Key Points
Positron emission tomography avidity after salvage predicts for suboptimal long-term outcomes with conventional therapies in patients with HL.
Allo-HSCT is associated with low relapse rates and encouraging longer-term survival outcomes in these patients.
Abstract
We evaluated the role of allogeneic hematopoietic stem cell transplantation (allo-HSCT) in transplant-naïve patients with relapsed/refractory Hodgkin lymphoma (HL) who failed to attain metabolic complete response (mCR) to 1 to 2 lines of salvage chemotherapyThose with residual but nonprogressive disease assessed by positron emission tomography/computed tomography scanningwere eligible. An additional 1 to 2 cycles of salvage therapy were permissible in those with progressive disease or when required to bridge to allo-HSCT, with additional imaging at baseline before transplantation. Conditioning consisted of carmustine, etoposide, cytarabine, melphalan, and alemtuzumab. Donor lymphocyte infusions (DLI) were administered for mixed chimerism or residual or relapsed disease. Eleven patients had sibling donors, 13 had HLA-matched unrelated donors, and 7 had HLA-mismatched unrelated donors. There were no graft failures, and no episodes of grade 4 acute graft-versus-host disease (GVHD); only 19.4% of patients had grade 2 to 3 GVHD, and 22.2% had extensive chronic GVHD. The non-relapse mortality rate was 16.1% (95% confidence interval [CI], 7.1%-34.5%). Relapse incidence was 18.7% (95% CI, 8.2%-39.2%). The study met its primary objective, with a 3-year progression-free survival of 67.7% (95% CI, 48.4%-81.2%). Survival outcomes were equivalent in those with residual metabolically active disease immediately before transplantation (n = 24 [70.8%; 95% CI, 17.2%-83.7%]). Two of the 5 patients who relapsed received DLI and remained in mCR at latest follow-up, with a 3-year overall survival of 80.7% (95% CI, 61.9%-90.8%). We demonstrate encouraging results that establish a potential role for allo-HSCT in selected high-risk patients with HL. This trial was registered at www.clinicaltrials.gov as #NCT00908180.
Visual Abstract
Introduction
Algorithms for the initial treatment of Hodgkin lymphoma (HL) have evolved to using response-adjusted strategies that reduce overall treatment burden while maintaining excellent survival outcomes. For the cohort of patients for whom primary treatment has failed (those with primary refractory disease or those who relapse after initial complete response [CR]), new therapies have emerged that offer high response rates (RRs). Establishing how these therapies integrate into current treatment pathways remains challenging in such a rapidly evolving field. Until relatively recently, patients with relapsed/refractory disease would have received either full-course multiagent chemotherapy or combined modality therapy as first-line treatment. At the point of treatment failure, they would be offered salvage chemotherapy with the aim of consolidation with autologous stem cell transplantation (ASCT). This was the established standard of care in chemotherapy-sensitive patients based on improved progression-free survival (PFS) compared with conventional chemotherapy.1 Nevertheless, there are some patients whose outcomes are predicted to be relatively poor after ASCT. Presentation with stage IV disease, the presence of extranodal disease, primary refractoriness, bulk ≥5 cm, Eastern Cooperative Oncology Group performance status ≥1, or inadequate response to salvage chemotherapy have all been linked to worse outcomes.2-4 Notably, those with residual metabolically avid disease assessed by 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) before ASCT had 10-year survival of 30% compared with 75% for those with a negative scan.5,6 Within the latter cohort, patients with nodal-only disease in remission at the time of ASCT have an 80% to 90% cure rate compared with 55% to 65% for patients with extranodal disease.7
On the basis of these considerations, we explored whether allogeneic hematopoietic stem cell transplantation (allo-HSCT) may have a role in the management of transplant-naïve patients with residual FDG-avid disease after conventional first- or second-line salvage chemotherapy. The role of allo-HSCT in the management of HL remains controversial, particularly in transplant-naïve patients. The emergence of more encouraging data on allograft outcomes after ASCT provided the rationale for evaluating patients earlier in the treatment pathway,8-12 which allows the use of more intensive conditioning chosen to match the standard used in the autologous setting (carmustine, etoposide, cytarabine, melphalan [BEAM]) with the addition of alemtuzumab, an agent that may both disrupt the immunosuppressive tumor microenvironment that characterizes HL and reduce the incidence of graft-versus-host disease (GVHD).13 The latter facilitates transplantation in the unrelated donor setting, particularly with HLA-mismatched grafts. Single-center data with this approach were encouraging13 but required confirmation in a multicenter prospective trial setting that incorporated stringent quality control and central review of combined modality PET/computed tomography (PET/CT) imaging at baseline and after transplantation.
