Abstract
Background
Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and mortality in China. However, identifying patients has proved challenging, resulting in widespread under-diagnosis of the condition. We examined the prevalence of COPD diagnosis and COPD risk among adults in urban mainland China, the factors associated with having a COPD diagnosis or COPD risk, and the healthcare resource use and health outcomes of these groups compared with controls.
Methods
Respondents to the 2017 National Health and Wellness Survey in China (n = 19,994) were classified into three groups: ‘COPD Diagnosed’, ‘COPD Risk (undiagnosed)’, and Control (unaffected), based on their self-reported diagnosis and Lung Function Questionnaire (LFQ) score. The groups were characterised by sociodemographic, health-related quality of life (HRQoL), productivity impairment, and healthcare resource use. Pairwise comparisons (t tests and chi-squared tests) and multivariable regression analyses were used to investigate factors associated with being at risk of, or diagnosed with, COPD.
Results
3320 (16.6%) respondents had a suspected risk of COPD but did not report receiving a diagnosis. This was projected to 105.3 million people, or 16.9% of adult urban Chinese. Of these respondents with an identified risk, only 554 (16.7%) were aware of COPD by name. Relative to those without COPD, those with a risk of COPD (undiagnosed) had significantly greater healthcare resource use, lower productivity and lower HRQoL not only compared to those without COPD, but also compared to people with a COPD diagnosis. Factors associated with increased odds of being at risk of COPD were older age, smoking, alcohol consumption, overweight BMI, occasional exercise, higher comorbidities, asthma diagnosis, being female, lower education, not being employed, and living in a high pollution province (p < 0.05).
Conclusions
There is a substantial group of individuals, undiagnosed, but living with a risk of COPD, who have impaired HRQoL, lower productivity and elevated healthcare resource use patterns. Case-detection tools such as the LFQ may prove a quick and cost-effective approach for identifying these at-risk individuals for further definitive testing and appropriate treatment in China.
Keywords: Chronic obstructive pulmonary disease, China, National health and wellness survey, Health-related quality of life, Resource use, Productivity, Case detection, Screening, Lung function questionnaire
Background
Chronic Obstructive Pulmonary Disease (COPD) is characterised by progressive, partially irreversible airflow limitation [1]. The disease is one of the leading causes of death and disability globally, accounting for an estimated three million deaths per year and 2.6% of global disability-adjusted life years (DALYs) [2]. The burden of disease is most severe in low- and middle-income countries (LMICs) and people in these countries may have a different trajectory of disease due to exposure to different risk factors earlier in life [3, 4].
In China, COPD is the third leading cause of death and was responsible for over 0.9 million deaths in 2013 [5]. Several public health issues associated with COPD including pollution, smoking and an aging population are particularly acute in China [6]. The prevalence of spirometry-defined COPD was estimated to be 8.6% in adults 20 years and older by the China Pulmonary Health (CPH) study, equating to 99.9 million people [7]. However, COPD is underdiagnosed in China: only 2.6% of this spirometry-defined prevalent population were aware of their condition and 9.7% had a prior pulmonary function test [7]. Consequently, the overall burden of COPD in the community may be considerably underestimated.
The prognosis of diagnosed COPD patients can be improved through pharmacological and rehabilitative treatment and through the management of risk-factors [7]. Early diagnosis is important to achieve good outcomes. Forced expiratory volume1 (FEV1) declines at a faster rate in the early stages of the COPD and comorbidities are present from early in the disease [8, 9].
Although testing via spirometry is recommended for diagnosis of COPD, implementing a spirometry-based public screening programme in China faces considerable challenges including low awareness of the condition among the general population and non-specific symptoms [10]. Furthermore, spirometry is not widely available and would require a major investment and behavioural change in China’s large healthcare system [11].
Pre-screening questionnaires may offer a rapid, cost-effective approach to identify high-risk patients for screening spirometry and direct them for appropriate treatment and advice on minimising risk. The Lung Function Questionnaire (LFQ) is one such validated questionnaire, which has shown a sensitivity of 83–88% and specificity of 25–48% in international settings [12]. The LFQ has previously identified a large COPD risk group in Japan whom had poorer health outcomes and higher healthcare resource use compared with controls [13].
Since many people at risk of COPD are believed to reside in the community, and may never present in hospital, a representative general population survey containing a validated screening questionnaire offers an opportunity to investigate this population. To the best of our knowledge, this is the first study to characterise the diagnosed COPD population and those at-risk of COPD in China using a large, representative survey of the urban population. This study estimates the prevalence of adults diagnosed with COPD and those at risk for COPD (undiagnosed) based on the LFQ in urban China. It investigates the factors associated with having a COPD diagnosis or COPD risk and characterises the health outcomes and healthcare resource use of these groups.
Methods
Data source
The 2017 China National Health and Wellness Survey (NHWS) by Kantar Health is a cross-sectional, Internet-based survey self-administered in Chinese language to a nationwide sample of adults (aged 18 or older) who reside in an urban settlement. The sample (n = 19,994) is stratified by gender, age and region to represent the demographic composition of the urban adult population in China. Representativeness of the data has been validated and weighted against the official Chinese Statistical Yearbook [14]. Further methodologic details are provided in Additional file 1.
All respondents from the 2017 NHWS were included in the analysis and classified according to their self-reported physician diagnosis or at-risk status of COPD. In addition to the variables recorded in the NHWS, respondents were assigned an air pollution exposure status based on their province of residence, using provincial Air Quality Index (AQI) levels from the China Air Quality Online Monitoring and Analysis Platform.
