Abstract
Therapeutic manipulation of regulatory T cells (Tregs) has been regarded as a promising approach for the treatment of immune disorders. However, a better understanding of the immunomodulatory mechanisms of Tregs, and new safe and effective methods to improve the therapeutic effects of Tregs are highly desired. Here, we have identified the key roles of a cAMP-adenosine positive feedback loop in the immunomodulatory function of Tregs. Adult male C57BL/6J mice were used for experimental autoimmune uveitis (EAU) model, Tregs and uveitogenic T cells (UTs). In established EAU, induced Tregs (iTregs) administration ameliorated the inflammatory response. In vitro, iTregs inhibited UTs proliferation and inflammatory cytokine production. Mechanistically, cAMP is partially responsible for iTregs-mediated inhibition on UTs. Importantly, intracellular cAMP regulates CD39 expression and CD39-dependent adenosine production in iTregs, and cAMP directly participates in iTreg-derived adenosine production by a CD39 signaling-independent extracellular cAMP-adenosine pathway. Moreover, extracellular adenosine increases the intracellular cAMP level in Tregs. More importantly, increasing the cAMP level in iTregs before transfer improves their therapeutic efficacy in established EAU. Notably, the cAMP-adenosine loop exists in both iTregs and naturally occurring Tregs. These findings provide new insights into the immunosuppressive mechanisms of Tregs and suggest a new strategy for improving the therapeutic efficacy of Tregs in established autoimmune disease.
Keywords: Regulatory T cells, Autoimmune diseases, cAMP, Adenosine, CD39
Introduction
Autoimmune diseases remain a therapeutic challenge worldwide. The numbers and functions of CD4+CD25+ forkhead box protein 3 (Foxp3)+ regulatory T cells (Tregs), which are crucial in maintaining immune homeostasis, are abnormal in autoimmune diseases (1). Therefore, the therapeutic manipulation of Tregs has been regarded as a promising approach for the treatment of autoimmune diseases (2,3,4,5,6,7,8). However, several hurdles remain in the clinical use of Treg therapy. Thus, a better understanding of the immunomodulatory mechanisms of Tregs and the development of safe and effective methods that will improve the therapeutic effects of Tregs are highly desired.
Tregs are heterogeneous and can be divided into at least two populations: thymus-derived naturally occurring Tregs (nTregs) and induced Tregs (iTregs), which are generated by IL-2 and TGF-β stimulation ex vivo or outside the thymus in vivo. nTregs and iTregs share the primary phenotypic and functional characteristics of Tregs, but the phenotype and function of nTregs and iTregs are clearly different(2,9,10). For example, CD28 is required for the costimulation of nTregs, whereas cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) is required for the costimulation of iTregs (2,11). In addition, Helios is expressed by nTregs but not by iTregs (2). Moreover, nTregs are able to be converted into Th17 cells in the presence of IL-6 and IL-1β (8,12,13,14), whereas iTregs are resistant to IL-6-driven Th17 cell conversion (15). Notably, a recent study found that iTregs but not nTregs suppress the function of type 2 innate lymphoid cells both in vitro and in vivo (4). Currently, a larger number of studies has investigated the immunomodulatory mechanisms of nTregs, and these studies have shown that the immunomodulatory properties of nTregs are associated with their expression of CD39, cAMP, and CTLA-4 and with their secretion of TGF-β, IL-10, and so on. However, the immunomodulatory mechanisms of Tregs remain largely unclear. The knowledge of the immunomodulatory mechanisms of iTregs is far less developed and understood than that of nTregs. For example, CD39 and cAMP, which were firmly established in 2007 to play key roles in the suppressive function of nTregs (16,17), still have unknown roles in the suppressive function of iTregs.
As a sight-threatening autoimmune eye disease, uveitis remains a therapeutic challenge for ophthalmologists. However, to date, the role of iTregs in uveitis has not been explored. Given that iTregs and nTregs display distinct features, in the present study, we evaluated the therapeutic effects of iTregs and nTregs in the setting of established uveitis and explored their potential immunomodulatory mechanisms.
MATERIALS AND METHODS
Mice and reagents
Six-to-eight-week-old female C57BL/6J mice were purchased from Guangzhou Animal Testing Center (Guangzhou, China) and maintained in an air-conditioned room with a 12-h light-dark cycle. The animals were provided access to food and water ad libitum until they were used for experiments. All animal experiments were performed according to a protocol approved by the Institutional Animal Care and Use Committee of Zhongshan Ophthalmic Center, Sun Yat-sen University, and all procedures were performed in compliance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research. Interphotoreceptor retinoid-binding protein (IRBP) peptide 1–20 (IRBP1–20; GPTHLFQPSLVLDMAKVLLD) was obtained from AnaSpec (Fremont, CA). All antibodies used for flow cytometry were obtained from BD Biosciences (San Jose, CA). All cytokines and TGF-β1 were purchased from R&D Systems (Minneapolis, MN).
Mouse naïve CD4+ cell isolation, nTreg expansion and iTreg induction
Naive CD4+ T cells were isolated by an EasySep™ Mouse Naïve CD4+ T Cell Isolation kit (STEMCELL Technologies, Canada). CD4+CD25+ nTregs sorted from the thymus of C57BL/6 mice were expanded with anti-mouse CD3/CD28 beads (1:3) and IL-2 (200 IU/ml) for 7 days and were then harvested. For iTreg induction, naive CD4+ T cells (3×105 cells/well) were stimulated with anti-mouse CD3/CD28 beads (1:5, Miltenyi Biotec) in the presence of rhIL-2 (50 IU/ml, BioLegend), all-trans retinoic acid (atRA) (5 nM) and 100 μg/ml vitamin C with rhTGF-β (5 ng/ml, BioLegend) in 96-well plates for 7 days as previously described (6). RPMI 1640 medium (Gibco) was supplemented with 100 U/ml penicillin, 100 mg/ml streptomycin, 10 mM N-2-Hydroxyethylpiperazine-N’−2’-ethanesulfonic Acid (Invitrogen Life Technologies), 1×105 M 2-mercaptoethanol (Sigma-Aldrich), and 10% heat-inactivated FBS (Hy Clone) and was used for all cultures. Foxp3 expression was evaluated using a Fortessa (BD Biosciences).
Induction of EAU and cell treatment protocol
Induction of EAU by active immunization was performed as described previously (18). In brief, C57BL/6 mice were immunized subcutaneously with a mixture of 200 µg of human IRBP1–20 (GPTHLFQPSLVLDMAKVLLD, Shengong, Shanghai, China) emulsified in an equal volume of complete Freund’s adjuvant (CFA) containing 2.5 mg/ml Mycobacterium tuberculosis H37RA (Difco, Detroit, MI, USA). In addition, these mice also received 200 ng of Bordetella pertussis toxin (Sigma-Aldrich Corp., St. Louis, MO, USA) intraperitoneally on the day of immunization and on the 2nd day after immunization. After immunization with IRBP/CFA, 3×106 nTregs or iTregs in 300 µl of phosphate-buffered saline (PBS) were intravenously transferred to the mice. Mice receiving 300 µl of PBS were used as the vehicle control group.
Clinical disease was scored at regular intervals by fundus examination using a Micron III retina imaging system (Phoenix Research Labs, Pleasanton, CA). Clinical inflammation scores ranged from 0 to 4 according to the following criteria: 0 = no inflammation; 0.5 = trace disease; 1 = minimally active, localized disease; 2 = moderately active disease with multiple lesions; 3 = active disease with multiple diffuse lesions; and 4 = very active disease often accompanied by retinal detachment or hemorrhage. Pathological scores were determined in accordance with previously published criteria (18).
In vitro Treg suppression assay
To determine the suppressive activity of iTregs, we measured their ability to inhibit the proliferation of IRBP-specific T cells. In brief, responder T cells were labeled with 1.5 μM carboxyfluorescein diacetate succinimidyl ester (CFSE, BioLegend, CA) and cocultured with iTregs in complete RPMI 1640 medium with or without IRBP1–20 (20 µg/ml). Tregs were harvested and washed 3 times in PBS before being used in the suppression assay. iTregs were added to responder T cells at the indicated ratio. Cocultures were incubated for 3–5 days (37 °C, 5% CO2). For mechanistic studies, neutralizing antibodies or inhibitors were added to the culture systems. After cells were harvested, the suppression of CFSE-labeled T cell proliferation was analyzed.
