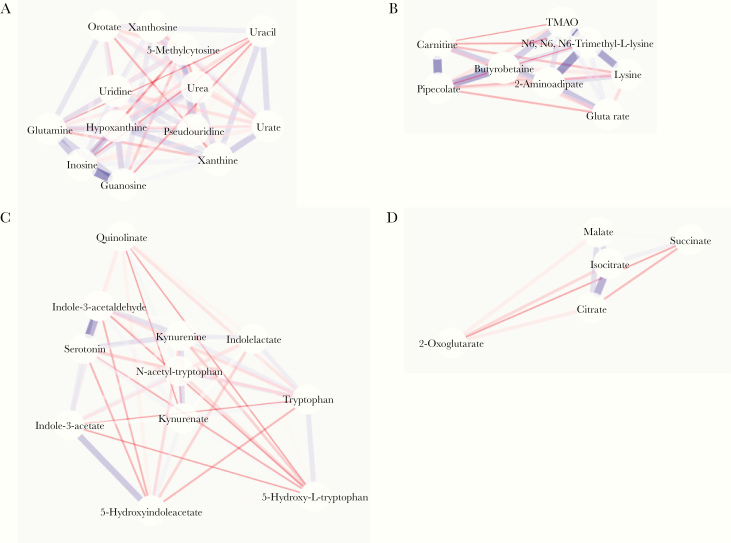
In the purine/pyrimidine metabolism pathway (Figure 2A), a strong correlation was observed between inosine and guanosine in HIV+ ART+ individuals (rho = .836; P < .001), which remained in HIV- individuals (Supplementary Figure 1A). In the lysine and dietary choline catabolism pathway (Figure 2B), strong correlations were observed between pipecolate and carnitine (rho = .408; P = .004), N6,N6,N6-trimethyl-L-lysine and lysine (rho = .322; P = .02), and N6,N6,N6-trimethyl-L-lysine and 2-aminoadipate (rho = .391; P = .005) in HIV+ ART+ individuals, whereas these correlations were not apparent in HIV- individuals (Supplementary Figure 1B). In the tryptophan metabolism pathway (Figure 2C), fairly weak correlations were observed between metabolites in HIV+ ART+ individuals, with the exception of indole-3-acetaldehyde and serotonin (rho = .989; P < .001), and while this correlation held in HIV- individuals (Supplementary Figure 1C), other correlations emerged. In the citrate cycle pathway (Figure 2D), only citrate and isocitrate were significantly correlated (rho = .439; P = .002), whereas this correlation and 1 between malate and isocitrate emerged in HIV- individuals (Supplementary Figure 1D).
Figure 2.
Correlation networks between metabolites in HIV-positive patients treated with antiretroviral therapy. Correlation networks between metabolites of the purine/pyrimidine metabolism (A), lysine degradation (B), tryptophan metabolism (C), and citrate cycle (D) pathways. Positive and negative correlations are depicted in blue and red, respectively. Stronger correlations have thicker lines and colored shading. Metabolites are arranged in an edge-weighted, spring-embedded layout.
Metabolites Associated With IMT and its Progression
Focusing on HIV+ ART+ individuals, we identified 4 metabolites associated with higher c-IMT levels (xanthosine, uridine, indole-3-acetate, and quinolinate) and 2 with lower c-IMT (pipecolate and g-butyrobetaine) in univariable analysis (Table 1). All except pipecolate remained significant in multivariable analysis, with additional adjustment for age, prior hypertension, diabetes, and creatinine clearance. Metabolites that were not associated with c-IMT in univariable analysis are presented in Supplementary Table 2.
Table 1.