Measures
Diagnosis of COPD
To establish the diagnosis of a COPD-related condition, all respondents first indicated whether they ever had experienced “Chronic obstructive pulmonary disease (COPD)”, “Chronic bronchitis (cough up phlegm 4 of 7 days a week over last 3 months)” or “Emphysema”, among other diseases, and then whether their condition had been diagnosed by a physician. Respondents who self-reported a diagnosis of COPD, chronic bronchitis or emphysema were classified as ‘COPD Diagnosed’.
At-risk status of COPD
The LFQ is a five-item, disease-specific, self-report measure of COPD symptoms and risk factors [15]. It was developed as a convenient COPD pre-screening tool to determine in a primary-care setting which individuals may be at-risk for COPD and should therefore receive follow-up spirometry to confirm a diagnosis. The LFQ has been validated in COPD patients and has been previously used in several international settings [13, 16]. The LFQ was translated to Chinese by the NHWS study team.
Scores on the LFQ range from 5 (highest risk) to 25 (lowest risk). According to LFQ working group guidelines, respondents with LFQ scores between 5 and 18 may be considered at risk for COPD [16]. All respondents completed the LFQ. Those with a sum score of 18 or lower, but no self-reported COPD diagnosis, were classified as ‘COPD Risk (undiagnosed)’. Those with an LFQ sum score of 19 or greater, and no self-reported COPD diagnosis, were classified as ‘Control’. Those who reported a COPD diagnosis, regardless of risk score, were classified as ‘COPD Diagnosed’.
Awareness and subjective risk of COPD
All respondents were asked about their awareness of “Chronic Obstructive Pulmonary Disease (COPD)”, among a list of 22 conditions. Respondents also indicated which conditions out of a list they believed to be at risk for developing in the future. Those who selected any of the three COPD-related conditions (COPD, Chronic Bronchitis or Emphysema) were classified to have a “subjective risk of COPD”.
Demographics
Demographic variables for analysis included age, gender, marital status, education, employment status, monthly household income, and medical insurance type.
Health characteristics and comorbidities
Health history variables included smoking, alcohol consumption, exercise level, and body mass index (BMI). Under/overweight was defined by BMI levels of ≤18.5 and > 25 respectively, which reflect the international norms, although it should be noted that some have suggested different BMI levels be used for the Chinese population [17].
Health-related behaviours, such as presentation for an annual check-up with a healthcare provider (HCP) and usage of digital health & wellness tools (Apps, websites, wearables), were also assessed.
The burden from comorbidities was established using the Charlson comorbidity index (CCI) [18]. Higher total CCI scores indicate a greater burden. Respondents also reported whether they had a physician diagnosis of asthma. Since asthma is a commonly co-existing condition to COPD, [19, 20] and not part of the CCI, it was examined separately.
Health-related quality of life
HRQoL was assessed using the SF-12 and EQ-5D, which have been shown to be appropriate for use among people with COPD [21]. The SF-12 version 2 consists of twelve questions measuring eight health domains to assess physical and mental health. Physical (PCS) and mental (MCS) component summary scores can be derived [22]. The questionnaire and its component summary scores have been shown to be valid in the Chinese population [23].
The EQ-5D-5L is a standardised HRQoL instrument developed by the EuroQol Group. It comprises a descriptive system and a visual analogue scale (VAS) [24]. For analysis, the ratings of the five individual dimensions were combined to generate a single index utility value anchored by 0 (equivalent to dead) and 1 (full health) using the China value set.
Higher scores on PCS, MCS and EQ-5D indicate better health status. Differences in PCS and MCS scores exceeding 3 points, and differences in EQ-5D health utility exceeding 0.074 points, respectively, were considered minimally important differences (MID) from a clinical perspective [25].
Productivity impairment
Productivity impairment was assessed using the Work Productivity and Activity Impairment (WPAI) questionnaire [26]. Among those currently employed, the WPAI measured the effect of respondents’ general health on absenteeism (% of work time missed due to health problems), presenteeism (% of impairment while working) and overall work impairment (% of total combined work productivity loss) in the past seven days. Among all respondents, WPAI also assessed the perceived overall activity impairment (% of impairment in daily activities due to health problems in the past seven days). Higher values of the WPAI indicate greater impairment.
Healthcare resource use
Healthcare resource use was assessed by self-reported number of HCP visits (by specialty), emergency room (ER) visits and hospitalisations in the past six months for respondents’ “own medical condition”. The phrasing was intentionally not disease-specific, to capture total resource use due to any medical condition. The number of respiratory specialist HCP visits (pulmonologist, respiratory therapist and otolaryngologist visits) was calculated in addition to the total number of HCP visits.
Air pollution exposure
Average monthly provincial Air Quality Index (AQI) levels were taken from the China Air Quality Online Monitoring and Analysis Platform (www.aqistudy.cn) for the period 2014 to 2017 (48 months). The AQI is a summary measure of daily air quality widely used by national governments. In China, the total AQI score combines levels of six atmospheric pollutants: sulphur dioxide (SO2), nitrogen dioxide (NO2), suspended particulates smaller than 10 μm in aerodynamic diameter (PM10), suspended particulates smaller than 2.5 μm in aerodynamic diameter (PM2.5) carbon monoxide (CO), and ozone (O3) The AQI is calculated on a non-linear scale where a higher score indicates worse air pollution (greater danger to health).
We calculated the number of months with AQI levels of at least 101 (‘unhealthy for sensitive groups’). The five highest pollution provinces were Beijing (31 months AQI ≥ 101), Henan (27), Hebei (28), Tianjin (25), and Xinjiang (23). We also calculated average AQI levels between 2014 and 2017 and the same five provinces had the highest pollution levels: Hebei (average AQI 115), Beijing (114), Henan (112), Tianjin (108), and Xinjiang (104). Residents of these provinces were exposed to more ‘dangerous’ air months and higher average air pollution, both of which may contribute to COPD. Respondents residing in one of these provinces were classified as living in a high-pollution area.
