Key Points
Children with PCNSL and no immunodeficiency have a good outcome when treated by a histological subtype–driven and radiation-free protocol.
New treatment guidelines are needed for PCNSL in children and adolescents with an underlying immunodeficiency.
Introduction
Primary central nervous system lymphoma (PCNSL) is a rare subtype of non-Hodgkin lymphoma (NHL) in childhood and adolescence, with an increased risk among patients with immunodeficiency.1-5 Given the rarity and lack of any prospective trials, reliable data on PCNSL are lacking, with most data coming from case reports or small series. Thus, 2 of the largest childhood NHL consortia, the European Intergroup for Childhood Non-Hodgkin Lymphoma (EICNHL) and the international Berlin-Frankfurt-Münster (i-BFM) Group, as well as selected North American centers, designed a retrospective multinational study on pediatric PCNSL. Here, we present data from the largest series of pediatric patients with PCNSL reported to date.
Methods
We conducted a retrospective survey of children and adolescents up to 19 years of age who were diagnosed with PCNSL between 1991 and 2019. Patient data were retrieved from 21 EICNHL and/or i-BFM Group members or selected North American institutions. The survey included questions about demographics, disease, treatment, outcome, and late effects. PCNSL was defined by intracerebral/intraspinal mass(es) and/or cranial nerve palsies not caused by an extradural tumor and/or tumor cells in the cerebrospinal fluid and/or involvement of the eye(s). Although the 2016 World Health Organization Classification now recognizes diffuse large B-cell lymphoma (DLBCL) of the central nervous system (CNS) as a distinct lymphoma, PCNSL is an accepted clinical term that is independent from the histopathology.1,2,5-7 For this reason, we included all histologic subtypes. For patients enrolled in national trials/registries, data collection was approved by the respective ethics committees.8-23 In nontrial/nonregistry patients, the retrospective data collection was performed with local Institutional Review Board approval.
Seventy-five patients with PCNSL were identified. Diagnosis of the NHL subtype was based on contemporaneous pathological criteria.6,24-27 Event-free survival (EFS) was calculated from date of diagnosis to date of first event. Events considered were relapse, progression, and death. Overall survival (OS) was defined as the time from diagnosis to death from any cause or date of last follow-up. EFS and OS curves were estimated according to the Kaplan-Meier method. The Cox proportional-hazard model was used for multivariate analyses of EFS and OS.
Results and discussion
The study cohort is shown in Tables 1-3 and supplemental Tables 1-3. The male/female ratio was 1.7:1, and median age was 12.5 years. Three histopathological subgroups were identified: (1) mature B-cell NHL (B-NHL; n = 51; 68%, including DLBCL [n = 37], Burkitt lymphoma [n = 9], and B-NHL, not otherwise specified [n = 5]), (2) anaplastic large cell lymphoma (ALCL; n = 17; 23%), and (3) other NHL (n = 7; 9%, including B-cell precursor lymphoblastic lymphoma [LBL; n = 4], peripheral T-cell lymphoma [PTCL; n = 2], and extranodal marginal zone lymphoma [n = 1]). Fourteen patients (19%) had preexisting disorders, including immunodeficiency (n = 11), hematologic malignancy (n = 2), and factor VII deficiency (n = 1). Epstein-Barr virus (EBV) status was documented in 47 patients, among whom 9 (19%) were EBV+.
Table 1.