Metabolites Associated With Carotid Intima-Media Thickness and Common Carotid Artery–IMT in HIV-Positive Patients Treated With Antiretroviral Therapy
| Univariablea | Multivariableb | |||
|---|---|---|---|---|
| Total c-IMT Cross-sectionalc (n = 49) | Diff (95% CI), mm | P | Diff (95% CI), mm | P |
| Purine/pyrimidine metabolism | ||||
| Xanthosine | 0.160 (0.005 to 0.314) | .04 | 0.128 (0.040 to 0.216) | .005 |
| Uridine | 0.283 (0.069 to 0.497) | .01 | 0.165 (0.041 to 0.289) | .01 |
| Lysine degradation | ||||
| Pipecolate | –0.234 (–0.337 to –0.130) | <.001 | ||
| g-butyrobetaine | –0.344 (–0.577 to –0.110) | .004 | –0.190 (–0.314 to –0.067) | .003 |
| Tryptophan metabolism | ||||
| Indole-3-acetate | 0.102 (0.009 to 0.194) | .03 | 0.056 (0.007 to 0.106) | .03 |
| Quinolinate | 0.169 (0.011 to 0.327) | .04 | 0.115 (0.018 to 0.211) | .02 |
| cca-IMT cross-sectionalc (n = 49) | Diff (95% CI), mm | P | Diff (95% CI), mm | P |
| Purine/pyrimidine metabolism | ||||
| Xanthosine | 0.108 (–0.032 to 0.248) | .13 | ||
| Uridine | 0.218 (0.024 to 0.413) | .03 | ||
| Lysine degradation | ||||
| Pipecolate | –0.193 (–0.288 to –0.099) | <.001 | ||
| g-butyrobetaine | –0.293 (–0.504 to –0.082) | .007 | –0.227 (–0.399 to –0.054) | .01 |
| Tryptophan metabolism | ||||
| Indole-3-acetate | 0.118 (0.035 to 0.201) | .006 | 0.078 (0.010 to 0.145) | .03 |
| Quinolinate | 0.099 (–0.046 to 0.243) | .18 | ||
| cca-IMT progressiond (n = 31) | Diff (95% CI), mm ∆/y | P | Diff (95% CI), mm ∆/y | P |
| Purine/pyrimidine metabolism | ||||
| Xanthosine | 0.062 (–0.161 to 0.285) | .6 | ||
| Uridine | 0.235 (–0.098 to 0.569) | .16 | ||
| Lysine degradation | ||||
| Pipecolate | –0.105 (–0.307 to 0.098) | .3 | ||
| g-butyrobetaine | –0.189 (–0.560 to 0.183) | .3 | ||
| Tryptophan metabolism | ||||
| Indole-3-acetate | 0.148 (0.014 to 0.282) | .03 | 0.182 (0.053 to 0.311) | .008 |
| Quinolinate | 0.341 (0.083 to 0.599) | .01 | 0.400 (0.149 to 0.651) | .003 |
Data were obtained from HIV-infected patients treated with antiretroviral therapy.
Abbrieviations: ∆, change; c-IMT, carotid intima-media thickness; cca, common carotid artery; diff, difference.
aOnly metabolites with P values <.1 in univariable analysis (for the c-IMT cross-sectional analysis) are provided; all other estimates are provided in Supplementary Table 2. The metabolites in the c-IMT cross-sectional univariable analysis were used as candidates for the cca-IMT cross-sectional and progression analysis.
bMultivariable models were adjusted for age, hypertension, diabetes, and creatinine clearance. The following metabolites were excluded: c-IMT cross-sectional—pipecolate (P = .899); cca-IMT cross-sectional—uridine (P = .131), pipecolate (P = .432); cca-IMT progression—none.
cAn evaluation of metabolite levels and c-IMT/cca-IMT was performed at the same moment in the cross-sectional studies. “Diff” represents the mm change in IMT for each log10 (area) increase in metabolite level.
dIn a subset of patients with a second cca-IMT measure (occurring a median [interquartile range] of 5.1 [4.8–5.3] years) after the first measure), metabolite levels at the time of first IMT measure were used to model mm change in cca-IMT per year. “Diff in mm ∆/y” represents the mm change in slope of IMT regression/progression for each log10 (area) increase in metabolite level.
We used the metabolites associated with c-IMT in univariable analysis as candidates for 2 subsequent multivariable analyses. First, cca-IMT was used as an end point and was found to be significantly associated with only g-butyrobetaine and indole-3-acetate (Table 1). Second, when examining the evolution of cca-IMT in the subset of participants with 2 measures, significantly faster progression of IMT was observed with higher baseline indole-3-acetate and quinolinate levels.