Statistical analysis
Descriptive and bivariate analyses
Prevalence estimates of a COPD diagnosis or an undiagnosed COPD risk status were calculated on the raw data and weighted data. The weighting procedure was performed in a multi-staged process accounting for age, gender and region (see Additional file 1). The weighted total sample was 621,668,160 adults in urban China, based on the Chinese Statistical Yearbook 2016 [14].
Descriptive statistics were used to examine the three groups (‘COPD Diagnosed’, ‘COPD Risk (undiagnosed)’ and ‘Control’) on demographics, health characteristics, and healthcare use. Those variables were also examined in bivariate comparisons between the three groups to test for differences, using independent-samples t-tests for continuous variables and chi-square tests for categorical variables. The results were the basis for identifying covariates for the multivariable models.
Multivariable analyses
Logistic regression was used to investigate demographic and health-related factors associated with a COPD diagnosis or an undiagnosed COPD risk status, respectively, compared to controls.
To assess the burden of COPD diagnosis or an undiagnosed COPD risk with respect to HRQoL, productivity impairment and healthcare resource use, generalized linear models specifying the appropriate distribution (normal for HRQoL variables, negative binomial for other variables) and link function (identity for HRQoL variables, log for other variables) were constructed. Adjusted means were calculated from the respective models for each outcome variable.
Statistical analyses were performed using R Version 3.5.2 (RStudio).
Results
Out of 19,994 adults in NHWS, 513 (2.6%) self-reported a physician diagnosis of a COPD condition (among them 453 claiming a diagnosis with chronic bronchitis, 57 with emphysema, and 19 with COPD). 3534 (17.7%) respondents were identified with a suspected risk of COPD (measured by an LFQ score of 18 points or less). After excluding those with a self-reported diagnosis from the at-risk population, the ‘COPD Risk (undiagnosed)’ group equalled 3320 (16.6%).
Projecting these groups to the Chinese urban adult population of 622 million people, the diagnosed COPD population was estimated at 15.9 million (2.6%) and the undiagnosed COPD risk population at 105.3 million (16.9%).
Among all respondents, 12.4% (n = 2478) reported to be aware of COPD by name. Within the ‘COPD Diagnosed’, ‘COPD Risk (undiagnosed)’ and Control group, respectively, the awareness was at 21.8% (112), 16.7% (555) and 11.2% (1811). For comparison, awareness of the condition asthma in the three groups was at 66.5, 53.0 and 51.9%, respectively, and at 52.4% overall.
Only 3.9% (781) of all respondents felt to be at risk for developing a COPD condition in future ("subjective risk of COPD"). In the ‘COPD Risk (undiagnosed)’ group, 6.7% (222) felt to be at risk. Among the ‘COPD Diagnosed’ population, 55.4% (282) reported to be currently on a prescription medication for their COPD condition.
The ‘COPD Risk (undiagnosed)’ group was significantly more likely to be male, older, married, and state-health insured than controls, while likely being less educated, unemployed (retired: 44.6% versus 13.6%), and with a lower household income (Table 1). The ‘COPD Diagnosed’ population had a similar profile to the ‘COPD Risk (undiagnosed)’ group, but was generally less pronounced in their difference to the Control group, with marital status and income level differences being non-significant.
Table 1.
Demographics of Control, ‘COPD Risk (undiagnosed)’ and ‘COPD Diagnosed’ groups
| All | Control group | ‘COPD Risk (undiagnosed)’ group | ‘COPD Diagnosed’ group | Risk vs. Control, p value | Diagnosed vs. Control, p value | Risk vs. Diagnosed, p value | |
|---|---|---|---|---|---|---|---|
| (n = 19,994) | (n = 16,161) | (n = 3320) | (n = 513) | ||||
| Gender, n (%) | < 0.001 | < 0.001 | < 0.001 | ||||
| Female | 9994 (50.0) | 8628 (53.4) | 1135 (34.2) | 231 (45.0) | |||
| Male | 10,000 (50.0) | 7533 (46.6) | 2185 (65.8) | 282 (55.0) | |||
| Age in years, n (%) | 0.000 | < 0.001 | < 0.001 | ||||
| 18–39 | 8369 (41.9) | 7717 (47.8) | 462 (13.9) | 190 (37.0) | |||
| 40–59 | 7942 (39.7) | 6491 (40.2) | 1254 (37.8) | 197 (38.4) | |||
| 60+ | 3683 (18.4) | 1953 (12.1) | 1604 (48.3) | 126 (24.6) | |||
| Marital status, n (%) | < 0.001 | 0.413 | < 0.001 | ||||
| Married or living with partner | 16,004 (80.0) | 12,640 (78.2) | 2951 (88.9) | 413 (80.5) | |||
| Not married | 3954 (19.8) | 3496 (21.6) | 358 (10.8) | 100 (19.5) | |||
| Decline to answer | 36 (0.18) | 25 (0.15) | 11 (0.33) | 0 (0.00) | |||
| Education, n (%) | < 0.001 | < 0.001 | < 0.001 | ||||
| Less than university degree | 8312 (41.6) | 5849 (36.2) | 2200 (66.3) | 263 (51.3) | |||
| University (4 years) degree or higher | 11,635 (58.2) | 10,277 (63.6) | 1109 (33.4) | 249 (48.5) | |||
| Decline to answer | 47 (0.24) | 35 (0.22) | 11 (0.33) | 1 (0.19) | |||
| Household income (after tax/welfare), n (%) | < 0.001 | 0.880 | < 0.001 | ||||
| CNY 7999 or below | 13,606 (68.1) | 10,718 (66.3) | 2544 (76.6) | 344 (67.1) | |||
| CNY 8000–15,999 | 4809 (24.1) | 4083 (25.3) | 598 (18.0) | 128 (25.0) | |||
| CNY 16,000 or above | 1321 (6.61) | 1136 (7.03) | 149 (4.49) | 36 (7.02) | |||
| Decline to answer | 258 (1.29) | 224 (1.39) | 29 (0.87) | 5 (0.97) | |||
| Employment, n (%) | < 0.001 | < 0.001 | < 0.001 | ||||
| Not employed | 5105 (25.5) | 3276 (20.3) | 1661 (50.0) | 168 (32.7) | |||
| Currently employed | 14,889 (74.5) | 12,885 (79.7) | 1659 (50.0) | 345 (67.3) | |||
Abbreviations: CNY Chinese Yuan (currency of the People’s Republic of China);
Both the ‘COPD Risk (undiagnosed)’ and ‘COPD Diagnosed’ groups were more likely to be smokers, drink more alcohol, exercise less, be more overweight, have more comorbidities (especially asthma), and attend annual health check-ups (Table 2). While the COPD Risk (undiagnosed) group less often used digital health tools, compared to controls, the COPD Diagnosed group used them more often.