Initial characteristics and therapy of the 75 PCNSL study patients
| Variable | Patients |
|---|---|
| Patients, n | 75 |
| Sex | |
| Female | 29 (39) |
| Male | 46 (61) |
| Age, y | |
| Median (range) | 12.5 (1.25-18.87) |
| <10 | 26 (35) |
| ≥10 to <15 | 31 (41) |
| ≥15 | 18 (24) |
| Preexisting disorder | |
| Yes | 14 (19) |
| No | 56 (75) |
| Unknown | 5 (6) |
| Lansky score | |
| <40% | 14 (19) |
| ≥40-79% | 27 (36) |
| ≥80% | 19 (25) |
| Unknown/not applicable | 15 (20) |
| B symptoms | |
| Yes | 9 (12) |
| No | 59 (79) |
| Unknown | 7 (9) |
| Cranial nerve palsies | |
| Yes | 33 (44) |
| No | 39 (52) |
| Unknown | 3 (4) |
| Histopathologic subtype | |
| DLBCL | 37 (49) |
| Burkitt lymphoma | 9 (12) |
| Mature B-NHL NOS | 5 (7) |
| ALCL | 17 (23) |
| Other NHL | 7 (9) |
| BCP LBL, n | 4 |
| EN MZL, n | 1 |
| PTCL, n | 2 |
| Localization | |
| Intracranial | 70 (93) |
| Intraspinal only | 2 (3) |
| Leptomeningeal only | 3 (4) |
| No. of lesions | |
| 1 | 36 (48) |
| ≥2 | 32 (43) |
| Unknown/not applicable | 7 (9) |
| Initial LDH level, U/L | |
| <500 | 53 (71) |
| ≥500 | 10 (13) |
| Unknown | 12 (16) |
| Initial therapy | |
| Chemo only | 31 (41) |
| Chemo and rituximab | 15 (20) |
| Chemo and RT | 24 (32) |
| Chemo and rituximab and RT | 2 (3) |
| Other | 3 (4) |
| Therapy | |
| Pediatric NHL-type* | 57 (76) |
| Adult NHL-type† | 1 (1) |
| Miscellaneous‡ | 17 (23) |
| Chemotherapeutic drugs, n§ | |
| High-dose methotrexate | 68 |
| High-dose cytarabine | 55 |
| Anthracyclines | 59 |
| Alkylating agents | 64 |
| Intrathecal therapies | 69 |
| RT | |
| Yes | 26 (35) |
| No | 49 (65) |
| Stem cell therapy in CR1 | |
| Autologous | 3 (4) |
| Allogeneic | 1 (1) |
| Rituximab in mature B-NHL | 17 (32) |
Unless otherwise noted, data are shown as n (%).
BCP, B-cell precursor; Chemo, chemotherapy; CR1, first complete remission; EN MZL, extranodal marginal zone lymphoma; LDH, lactate dehydrogenase; NOS, not otherwise specified; RT, radiotherapy.
NHL Berlin-Frankfurt-Münster therapy, n = 30; Lymphomes Malins B therapy, n = 14; Inter–B-NHL therapy, n = 5; Associazione Italiana Ematologia Oncologia Pediatrica linfoma non-Hodgkin (AIEOP LNH) therapy, n = 2; EICNHL therapy, n = 2; Sociedade de Hematologia e Oncologia Pediatrica LNH therapy, n = 1; Japanese Pediatric Leukemia/Lymphoma Study Group B-NHL therapy, n = 2;, Children's Oncology Group AALL1131 trial, n = 1, including 17 of the 26 (65%) patients with RT.
Cancer and Leukemia Group B 50202 trial, n = 1.
Including 9 of the 26 (35%) patients with RT.
High-dose-methotrexate (n = 68): <5 g/m2, n = 5; 5 g/m2, n = 38; 8 g/m2, n = 25. High-dose-cytarabine (n = 55): ≤6 g/m2, n = 13; 8 g/m2, n = 1; 12 to 12.5 g/m2, n = 41. Anthracyclines (n = 59) with doxorubicin in 57 patients and a median cumulative dose of 100 mg/m2 (range: 25-240 mg/m2).
Table 2.