Of note, these associations were not observed in HIV+ ART-naïve (data not shown) or HIV-negative participants (Supplementary Table 3).
Metabolites Associated With Immune and Inflammatory Markers
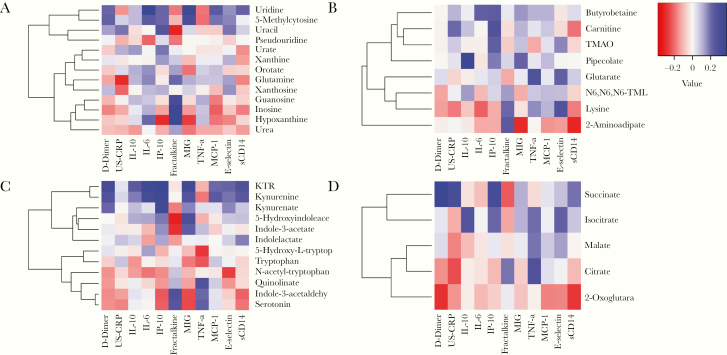
Of the metabolites associated with c-IMT levels, most were not associated with profiles of high inflammation levels (Figure 3). However, uridine of the purine/pyrimidine metabolism pathway was strongly correlated with several markers of inflammation and cytokines (Figure 3A), and there was a significant correlation between quinolinate of the tryptophan metabolism pathway and TNF-α levels (rho = .334; P = .02) (Figure 3C). Of all the candidate metabolites, g-butyrobetaine was significantly correlated with percentage of naïve CD4+CD27+CD45RA+ (rho = .409; P = .03), memory CD4+ (rho = –.469; P = .009), naïve CD8+CD27+CD45RA+ (rho = .469; P = .009), and memory CD8+CD57+ senescent cells (rho = .422; P = .02) (Supplementary Figure 2). Pipecolate was also significantly correlated with percentage of CD4+CD57+ cells (rho = .378; P = .04).
Figure 3.
Correlation between metabolites and inflammatory markers in HIV-positive patients treated with antiretroviral therapy. Spearman correlations between metabolites and inflammatory markers are depicted in heatmaps for metabolites involved in purine/pyrimidine metabolism (A), lysine degradation (B), tryptophan metabolism (C), and the citrate cycle (D). Abbreviations: IL, interleukin; IP, inducible protein; MCP, monocyte chemoattractant protein; MIG, Monokine induced by gamma interferon; TMAO, trimethylamineoxide; TNF, tumor necrosis factor; US-CRP, ultrasensitive C-reactive protein.
Of note, 4 of the 5 metabolites involved in the citrate cycle were significantly associated with TNF-α and/or D-dimer (Figure 3D).
Metabolites Associated With Markers of HIV Infection Severity
As shown in Table 2, none of the metabolites associated with c-IMT in univariable analysis were significantly correlated with CD4+ T-cell count, nadir CD4+, or CD4:CD8 ratio in HIV+ ART+ participants. Nevertheless, higher levels of xanthine were significantly correlated with lower CD4+ nadir, and higher orotate and L-carnitine levels with higher CD4+ nadir (Table 2). Higher indolelactate levels were significantly correlated with a lower CD4:CD8 ratio and kynurenate with lower CD4+ T-cell count. All other correlations are provided in Supplementary Table 4.
Table 2.
Association Between Metabolites and Immunological Parameters in HIV-Positive Patients Treated With Antiretroviral Therapy
| Correlation | |||
|---|---|---|---|
| CD4+ T Cell | CD4+ Nadir | CD4:CD8 Ratio | |
| Purine/pyrimidine metabolism | |||
| Xanthine | –0.0486 | –0.2876 | –0.0912 |
| Xanthosine | 0.1053 | 0.1359 | 0.2084 |
| Orotate | 0.0398 | 0.2889 | –0.0345 |
| Uridine | 0.1494 | 0.1091 | 0.2433 |
| Lysine degradation | |||
| Pipecolate | 0.0510 | 0.0304 | –0.0441 |
| g-butyrobetaine | 0.0844 | 0.0541 | –0.0733 |
| Carnitine | 0.0848 | 0.2886 | 0.2886 |
| Tryptophan metabolism | |||
| Indole-3-acetate | 0.0310 | 0.0310 | 0.0310 |
| Kynurenate | –0.2970 | –0.0900 | –0.0647 |
| Indolelactate | –0.1000 | –0.0814 | –0.3054 |
| Quinolinate | 0.0992 | –0.0200 | 0.0825 |
Only metabolites with significant Spearman’s correlations (as well as metabolites identified in the c-IMT cross-sectional analysis of Table 1) are provided; those with nonsignificant correlations are provided in Supplementary Table 4. Significant correlations are in bold.