Other reagents used in the research
The following reagents were used: TGFβ antibody (Ab) (10 μg/ml, BioLegend, catalog 521704), CTLA-4 Ab (10 μg/ml, Selleck, catalog A2001), adenosine-A2A receptor (A2AR)-specific antagonist (ZM 241385, 0.5 μM, Selleck, catalog 8105), and a gap junction inhibitor (GAP-27, 300 μM, Sigma-Aldrich, catalog G1794), Sodium polyoxotungstate (POM-1, 100 μM, Torics, catalog 2689), Rp-Adenosine 3′,5′-cyclic monophosphorothioate triethylammonium salt (Rp-cAMPS, 4 mM, Sigma-Aldrich, catalog A165), exogenous cAMP (50 μM, Sigma-Aldrich, catalog A9501), Cholera toxin (CT, 1 μg/ml, Sigma-Aldrich, catalog C8052), Adenosine 5′-(α,β-methylene) diphosphate (APCP, 100 μM, Sigma-Aldrich, catalog M3763), 1,3-Dipropyl-8-p-sulfophenylxanthine (DPSPX, 100 μM, Santa Cruz Biotechnology, catalog sc-208778), 2-p-(2-Carboxyethyl)phenethylamino-5′-N-ethylcarboxamidoadenosine (CGS, 1μM, Selleck, catalog S2153), and adenosine (Sigma-Aldrich, catalog A9251).
Cell transfection
CD39 siRNA (sc-42786), CD73 siRNA (sc-42863), their respective negative control siRNAs, and transfection reagents were purchased from Santa Cruz Biotechnology (19). The transfection of iTregs with siRNA was performed according to the manufacturer’s instructions.
Flow cytometry
Cytokines and surface markers were evaluated using a Fortessa (BD Biosciences). In brief, for cytokine detection, cells were stimulated for 5 hours with PMA and ionomycin supplemented with brefeldin A (BFA) (Sigma-Aldrich Corp., St. Louis, MO, USA). Then, cells were incubated on ice for 30 minutes in the dark with antibodies against surface markers. For intracellular staining, cells were fixed and permeabilized with a Human Foxp3 Buffer Set (BioLegend, CA) and were stained with fluorescent antibodies for an additional 30 minutes on ice in the dark. Events were collected and analyzed with FlowJo software (Tree Star, Ashland, OR). The following antibodies were used: CD4 (BioLegend, catalog 100434), CD62L (BioLegend, catalog 104406), CD44 (BioLegend, catalog 103012), CD25 (BioLegend, catalog 101918), Foxp3 (BioLegend, catalog 320014), IL-17A (BioLegend, catalog 506912), and IFN-γ (BioLegend, catalog 505808). All antibodies were used at the manufacturers’ recommended concentrations.
ELISAs
ELISAs were performed to detect mouse cytokines following the manufacturer’s instructions. Mouse IFN-γ and IL-17A ELISA Ready-SET-Go (RSG) standard kits were purchased from Thermo Fisher Scientific (eBioscience, Invitrogen). To assess cAMP release by iTreg cells, culture system supernatants were collected and washed, and a cAMP ELISA (ab65355, Abcam) was performed. Adenosine was detected by an adenosine assay kit (BioVision Inc, CA). In brief, erythro-9-Amino-β-hexyl-α-methyl-9H-purine-9-ethanol (EHNA) was added into the supernatant to inhibit adenosine deaminase (final concentration 10 μM). For adenosine measure, 100 μl mixed liquor (50 supernatant and 50 μl Reaction Mix) was incubated at room temperature for 15 min., protected from light.
Histology
Eyes harvested from control mice or from mice with EAU were fixed with 10% neutral-buffered formalin and embedded in paraffin for sectioning (5-μm thickness) followed by H&E staining using standard procedures. The sections were observed and imaged under a microscope (Leica DM4000, Germany). Histopathological changes in the retina were graded according to previously published scoring criteria using 4 sections from each eye [2]. The samples were assessed by a pathologist (DSR) who was blinded to the sample group assignments during the initial scoring.
qPCR analysis
RNA was extracted from cells using an RNeasy Mini kit (QIAGEN) according to the manufacturer’s protocol. First-strand cDNA was synthesized using SuperScript III reverse transcription reagents. Quantitative real-time PCR was performed to analyze transcripts encoding the IL-17A, IFN-γ and CD39 protein using Fast Start universal probe master mix (Roche) and TaqMan probes on a Stratagene MxPro 3005p system. Transcripts from the mouse samples were normalized using GAPDH primer/probe sets (05046211001, Roche). The primers for GAPDH, IL-17A, IFN-γ and CD39 were purchased from Invitrogen, and the sequences for these primers are provided in previous studies (20,21).
Statistical analyses
The statistical analyses were performed with Student’s t-test or one-way ANOVA followed by Bonferroni post hoc tests as appropriate using SPSS software (17.0; SPSS, Chicago, IL). A P value less than 0.05 was considered significant.
RESULTS
iTregs ameliorate established experimental autoimmune uveitis (EAU)
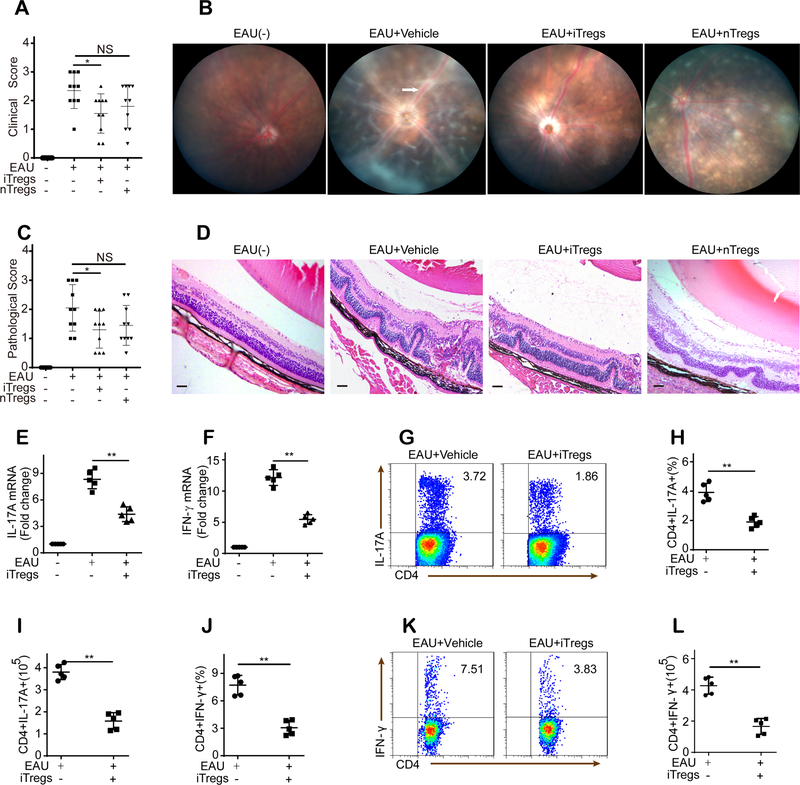
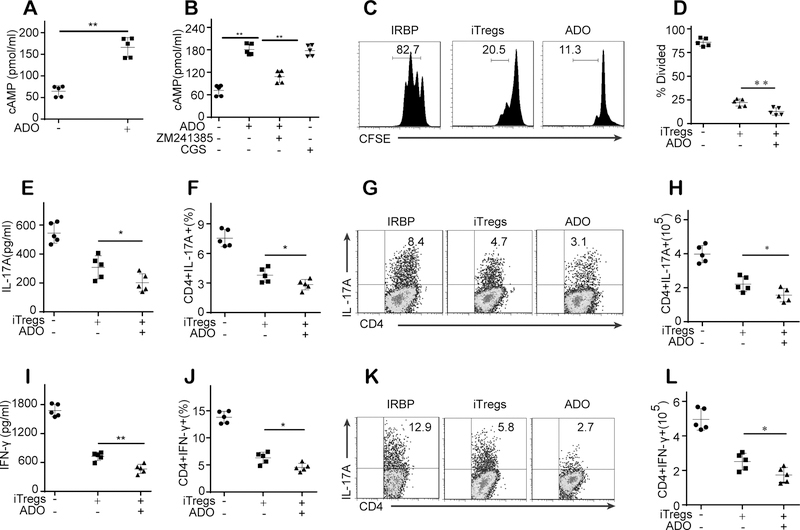
Most of the interphotoreceptor retinoid-binding protein (IRBP)-immunized mice showed clinical signs of uveitis on day 9 (9 days after immunization). Thus, to examine the therapeutic effect of different Treg subsets in established uveitis, nTregs or iTregs were administered to mice with EAU on day 11, when mice were suffering from severe uveitis. The effect of Tregs on the clinical inflammation scores and retinal inflammation pathology of EAU was evaluated on day 21 after immunization. Control mice that received PBS showed severe signs of uveitis with clinical symptoms and retinal inflammation (Fig. 1A–D). In contrast, mice with established EAU that received intravenous administration of iTregs exhibited an alleviation of inflammatory symptoms and retinal inflammation (Fig. 1A–D). However, the inflammatory symptom scores and retinal inflammation after nTreg administration were not significantly different from those of control mice with EAU (Fig. 1A–D).