Table 2.
Health characteristics of Control, ‘COPD Risk (undiagnosed)’ and ‘COPD Diagnosed’ groups
| All | Control group | ‘COPD Risk (undiagnosed)’ group | ‘COPD Diagnosed’ group | Risk vs. Control, p value | Diagnosed vs. Control, p value | Risk vs. Diagnosed, p value | |
|---|---|---|---|---|---|---|---|
| (n = 19,994) | (n = 16,161) | (n = 3320) | (n = 513) | ||||
| Smoking, n (%) | 0.000 | < 0.001 | < 0.001 | ||||
| Never smoked | 15,019 (75.1) | 13,342 (82.6) | 1367 (41.2) | 310 (60.4) | |||
| Former smoker | 1246 (6.23) | 868 (5.37) | 332 (10.0) | 46 (8.97) | |||
| Current smoker | 3729 (18.7) | 1951 (12.1) | 1621 (48.8) | 157 (30.6) | |||
| Alcohol use, n (%) | < 0.001 | < 0.001 | 0.030 | ||||
| 2–3 times a week or more | 3726 (18.6) | 2542 (15.7) | 1050 (31.6) | 134 (26.1) | |||
| Once a week or less often | 7895 (39.5) | 6327 (39.1) | 1351 (40.7) | 217 (42.3) | |||
| I do not drink alcohol | 8373 (41.9) | 7292 (45.1) | 919 (27.7) | 162 (31.6) | |||
| BMI, n (%) | < 0.001 | 0.008 | 0.023 | ||||
| Overweight/ Obese (BMI ≥ 25) | 4272 (21.4) | 3141 (19.4) | 1006 (30.3) | 125 (24.4) | |||
| Normal (18.5 ≥ BMI < 25) | 13,257 (66.3) | 10,884 (67.3) | 2032 (61.2) | 341 (66.5) | |||
| Underweight (BMI < 18.5) | 1826 (9.13) | 1595 (9.87) | 194 (5.84) | 37 (7.21) | |||
| Decline to answer | 639 (3.20) | 541 (3.35) | 88 (2.65) | 10 (1.95) | |||
| Exercise level (per month), n (%) | < 0.001 | 0.032 | 0.003 | ||||
| No exercise (0 days) | 7272 (36.4) | 5795 (35.9) | 1281 (38.6) | 196 (38.2) | |||
| Occasional (1–10 days) | 7388 (37.0) | 6063 (37.5) | 1118 (33.7) | 207 (40.4) | |||
| Frequent (11+ days) | 5334 (26.7) | 4303 (26.6) | 921 (27.7) | 110 (21.4) | |||
| Charlson Comorbidity Index, mean ± SD | 0.000 | 0.000 | 0.000 | ||||
| CCI | 0.17 ± 0.52 | 0.10 ± 0.38 | 0.32 ± 0.74 | 1.37 ± 0.78 | |||
| Diagnosed of asthma, n (%) | < 0.001 | < 0.001 | < 0.001 | ||||
| Yes | 357 (1.79) | 191 (1.18) | 117 (3.52) | 49 (9.55) | |||
| Annual physical check-up, n (%) | < 0.001 | < 0.001 | < 0.001 | ||||
| Yes | 6451 (32.3) | 4809 (29.8) | 1378 (41.5) | 264 (51.5) | |||
| Digital health tools usage, n (%) | < 0.001 | < 0.001 | < 0.001 | ||||
| 1+ tools used | 8886 (44.4) | 7337 (45.4) | 1256 (37.8) | 293 (57.1) | |||
| None used | 11,108 (55.6) | 8824 (54.6) | 2064 (62.2) | 220 (42.9) | |||
| COPD symptom scores (LFQ), mean ± SD | |||||||
| Frequency of mucus productiona | 4.23 ± 0.83 | 4.43 ± 0.67 | 3.34 ± 0.87 | 3.57 ± 0.88 | 0.000 | 0.000 | 0.000 |
| Frequency of wheezinga | 4.55 ± 0.71 | 4.73 ± 0.50 | 3.74 ± 0.92 | 4.00 ± 0.86 | 0.000 | 0.000 | 0.000 |
| Frequency of dyspnoeaa | 4.07 ± 0.91 | 4.27 ± 0.79 | 3.17 ± 0.86 | 3.58 ± 0.94 | 0.000 | 0.000 | 0.000 |
| LFQ sum score, mean ± SD | |||||||
| LFQ score | 21.1 ± 3.00 | 22.2 ± 1.88 | 16.1 ± 1.89 | 18.8 ± 3.37 | 0.000 | 0.000 | 0.000 |
a Scale 1 = very often to 5 = never
Abbreviations: BMI Body Mass Index; COPD Chronic Obstructive Pulmonary Disease; LFQ Lung Function Questionnaire; SD standard deviation
The three core symptoms of COPD captured in the LFQ - mucus production, wheezing and dyspnoea - were all significantly more frequent in the Diagnosed group than among controls, but even more frequent in the COPD Risk group (undiagnosed). The same pattern emerged regarding the LFQ sum score.