Methods
Study design
The Pilot of Allogeneic Immunotherapy in Relapsed/Refractory Disease (PAIReD) trial was conducted according to the Declaration of Helsinki and relevant International Conference on Harmonization Good Clinical Practice Guidelines and was approved by an independent national ethics committee.
Patients were required to have a confirmed diagnosis of HL, to have either primary refractory or relapsed disease failing to achieve metabolic CR (mCR) as assessed by combined-modality PET-CT imaging after 1 to 2 lines of multiagent salvage chemotherapy, and have an HLA-compatible sibling or unrelated donor (at least a 9/10 match). Patients were ineligible for trial entry if they had achieved mCR (defined as a Deauville score of 2 or lower) or had progressive disease (PD) at the time of screening. Those with PD could, however, receive an additional line of salvage chemotherapy before rescreening. In view of the logistics of scheduling allo-HSCT, patients could receive 1 to 2 additional cycles of salvage chemotherapy after trial entry, using either the same regimen they had received most recently or an alternative regimen based on their clinical status. A repeat PET-CT scan was performed at baseline before allo-HSCT in these patients to allow comparison after transplant. General inclusion criteria were age 11 to 65 years, World Health Organization performance status of 0 to 1, creatinine clearance >50 mL/minute, cardiac ejection fraction >40%, negative pregnancy test, no relevant comorbidities, and signed informed consent. Exclusion criteria included severe hepatic impairment (serum bilirubin >1.5 times or alkaline phosphatase >2 times the upper limit of normal), previous malignancy within 5 years (excluding nonmelanoma skin tumor or curatively treated in situ carcinoma of the cervix), previous SCT, or history of HIV infection.
Transplantation platform
Allo-HSCT was performed using BEAM-alemtuzumab conditioning (carmustine 300 mg/m2 on day −6, etoposide 200 mg/m2 on days −5 to −2, cytarabine 200 mg/m2 twice per day on days −5 to −2, melphalan 140 mg/m2 on day −1, and alemtuzumab 10 mg intravenously on days −5 to −1). Because of global supply shortages, 4 patients received lomustine 200 mg/m2 instead of carmustine. The graft source was mobilized stem cells (target dose 4 × 106 CD34+ cells per kg). Additional GVHD prophylaxis consisted of cyclosporine A from day −1 with a target level of 200 to 300 ng/mL, tapered from day 60 after transplant. Growth factor (lenograstim 263 μg subcutaneously) was recommended once per day from day 6 posttransplant until neutrophil recovery (>0.5 × 109/L). Anti-infection prophylaxis against fungi, Varicella zoster, and Pneumocystis jiroveci (previously P carinii) was given according to local standards. Surveillance for and management of other emergent infections (eg, cytomegalovirus [CMV]) was performed according to local standards, with guidance that once-per-week surveillance for CMV infection should be performed for the first 3 months posttransplantation. Disease assessment and chimerism studies were performed at protocol-defined time points, and donor lymphocyte infusions (DLIs) were administered as per standard protocol.
DLIs
DLIs were administered in 3 settings: (1) evidence of persistent stable or increasing recipient chimerism from 6 months posttransplantation and after discontinuation of cyclosporine A for 2 months, (2) evidence of residual disease from 6 months posttransplantation and after discontinuation of cyclosporine A for 2 months, and (3) evidence of PD or relapse at any time point posttransplantation as assessed by PET-CT (Deauville score of 4-5), in which case cyclosporine A was discontinued and debulking chemotherapy could be administered at the discretion of the treating physician before DLI. DLIs were administered in an escalating dose protocol, with the initial dose determined by the indication for intervention and the donor source (Table 1). After the initial dose, additional infusions were administered once every 3 months until the desired end point was achieved or GVHD developed. For patients with sibling donors, dose escalation proceeded according to the following schedule, which was dependent on initial dose: 3 × 106, 1 × 107, 3 × 107, and 1 × 108 CD3+ T cells per kg. For patients with unrelated donors, the scheduling was one increment lower: 1 × 106, 3 × 106, 1 × 107, 3 × 107 CD3+ T cells per kg. DLIs were not administered in the presence of active GVHD.
Table 1.