Figure 1. iTregs ameliorate established EAU.
(A-B): Clinical inflammation scores (n = 10) and representative images from fundoscopic examination (white arrow: vasculitis) of mice with EAU were obtained 21 days after immunization. (C-D): Pathological inflammation scores (n = 10) and representative images from histopathologic examination of retinal tissue from mice with EAU (scale bars represent 200 μm) were obtained 21 days after immunization. (E-F): Compared with vehicle control administration, iTreg administration reduced IL-17A and IFN-γ mRNA expression in retinas 21 days after immunization (n = 5). (G-L): iTreg administration decreased the number and frequency of CD4+IL-17A+ T cells and CD4+IFN-γ+ T cells in the dLNs of mice with EAU 21 days after immunization (n = 5, gated on CD4). The results were representative of three independent experiments. The data are presented as the means ± SDs. NS: P > 0.05; *: P < 0.05; **P < 0.01 (between the indicated groups). Data were analyzed using one-way ANOVA with Bonferroni correction (A, C, E, and F) or independent unpaired two-tailed Student’s t tests (H-L).
We next investigated the in vivo effects of iTregs on the production of local inflammatory cytokines in the retinas of mice with EAU. Our results revealed a significant increase in the expression of IL-17 and IFN-γ in mice with EAU, whereas treatment with iTregs significantly suppressed this upregulation of IL-17 and IFN-γ (Fig. 1E–F). Further analysis of regional draining lymph nodes (dLNs) harvested 21 days after challenge showed that compared with PBS-treated control mice, iTreg-treated mice exhibited a marked decrease in the frequency and number of Th17 (CD4+IL-17+) and Th1 (CD4+IFN-γ+) cells (Fig. 1G–L).
Intercellular transport via gap junctions and A2AR signaling is responsible for the iTreg-mediated inhibitory effects on uveitogenic T cells (UTs) obtained from mice with EAU
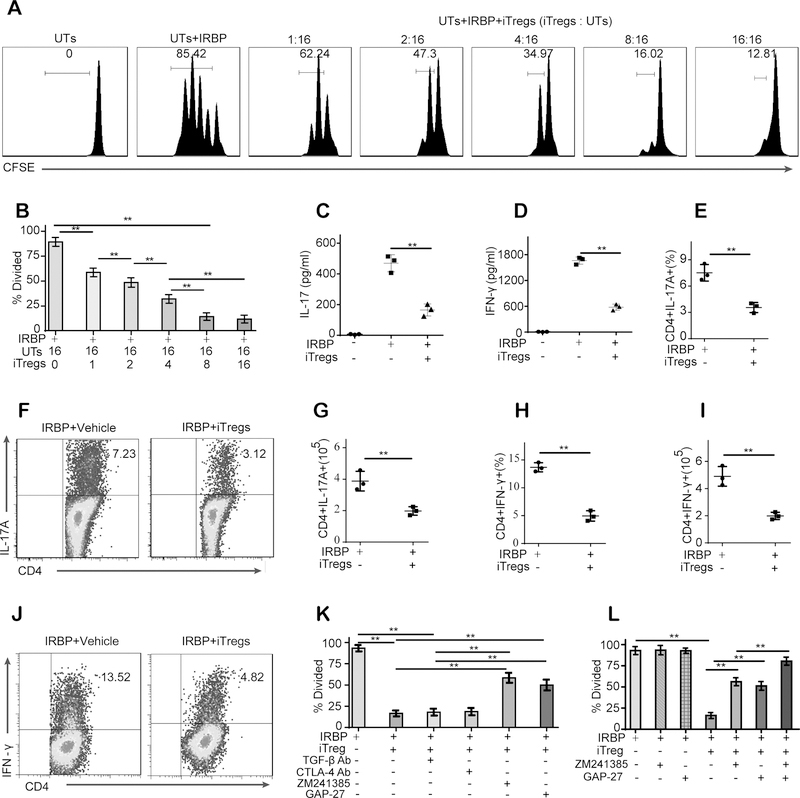
We next investigated whether iTregs can suppress the proliferation and function of UTs obtained from mice with EAU. For this purpose, UTs derived from mice with established EAU were cultured with iTregs for 72 hours for assay of proliferation. Coculture with iTregs led to a cell number-dependent inhibition of UT proliferation (Fig. 2A–B). Next, UTs were cultured alone or with iTregs at a UT:iTreg ratio of 2:1 to determine whether iTregs inhibit inflammatory cytokine release. The ELISA results showed that coculture with iTregs significantly inhibited the release of IL-17 and IFN-γ into the culture supernatants (Fig. 2C–D). These results were further supported by flow cytometric analysis (Fig. 2E–J).
Figure 2. Intercellular transport of cAMP through gap junctions and adenosine-A2A receptor (A2AR) signaling are responsible for the iTreg-mediated inhibitory effects on uveitogenic T cells (UTs) obtained from mice with EAU.
(A-B): Coculture with iTregs led to a cell number-dependent inhibition of UT proliferation. (C-D): Coculture with iTregs inhibited the release of IL-17 and IFN-γ into the culture supernatants. (E-J): The expression of IL-17A and IFN-γ in retinas 21 days after immunization was measured by real-time PCR. (G-L): Coculture with iTregs decreased the number and frequency of CD4+IL-17A+ T cells and CD4+IFN-γ+ T cells in UTs (Gated on CD4). (K): Both the A2AR antagonists (ZM 241385) and the gap junction inhibitor (GAP-27) significantly reversed the iTreg-mediated inhibitory effect on UT proliferation. (L): The combination of the A2AR antagonist and the gap junction inhibitor was more effective than either alone. These results were representative of three independent experiments. The data are presented as the means ± SDs. **: P < 0.01 (between the indicated groups). Abbreviations: TGF-β Ab, TGF-β antibody; CTLA-4 Ab, CTLA-4 antibody. Data were analyzed using one-way ANOVA with Bonferroni correction (B-D, K, and L) or independent unpaired two-tailed Student’s t tests (E-I).