Disease outcome and long-term side effects of the 75 PCNSL study patients
| Variable | Patients |
|---|---|
| Treatment failure | |
| Median (range) time to, y | 0.77 (0.27-6.78) |
| Relapse* | 12 (16) |
| Progression | 2 (3) |
| Localization of failure, n | |
| CNS only | 8 |
| CNS and other | 2 |
| Outside CNS only | 2 |
| Unknown | 1 |
| Multiple sites n.f.s. | 1 |
| Death | 12 (16) |
| Relapse/progression, n | 6 |
| Without any therapy, n | 1 |
| Therapy-related toxicity, n | 4 |
| Unknown, n | 1 |
| Second malignancy, n | 0 |
| Continuous CR | 58 (75) |
| Follow-up | |
| Median (range), y | 5.22 (0.19-25.29) |
| In CR1 | 53 (91) |
| In CR2 | 5 (9) |
| CR not yet achieved at LFU, n | 5 |
| Long-term side effects | |
| Neurological | 22 (29) |
| Endocrine | 3 (4) |
| Cardiac | 0 (0) |
| Other | 7 (9) |
| Osteonecrosis, n | 5 |
| Psychosocial, n | 1 |
| GVHD, CMV-associated retinitis, n | 1 |
Unless otherwise noted, data are shown as n (%).
CMV, cytomegalovirus; CR, complete remission; CR1, first complete remission; CR2, second complete remission; GVHD, graft-versus-host disease; LFU, last follow-up; n.f.s., not further specified.
ALCL, n = 6; DLBCL, n = 5; Burkitt lymphoma, n = 1; extranodal marginal zone lymphoma, n = 1; LBL, n = 1.
Table 3.
Characteristics of the 14 PCNSL patients with preexisting disorders
| Type of preexisting disorder | Patients, n | Histology | Event |
|---|---|---|---|
| Immunodeficiency | |||
| HIV infection | 2 | Burkitt lymphoma/DLBCL | No/dead: TRM (sepsis) |
| St. p. heart transplantation | 1 | DLBCL | Dead: TRM (graft failure) |
| St. p. kidney transplantation | 1 | DLBCL | No |
| St. p. liver transplantation | 1 | DLBCL | No |
| SLE | 1 | DLBCL | Dead (cause unknown) |
| CLIPPERS | 1 | DLBCL | No |
| Colitis ulcerosa | 1 | DLBCL | Dead: TRM (details NA) |
| PID n.f.s. | 2 | DLBCL/DLBCL | No/dead of disease |
| CVID | 1 | ALCL | Dead: TRM (pneumonia) |
| Other disorders | |||
| B-cell precursor LBL | 1 | DLBCL | No |
| JMML | 1 | DLBCL | Relapse |
| Factor VIII deficiency | 1 | ALCL | No |
CLIPPERS, chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids; CVID, common variable immunodeficiency; JMML, juvenile myelomonocytic leukemia; NA, not available; PID n.f.s., primary immunodeficiency not further specified; SLE, systemic lupus erythematosus; St. p., status post; TRM, treatment-related mortality.
Median time to diagnosis was 6.4 weeks, with 31 patients (41%) receiving a diagnosis within 4 weeks of symptom onset. Thirty-three patients (44%) presented with cranial nerve palsies. Seventy patients (93%) had solid intracranial masses, 2 patients (3%) had intraspinal mass(es) only, and 3 patients (4%) had leptomeningeal disease with involvement of the cerebrospinal fluid only. The Lansky score was available in 60 patients: 19 had a score ≥ 80%, and 14 had a score < 40%.28
Fifty-eight patients (77%) were treated according to NHL-subtype–directed protocols (pediatric type, n = 57; adult type, n = 1; B-NHL–block therapy: B-NHLs, n = 38, ALCL, n = 14; leukemia-type therapy: LBL, n = 4, PTCL, n = 2), and 17 patients (23%) received miscellaneous therapies.8-23 Thirty-one patients (41%) were treated with chemotherapy only, 15 (20%) were treated with chemotherapy + rituximab, 24 (32%) were treated with chemotherapy + radiotherapy, and 2 (3%) were treated with chemotherapy + rituximab + radiotherapy. One patient underwent observation alone after total resection, 1 received no therapy for 3 months for logistic reasons, and 1 patients remained without therapy.