Discussion
We report that in ART-controlled HIV-positive males but not HIV-positive ART-naïve or HIV-negative controls, c-IMT and its progression were positively associated with metabolites derived from the Trp catabolism pathway, indole-3-acetate and quinolinate. Xanthosine and uridine metabolites derived from purine/pyrimidine metabolism were positively associated with c-IMT, whereas the derivative g-butyrobetaine from the lysine and dietary choline degradation was inversely associated with c-IMT.
Of all the pathways studied, metabolites of Trp catabolism were the only ones consistently associated with c-IMT/cca-IMT at the cross-sectional visit, as well as cca-IMT progression. Kynurenine and, to a lesser extent, kynurenic acid (KA) have already been identified as correlates with c-IMT and CVD, but these associations were observed mostly in patients with end-stage renal disease [15]. Few studies have evaluated the association between these metabolites and CVD outcomes in HIV-positive individuals. For instance, a study in 105 ART-naïve HIV-positive individuals from Uganda demonstrated that lower absolute values of KTR 6 months after ART initiation, specifically among those achieving an undetectable HIV viral load, were associated with lower c-IMT levels when measured 7 years after treatment initiation [22]. In other cohorts, HIV-positive individuals, compared with HIV-negative, were found to have lower levels of Trp but comparable levels of KA, whereas higher levels of Trp and lower KA or KA/Trp ratio were associated with a decreased risk of carotid artery plaque formation after a median of 7 years of follow-up [23]. Although this study importantly stresses the role of Trp catabolites in increased CVD risk among HIV-positive patients, the relationship with c-IMT was not provided, and other classical Trp catabolites, such as kynurenine, were not measured. Furthermore, this previous observation stemmed from study populations with a high prevalence of comorbidities and smokers, thereby increasing the risk of residual confounding bias.
In our study, we observed that Trp and KA were never identified as metabolite features between HIV/ART groups, yet 5-hydroxy-L-tryptophan was a feature with lower levels in HIV-positive individuals, regardless of ART status. We also confirmed the higher levels of kynurenine in HIV+ ART- individuals compared with HIV-negative individuals [10, 11, 24]. None of these metabolites, however, were associated with c-IMT in our study, and instead only indoleacetate and quinolinate were positively associated with c-IMT after adjusting for age, hypertension, diabetes, and creatinine clearance. Importantly, this association was not apparent in HIV+ ART-naïve and HIV-negative controls, implying that the underlying role of these metabolites in CVD is specific to ART-controlled HIV infection. It should be mentioned that indole-3-acetate and quinolinate have been associated with CVD and/or c-IMT in patients with end-stage renal disease [25, 26]. As all but 2 patients in our cohort had normal creatinine clearance levels, any impact of renal function on our results was likely minimal.
How these metabolite imbalances could consequently impact CVD remains debatable. In a previous study, the KTR largely explained the dysbiosis in gut microbiota observed in HIV+ ART+ vs HIV-negative individuals [27], whereas another study found a close link between Trp or KA levels and several taxa of gut microbiota in HIV-positive individuals [28]. Meanwhile, the KTR has been associated with both altered gut microbiota and endothelial dysfunction in the HIV-positive population [29], suggesting some indirect mechanism associated with intestinal flora. We did observe a strong correlation between KTR and kyurenine levels with sCD14, a marker of active microbial translocation but also innate immune activation [30]. However, their levels were not associated with c-IMT. Systemic inflammation from metabolite imbalances could be another reason for increases in c-IMT, as reported mainly in ART-naïve patients [10]. In our study, kynurenine and KA were strongly associated with D-dimer, whereas quinolinate was only correlated with TNF-α and no other markers of inflammation. Furthermore, there was no correlation between quinolinate or indole-3-acetate and activated or senescent CD4+/CD8+ cells, which could promote systemic inflammatory responses [31]. These observations overall do not support the influence of low-grade inflammation as an explanation for these findings.