Starting dose for DLI, depending upon indication and donor source
| Indication | CD3+ T cells per kg | |
|---|---|---|
| Sibling donor | Unrelated donor | |
| Mixed chimerism | 1 × 106 | 5 × 105 |
| Residual SD | 1 × 106 | 5 × 105 |
| Progression at: | ||
| <12 mo | 3 × 106 | 1 × 106 |
| >12 mo | 1 × 107 | 1 × 106 |
PET imaging
Patients underwent PET-CT scanning with low-dose unenhanced CT using full-ring dedicated PET-CT cameras with quality control overseen by a core team based at University College London Hospitals National Health Service (NHS) Trust. Subsequent PET-CT scanning for individual patients was performed under the same conditions and on the same scanner as baseline scanning. Scans were centrally reported, dictating study entry and subsequent study-directed interventions with DLIs. All scans were assigned a Deauville grade. Since Deauville score in isolation gives limited information regarding overall response to intervention in those achieving less than an mCR, additional response parameters were defined for the purposes of trial reporting and guidance for posttransplantation interventions. Partial response (PR) was defined as ≥50% decrease in tumor size, with residual FDG avidity at sites of previous disease, with no increase in any mass or new mass. PD was defined as ≥50% increase in disease or development of new lesions that were FDG avid. Stable disease (SD) was defined as neither PR nor progression, with FDG avidity only at sites of previous disease. If new PET findings could have been a result of inflammatory or infective pathology, a decision on classification of disease status was made by the PET review panel and the chief investigator if biopsy of the lesion was not possible. Scans were performed at initial baseline for study entry, repeated before transplantation as a baseline for posttransplant comparison in those patients receiving further chemotherapy between study entry and transplantation, and then for routine restaging at 3 and 6 months and at 1, 2, and 3 years posttransplant. Additional scans were performed to assess response once every 3 months if patients progressed or relapsed and received further interventional therapy with DLIs.
Trial end points
The primary end point of the study was 3-year PFS. Key secondary end points included engraftment rates, chimerism at 3 and 6 months, non-relapse mortality (NRM), incidence of acute GVHD (aGVHD) and chronic GVHD (cGVHD), RR, and overall survival (OS).
Statistical methods