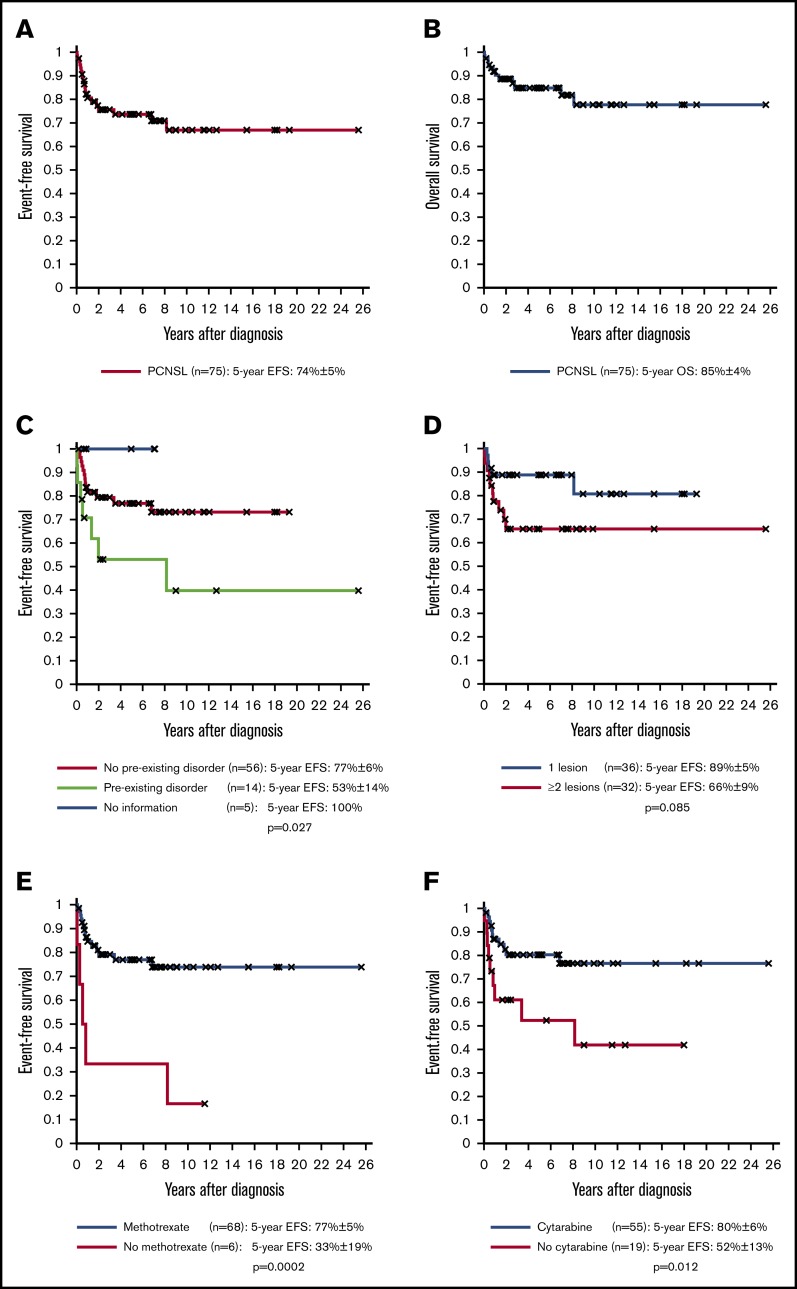
The chemotherapeutic cycles included high-dose methotrexate in 68 patients (91%), high-dose cytarabine in 55 patients (73%), and anthracyclines in 59 patients (79%). Alkylating agents were used in 64 patients (85%), with cyclophosphamide used in 61 patients. Intrathecal therapies were used in 69 patients (92%) including triple therapies in 56 patients. Twenty-six patients underwent irradiation (35%; whole brain, n = 22) with a dose range of 18 to 45 Gy (ALCL, n = 13; DLBCL, n = 9; other NHL, n = 4). Four patients (5%) completed therapy with a stem cell transplantation (autologous, n = 3; allogeneic, n = 1). After a median time of 5.22 years, 5-year EFS and OS were 74% ± 5% and 85% ± 4%, respectively (Figure 1A-B). Of the 75 patients, 14 (19%) had primary treatment failure (relapse; n = 12; progression, n = 2) after a median time of 0.77 years, including only 1 patient with a preexisting disorder. Twelve patients (16%) died, including 6 after relapse, 1 from PCNSL without any therapy, 4 from treatment-related toxicity as a first event, and 1 from unknown causes. Of the latter 6 patients, all had preexisting disorders.