Among metabolites of the lysine, carnitine, and dietary choline catabolic pathways, TMAO was not identified as a metabolite feature of HIV+ ART+ compared with HIV- individuals and was not associated with c-IMT [20]. This result is in line with previous findings on coronary artery plaque level [32]. Nevertheless, TMAO might not be an adequate predictor of cardiovascular disease in HIV-positive individuals [20]. We did identify another metabolite from this pathway, g-butyrobetaine, which was negatively associated with c-IMT and cca-IMT. Others have shown the proatherogenic properties of g-butyrobetaine [33], and its levels have already been associated with both carotid artery atherosclerosis and cardiovascular mortality in patients with carotid artery stenosis [13]. However, levels of this metabolite could be reflective of lacking synthesis to carnitine, which leads to TMAO production and further vascular damage [34] or imbalances in gut microbiota [33]. G-butyrobetaine was significantly and positively correlated with naïve T cells, but also senescent CD8+ cells, suggestive of highly active adaptive immune responses in individuals with higher g-butyrobetaine levels. However, the indirect relationship of these metabolites to immunity is unclear. The mechanisms driving the association between g-butyrobetaine and c-IMT require further study in HIV+ ART+ individuals.
Of the metabolites derived from the purine/pyrimidine metabolism pathway, higher xanthosine and uridine levels were associated with higher c-IMT in the present study. Such an association has not been reported previously. Nonetheless, altered levels of metabolites in these pathways, particularly xanthosine and pseudouridine, have been reported in older individuals, with high expression of genes linked to inflammasome and in turn associated with increased risk of all-cause mortality [35]. A recent study has reported that glutamine levels were increased in matched HIV-positive individuals with vs without coronary artery disease [36], but it only evaluated 15 amino acids. Glutamine levels were decreased in HIV+ ART+ vs HIV+ ART- in our study, yet they were not associated with c-IMT.
Considering the significant differences in metabolite levels between HIV/ART groups observed in our study population and others [8, 24, 37, 38], it is possible that some metabolites are related to the severity of HIV infection [8, 37]. Accordingly, we observed that carnitine from lysine and dietary choline catabolism and xanthine and orotate from the purine/pyrimidine pathway were correlated with nadir CD4+ T-cell count. Higher levels of metabolites from the Trp catabolism pathway were more strongly correlated with lower CD4+ T-cell counts and CD4:CD8 ratio.
HIV infection has been previously associated with mitochondrial dysfunction [8, 37, 38], whereby mitochondrial content within cells increases and results in higher KTR and levels of metabolites from the TCA cycle [38]. Indeed, most metabolites derived from the TCA cycle were identified as metabolite features with higher levels in HIV+ ART- participants, and some were strongly correlated with several inflammatory markers in HIV+ ART+ participants, arguing for mitochondrial dysfunction in the presence of low-grade inflammation during effective ART. Nonetheless, this seemed unrelated to c-IMT.
There are certain limitations of our study. First, the analysis was limited to 48–50 participants in each HIV/ART group. The small sample sizes precluded any in-depth analysis of other factors, such as specific classes of antiretroviral agents. Second, aside from the reduced confounding bias of important factors, namely smoking and gender, due to the exposure-matched design, our results might not be generalizable to other populations. Third, the strict selection of this population likely reduced the variability of metabolite levels, explaining why the criteria for metabolite features included a 0.25-fold log2 mean change compared with the more commonly used 2-fold change. Nevertheless, the 2-fold cutoff has been criticized as arbitrary and not entirely applicable to metabolomics data [39]. Fourth, there was a significantly higher proportion of HIV+ ART+ individuals with illicit drug use, higher HDL, and lower triglyceride levels than HIV- individuals. These differences could have confounded our findings, yet it is unclear how drug use or lipid parameters influence metabolite concentrations. Finally, analyses of cellular markers of immunity and cca-IMT progression were conducted in a subset of the original study population.