TGF-β1, CTLA-4, adenosine and the intercellular transport of cAMP via gap junctions have all been reported to play important roles in Treg-mediated immunomodulation (16,17,22,23,24,25,26). Thus, to identify the specific mechanism or mechanisms that could potentially be contributing to the iTreg-mediated inhibition of UTs, we used TGF-β1-specific and CTLA-4-specific neutralizing antibodies, an A2AR-specific antagonist (ZM 241385) and a gap junction inhibitor (GAP-27) as indicated. We observed that although the addition of the anti-TGF-β1 or anti-CTLA-4 antibodies did not affect the suppressive effects of iTregs on UTs, the addition of the A2AR antagonist or the gap junction inhibitor significantly reversed the iTreg-mediated inhibition of UT proliferation and proinflammatory cytokine production (Fig. 2K, Fig. S1A–C). Moreover, the combination of the A2AR antagonist and the gap junction inhibitor was more effective than either was alone at reversing the iTreg-mediated suppression of UTs (Fig. 2L, Fig. S1D–E).
cAMP regulates the CD39-adenosine pathway in iTregs
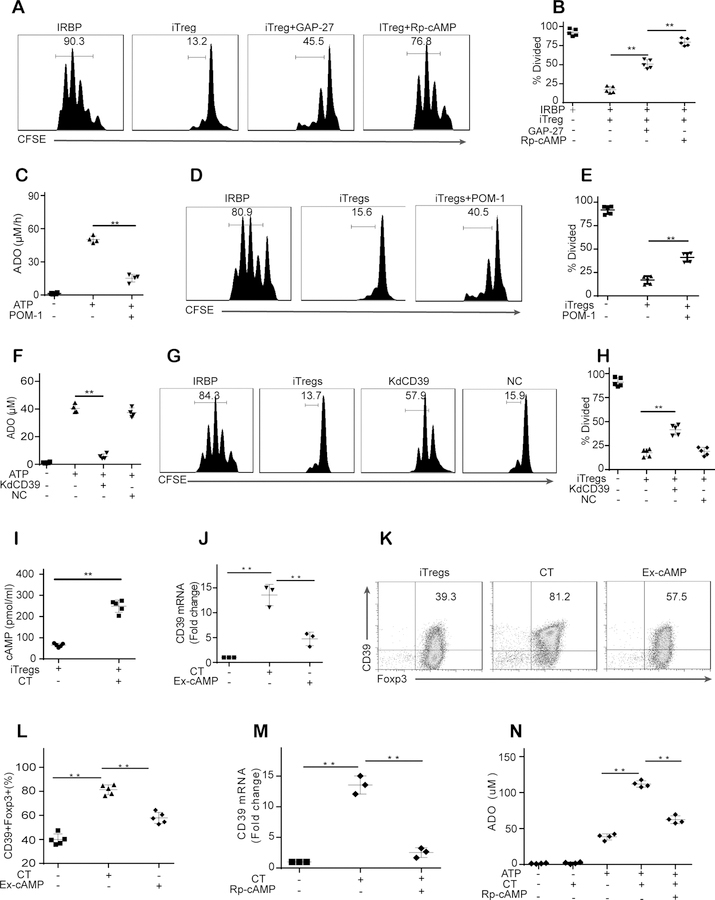
The above results showed that the inhibition of gap junctions by GAP-27 partially reversed the iTreg-mediated inhibitory effects on UTs. We next examined the role that the intracellular cAMP in iTregs played in the inhibitory effects of iTregs on UTs. To this end, iTregs were preincubated with a cAMP antagonist (Rp-cAMPS, 4 mM) before coculture with UTs. Strikingly, pretreatment of iTregs with Rp-cAMPS was more effective than was pretreatment with the gap junction inhibitor and produced a reversal of the iTreg-mediated suppression of UTs that was similar to that produced by the combination of the A2AR antagonist and the gap junction inhibitor (Fig. 3A–B, Fig. S2A–C). These results indicate that the intracellular cAMP in iTregs may not only direct cAMP transport through gap junctions but may also be related to adenosine signaling in iTregs.
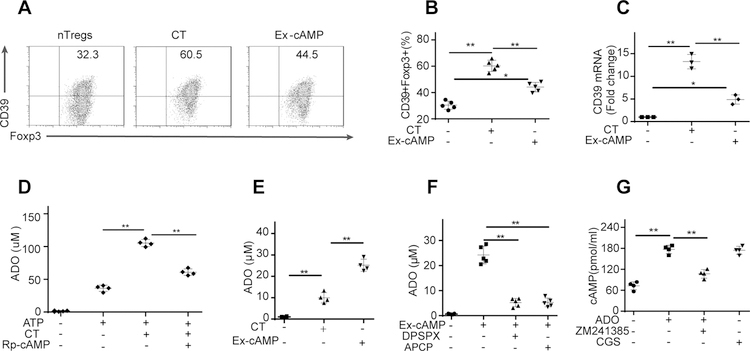
Figure 3. cAMP regulates the CD39-adenosine pathway in iTregs.
(A-B): Pretreatment of iTregs with Rp-cAMPS (a cAMP antagonist, 4 mM) was more effective than pretreatment with the gap junction inhibitor in reversing the iTreg-mediated suppression of UTs. (C): POM-1 (a CD39 inhibitor) pretreatment significantly reduced adenosine (ADO) release by iTregs in the presence of ATP. (D-E): POM-1 pretreatment partially reversed the iTreg-mediated suppression of UTs. (F-H): CD39 siRNA but not control siRNA decreased adenosine release by iTregs and the capability of iTregs to inhibit UT proliferation. (I-L): CT (a potent cAMP antagonist) increased the intracellular cAMP level in iTregs and upregulated CD39 expression in iTregs (Gated on Foxp3). (M): Rp-cAMPS pretreatment almost abolished the CT-induced CD39 expression in iTregs. (N): In the presence of ATP, CT increased the production of adenosine by iTregs, whereas Rp-cAMPS pretreatment weakened this increase. The results were representative of three independent experiments. The data are presented as the means ± SDs. **: P < 0.01 (between the indicated groups). Data were analyzed using one-way ANOVA with Bonferroni correction (B-H, and J-N) or independent unpaired two-tailed Student’s t tests (I).
The typical mechanism of adenosine production is the extracellular conversion of adenine nucleotides to adenosine by ectonucleotidases. During this process, CD39, an ectonucleotidase, mainly degrades adenosine triphosphate (ATP) and adenosine diphosphate (ADP) to produce adenosine monophosphate (AMP), which is then dephosphorylated by CD73 to form adenosine (27). Thus, to further elucidate the roles of adenosine in the iTreg-mediated suppression of UTs, we used POM-1 (a nonselective CD39 inhibitor) as indicated. As shown in Fig. 3C, POM-1 pretreatment significantly reduced adenosine release by iTregs in the presence of ATP. As expected, POM-1 pretreatment partially reversed the iTreg-mediated suppression of UTs (Fig. 3D–E, Fig. S2D–E). Next, we confirmed the role of the CD39-adenosine pathway in regulating the iTreg-mediated suppression of UTs by using CD39 siRNA to knock down CD39 expression in iTregs. CD39 siRNA but not control siRNA significantly decreased adenosine release by iTregs (Fig. 3F) and the capability of iTregs to inhibit UT proliferation and proinflammatory cytokine production (Fig. 3G–H, Fig. S2F–H). These results suggested that CD39-dependent adenosine release contributed to the iTreg-mediated inhibition of UTs.
We next explored whether intracellular cAMP regulated CD39-adenosine signaling in iTregs. CT (a potent cAMP inducer), which elevated the intracellular cAMP level in iTregs (Fig. 3I), significantly upregulated CD39 expression in iTregs (Fig. 3J–L). The effect of exogenous cAMP on CD39 expression was not as substantial as that of CT (Fig. 3J–L). In contrast, Rp-cAMPS pretreatment almost abolished the CT-induced CD39 expression in iTregs (Fig. 3M). As expected, in the presence of ATP, CT increased the production of adenosine by iTregs, whereas Rp-cAMPS pretreatment weakened this increase (Fig. 3N). These findings suggest that intracellular cAMP modulated the CD39-adenosine pathway in iTregs.