Figure 1.
EFS and OS rates of the 75 PCNSL patients according to risk factors. EFS (A) and OS (B) of the 75 PCNSL patients. (C) EFS of the 56 PCNSL patients without a preexisting disorder vs 14 PCNSL patients with a preexisting disorder. (D) EFS of the 36 PCNSL patients with 1 lesion vs 32 PCNSL patients with ≥2 lesions. (E) EFS of the 68 PCNSL patients who received therapies that included high-dose methotrexate vs 6 PCNSL patients who did not receive high-dose methotrexate. (F) EFS of the 55 PCNSL patients who received therapies with high-dose cytarabine vs 19 PCNSL patients who did not receive high-dose cytarabine.
Univariate analysis showed that preexisting disorders (EFS, 77% ± 6% vs 53% ± 14%, P = .027; OS, 89% ± 5% vs 62% ± 14%, P = .001) and number of lesions (1 vs ≥2 lesions: EFS, 89% ± 5% vs 66% ± 9%, P = .085; OS, 97% ± 3% vs 76% ± 8%, P = .043) were associated with inferior outcome (Figure 1C-D). In contrast, high-dose methotrexate (P = .0002), high-dose cytarabine (P = .012), and alkylating agents (P = .033) were associated with improved EFS (Figure 1E-F). Other factors, including age, sex, Lansky score, B symptoms, histopathology, type of therapy, rituximab in B-NHLs, or radiotherapy, had no significant impact on EFS or OS (supplemental Table 4). In multivariate analysis, high-dose methotrexate remained significant for EFS, and preexisting disorders remained significant for OS. Analysis of 60 patients without preexisting disorders showed an EFS of 83% ± 5% for pediatric NHL therapy vs 57% ± 19% for miscellaneous therapy (P = .044; supplemental Figure 1), whereas, among 14 patients with a preexisting disorder, 5 of 6 patients with NHL therapy had an event but only 2 of 8 patients with miscellaneous therapy had an event.
Long-term sequelae were primarily neurological (n = 22, 29%; including 8/26 with radiotherapy) and rarely affected endocrine organs (n = 3, 4%), heart (n = 0), or other organs/systems (n = 7, 9%).
This report on 75 patients with PCNSL represents the largest series of PCNSL in childhood and adolescence reported to date.1-3,29-31 Our results show that PCNSL is associated with male sex, age ≥ 10 years, solid intracerebral disease, and immunodeficiency. Among adults with PCNSL, the majority of cases are DLBCL (90%).7 In our cohort, DLBCL was the most common histology, but it was observed in only 49% of cases, suggesting that histopathology of PCNSL in pediatrics might be distinct.