In conclusion, our data suggest that disturbances in the Trp, nucleoside, and Lys-dietary choline catabolic pathways could be involved in HIV-related atherosclerosis. Given that the Trp catabolites TMAO and butyrobetaine are also products of the gut microbiota, an attractive explanation would be that altered gut microbiota observed in HIV-infected individuals, occurring during ART-induced viral suppression, could contribute to increased risk of atherosclerosis via alterations in the Trp catabolism and lysine and dietary choline degradation pathways. Further research would be needed to confirm this hypothesis.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We are grateful to the participants. We acknowledge Ludivine Laurans, Lydie Houssou, and Marie-Christine Rincon for their technical assistance with quantifying inflammatory and metabolic markers; Manuela Sebire, Alina Ciuchete, Loïc Desquilbet, Nelly Desplanque, Catherine Lupin, and Eveline Deneer for participant recruitment, management, and data collection; Dr. Jürgen Tredup, Dr. Pauline Campa, Dr. Diane Bollens, Dr. Bénédicte Lefebvre, Dr. Marie-Caroline Meyohas, Dr. Jérôme Pacanowski, Dr. Zineb Ouazene, Dr. Gilles Raguin, Dr. Phillippe Tangre, Dr. Laurent Fonquernie, and Dr. Karine Lacombe for patient recruitment; and Sandrine Couffin-Cadiergues and Marcia Trumeau for administrative assistance.
Financial support. This work was supported by the Agence Nationale de Recherche sur le Sida et les Hépatites (ANRS grant 2007/303), Sidaction (grant AI 20), and IHU-ICAN.
Potential conflicts of interest. The authors report no conflicts of interest with the present publication. Dr. Boccara reports receiving research grants from Amgen and lecture fees from Janssen, Gilead, ViiV Healthcare, Amgen, Sanofi, MSD, and Servier outside the submitted work. Dr. Cohen reports receiving consultant and lecture fees from Amgen, AstraZeneca, Bayer Pharma, BMS-Pfizer Alliance, Boehringer-Ingelheim, and Novartis and research grants from ARS, RESICARD, Bayer, and Boehringer-Ingelheim outside the submitted work. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. A.B. and J.C. were responsible for statistical analysis, data interpretation, and drafting the manuscript. F.B., J.-L.M., and P.-M.G. helped design, conceptualize, and obtain funding for the ANRS CHIC study, coordinated data collection and data analysis, and provided critical revisions of the manuscript. F.I. coordinated data collection on metabolite analysis, helped with data interpretation, drafted parts of the manuscript, and provided critical revisions of the manuscript. J.-P.B. and S.F. were responsible for quantification of inflammatory markers and provided critical revisions of the manuscript. A.S., D.S., and B.A. were responsible for quantification of cellular and some inflammatory markers and provided critical revisions of the manuscript. N.H. and A.C. (along with F.B.) measured cardiovascular parameters and provided critical revisions of the manuscript. All authors have approved the final version of the manuscript.
Prior presentations. This work was presented in part as an oral presentation at the 19th International Workshop on Comorbidities and Adverse Drug Reactions in HIV, Milan, Italy, October 2017, and as a poster at the European AIDS Clinical Society Meeting in Milan, Italy, October 2017.