cAMP directly participates in iTreg-derived adenosine production through the cAMP-adenosine pathway
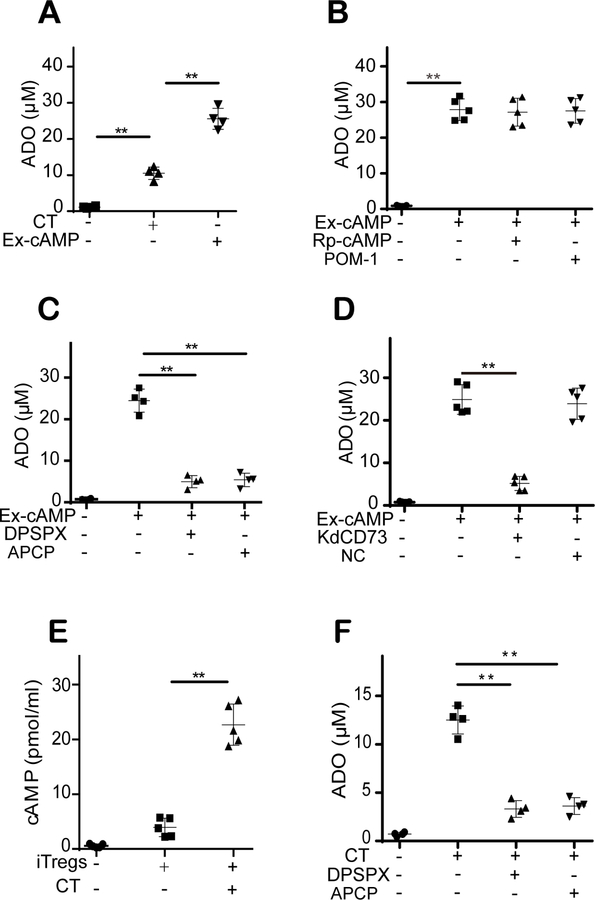
The above results showed the effects of intracellular cAMP on the CD39-adenosine pathway. Notably, in the absence of ATP, CT induced adenosine release by iTregs (Fig. 4A). In addition, exogenous cAMP was more effective than CT was at inducing adenosine release by iTregs in the absence of ATP (Fig. 4A). Moreover, pretreatment with Rp-cAMPS or POM-1 had no effect on the exogenous cAMP-induced adenosine release by iTregs (Fig. 4B). These results indicate that CD39-independent cAMP-derived adenosine production mechanisms exist in iTregs.
Figure 4. cAMP directly participates in iTreg-derived adenosine production through the cAMP-adenosine pathway.
(A): In the absence of ATP, CT induced adenosine release by iTregs, and exogenous cAMP showed a greater effect than CT. (B): Rp-cAMPS and POM-1 pretreatment had no effect on exogenous cAMP-induced adenosine (ADO) release by iTregs. (C): The conversion of exogenous cAMP into adenosine was significantly prevented by treatment with DPSPX (a selective ecto-PDE inhibitor) or APCP (a selective CD73 inhibitor). (D): CD73 siRNA but not control siRNA significantly decreased adenosine release by iTregs. (E): CT treatment, which increased the intracellular cAMP level, significantly induced extracellular cAMP release by iTregs. (F): CT-induced adenosine release by iTregs was prevented by DPSPX or APCP treatment. The results were representative of three independent experiments. The data are presented as the means ± SDs. **: P < 0.01 (between the indicated groups). Data were analyzed using one-way ANOVA with Bonferroni correction.
A literature search revealed that the extracellular cAMP-adenosine pathway has been studied in several cells and tissues (28). However, the existence and roles of this pathway in Treg-mediated immunomodulation remain unknown. Extracellular cAMP is generally converted into AMP by ecto-phosphodiesterase (ecto-PDE), and AMP is subsequently dephosphorylated by CD73 to form adenosine. Thus, DPSPX (a selective ecto-PDE inhibitor) and APCP (a selective CD73 inhibitor were used to confirm the existence of the cAMP-adenosine pathway in iTregs). As shown in Fig. 4C, the conversion of exogenous cAMP into adenosine was significantly prevented by DPSPX or APCP treatment. In addition, our results showed that CD73 siRNA but not control siRNA significantly decreased adenosine release by iTregs (Fig. 4D and Fig. S2I). Moreover, we found that CT treatment, which increased the intracellular cAMP level, significantly induced extracellular cAMP release by iTregs (Fig. 4E). In addition, CT-induced adenosine release by iTregs was prevented by DPSPX or APCP treatment (Fig. 4F). These results showed that the extracellular cAMP-adenosine pathway exists in iTregs and contributes to adenosine production.
Adenosine/A2AR signaling increases the intracellular cAMP level in iTregs and the suppressive effects of iTregs on UTs
Fig. 3 showed that intracellular cAMP regulated the CD39-adenosine pathway in iTregs. Therefore, we next asked whether adenosine also plays roles in cAMP signaling in iTregs. Our results revealed that exogenous adenosine significantly increased the intracellular cAMP level in iTregs (Fig. 5A). As shown in Fig. 5B, treatment with the A2AR antagonist reversed the adenosine-mediated increase in the intracellular cAMP level in iTregs. In addition, the effects of the A2AR agonist CGS on the intracellular cAMP level in iTregs mimic those of adenosine (Fig. 5B). Importantly, adenosine pretreatment enhanced the iTreg-mediated suppressive effects on UT proliferation and inflammatory cytokine release (Fig. 5C–F). Flow cytometric analysis showed that compared with iTregs, iTreg pretreated with adenosine exhibited an enhanced inhibitory effect on the frequency and number of CD4+IL-17+ and CD4+IFN-γ+cells (Fig. 5G–L).
Figure 5. Adenosine/A2AR signaling increases the intracellular cAMP level in iTregs and the suppressive effects of iTregs on UTs.
(A): Exogenous adenosine (ADO) significantly increased the intracellular cAMP level in iTregs. (B): The A2AR antagonist reversed the adenosine-mediated increase in the intracellular cAMP level in iTregs, and the effects of the A2AR agonist CGS mimic the adenosine-mediated increase in the intracellular cAMP level in iTregs. (C-D): Adenosine pretreatment enhanced the iTreg-mediated suppressive effects on the proliferation of UTs. (E-F): Adenosine pretreatment enhanced the iTreg-mediated suppressive effects on inflammatory cytokine release by UTs. (G-L): Flow cytometric analysis showed that compared with iTregs, iTreg pretreated with adenosine exhibited an enhanced inhibitory effect in the frequency and number of CD4+IL-17+ and CD4+IFN-γ+cells (Gated on CD4). The results were representative of three independent experiments. The data are presented as the means ± SDs. *: P < 0.05; **: P < 0.01 (between the indicated groups). Data were analyzed using one-way ANOVA with Bonferroni correction.
The cAMP-adenosine loop also exists in nTregs
The above results demonstrated that cAMP regulates and directly participates in iTreg-derived adenosine production; in turn, adenosine increases the intracellular cAMP level in iTregs. Thus, we next asked whether this cAMP-adenosine loop also exists in nTregs. For this purpose, CT or exogenous cAMP was added into the culture medium of nTregs. As expected, treatment with CT or exogenous cAMP significantly upregulated CD39 expression in nTregs, although the effect of exogenous cAMP was minimal (Fig. 6A–C). Similarly, in the presence of ATP, CT treatment significantly increased adenosine production by nTregs (Fig. 6D). In addition, in the absence of ATP, exogenous cAMP induced adenosine release by nTregs (Fig. 6E). Moreover, our results demonstrated that the conversion of exogenous cAMP into adenosine in nTregs was significantly prevented by treatment with DPSPX or APCP (Fig. 6F). As shown in Fig. 6G, exogenous adenosine significantly increased the intracellular cAMP level in nTregs, and the A2AR antagonist blocked the exogenous adenosine-mediated effects in nTregs. These findings indicate that the cAMP-adenosine loop also exists in nTregs.
Figure 6. The cAMP-adenosine loop also exists in nTregs.
(A-C): Treatment with CT or exogenous cAMP significantly upregulated CD39 expression in nTregs (Gated on Foxp3), but the effect of exogenous cAMP was minimal. (D): In the presence of ATP, CT treatment significantly increased adenosine production by nTregs. (E): In the absence of ATP, exogenous cAMP induced adenosine release by nTregs. (F): The conversion of exogenous cAMP into adenosine in nTregs was significantly prevented by treatment with DPSPX or APCP. (G): Exogenous adenosine significantly increased the intracellular cAMP level in nTregs, and the A2AR antagonist blocked the exogenous adenosine-mediated effects on nTregs. The results were representative of three independent experiments. The data are presented as the means ± SDs. **: P < 0.01 (between the indicated groups). Data were analyzed using one-way ANOVA with Bonferroni correction.