EFS of 74% ± 5% for the entire cohort is comparable to that of children with CNS+ systemic NHL and similar to the 2-year progression-free survival of 61% that was reported by the International PCNSL Collaborative Group in 29 2- to 21-year-old PCNSL patients.11-13,15,16,23,29 Our favorable outcome has been achieved by histological subtype–directed polychemotherapy in most patients, suggesting that pediatric PCNSL can be successfully managed by protocols designed for children with CNS+ systemic NHL, usually including high-dose methotrexate and high-dose cytarabine, steroids, and no irradiation (except for ALCL).11-13,15,16,23,29 Our evaluation of prognostic factors found preexisting disorders to be associated with inferior EFS and OS. In contrast, usage of high-dose methotrexate, high-dose cytarabine, and alkylators, which are fundamental drugs of all pediatric NHL-subtype protocols/trials, were associated with improved prognosis.11-13,15,16,21,23,29
Our study has limitations, including its retrospective nature, with potential for reporting bias. For subanalyses, patient numbers were small and included different histologic entities. Nevertheless, given the rarity of this entity, these data are likely to be the best available to determine important factors in the treatment and outcome of pediatric PCNSL. This international collaboration allowed us to conclude that treatment recommendations are especially needed for PCNSL patients with immunodeficiencies, who are at risk for treatment-related toxicity/mortality, whereas immunocompetent PCNSL patients receiving pediatric histological subtype–directed polychemotherapy and no irradiation do well.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank all participating institutions and physicians for their support of the study. This EICNHL and i-BFM article was written on behalf of the Berlin-Frankfurt-Münster (BFM) Study Group (Austria, Germany, Switzerland, Czech Republic), Associazione Italiana Ematologia Oncologia Pediatrica, Société Française de Lutte contre les Cancers et Leucémies de l’Enfant, United Kingdom Children’s Cancer and Leukemia Study Group, Belgian Society of Pediatric Hematology and Oncology, Dutch Childhood Oncology Group, Slovenian Society of Hematology and Oncology, Serbian Society of Hematology and Oncology, Polish Society of Pediatric Oncology and Hematology, Japanese Pediatric Leukemia/Lymphoma Study Group, Hong Kong Pediatric Hematology and Oncology Study Group, Spanish Society of Pediatric Hematology and Oncology, Hellenic Society of Pediatric Hematology and Oncology, and 5 single institutions from Armenia (Yerevan), Australia (Perth), Canada (Toronto), Belarus (Minsk), and Russia (Moscow) and 2 centers from the United States (Phoenix, Washington, DC).
This work was supported by Cancer Research United Kingdom, the Deutsche Kinderkrebsstiftung (DKS 2014.11A/B, DKS 2016.24A/B, BFM Germany) (B.B. and W.W.), the St. Anna Kinderkrebsforschung (BFM Austria) (A.A.), Project No. LQ1605 of the National Program of Sustainability II and Project Nos. 00064203 and 65269705 of the Czech Ministry of Health (Czech Republic) (E.K.), and the Ministry of Health, Labour and Welfare of Japan (Japanese Pediatric Leukemia/Lymphoma Study Group) (T.O.). L.G.-R. is supported by National Institutes of Health, National Cancer Institute grant K08 CA219473.
Footnotes
Data sharing requests should be sent to Andishe Attarbaschi (andishe.attarbaschi@stanna.at).
Authorship
Contribution: A.A., O.A., and L.G.-R. designed and planned the study and wrote the manuscript; A.A., S. Bansil, S.T., and L.R. pooled data, checked data, and performed statistical analyses; S. Bomken, B.B., F.C., A.K.S.C., H.D., A.F., M.H., J.J., E.K., R.S.K., J. Lazic, J. Loeffen, N.M., T.O., M.P., A.P., C.R., G.T., A.U., J.V.-A., W.W., G.W., A.A., O.A., and L.G.-R. were principal investigators or coinvestigators in their study groups and institutions, coordinated the national trials in their countries, provided study materials, and recruited patients; and all authors read and approved the final version of the manuscript.
Conflict-of-interest disclosure: L.G.-R. has acted as a consultant for Janssen, ADC Therapeutics, and Celgene. The remaining authors declare no competing financial interests.
Correspondence: Andishe Attarbaschi, St. Anna Children’s Hospital, Kinderspitalgasse 6, 1090 Vienna, Austria; e-mail: andishe.attarbaschi@stanna.at.