The trial used an A’Hern design (1-sided 10% significance level and 80% power) aiming to show a 3-year PFS of 45%, with a lower limit for acceptability of 25%. Sample size was calculated at 26 patients and was increased to 32 patients to allow for dropouts. PFS and OS were measured from the date of transplantation until the date of first progression or death (PFS) or death (OS). Patients without an event were censored at the date last seen. PFS and OS rates were calculated by using Kaplan-Meier survival analysis, and the cumulative incidences of relapse and NRM were calculated by using competing risk survival analysis with NRM and relapse as competing risks, respectively. All analyses were performed using STATA version 15.1 (STATA, College Station, TX).
Results
Patient characteristics
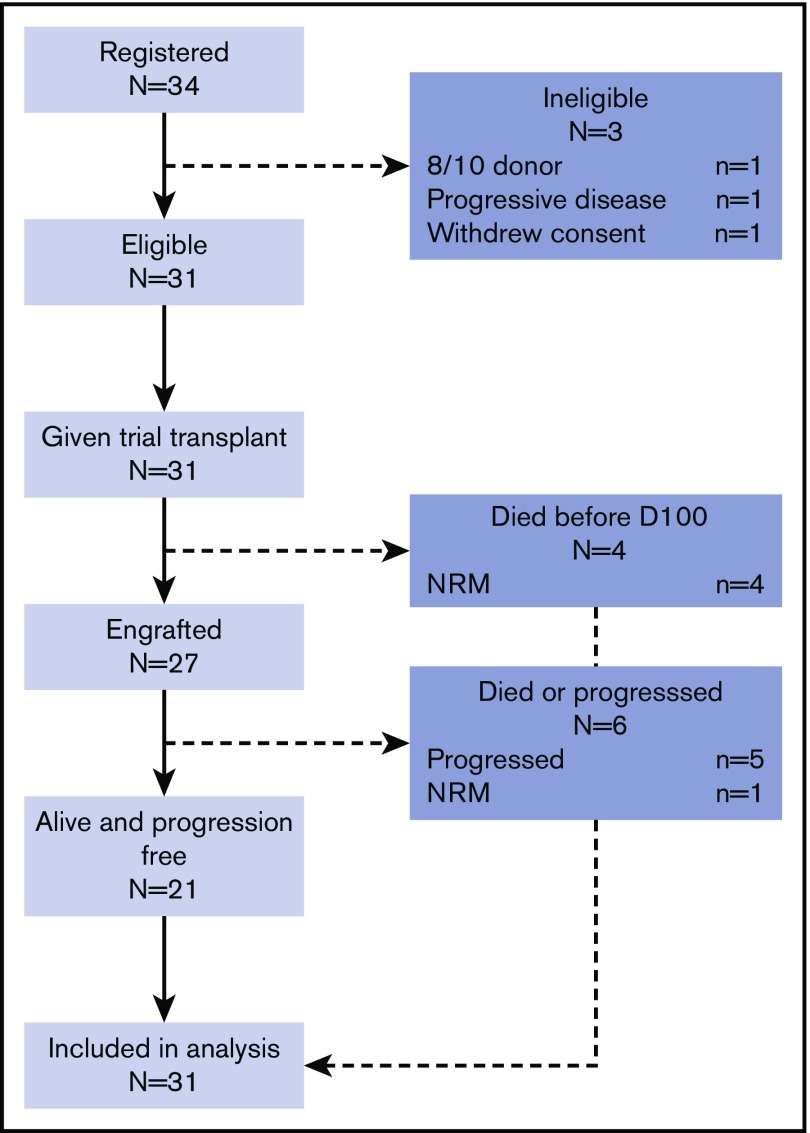
Thirty-one trial-eligible patients were recruited between May 2010 and February 2014 from 8 transplantation centers in the United Kingdom (Figure 1). Median age at transplantation was 31 years (range, 15-62 years). Major risk factors for poor outcome are listed in Table 2. The majority (77.4%) received doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) with or without bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPP) as first-line therapy, with smaller numbers receiving vincristine, etoposide, prednisone, and doxorubicin (OEPA)–based therapy. First-line salvage regimens were mainly cisplatin-based (77.4%) (either etoposide, methylprednisolone, cisplatin, and cytarabine [ESHAP] or dexamethasone, cisplatin, and cytarabine [DHAP]) or ifosfamide-containing (19.3%). Two or more lines of salvage therapy were administered in 24 patients (77.4%) before transplantation, with 12 (38.7%) receiving brentuximab vedotin. Although all patients had residual FDG-avid lesions at study entry, 7 patients (22.6%) had achieved mCR immediately before transplantation after additional chemotherapy, 15 (48.4%) achieved PR, and 9 (29.0%) had SD. Median time from diagnosis to transplantation was 15.9 months (range, 7.5-131.1 months).
Figure 1.
CONSORT diagram. D110, day 100.
Table 2.
Patient characteristics (risk factors for poor outcome)
| Factor | Incidence (N = 31) | |
|---|---|---|
| No. | % | |
| Stage IV disease | 10 | 32.3 |
| Primary refractory disease* | 10 | 32.3 |
| Extranodal disease | 16 | 51.6 |
| ≥3 lines of previous treatment | 24 | 77.4 |
| Metabolically active disease at transplant | 24 | 77.4 |
| WHO performance status | ||
| 0 | 29 | 93.5 |
| 1 | 2 | 6.5 |
Median time from diagnosis to relapse in the cohort of 21 patients with relapsed disease was 10.5 months (range, 3.4-121.9 months).
Donor characteristics
Eleven donors (35.5%) were siblings (2 mismatched at a single class I locus), 13 (41.9%) were 10/10 HLA-matched unrelated donors, and 7 (22.6%) were 9/10 HLA-mismatched unrelated donors. Of the latter, 6 were single-allele class I mismatches (2A, 1B, 3C) and 1 was a class II mismatch (DQ). Five mismatches were bidirectional and 2 were unidirectional (1 host-versus-graft, 1 graft-versus-host). In 18 cases, both donor and recipient were CMV seronegative, in 10 both were seropositive, and in 1, the donor was seropositive and the recipient was seronegative.