Both the ‘COPD Risk (undiagnosed)’ and ‘COPD Diagnosed’ groups showed significantly higher healthcare resource use, higher productivity impairment and worse HRQoL scores than the Control group (Table 3). Again, the gaps for most health outcome variables versus controls were more pronounced in the ‘COPD Risk’ population, compared to the ‘COPD Diagnosed’ population.
Table 3.
Health outcomes of Control, ‘COPD Risk (undiagnosed)’ and ‘COPD Diagnosed’ groups
| All | Control group | ‘COPD Risk (undiagnosed)’ group | ‘COPD Diagnosed’ group | Risk vs. Control, p value | Diagnosed vs. Control, p value | Risk vs. Diagnosed, p value | |
|---|---|---|---|---|---|---|---|
| (n = 19,994) | (n = 16,161) | (n = 3320) | (n = 513) | ||||
| Healthcare resource use (in past 6 months), mean ± SD | |||||||
| ER visits | 0.32 ± 1.00 | 0.26 ± 0.87 | 0.53 ± 1.38 | 0.68 ± 1.42 | 0.000 | 0.000 | 0.004 |
| Hospitalisations | 0.13 ± 0.83 | 0.09 ± 0.81 | 0.31 ± 0.91 | 0.27 ± 0.74 | 0.000 | < 0.001 | 0.534 |
| HCP visits | 1.49 ± 3.53 | 1.30 ± 3.39 | 2.12 ± 3.86 | 3.28 ± 4.55 | 0.000 | 0.000 | 0.000 |
| Respiratory specialist visits | 0.08 ± 0.69 | 0.07 ± 0.56 | 0.11 ± 1.11 | 0.39 ± 0.93 | 0.003 | 0.000 | 0.000 |
| WPAI, mean ± SD | |||||||
| %Absenteeisma | 3.93 ± 10.8 | 3.22 ± 9.62 | 8.87 ± 16.2 | 6.75 ± 13.8 | < 0.001 | < 0.001 | 0.002 |
| %Presenteeisma | 21.5 ± 24.6 | 19.7 ± 23.9 | 34.0 ± 27.1 | 26.4 ± 22.7 | < 0.001 | < 0.001 | < 0.001 |
| %Overall work impairmenta | 23.4 ± 26.0 | 21.4 ± 25.0 | 37.7 ± 28.9 | 29.7 ± 25.1 | < 0.001 | < 0.001 | < 0.001 |
| %Activity impairment | 20.6 ± 23.1 | 18.2 ± 22.0 | 31.1 ± 25.0 | 27.0 ± 23.1 | 0.000 | 0.000 | < 0.001 |
| HRQoL, mean ± SD | |||||||
| MCS (SF-12v2) | 48.5 ± 7.92 | 49.0 ± 7.82 | 46.6 ± 7.99 | 45.6 ± 7.89 | 0.000 | 0.000 | 0.029 |
| PCS (SF-12v2) | 50.7 ± 6.83 | 51.7 ± 6.28 | 46.3 ± 7.44 | 47.5 ± 6.96 | 0.000 | 0.000 | < 0.001 |
| Health Utility (EQ-5D) | 0.93 ± 0.12 | 0.94 ± 0.10 | 0.87 ± 0.18 | 0.88 ± 0.15 | 0.000 | 0.000 | 0.067 |
a Absenteeism, Presenteeism and Overall Work Impairment calculated for the employed population only (n = 14,889)
Abbreviations: ER Emergency Room; HCP Healthcare provider; HRQoL Health-related quality of life; SF-12v2 Short Form-12 version 2; MCS mental component summary; PCS physical component summary; SD Standard Deviation; WPAI Work Productivity and Activity Impairment
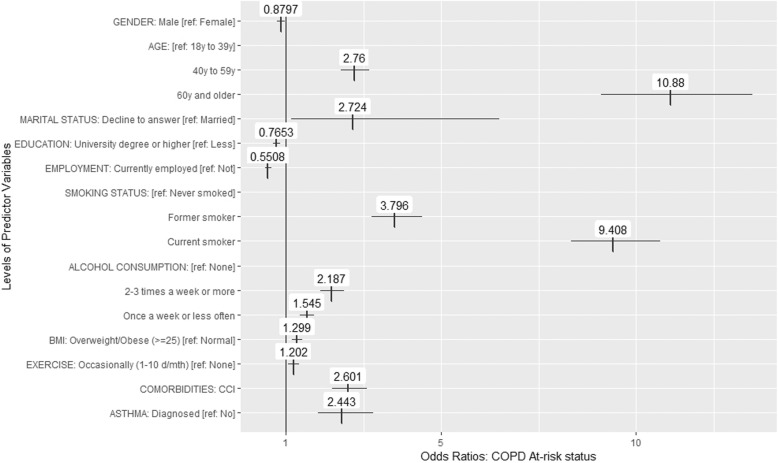
Figure 1 shows factors that are associated with being at an undiagnosed risk of COPD. Logistic regression models showed that older age (40–59 & 60+ vs. 18–39), smoking, alcohol consumption, overweight BMI level, occasional exercise (1–10 days per month vs. no exercise), higher CCI score, diagnosis of asthma, being female, having less than a university degree, and not being employed were all significantly, independently associated with being at an undiagnosed risk of COPD, relative to controls.