cAMP is critical for iTreg-mediated amelioration of established EAU, and increasing the intracellular cAMP level in iTregs before transfer enhanced their therapeutic effects on established EAU
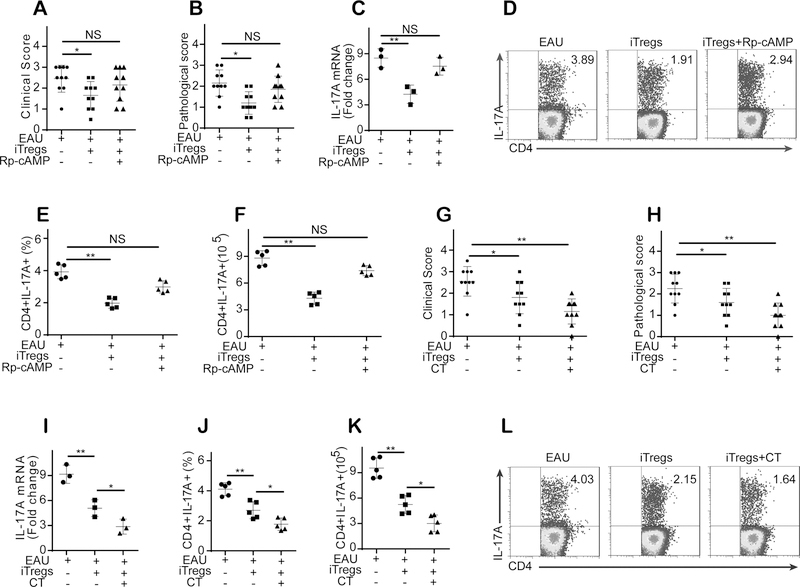
Because our in vitro studies showed that cAMP plays essential roles in the iTreg-mediated suppression of UTs, we next asked whether cAMP is also implicated in the iTreg-mediated attenuation of established EAU. To this end, we treated mice with EAU with iTregs that had been preincubated with Rp-cAMPS and found that iTregs pretreated with Rp-cAMPS no longer suppressed EAU in mice (Fig. 7A–B). In addition, the preincubation of iTregs with Rp-cAMPS significantly decreased their capability to inhibit IL-17 and IFN-γ expression in mice with EAU (Fig. 7C–F, Fig. S3A–D). Importantly, we found that the pretreatment of iTregs with CT, which increases the intracellular cAMP level in iTregs, improved the therapeutic effects of iTregs on EAU in mice (Fig. 7G–H). In addition, CT pretreatment also improved the capability of iTregs’ to inhibit IL-17 and IFN-γ expression in mice with EAU (Fig. 7I–L, Fig. S3E–H).
Figure 7. cAMP is critical for iTreg-mediated amelioration of established EAU, and increasing the intracellular cAMP level in iTregs before transfer enhanced their therapeutic effects on established EAU.
(A-B): iTregs pretreated with Rp-cAMPS did not attenuate EAU in mice (n = 10). (C-F): The preincubation of iTregs with Rp-cAMPS significantly decreased their capability to inhibit IL-17 expression and decreased the number and frequency of CD4+CD17A+ T cells in mice with EAU (n = 6). (G-H): The pretreatment of iTregs with CT, which increases the intracellular cAMP level in iTregs, improved the therapeutic effects of iTregs on EAU in mice (n = 6). (I-L): CT pretreatment improved iTregs’ capability to inhibit IL-17 expression and decreased the number and frequency of CD4+CD17A+ T cells in EAU mice (n = 3, gated on CD4). The results were representative of three independent experiments. The data are presented as the means ± SDs. NS: P > 0.05; *: P <0.05; **P <0.01 (between the indicated groups). Data were analyzed using one-way ANOVA with Bonferroni correction.
Discussion
Tregs maintain immune tolerance through immunomodulatory mechanisms. However, to date, the immunomodulatory mechanisms of Tregs remain poorly understood. This study shows that the adoptive transfer of iTregs is a feasible and effective strategy for the treatment of established uveitis and identifies the key roles of the cAMP-adenosine feedback loop in the immunomodulatory function of both iTregs and nTregs. In established EAU, the administration of iTregs ameliorates the inflammatory response, significantly decreasing both the expression of IL-17 and IFN-γ in retinas and the number and frequency of Th17 and Th1 cells in dLNs. In vitro, iTregs inhibited IRBP-induced T cell proliferation and IL-17 and IFN-γ production. Mechanistically, both cAMP and adenosine are responsible for the iTreg-mediated inhibitory effect on UTs. Furthermore, we found that cAMP regulates CD39 expression and CD39-dependent adenosine production in iTregs. Importantly, our results revealed that extracellular cAMP directly contributes to adenosine production by iTregs in a CD39-independent manner and that the cAMP-adenosine loop plays a critical role in the immunomodulatory function of not only iTregs but also nTregs. More importantly, cAMP is critical for the iTreg-mediated amelioration of established EAU, and increasing the intracellular cAMP level in iTregs before intravenous administration enhanced the therapeutic effects of iTregs on established EAU.
Ectonucleoside triphosphate diphosphohydrolase 1, commonly known as CD39, is an integral membrane protein expressed on the surface of vascular and immune cells (29). CD39 degrades mainly ATP and ADP to produce AMP, and CD73 then dephosphorylates AMP to form adenosine (30,31). In 2007, Deaglio et al. first reported that the expression of CD39 in nTregs plays crucial roles in nTreg-mediated immune suppression (16). Since 2007, many investigations have firmly established a key role for CD39 in the suppressive function of nTregs (32,33). However, to date, little is known concerning the molecular mechanisms responsible for regulating CD39 expression in iTregs and the role of CD39 in the immunosuppressive function of iTregs. In this study, we found that CD39 expressed by iTregs contributes to iTreg-mediated immunosuppression both in vitro and in vivo. Importantly, we also showed that intracellular cAMP is responsible for regulating CD39 expression in iTregs and nTregs. To our knowledge, this is a novel observation regarding the molecular mechanisms that regulate CD39 expression in Tregs.
cAMP was first identified as a second messenger that mediates various biological processes. To date, the role of cAMP in immune cells, especially in the development, differentiation and proliferation of T cells, has been widely noted. In 2007, Bopp et al. proposed that cAMP transfer through gap junctions plays a pivotal role in nTreg-mediated immunosuppression (17), and this notion has been supported by a larger number of studies since that time (34,35). However, the link between cAMP signaling and the CD39-adenosine pathway in Tregs remained unknown, and the role of cAMP in the immunosuppressive function of iTregs was also unclear. The present study showed that cAMP is partially responsible for the iTreg-mediated inhibitory effects on UTs. Moreover, we found that intracellular cAMP regulated CD39-adenosine signaling in iTregs. Importantly, cAMP was found to directly participate in iTreg-derived adenosine production by an extracellular cAMP-adenosine pathway that is independent of CD39 signaling. Collectively, these findings indicate that cAMP contributes to Treg-mediated immunosuppression by three major mechanisms. This knowledge helps to improve our understanding of the immunosuppressive mechanisms of Tregs.
As mentioned above, adenosine and cAMP have been identified to play important roles in the immunosuppressive function of nTregs, but the link between adenosine and cAMP in Tregs remains unknown. The present study showed not only that cAMP participates in Treg-derived adenosine production but also that adenosine increases the intracellular cAMP level by interacting with A2AR. These findings indicate that the cAMP-adenosine feedback loop exists and plays critical roles in maintaining the immunosuppressive function of Tregs. The discovery of this feedback loop deepens our insight into the molecular mechanisms that modulate the immunosuppressive function of Tregs.
The manipulation of nTregs has been regarded as a promising strategy for the treatment of GVHD, organ transplantation, and autoimmune diseases (36). However, some hurdles remain in the clinical use of nTreg therapy. First, the stability of Tregs has become a major issue in clinical nTreg therapy. nTregs, which retain a measure of plasticity, may be converted into various classes of Th cells under inflammatory conditions (12,15,37,38,39). Moreover, the acquisition of sufficient numbers of nTregs for cell therapy remains a substantial challenge due to the low frequency of nTregs. Currently, nTreg expansion in vitro can resolve this problem, but repeated stimulation has been found to result in diminishing Foxp3 expression and the suppressive function of nTregs (40). Hence, iTregs have been of interest to treat autoimmune diseases and GVHD in humans since 2012 (41,42). Unlike nTregs, which can be converted into Th17 cells in the presence of IL-6, iTregs are resistant to IL-6-driven Th17 cell conversion (15). Furthermore, iTregs are available in sufficient numbers and can be induced to differentiate into antigen-specific Treg subsets (43). Therefore, iTregs may be another potential therapeutic choice for established autoimmune diseases. However, the instability of Foxp3 expression in iTregs has also become a serious concern for the clinical use of iTreg therapy. Koenecke et al. reported that CD4+ iTregs are not stable in vivo and failed to prevent acute GVHD in the B6 to BALB/c model (44,45). In contrast, we recently demonstrated in a comparison study that iTregs are sufficiently stable to successfully prevent acute GVHD and remain functional activities when the appropriate protocol for the differentiation of iTregs is used (6). The current study, in which the appropriate protocol for the differentiation of iTregs was used, showed that iTregs attenuate established EAU to a slightly greater degree than do nTregs. The therapeutic efficacy of Tregs is another critical factor in the clinical use of Treg therapy. The present study found that increasing the intracellular cAMP level in iTregs before intravenous administration enhanced the therapeutic effects of iTregs on established EAU. This finding provides an innovative strategy for improving the therapeutic efficacy of iTregs in established autoimmune diseases.