Engraftment, NRM, and GVHD
Twenty-seven patients survived until day 100. There were no cases of graft rejection or secondary graft failure. Delayed engraftment (beyond 28 days to neutrophils >0.5 × 109/L or platelets >20 × 109/L) was reported only in the platelet lineage and occurred in 5 cases (range, 42-356 days). Five patients died as a result of NRM, giving a 3-year cumulative incidence of 16.1% (95% confidence interval [CI]: 7.1%-34.5%; Figure 1). Four had unrelated donors (2 matched, 2 mismatched), and 1 had a sibling donor. Four died before day 100, 3 with respiratory failure or infection as a major contributing factor, and 1 with unexplained acute hepatic failure at day 20 (no evidence of GVHD). The latest NRM death occurred at 8.8 months. This patient developed grade 3 aGVHD, followed by extensive cGVHD affecting the gastrointestinal tract, which was treated with systemic steroids. She developed multiple viral infections, including both CMV and BK virus, initially receiving foscarnet and then cidofovir, with subsequent deteriorating renal function. Renal biopsy was consistent with BK nephropathy, and she died of progressive renal and multi-organ failure. Grades 2 to 3 aGVHD occurred in 6 patients (19.4%). Of these, 4 (12.9%) developed grade 2 GVHD (1 isolated cutaneous involvement, 2 with cutaneous and gastrointestinal tract involvement, and 1 with cutaneous, gastrointestinal, and liver involvement), and 2 patients (6.5%) developed grade 3 GVHD (both involving cutaneous and gastrointestinal systems). There were no cases of grade 4 GVHD. Extensive cGVHD occurred in 6 (22.2%) of 27 patients surviving beyond 100 days posttransplantation, although none remained on immune suppression at latest follow-up.
Adverse events
All patients experienced at least one grade 3 adverse event (AE), most commonly relating to cytopenias, which is in keeping with the treatment modality. Of the nonhematologic AEs, the most common were related to gastrointestinal disorders (mucositis, 51.6%; diarrhea, 25.8%; nausea, 6.5%) and infections (overall, 45.2%; neutropenic sepsis, 32.3%; fungal infection, 9.7%). One patient (3.2%) developed a pericardial effusion, and 2 (6.5%) developed reversible posterior leukoencephalopathy syndrome secondary to use of cyclosporin. All 3 remain alive and progression free.
Chimerism
All 27 patients who survived until day 100 were tested for chimerism at least once. The median time to first test was 99 days, at which point 14 had full-donor status and 13 were mixed chimeras. Four patients converted to full-donor status after immune suppression withdrawal and an additional seven patients converted after DLI (5 patients after a single dose, and 2 patients after 2 doses). Of the remaining 2, 1 had PD at 6.2 months and died at 7.7 months from disease after receiving a single dose of brentuximab vedotin; the other progressed at 5.9 months, received 3 DLIs, and is currently alive and subsequently progression-free 3 years posttransplant (and remains a mixed chimera).
Relapse and survival outcomes
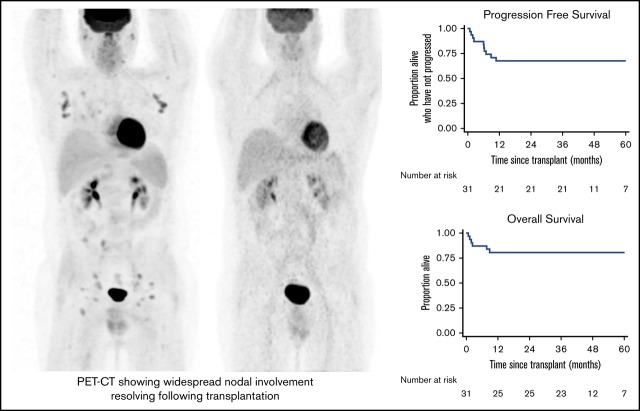
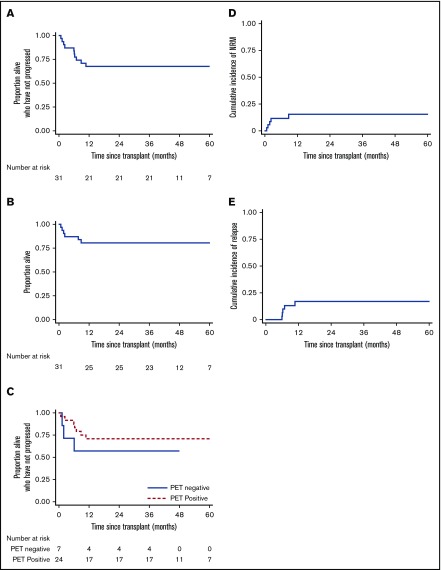
Five patients had disease relapse or progression, giving a 3-year cumulative relapse incidence of 16.1% (95% CI, 7.1%-34.5%) and a 3-year PFS rate of 67.7% (95% CI, 48.4%-81.2%; Figure 2). Notably, 24 patients had residual disease at the time of transplantation. Their outcomes seemed no worse than outcomes for those who had achieved mCR, with a 3-year PFS of 70.8% (95% CI, 48.4%-84.8%; Figure 2). Only 1 patient with relapse died. One received DLI only and achieved mCR as previously noted. One relapsed at 10.7 months and received salvage chemotherapy followed by DLIs, achieving an mCR that was maintained at the latest follow-up (46.9 months). One patient relapsed at 6.9 months, received a combination of brentuximab vedotin and bendamustine, and remains free from further relapse at 48.3 months. The final patient relapsed at 6.0 months, received radiotherapy, and remains progression free at 35.5 months. Thus 25 patients remain alive at latest follow-up (3-year OS 80.7%; 95% CI, 61.9%-90.8%; Figure 2), 21 of whom remain event free and 4 in CR after additional salvage chemotherapy.