Fig. 1.
Logistic regression showing odds of being part of the ‘COPD Risk (undiagnosed)’ group (vs. Control group) as a function of predictors, adjusting for covariates. All predictors shown in the graphic reached statistical significance at p < 0.05. Tested predictors that did not reach significance include: Not married [ref. Married/living with partner]; Education level Decline to answer [ref. Less than university degree]; Monthly household income after deducting employer welfare benefits: All levels [ref. CNY 7999 or less]; BMI Underweight [ref. BMI Normal]; BMI Decline to answer [ref. BMI Normal]; Frequent exercise (11+ days/month) [ref. No exercise]
Table 4 shows the results of regression models investigating the association of ‘COPD Risk’ status with HRQoL, healthcare resource use, and productivity impairment, controlled for the independent predictors of ‘COPD Risk’ status (details: see table footnote).
Table 4.
Health-related quality of life, healthcare resource use and productivity outcome measures as a function of ‘COPD Risk (undiagnosed)’ status
| Outcome | Group | Coefficient (β/ IRR) | P value | Adjusted mean | SE |
|---|---|---|---|---|---|
| MCS (SF-12v2) | Controls (ref.) | 47.44 | 0.59 | ||
| (n = 19,481) | COPD Risk | β: − 3.75 | < 0.000 | 43.70 | 0.59 |
| PCS (SF-12v2) | Controls (ref.) | 47.01 | 0.49 | ||
| (n = 19,481) | COPD Risk | β: −4.28 | < 0.000 | 42.73 | 0.50 |
| Health utility (EQ-5D) | Controls (ref.) | 0.86 | 0.01 | ||
| (n = 19,481) | COPD Risk | β: −0.08 | < 0.000 | 0.61 | 0.01 |
| ER visits | Controls (ref.) | 0.41 | 0.03 | ||
| (n = 18,616) | COPD Risk | IRR: 1.71 | < 0.000 | 0.70 | 0.06 |
| Hospitalisations | Controls (ref.) | 0.11 | 0.01 | ||
| (n = 18,616) | COPD Risk | IRR: 2.92 | < 0.000 | 0.31 | 0.04 |
| HCP visits | Controls (ref.) | 2.16 | 0.11 | ||
| (n = 18,616) | COPD Risk | IRR: 1.31 | < 0.000 | 2.83 | 0.15 |
| Respiratory specialist visits | Controls (ref.) | 0.17 | 0.03 | ||
| (n = 18,616) | COPD Risk | IRR: 1.38 | 0.008 | 0.23 | 0.04 |
| Absenteeism | Controls (ref.) | 4.26 | 0.58 | ||
| (n = 13,658) | COPD Risk | IRR: 2.46 | < 0.000 | 10.48 | 1.55 |
| Presenteeism | Controls (ref.) | 21.57 | 1.52 | ||
| (n = 13,658) | COPD Risk | IRR: 1.74 | < 0.000 | 37.51 | 2.85 |
| Overall work impairment | Controls (ref.) | 24.10 | 1.66 | ||
| (n = 13,658) | COPD Risk | IRR: 1.74 | < 0.000 | 42.01 | 3.13 |
| Activity impairment | Controls (ref.) | 20.85 | 1.12 | ||
| (n = 18,616) | COPD Risk | IRR: 1.71 | < 0.000 | 35.69 | 2.02 |
Note: All models adjusted for gender, age, marital status, education level, household income, employment status, smoking status, alcohol consumption level, body mass index, exercise level, Charlson Comorbidity Index and self-reported asthma diagnosis. Betas (β) were from normal regression models, whereas Incident Rate Ratios (IRR) were from negative binomial models. Models predicting Absenteeism, Presenteeism and Overall Work Impairment are limited to the employed population, while employment status has been dropped as a control variable in these models accordingly. Abbreviations: SF-12v2 Short Form-12 version 2; MCS mental component summary; PCS physical component summary; ER emergency room; HCP healthcare provider; SE standard error
Relative to controls, COPD risk was a significant predictor of poorer HRQoL in all models. COPD risk was associated with lower MCS, lower PCS, and lower EQ-5D health utility score.
Relative to controls, COPD risk was a significant independent predictor of higher healthcare resource use in the past six months, namely a higher frequency of ER visits, hospitalisations, and healthcare provider visits, with a higher frequency of respiratory specialist visits in particular.
Relative to controls, COPD risk was a significant predictor of lower productivity, with higher levels of absenteeism, presenteeism, overall work impairment, and activity impairment.
We also investigated the association of ‘COPD Diagnosed’ status with health outcomes (see Additional file 2). Relative to controls, COPD diagnosis was a significant predictor of lower MCS and EQ-5D scores, as well as a higher frequency of respiratory specialist visits. However, no significant associations were found for other resource use variables, nor for the productivity measures or the PCS.
Given the high levels of air pollution exposure faced by many urban Chinese residents, we additionally investigated the impact of living in a high pollution area on ‘COPD Risk’ status. 3520 (17.6%) respondents lived in one of the five provinces defined as ‘high-pollution areas’. Pairwise comparisons exhibited no significant differences in COPD status versus the Control group (‘COPD Risk (undiagnosed)’ vs. Control, p = 0.192; ‘COPD Diagnosed’ vs. Control, p = 0.154). However, after controlling for covariates, a logistic regression model showed that living in a province with high AQI levels was a significant independent predictor of being at an undiagnosed risk for COPD (OR 1.203, p = 0.002). (see Additional file 3 for details).