References
- 1. Boccara F, Lang S, Meuleman C, et al. HIV and coronary heart disease: time for a better understanding. J Am Coll Cardiol 2013; 61:511–23. [DOI] [PubMed] [Google Scholar]
- 2. Kaplan RC, Hanna DB, Kizer JR. Recent insights into cardiovascular disease (CVD) risk among HIV-infected adults. Curr HIV/AIDS Rep 2016; 13:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hanna DB, Guo M, Bůžková P, et al. HIV infection and carotid artery intima-media thickness: pooled analyses across 5 cohorts of the NHLBI HIV-CVD collaborative. Clin Infect Dis 2016; 63:249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Desvarieux M, Boccara F, Meynard JL, et al. Infection duration and inflammatory imbalance are associated with atherosclerotic risk in HIV-infected never-smokers independent of antiretroviral therapy. AIDS 2013; 27:2603–14. [DOI] [PubMed] [Google Scholar]
- 5. Hsue PY, Hunt PW, Sinclair E, et al. Increased carotid intima-media thickness in HIV patients is associated with increased cytomegalovirus-specific T-cell responses. AIDS 2006; 20:2275–83. [DOI] [PubMed] [Google Scholar]
- 6. Lang S, Mary-Krause M, Cotte L, et al. ; Clinical Epidemiology Group of the French Hospital Database on HIV Impact of individual antiretroviral drugs on the risk of myocardial infarction in human immunodeficiency virus-infected patients: a case-control study nested within the French Hospital Database on HIV ANRS cohort CO4. Arch Intern Med 2010; 170:1228–38. [DOI] [PubMed] [Google Scholar]
- 7. Ryom L, Lundgren JD, El-Sadr W, et al. ; D:A:D study group Cardiovascular disease and use of contemporary protease inhibitors: the D:A:D international prospective multicohort study. Lancet HIV 2018; 5:e291–300. [DOI] [PubMed] [Google Scholar]
- 8. Sitole LJ, Williams AA, Meyer D. Metabonomic analysis of HIV-infected biofluids. Mol Biosyst 2013; 9:18–28. [DOI] [PubMed] [Google Scholar]
- 9. Peltenburg NC, Schoeman JC, Hou J, et al. Persistent metabolic changes in HIV-infected patients during the first year of combination antiretroviral therapy. Sci Rep 2018; 8:16947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jenabian MA, El-Far M, Vyboh K, et al. ; Montreal Primary infection and Slow Progressor Study Groups Immunosuppressive tryptophan catabolism and gut mucosal dysfunction following early HIV infection. J Infect Dis 2015; 212:355–66. [DOI] [PubMed] [Google Scholar]
- 11. Gaardbo JC, Trøsied M, Stiksrud B, et al. Increased tryptophan catabolism is associated with increased frequency of CD161+Tc17/MAIT cells and lower CD4+ T-Cell count in HIV-1 infected patients on cART after 2 years of follow-up. J Acquir Immune Defic Syndr 2015; 70:228–35. [DOI] [PubMed] [Google Scholar]
- 12. Byakwaga H, Boum Y 2nd, Huang Y, et al. The kynurenine pathway of tryptophan catabolism, CD4+ T-cell recovery, and mortality among HIV-infected Ugandans initiating antiretroviral therapy. J Infect Dis 2014; 210:383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Skagen K, Trøseid M, Ueland T, et al. The carnitine-butyrobetaine-trimethylamine-N-oxide pathway and its association with cardiovascular mortality in patients with carotid atherosclerosis. Atherosclerosis 2016; 247:64–9. [DOI] [PubMed] [Google Scholar]
- 14. Schiattarella GG, Sannino A, Toscano E, et al. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: a systematic review and dose-response meta-analysis. Eur Heart J 2017; 38:2948–56. [DOI] [PubMed] [Google Scholar]
- 15. Wang Q, Liu D, Song P, Zou MH. Tryptophan-kynurenine pathway is dysregulated in inflammation, and immune activation. Front Biosci 2015; 20:1116–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goulenok T, Boyd A, Larsen M, et al. ; CHIC study group Increased carotid intima-media thickness is not associated with T-cell activation nor with cytomegalovirus in HIV-infected never-smoker patients. AIDS 2015; 29:287–93. [DOI] [PubMed] [Google Scholar]
- 17. Aron-Wisnewsky J, Prifti E, Belda E, et al. Major microbiota dysbiosis in severe obesity: fate after bariatric surgery. Gut 2019; 68:70–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Garali I, Adanyeguh IM, Ichou F, et al. A strategy for multimodal data integration: application to biomarkers identification in spinocerebellar ataxia. Brief Bioinform 2018; 19:1356–69. [DOI] [PubMed] [Google Scholar]