References
- 1.Abla O, Sandlund JT, Sung L, et al. A case series of pediatric primary central nervous system lymphoma: favorable outcome without cranial irradiation. Pediatr Blood Cancer. 2006;47(7):880-885. [DOI] [PubMed] [Google Scholar]
- 2.Giulino-Roth L, Abla O, Batchelor TT. Management of primary central nervous system lymphoma in children. Hematology Am Soc Hematol Educ Program. 2016;2016:386-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thorer H, Zimmermann M, Makarova O, et al. Primary central nervous system lymphoma in children and adolescents: low relapse rate after treatment according to Non-Hodgkin-Lymphoma Berlin-Frankfurt-Münster protocols for systemic lymphoma. Haematologica. 2014;99(11):e238-e241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Attarbaschi A, Carraro E, Abla O, et al. ; European Intergroup for Childhood Non-Hodgkin Lymphoma (EICNHL) and the International Berlin-Frankfur t-Münster (i-BFM) Study Group . Non-Hodgkin lymphoma and pre-existing conditions: spectrum, clinical characteristics and outcome in 213 children and adolescents. Haematologica. 2016;101(12):1581-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batchelor T, Loeffler JS. Primary CNS lymphoma. J Clin Oncol. 2006;24(8):1281-1288. [DOI] [PubMed] [Google Scholar]
- 6.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grommes C, DeAngelis LM. Primary CNS Lymphoma. J Clin Oncol. 2017;35(21):2410-2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brugières L, Le Deley MC, Rosolen A, et al. Impact of the methotrexate administration dose on the need for intrathecal treatment in children and adolescents with anaplastic large-cell lymphoma: results of a randomized trial of the EICNHL Group. J Clin Oncol. 2009;27(6):897-903. [DOI] [PubMed] [Google Scholar]
- 9.Le Deley MC, Rosolen A, Williams DM, et al. Vinblastine in children and adolescents with high-risk anaplastic large-cell lymphoma: results of the randomized ALCL99-vinblastine trial. J Clin Oncol. 2010;28(25):3987-3993. [DOI] [PubMed] [Google Scholar]
- 10.Seidemann K, Tiemann M, Schrappe M, et al. Short-pulse B-non-Hodgkin lymphoma-type chemotherapy is efficacious treatment for pediatric anaplastic large cell lymphoma: a report of the Berlin-Frankfurt-Münster Group Trial NHL-BFM 90. Blood. 2001;97(12):3699-3706. [DOI] [PubMed] [Google Scholar]
- 11.Williams D, Mori T, Reiter A, et al. ; European Intergroup for Childhood Non-Hodgkin Lymphoma, the Japanese Pediatric Leukemia/Lymphoma Study Group . Central nervous system involvement in anaplastic large cell lymphoma in childhood: results from a multicentre European and Japanese study. Pediatr Blood Cancer. 2013;60(10):E118-E121. [DOI] [PubMed] [Google Scholar]
- 12.Burkhardt B, Woessmann W, Zimmermann M, et al. Impact of cranial radiotherapy on central nervous system prophylaxis in children and adolescents with central nervous system-negative stage III or IV lymphoblastic lymphoma. J Clin Oncol. 2006;24(3):491-499. [DOI] [PubMed] [Google Scholar]
- 13.Cairo MS, Gerrard M, Sposto R, et al. ; FAB LMB96 International Study Committee . Results of a randomized international study of high-risk central nervous system B non-Hodgkin lymphoma and B acute lymphoblastic leukemia in children and adolescents. Blood. 2007;109(7):2736-2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forns M, Javier G, Estella J, et al. ; en representación del grupo SHOP de las Sociedades Españolas de Hematología (SEHP) y Oncología Pediátricas (SEOP) . [Results of the SHOP LNHB98 (LMB89) trial in pediatric patients with B-cell non-Hodgkin’s lymphoma]. Med Clin (Barc). 2007;128(17):641-646. [DOI] [PubMed] [Google Scholar]