Figure 2.
Survival outcomes. PFS (A) and OS (B) for the cohort of 31 patients. (C) PFS according to pretransplant disease status. NRM (D) and relapse incidence (E).
Discussion
The most appropriate therapeutic strategy for patients with relapsed/refractory HL who did not respond adequately to initial salvage remains unclear. Our data help to inform this debate and represent the only prospective multicenter trial experience of a modern allo-HSCT platform in a transplant-naïve population. By comparison, 86% of the patients on the HDR-ALLO prospective study had relapsed after a previous ASCT.12 Although our study was relatively small, the data support the outcomes reported in single-center retrospective cohorts. They confirm that by using a more intensive T-depletion transplantation platform, it is possible to combine relatively low procedural mortality (NRM, 16.1%) with an encouragingly low relapse incidence (16.1% at 3 years), to achieve a 3-year PFS of 67.7% (95% CI, 48.4%-81.2%). Procedural mortality and ongoing morbidity are both important factors when considering alternative treatment options for these patients. Notably, more severe (grade 3-4) GVHD occurred in only 2 of the patients (6.5%), extensive cGVHD occurred in 6 patients (22.2%) despite inclusion of 20 (64.5%) patients with unrelated donor transplants, and 9 (29.0%) HLA-mismatched donors. Furthermore, no surviving patient remained on immune suppression.
Putting these data into the context of current clinical practice remains challenging. The study design was informed by data on ASCT outcomes in patients with FDG-avid disease after a single line of salvage chemotherapy.6 Earlier data suggested similar outcomes for those who achieved mCR after 1 or 2 lines of salvage therapy.14,15 Our hypothesis was that improving PFS outcomes in this cohort to 45% or greater would be clinically valuable, and by those criteria, the trial met its primary end point. Emergent data clearly suggest, however, that patients achieving an mCR after a second line of salvage chemotherapy may be better candidates for ASCT.14,15 The majority of patients in this study had residual metabolically active disease (n = 24; 77.4%) immediately prior to transplantation, with no evidence of inferior outcomes (3-year PFS, 70.8%), supporting our previous demonstration that PET avidity before allo-HSCT has much less prognostic significance than in the setting of ASCT.
For patients with residual disease after 2 lines of salvage chemotherapy, there are several clinical options. First, response could be consolidated with ASCT alone, accepting that the majority will relapse and require further therapy.6,14,15 Outcomes of allo-HSCT in this setting of relapse after ASCT are generally less good. In the HDR-ALLO study, 15% of patients did not respond to salvage chemotherapy, and in those proceeding to allo-HSCT, relapse incidence was 59% (95% CI, 55%-63%) and 4-year PFS was 24% (95% CI, 22%-27%).12 Outcomes were particularly poor in those with SD who received a transplantation and in whom the incidence of relapse was more than 80%. Outcomes were best in those in CR who received a transplantation and in whom 4-year PFS was 50% (95% CI, 47%-53%).
Second, an additional line of salvage chemotherapy could be given with the aim of achieving mCR, on the assumption that ASCT outcomes would be similarly favorable regardless of the number of lines of therapy required to achieve an mCR. Although this may be the case, the number of patients achieving this level of response would be relatively small, because most will have already received brentuximab vedotin. It is likely that these patients would be considered for a checkpoint inhibitor targeting the programmed cell death protein 1/programmed death-ligand 1 (PD-1/PD-L1) axis. RRs for these patients are encouraging, although most responses are partial, and there is currently no consensus on the role, timing, or outcomes of ASCT in these patients, with many favoring the idea of ongoing treatment to progression rather than consolidation. The majority of such patients will progress within 18 months, and the impact on subsequent attempts at salvage chemotherapy and transplantation remains unknown. Because our study did not include any patients who had received these agents before allograft, it will be important to establish whether results of allograft in this patient group remain comparable to results demonstrated in this study or whether patients are potentially adversely impacted. Early experience of use of anti-PD-1 antibodies either before or after allo-HSCT has indicated a possible impact on risk of GVHD or sinusoidal obstruction syndrome,16-18 although it is notable that most of this experience is reported in the setting of T-replete transplants, and toxicity may potentially be modulated or abrogated by the incorporation of T-cell–depleting serotherapy such as alemtuzumab.