- 19. Kanehisa M, Furumichi M, Tanabe M, et al. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res 2017; 45:D353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Missailidis C, Neogi U, Stenvinkel P, et al. The microbial metabolite trimethylamine-N-oxide in association with inflammation and microbial dysregulation in three HIV cohorts at various disease stages. AIDS 2018; 32:1589–98. [DOI] [PubMed] [Google Scholar]
- 21. Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 2003; 13:2498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Siedner MJ, Kim JH, Nakku RS, et al. Persistent immune activation and carotid atherosclerosis in HIV-infected Ugandans receiving antiretroviral therapy. J Infect Dis 2016; 213:370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Qi Q, Hua S, Clish CB, et al. Plasma tryptophan-kynurenine metabolites are altered in human immunodeficiency virus infection and associated with progression of carotid artery atherosclerosis. Clin Infect Dis 2018; 67:235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cassol E, Misra V, Holman A, et al. Plasma metabolomics identifies lipid abnormalities linked to markers of inflammation, microbial translocation, and hepatic function in HIV patients receiving protease inhibitors. BMC Infect Dis 2013; 13:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pawlak K, Brzosko S, Mysliwiec M, Pawlak D. Kynurenine, quinolinic acid—the new factors linked to carotid atherosclerosis in patients with end-stage renal disease. Atherosclerosis 2009; 204:561–6. [DOI] [PubMed] [Google Scholar]
- 26. Dou L, Sallée M, Cerini C, et al. The cardiovascular effect of the uremic solute indole-3 acetic acid. J Am Soc Nephrol 2015; 26:876–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vujkovic-Cvijin I, Dunham RM, Iwai S, et al. Dysbiosis of the gut microbiota is associated with HIV disease progression and tryptophan catabolism. Sci Transl Med 2013; 5:193ra91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Qi Q, Hua S, Hanna DB, et al. Gut microbiota, tryptophan catabolism and athersclerosis in HIV infection. Paper presented at: Conference on Retroviruses and Opportunistic Infections; February 13–16 2017; Seattle, WA. Abstract 636LB. [Google Scholar]
- 29. Hoel H, Hove-Skovsgaard M, Hov JR, et al. Impact of HIV and type 2 diabetes on gut microbiota diversity, tryptophan catabolism and endothelial dysfunction. Sci Rep 2018; 8:6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marchetti G, Tincati C, Silvestri G. Microbial translocation in the pathogenesis of HIV infection and AIDS. Clin Microbiol Rev 2013; 26:2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sauce D, Elbim C, Appay V. Monitoring cellular immune markers in HIV infection: from activation to exhaustion. Curr Opin HIV AIDS 2013; 8:125–31. [DOI] [PubMed] [Google Scholar]
- 32. Srinivasa S, Fitch KV, Lo J, et al. Plaque burden in HIV-infected patients is associated with serum intestinal microbiota-generated trimethylamine. AIDS 2015; 29:443–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Koeth RA, Levison BS, Culley MK, et al. γ-butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of L-carnitine to TMAO. Cell Metab 2014; 20:799–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rydzik AM, Chowdhury R, Kochan GT, et al. Modulating carnitine levels by targeting its biosynthesis pathway - selective inhibition of γ-butyrobetaine hydroxylase. Chem Sci 2014; 5:1765–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Furman D, Chang J, Lartigue L, et al. Expression of specific inflammasome gene modules stratifies older individuals into two extreme clinical and immunological states. Nat Med 2017; 23:174–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Okeke NL, Craig DM, Muehlbauer MJ, et al. Metabolites predict cardiovascular disease events in persons living with HIV: a pilot case-control study. Metabolomics 2018; 14:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Scarpellini B, Zanoni M, Sucupira MC, et al. Plasma metabolomics biosignature according to HIV stage of infection, pace of disease progression, viremia level and immunological response to treatment. PLoS One 2016; 11:e0161920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cassol E, Misra V, Mukerji SS, et al. Early markers of renal dysfunction among cocaine users with HIV and HCV infection. Paper presented at: Conference on Retroviruses and Opportunistic Infections; February 22–25 2016; Boston, MA. Abstract 693. [Google Scholar]
- 39. Vinaixa M, Samino S, Saez I, et al. A guideline to univariate statistical analysis for LC/MS-based untargeted metabolomics-derived data. Metabolites 2012; 2:775–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.