In summary, this study identifies an innovative mechanism whereby cAMP regulates adenosine production and CD39 expression in Tregs. First, intracellular cAMP regulates CD39 expression and CD39-dependent adenosine production in Tregs. Second, extracellular cAMP directly participates in the production of Treg-derived adenosine by the cAMP-adenosine pathway, which is independent of CD39 signaling. Third, extracellular adenosine increases the intracellular cAMP level in Tregs via its interaction with A2AR. Therefore, both intracellular and extracellular cAMP promote adenosine production by Tregs. The produced extracellular adenosine, in turn, interacts with A2AR, thereby increasing the intracellular cAMP level. This cAMP-adenosine feedback loop may preserve the high intracellular cAMP level and adenosine-producing capability of Tregs in order to maintain their immunosuppressive function. Importantly, increasing the intracellular cAMP level in iTregs before transfer improves the therapeutic efficacy of iTregs in established EAU. These findings provide new insight into the immunosuppressive mechanisms of Tregs and suggest a new strategy for improving the therapeutic efficacy of Tregs in established autoimmune diseases or other inflammatory disorders.
Supplementary Material
Key points.
Intracellular cAMP regulates CD39-dependent adenosine production in Tregs.
Extracellular cAMP directly participates in Treg-derived adenosine production.
Increasing the intracellular cAMP level improves the therapeutic efficacy of iTregs.
Acknowledgments
Funds: This study was partially supported by grants from the National Key R&D Program of China (2017YFA0105800), the Natural Science Foundation of China (81670897, 81671611, 81871224 and U1601226), and Guangdong Natural Science Funds for Distinguished Young Scholar (2016A030306006) (all to W.R.S), and grants from National Institutes of Health (AR059103 to S.G.Z).
Abbreviations:
- iTregs
induced CD4+Foxp3+regulatory T cells
- nTregs
nature occurring CD4+CD25+ regulatory T cells
- Tregs
both Treg subsets (nTregs and iTregs)
- EAU
experimental autoimmune uveitis
- dLNs
regional draining lymph nodes
- A2AR
adenosine-A2A receptor
- UTs
uveitogenic T cells
- CTLA-4
cytotoxic T-lymphocyte-associated protein 4
- CT
Cholera toxin
- GVHD
graft vs host disease
- IRBP
Interphotoreceptor retinoid-binding protein
- CFSE
carboxyfluorescein diacetate succinimidyl ester
Footnotes
Disclosure of potential conflicts of interest: The authors declare no competing financial conflicts of interest.
References
- 1.Noack M, and Miossec P. 2014. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun Rev 13:668–677. [DOI] [PubMed] [Google Scholar]
- 2.Bilate AM, and Lafaille JJ. 2012. Induced CD4+Foxp3+ regulatory T cells in immune tolerance. Annu. Rev. Immunol 30:733–758. [DOI] [PubMed] [Google Scholar]
- 3.Waldmann H, Hilbrands R, Howie D, and Cobbold S. 2014. Harnessing FOXP3+ regulatory T cells for transplantation tolerance. J. Clin. Invest 124:1439–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rigas D, Lewis G, Aron JL, Wang B, Banie H, Sankaranarayanan I, Galle-Treger L, Maazi H, Lo R, Freeman GJ, et al. 2017. Type 2 innate lymphoid cell suppression by regulatory T cells attenuates airway hyperreactivity and requires inducible T-cell costimulator-inducible T-cell costimulator ligand interaction. J. Allergy Clin. Immunol 139:1468–1477.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trzonkowski P, Bacchetta R, Battaglia M, Berglund D, Bohnenkamp HR, ten BA, Bushell A, Cools N, Geissler EK, Gregori S, et al. 2015. Hurdles in therapy with regulatory T cells. Sci Transl Med 7:304ps18. [DOI] [PubMed] [Google Scholar]
- 6.Gu J, Lu L, Chen M, Xu L, Lan Q, Li Q, Liu Z, Chen G, Wang P, Wang X, et al. 2014. TGF-beta-induced CD4+Foxp3+ T cells attenuate acute graft-versus-host disease by suppressing expansion and killing of effector CD8+ cells. J. Immunol 193:3388–3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng SG, Wang JH, Koss MN, Quismorio F Jr, Gray JD, and Horwitz DA. 2004. CD4+ and CD8+ regulatory T cells generated ex vivo with IL-2 and TGF-beta suppress a stimulatory graft-versus-host disease with a lupus-like syndrome. J. Immunol 172:1531–1539. [DOI] [PubMed] [Google Scholar]
- 8.Kong N, Lan Q, Chen M, Wang J, Shi W, Horwitz DA, Quesniaux V, Ryffel B, Liu Z, Brand D, et al. 2012. Antigen-specific transforming growth factor beta-induced Treg cells, but not natural Treg cells, ameliorate autoimmune arthritis in mice by shifting the Th17/Treg cell balance from Th17 predominance to Treg cell predominance. Arthritis Rheum 64:2548–2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin X, Chen M, Liu Y, Guo Z, He X, Brand D, and Zheng SG. 2013. Advances in distinguishing natural from induced Foxp3(+) regulatory T cells. Int J Clin Exp Pathol 6:116–123. [PMC free article] [PubMed] [Google Scholar]
- 10.Horwitz DA, Zheng SG, and Gray JD. 2008. Natural and TGF-beta-induced Foxp3(+)CD4(+) CD25(+) regulatory T cells are not mirror images of each other. Trends Immunol 29:429–435. [DOI] [PubMed] [Google Scholar]
- 11.Zheng SG, Wang JH, Stohl W, Kim KS, Gray JD, and Horwitz DA. 2006. TGF-beta requires CTLA-4 early after T cell activation to induce FoxP3 and generate adaptive CD4+CD25+ regulatory cells. J. Immunol 176:3321–3329. [DOI] [PubMed] [Google Scholar]
- 12.Xu L, Kitani A, Fuss I, and Strober W. 2007. Cutting edge: regulatory T cells induce CD4+CD25-Foxp3- T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J. Immunol 178:6725–6729. [DOI] [PubMed] [Google Scholar]
- 13.Lu L, Lan Q, Li Z, Zhou X, Gu J, Li Q, Wang J, Chen M, Liu Y, Shen Y, et al. 2014. Critical role of all-trans retinoic acid in stabilizing human natural regulatory T cells under inflammatory conditions. Proc. Natl. Acad. Sci. U.S.A 111:E3432–3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koenen HJ, Smeets RL, Vink PM, van Rijssen E, Boots AM, and Joosten I. 2008. Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood 112:2340–2352. [DOI] [PubMed] [Google Scholar]
- 15.Zheng SG, Wang J, and Horwitz DA. 2008. Cutting edge: Foxp3+CD4+CD25+ regulatory T cells induced by IL-2 and TGF-beta are resistant to Th17 conversion by IL-6. J. Immunol 180:7112–7116. [DOI] [PubMed] [Google Scholar]
- 16.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, et al. 2007. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med 204:1257–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bopp T, Becker C, Klein M, Klein-Hessling S, Palmetshofer A, Serfling E, Heib V, Becker M, Kubach J, Schmitt S, et al. 2007. Cyclic adenosine monophosphate is a key component of regulatory T cell-mediated suppression. J. Exp. Med 204:1303–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agarwal RK, Silver PB, and Caspi RR. 2012. Rodent models of experimental autoimmune uveitis. Methods Mol. Biol 900:443–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cui M, Ding H, Chen F, Zhao Y, Yang Q, and Dong Q. 2016. Mdivi-1 Protects Against Ischemic Brain Injury via Elevating Extracellular Adenosine in a cAMP/CREB-CD39-Dependent Manner. Mol. Neurobiol 53:240–253. [DOI] [PubMed] [Google Scholar]