- 15.Frazer JK, Li KJ, Galardy PJ, et al. Excellent outcomes in children and adolescents with CNS+ Burkitt lymphoma or other mature B-NHL using only intrathecal and systemic chemoimmunotherapy: results from FAB/LMB96 and COG ANHL01P1. Br J Haematol. 2019;185(2):374-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Landmann E, Burkhardt B, Zimmermann M, et al. Results and conclusions of the European Intergroup EURO-LB02 trial in children and adolescents with lymphoblastic lymphoma. Haematologica. 2017;102(12):2086-2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minard-Colin V, Brugières L, Reiter A, et al. Non-Hodgkin lymphoma in children and adolescents: progress through effective collaboration, current knowledge, and challenges ahead. J Clin Oncol. 2015;33(27):2963-2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patte C, Auperin A, Michon J, et al. ; Société Française d’Oncologie Pédiatrique . The Société Française d’Oncologie Pédiatrique LMB89 protocol: highly effective multiagent chemotherapy tailored to the tumor burden and initial response in 561 unselected children with B-cell lymphomas and L3 leukemia. Blood. 2001;97(11):3370-3379. [DOI] [PubMed] [Google Scholar]
- 19.Pillon M, Mussolin L, Carraro E, et al. Detection of prognostic factors in children and adolescents with Burkitt and diffuse large B-cell lymphoma treated with the AIEOP LNH-97 protocol. Br J Haematol. 2016;175(3):467-475. [DOI] [PubMed] [Google Scholar]
- 20.Reiter A, Schrappe M, Tiemann M, et al. Improved treatment results in childhood B-cell neoplasms with tailored intensification of therapy: a report of the Berlin-Frankfurt-Münster Group Trial NHL-BFM 90. Blood. 1999;94(10):3294-3306. [PubMed] [Google Scholar]
- 21.Tsurusawa M, Mori T, Kikuchi A, et al. ; Lymphoma Committee of Japanese Pediatric Leukemia/Lymphoma Study Group . Improved treatment results of children with B-cell non-Hodgkin lymphoma: a report from the Japanese Pediatric Leukemia/Lymphoma Study Group B-NHL03 study. Pediatr Blood Cancer. 2014;61(7):1215-1221. [DOI] [PubMed] [Google Scholar]
- 22.Woessmann W, Lisfeld J, Burkhardt B; NHL-BFM Study Group . Therapy in primary mediastinal B-cell lymphoma. N Engl J Med. 2013;369(3):282-284. [DOI] [PubMed] [Google Scholar]
- 23.Woessmann W, Seidemann K, Mann G, et al. ; BFM Group . The impact of the methotrexate administration schedule and dose in the treatment of children and adolescents with B-cell neoplasms: a report of the BFM Group Study NHL-BFM95. Blood. 2005;105(3):948-958. [DOI] [PubMed] [Google Scholar]
- 24.Harris NL, Jaffe ES, Diebold J, et al. The World Health Organization classification of neoplasms of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting--Airlie House, Virginia, November, 1997. Hematol J. 2000;1(1):53-66. [DOI] [PubMed] [Google Scholar]
- 25.Harris NL, Jaffe ES, Stein H, et al. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994;84(5):1361-1392. [PubMed] [Google Scholar]
- 26.Stansfeld AG, Diebold J, Noel H, et al. Updated Kiel classification for lymphomas. Lancet. 1988;1(8580):292-293. [DOI] [PubMed] [Google Scholar]
- 27.Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011;117(19):5019-5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lansky SB, List MA, Lansky LL, Ritter-Sterr C, Miller DR. The measurement of performance in childhood cancer patients. Cancer. 1987;60(7):1651-1656. [DOI] [PubMed] [Google Scholar]
- 29.Abla O, Weitzman S, Blay JY, et al. Primary CNS lymphoma in children and adolescents: a descriptive analysis from the International Primary CNS Lymphoma Collaborative Group (IPCG). Clin Cancer Res. 2011;17(2):346-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Suoji C, Welch JJ, Perkins SL, et al. Rare pediatric non-Hodgkin lymphomas: a report from Children’s Oncology Group Study ANHL 04B1. Pediatr Blood Cancer. 2016;63(5):794-800. [DOI] [PubMed] [Google Scholar]
- 31.Yoon JH, Kang HJ, Kim H, et al. Successful treatment of primary central nervous system lymphoma without irradiation in children: single center experience. J Korean Med Sci. 2012;27(11):1378-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.