Discussion
This study found a large population at risk of COPD. We estimated an undiagnosed COPD risk population of 105.3 million adult urban residents. While we cannot reliably project this to the overall Chinese population due to different risk factors between rural and urban areas, given that urban residents make up just slightly more than half of the Chinese population (56.1% vs. 43.9% rural), and other studies have found higher rates of COPD in rural areas, [28] there may be a similar or greater number of people at risk of COPD living in rural China.
The ‘COPD Diagnosed’ group in our study was small, with only 2.6% reporting a COPD diagnosis from a physician. This prevalence rate is lower than other studies in clinical settings that calculated prevalence rates based on spirometer testing of patients. A systematic review reported the prevalence rate of COPD in China to be 5.87% [28]. We believe the difference is due to our study being conducted among the general population and therefore relying on people having received prior clinical testing for COPD. Studies in clinical settings have typically found low levels of prior diagnosis. In the CPH study, among participants with spirometry-defined COPD only 2.6% were aware of their condition [7].
The finding is consistent with other studies indicating that many patients who were found to have COPD via spirometry had not previously been diagnosed with COPD [7]. It is also consistent with our findings that there is a lack of awareness of the condition among the general population and the population at risk of COPD. Among the diagnosed population, awareness of the term “COPD” was also low, indicating that patients know COPD-related disease by a range of different terms.
Our findings that a range of sociodemographic factors are predictive of COPD are consistent with previous research that showed education and household income were risk factors for self-reported COPD in China [29]. It may allow for targeted screening to be undertaken to identify high risk individuals. For example, the finding that significantly more at-risk people reported undertaking annual health check-ups means that health centres performing these examinations may be a fruitful place to conduct screening programmes for COPD patients.
We identified some different features between the ‘COPD Diagnosed and ‘COPD Risk (undiagnosed)’ populations. Firstly, the overlap between the two groups was low, with 299 (58.3%) of ‘COPD Diagnosed’ patients being classified as ‘not-at-risk’. This may indicate that being diagnosed with COPD facilitates disease management and avoidance of risk factors that mean that the condition is better controlled and therefore the LFQ score is lowered. Supporting this hypothesis, we found that being at risk of COPD was a significant predictor of diminished patient outcomes and increased healthcare resource use and that these signals were stronger in the risk group than in the diagnosed group.
In the absence of widespread systematic spirometry screening for COPD and its risk factors in China, there is interest in approaches to raise disease awareness and diagnosis [10]. From a humanistic standpoint, while COPD prevalence is half that of asthma, it is considerably more deadly, accounting for eight times more deaths globally [2]. From a healthcare system perspective, estimates of direct medical costs between USD72 to USD3,565 per patient per year (CNY507 to CNY25,100; USD1.00:CNY7.04) [28]. Costs incurred during an acute exacerbation are about 40–70% of overall treatment costs while the average length of an inpatient hospital stay for each acute exacerbation episode was reported to be 20.7 days, with average costs of CNY22,691 (USD3,223; USD1.00:CNY7.04) [30].
There are several reasons that identification of COPD patients has proved challenging. Firstly, awareness levels among clinicians and patients are generally low. Many local physicians are not familiar with specific symptoms of COPD, and they typically mistake COPD as bronchiolitis or pneumococcal pneumonia [30]. There are significant regional variations in disease awareness, diagnosis and outcomes. One study found a tenfold difference in prevalence of airflow obstruction between different regions due to different risk factors and high levels of misdiagnosis [27].
In rural areas, patients may be lacking basic knowledge of COPD, its risk factors and treatment options. Among 8217 patients in a rural area of China who were identified as having COPD from their medical records, 96% had not heard of COPD when they were interviewed, while almost one third did not realise that smoking was a risk factor [31]. Introducing a standardised questionnaire such as the LFQ into the healthcare system may help improve case detection and reduce rates of misdiagnosis, particularly in resource- or knowledge-poor settings.
Secondly, perverse financial incentives have driven historic over- and under-use of diagnostics and treatments. Studies have found high use of antibiotics and corticosteroids in China including a level of antibiotic prescription twice the level recommended by the World Health Organization (WHO) [32, 33]. Among the 8217 COPD patients in rural China, none used common management options such as inhalers, nebuliser drugs and oxygen therapy. Instead, 51% took theophylline to relieve dyspnoea, and 42% used antibiotics to treat exacerbations [31]. Identifying signs of COPD and intervening early may help to manage patients in a better state of health reducing the need to resort to antibiotics and corticosteroids.
This study suggests that pre-screening tools such as the LFQ may provide a quick and cost-effective approach for identifying individuals at risk of COPD for further definitive testing. In parallel with measures to improve identification of patients, a program of prevention via pneumococcal vaccination, improving and standardising treatment is needed over the longer term to reduce regional disparities in treatment patterns and outcomes, such as that undertaken by the China National Health Development Research Center (CNHDRC) to reform the COPD clinical pathway in rural areas [30].
The findings of this study such as low awareness, underdiagnosis, and particular risk factors are common in many Asian countries, therefore this study may be generalisable to other countries in the Asia region [34]. There may be other specific issues related to COPD in Asian populations such as the relatively low BMI of patients, that means COPD studies may not be generalisable more widely.
Our study has several limitations. Outcomes were self-reported and not clinically verified. As such, the findings may be limited by recall issues. Further details on COPD-specific symptomatology are contained within the NHWS, however since these were only collected from those with a self-reported diagnosis, it was not possible to use these to further characterise the population at risk of COPD. Additionally, this was a cross-sectional study, and results may thus not represent how the relationships of interest change over time and neither can causal relationships be established. While the NHWS is broadly representative of the Chinese adult urban population, its representativeness of the diagnosed and at-risk COPD subpopulations is unknown. It is possible that low estimates of COPD diagnosis may reflect a bias in the study design towards relatively younger, healthier and/or wealthier respondents, who have easier access to online studies.