Third, response could be consolidated by ASCT with post-ASCT brentuximab vedotin, based on the outcomes of the AETHERA trial.19,20 Notably, these outcomes were delivered in the context of brentuximab vedotin–naïve patients, and it is likely that patients who are not able to achieve mCR with brentuximab vedotin before ASCT will experience less benefit. Our data support considering a fourth alternative: demonstrating the feasibility of delivering encouraging survival outcomes by using a T-cell–depleted allo-HSCT platform. Early mortality is undeniably higher with this approach, but relapse incidence is remarkably low. Although we would not recommend this approach for patients who achieve an mCR, the encouraging outcomes in the cohort with residual FDG-avid disease who received a transplantation suggest that further exploration of this strategy is worthwhile in the higher-risk patients (extranodal disease, primary refractory disease, SD after salvage chemotherapy, inability to achieve an mCR with previous brentuximab vedotin therapy) whose outcomes with alternative strategies remain suboptimal.
Acknowledgments
The authors acknowledge the following investigators at sites recruiting <10% of the total study population: Maria Gilleece (St. James’s University Hospital, Leeds, United Kingdom), Ram Malladi (Queen Elizabeth Hospital Birmingham, Birmingham, United Kingdom), Stephen Robinson (University Hospitals Bristol, Bristol, United Kingdom).
This work was supported by a grant from Chugai Pharma (partially covering the trial management costs), by grants from the Department of Health and from Cancer Research UK (CRUK) funding schemes for the National Institute for Health Research (NIHR) Biomedical Research Centres and Experimental Cancer Medicine Centres. K.S.P. was funded in part by the NIHR Blood and Transplant Research Unit in Stem Cells and Immunotherapy at University College London in partnership with the National Health Service Blood and Transplant Research Unit. The study was endorsed by CRUK (CRUK-E/09/005). Sanofi Genzyme provided alemtuzumab for use within the trial.
Footnotes
Requests for data may be addressed to Karl S. Peggs at k.peggs@ucl.ac.uk.
Authorship
Contribution: E.D.-G., N.H.R., D.C.L., and K.S.P. designed the research; E.D.-G., K.J.T., A.J.C.B., A.D.C., S.M., N.H.R., and K.S.P. served as principal investigator and sub investigators on the clinical study; I.K. performed the central PET-CT reviews; L.C.-H., P.P., N.E.-M., and A.L. performed the trial data collection, analysis, and oversight; A.A.K. was the trial statistician; K.S.P. wrote the manuscript; and all authors reviewed and commented on the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Karl S. Peggs, UCL Cancer Institute, Paul O’Gorman Building, 72 Huntley St, London WC1E 6BT, United Kingdom; e-mail: k.peggs@ucl.ac.uk.
References
- 1.Schmitz N, Pfistner B, Sextro M, et al. ; Lymphoma Working Party of the European Group for Blood and Marrow Transplantation . Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease: a randomised trial. Lancet. 2002;359(9323):2065-2071. [DOI] [PubMed] [Google Scholar]
- 2.Brice P, Bouabdallah R, Moreau P, et al. Prognostic factors for survival after high-dose therapy and autologous stem cell transplantation for patients with relapsing Hodgkin’s disease: analysis of 280 patients from the French registry. Société Française de Greffe de Moëlle. Bone Marrow Transplant. 1997;20(1):21-26. [DOI] [PubMed] [Google Scholar]
- 3.Sureda A, Arranz R, Iriondo A, et al. ; Grupo Español de Linformas/Transplante Autólogo de Médula Osea Spanish Cooperative Group . Autologous stem-cell transplantation for Hodgkin’s disease: results and prognostic factors in 494 patients from the Grupo Español de Linfomas/Transplante Autólogo de Médula Osea Spanish Cooperative Group. J Clin Oncol. 2001;19(5):1395-1404. [DOI] [PubMed] [Google Scholar]
- 4.Bröckelmann PJ, Müller H, Casasnovas O, et al. Risk factors and a prognostic score for survival after autologous stem-cell transplantation for relapsed or refractory Hodgkin lymphoma. Ann Oncol. 2017;28(6):1352-1358. [DOI] [PubMed] [Google Scholar]