- 20.Zhu W, Chen X, Yu J, Xiao Y, Li Y, Wan S, Su W, and Liang D. 2018. Baicalin modulates the Treg/Teff balance to alleviate uveitis by activating the aryl hydrocarbon receptor. Biochem. Pharmacol 154:18–27. [DOI] [PubMed] [Google Scholar]
- 21.Zhou Q, Gong X, Kuang G, Jiang R, Xie T, Tie H, Chen X, Li K, Wan J, and Wang B. 2018. Ferulic Acid Protected from Kidney Ischemia Reperfusion Injury in Mice: Possible Mechanism Through Increasing Adenosine Generation via HIF-1α. Inflammation 41:2068–2078. [DOI] [PubMed] [Google Scholar]
- 22.Schildknecht A, Brauer S, Brenner C, Lahl K, Schild H, Sparwasser T, Probst HC, and van den Broek M 2010. FoxP3+ regulatory T cells essentially contribute to peripheral CD8+ T-cell tolerance induced by steady-state dendritic cells. Proc. Natl. Acad. Sci. U.S.A 107:199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Su W, Fan H, Chen M, Wang J, Brand D, He X, Quesniaux V, Ryffel B, Zhu L, Liang D, et al. 2012. Induced CD4(+) forkhead box protein-positive T cells inhibit mast cell function and established contact hypersensitivity through TGF-beta1. J. Allergy Clin. Immunol 130:444–452.e7. [DOI] [PubMed] [Google Scholar]
- 24.Xu A, Liu Y, Chen W, Wang J, Xue Y, Huang F, Rong L, Lin J, Liu D, Yan M, et al. 2016. TGF-β-Induced Regulatory T Cells Directly Suppress B Cell Responses through a Noncytotoxic Mechanism. J. Immunol 196:3631–3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng SG, Wang JH, Gray JD, Soucier H, and Horwitz DA. 2004. Natural and induced CD4+CD25+ cells educate CD4+CD25- cells to develop suppressive activity: the role of IL-2, TGF-beta, and IL-10. J. Immunol 172:5213–5221. [DOI] [PubMed] [Google Scholar]
- 26.Lan Q, Zhou X, Fan H, Chen M, Wang J, Ryffel B, Brand D, Ramalingam R, Kiela PR, Horwitz DA, et al. 2012. Polyclonal CD4+Foxp3+ Treg cells induce TGFβ-dependent tolerogenic dendritic cells that suppress the murine lupus-like syndrome. J Mol Cell Biol 4:409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eltzschig HK, Sitkovsky MV, and Robson SC. 2012. Purinergic signaling during inflammation. N. Engl. J. Med 367:2322–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giron MC, Bin A, Brun P, Etteri S, Bolego C, Florio C, and Gaion RM. 2008. Cyclic AMP in rat ileum: evidence for the presence of an extracellular cyclic AMP-adenosine pathway. Gastroenterology 134:1116–1126. [DOI] [PubMed] [Google Scholar]
- 29.Bono MR, Fernández D, Flores-Santibáñez F, Rosemblatt M, and Sauma D. 2015. CD73 and CD39 ectonucleotidases in T cell differentiation: Beyond immunosuppression. FEBS Lett 589:3454–3460. [DOI] [PubMed] [Google Scholar]
- 30.Huang F, Chen M, Chen W, Gu J, Yuan J, Xue Y, Dang J, Su W, Wang J, Zadeh HH, et al. 2017. Human Gingiva-Derived Mesenchymal Stem Cells Inhibit Xeno-Graft-versus-Host Disease via CD39-CD73-Adenosine and IDO Signals. Front Immunol 8:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen M, Su W, Lin X, Guo Z, Wang J, Zhang Q, Brand D, Ryffel B, Huang J, Liu Z, et al. 2013. Adoptive transfer of human gingiva-derived mesenchymal stem cells ameliorates collagen-induced arthritis via suppression of Th1 and Th17 cells and enhancement of regulatory T cell differentiation. Arthritis Rheum 65:1181–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ring S, Oliver SJ, Cronstein BN, Enk AH, and Mahnke K. 2009. CD4+CD25+ regulatory T cells suppress contact hypersensitivity reactions through a CD39, adenosine-dependent mechanism. J. Allergy Clin. Immunol 123:1287–1296.e2. [DOI] [PubMed] [Google Scholar]
- 33.Sun X, Wu Y, Gao W, Enjyoji K, Csizmadia E, Müller CE, Murakami T, and Robson SC. 2010. CD39/ENTPD1 expression by CD4+Foxp3+ regulatory T cells promotes hepatic metastatic tumor growth in mice. Gastroenterology 139:1030–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moreno-Fernandez ME, Rueda CM, Rusie LK, and Chougnet CA. 2011. Regulatory T cells control HIV replication in activated T cells through a cAMP-dependent mechanism. Blood 117:5372–5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luckey U, Schmidt T, Pfender N, Romer M, Lorenz N, Martin SF, Bopp T, Schmitt E, Nikolaev A, Yogev N, et al. 2012. Crosstalk of regulatory T cells and tolerogenic dendritic cells prevents contact allergy in subjects with low zone tolerance. J. Allergy Clin. Immunol 130:781–797.e11. [DOI] [PubMed] [Google Scholar]
- 36.Leslie M 2011. Immunology. Regulatory T cells get their chance to shine. Science 332:1020–1021. [DOI] [PubMed] [Google Scholar]
- 37.Zhou X, Kong N, Wang J, Fan H, Zou H, Horwitz D, Brand D, Liu Z, and Zheng SG. 2010. Cutting edge: all-trans retinoic acid sustains the stability and function of natural regulatory T cells in an inflammatory milieu. J. Immunol 185:2675–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wan YY, and Flavell RA. 2007. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature 445:766–770. [DOI] [PubMed] [Google Scholar]
- 39.Tsuji M, Komatsu N, Kawamoto S, Suzuki K, Kanagawa O, Honjo T, Hori S, and Fagarasan S. 2009. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer’s patches. Science 323:1488–1492. [DOI] [PubMed] [Google Scholar]
- 40.Hoffmann P, Boeld TJ, Eder R, Huehn J, Floess S, Wieczorek G, Olek S, Dietmaier W, Andreesen R, and Edinger M. 2009. Loss of FOXP3 expression in natural human CD4+CD25+ regulatory T cells upon repetitive in vitro stimulation. Eur. J. Immunol 39:1088–1097. [DOI] [PubMed] [Google Scholar]
- 41.Tang Q, and Bluestone JA. 2013. Regulatory T-cell therapy in transplantation: moving to the clinic. Cold Spring Harb Perspect Med 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng SG, Gray JD, Ohtsuka K, Yamagiwa S, and Horwitz DA. 2002. Generation ex vivo of TGF-beta-producing regulatory T cells from CD4+CD25- precursors. J. Immunol 169:4183–4189. [DOI] [PubMed] [Google Scholar]
- 43.Zheng SG, Meng L, Wang JH, Watanabe M, Barr ML, Cramer DV, Gray JD, and Horwitz DA. 2006. Transfer of regulatory T cells generated ex vivo modifies graft rejection through induction of tolerogenic CD4+CD25+ cells in the recipient. Int. Immunol 18:279–289. [DOI] [PubMed] [Google Scholar]
- 44.Koenecke C, Czeloth N, Bubke A, Schmitz S, Kissenpfennig A, Malissen B, Huehn J, Ganser A, Förster R, and Prinz I. 2009. Alloantigen-specific de novo-induced Foxp3+ Treg revert in vivo and do not protect from experimental GVHD. Eur. J. Immunol 39:3091–3096. [DOI] [PubMed] [Google Scholar]
- 45.Zhang P, Tey SK, Koyama M, Kuns RD, Olver SD, Lineburg KE, Lor M, Teal BE, Raffelt NC, Raju J, et al. 2013. Induced regulatory T cells promote tolerance when stabilized by rapamycin and IL-2 in vivo. J. Immunol 191:5291–5303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.