- 5.Shah GL, Yahalom J, Matasar MJ, et al. Risk factors predicting outcomes for primary refractory Hodgkin lymphoma patients treated with salvage chemotherapy and autologous stem cell transplantation. Br J Haematol. 2016;175(3):440-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moskowitz AJ, Yahalom J, Kewalramani T, et al. Pretransplantation functional imaging predicts outcome following autologous stem cell transplantation for relapsed and refractory Hodgkin lymphoma. Blood. 2010;116(23):4934-4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moskowitz C. Novel agents and strategies in transplant-eligible patients with relapsed and refractory Hodgkin lymphoma. Hematology Am Soc Hematol Educ Program. 2016;2016:331-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peggs KS, Hunter A, Chopra R, et al. Clinical evidence of a graft-versus-Hodgkin’s-lymphoma effect after reduced-intensity allogeneic transplantation. Lancet. 2005;365(9475):1934-1941. [DOI] [PubMed] [Google Scholar]
- 9.Reyal Y, Kayani I, Bloor AJC, et al. Impact of pretransplantation (18)F-fluorodeoxyglucose-positron emission tomography on survival outcomes after T cell-depleted allogeneic transplantation for Hodgkin lymphoma. Biol Blood Marrow Transplant. 2016;22(7):1234-1241. [DOI] [PubMed] [Google Scholar]
- 10.Martínez C, Gayoso J, Canals C, et al. ; Lymphoma Working Party of the European Group for Blood and Marrow Transplantation . Post-transplantation cyclophosphamide-based haploidentical transplantation as alternative to matched sibling or unrelated donor transplantation for Hodgkin lymphoma: a registry study of the Lymphoma Working Party of the European Society for Blood and Marrow Transplantation. J Clin Oncol. 2017;35(30):3425-3432. [DOI] [PubMed] [Google Scholar]
- 11.Genadieva-Stavrik S, Boumendil A, Dreger P, et al. Myeloablative versus reduced intensity allogeneic stem cell transplantation for relapsed/refractory Hodgkin’s lymphoma in recent years: a retrospective analysis of the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. Ann Oncol. 2016;27(12):2251-2257. [DOI] [PubMed] [Google Scholar]
- 12.Sureda A, Canals C, Arranz R, et al. Allogeneic stem cell transplantation after reduced intensity conditioning in patients with relapsed or refractory Hodgkin’s lymphoma. Results of the HDR-ALLO study - a prospective clinical trial by the Grupo Español de Linfomas/Trasplante de Médula Osea (GEL/TAMO) and the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. 2012;97(2):310-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomson KJ, Kayani I, Ardeshna K, et al. A response-adjusted PET-based transplantation strategy in primary resistant and relapsed Hodgkin Lymphoma. Leukemia. 2013;27(6):1419-1422. [DOI] [PubMed] [Google Scholar]
- 14.Moskowitz CH, Matasar MJ, Zelenetz AD, et al. Normalization of pre-ASCT, FDG-PET imaging with second-line, non-cross-resistant, chemotherapy programs improves event-free survival in patients with Hodgkin lymphoma. Blood. 2012;119(7):1665-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moskowitz AJ, Schöder H, Yahalom J, et al. PET-adapted sequential salvage therapy with brentuximab vedotin followed by augmented ifosamide, carboplatin, and etoposide for patients with relapsed and refractory Hodgkin’s lymphoma: a non-randomised, open-label, single-centre, phase 2 study. Lancet Oncol. 2015;16(3):284-292. [DOI] [PubMed] [Google Scholar]
- 16.Merryman RW, Kim HT, Zinzani PL, et al. Safety and efficacy of allogeneic hematopoietic stem cell transplant after PD-1 blockade in relapsed/refractory lymphoma. Blood. 2017;129(10):1380-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herbaux C, Gauthier J, Brice P, et al. Efficacy and tolerability of nivolumab after allogeneic transplantation for relapsed Hodgkin lymphoma. Blood. 2017;129(18):2471-2478. [DOI] [PubMed] [Google Scholar]
- 18.Peggs KS. Should all patients with Hodgkin lymphoma who relapse after autologous SCT be considered for allogeneic SCT? Blood Adv. 2018;2(7):817-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moskowitz CH, Nademanee A, Masszi T, et al. ; AETHERA Study Group . Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin’s lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;385(9980):1853-1862. [DOI] [PubMed] [Google Scholar]
- 20.Moskowitz CH, Walewski J, Nademanee A, et al. Five-year PFS from the AETHERA trial of brentuximab vedotin for Hodgkin lymphoma at high risk of progression or relapse. Blood. 2018;132(25):2639-2642. [DOI] [PubMed] [Google Scholar]