Previous studies have attributed airborne PM2.5 to increased mortality from respiratory diseases including COPD [6]. The link between risk of COPD and air pollution is highly relevant to Chinese policymakers as urban Chinese residents are exposed to high levels of airborne pollutants. In this study we identified air pollution as an independent factor that contributes to COPD at-risk status. However, risk is likely to be a non-linear function including multiple sources of PM2.5 (principally air, tobacco and solid fuel), other particles such as PM10, exposure levels and historic exposure, [35, 36] therefore we would recommend further work to understand this association.
The LFQ is a well validated case detection tool for COPD. However, it was originally developed in the USA and therefore it may benefit from validation and/or adaptation to the Chinese context. For example, it may be appropriate to incorporate some risk factors that are more prevalent in China such as exposure to indoor air pollution from heating and cooking with solid fuel.
Conclusions
This study found a substantial population suspected to be at risk of COPD, but undiagnosed, who have impaired HRQoL and elevated healthcare resource use. The LFQ or an adapted version may prove a quick and cost-effective approach for identifying these at-risk individuals for further definitive testing.
Supplementary information
Additional file 1. Further details on study methodology. Additional information on study methodology including study recruitment, covariates, the Lung Function Questionnaire, and sample weighting.
Additional file 2. Health-related quality of life, healthcare resource use and productivity outcome measures as a function of ‘COPD Diagnosed’ status. Table showing logistic regression for the ‘COPD Diagnosed’ group.
Additional file 3. Supplemental analyses on the impact of provincial air pollution exposure on COPD status. Table and figure showing the results of group comparisons by air pollution exposure status and an odds ratio model where pollution exposure status was added to the list of predictors.
Acknowledgements
Permission to use the NHWS data for this study was granted by Kantar Health (New York, NY, USA). The authors would like to thank Lulu Lee, PhD, for her feedback on earlier versions of this manuscript.
Authors’ information (optional)
Not applicable
Abbreviations
- AQI
Air quality index
- BMI
Body mass index
- CCI
Charlson comorbidity index
- CNY
Chinese yuan
- COPD
Chronic obstructive pulmonary disease
- DALY
Disability-adjusted life year
- ER
Emergency room
- FEV
Forced expiratory volume
- HCP
Health care provider
- HRQoL
Health-related quality of life
- LFQ
Lung Function Questionnaire
- LMIC
Low- and middle-income countries
- NHWS
National Health and Wellness Survey
- VAS
Visual analogue scale
- WHO
World Health Organization
- WPAI
Work Productivity and Activity Impairment
Authors’ contributions
MK and TB developed the study concept with input from WG, XL, YC, DT, and GGL. YC and DT curated the dataset. MK and YC conducted the analysis with input from TB, WG, XL, DT, and GGL. MK and TB wrote the manuscript with critical input from WG, XL, YC, DT, and GGL. All authors reviewed and approved the final manuscript.
Funding
The authors received no specific funding for this study. TB was supported by a Boya fellowship from Peking University.
Availability of data and materials
The data that support the findings of this study are available from Kantar Health but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Kantar Health.
Ethics approval and consent to participate
The NHWS received Pearl Institutional Review Board (Indianapolis, IN, USA) approval, and all respondents were informed about confidentiality in the statement of informed consent. Respondents provided written consent to participate.
Consent for publication
Not applicable.
Competing interests
MK, WG, XL and GGL have nothing to disclose. YC and DT report that they are employees of Kantar Health, which is the owner of NHWS. TB is an Editorial Board member for BMC Public Health, but had no role in the editorial process.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12889-019-8071-8.
References
- 1.Rabe KF, Watz H. Chronic obstructive pulmonary disease. Lancet (London, England) 2017;389(10082):1931–1940. doi: 10.1016/S0140-6736(17)31222-9. [DOI] [PubMed] [Google Scholar]
- 2.GBD 2015 Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med. 2017;5(9):691–706. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5573769/. [DOI] [PMC free article] [PubMed]
- 3.Brakema EA, van Gemert FA, der Kleij RMJJ V, Salvi S, Puhan M, Chavannes NH, et al. COPD’s early origins in low-and-middle income countries: what are the implications of a false start? NPJ Prim Care Respir Med. 2019;29(1):6. doi: 10.1038/s41533-019-0117-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinez FD. Early-life origins of chronic obstructive pulmonary disease. N Engl J Med. 2016;375(9):871–878. doi: 10.1056/NEJMra1603287. [DOI] [PubMed] [Google Scholar]
- 5.Zhou M, Wang H, Zhu J, Chen W, Wang L, Liu S, et al. Cause-specific mortality for 240 causes in China during 1990–2013: a systematic subnational analysis for the Global Burden of Disease Study 2013. Lancet (London, England) 2016;387(10015):251–272. doi: 10.1016/S0140-6736(15)00551-6. [DOI] [PubMed] [Google Scholar]
- 6.Guan WJ, Zheng XY, Chung KF, Zhong NS. Impact of air pollution on the burden of chronic respiratory diseases in China: time for urgent action. Lancet (London, England) 2016;388(10054):1939–1951. doi: 10.1016/S0140-6736(16)31597-5. [DOI] [PubMed] [Google Scholar]
- 7.Wang C, Xu J, Yang L, Xu Y, Zhang X, Bai C, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet (London, England) 2018;391(10131):1706–1717. doi: 10.1016/S0140-6736(18)30841-9. [DOI] [PubMed] [Google Scholar]
- 8.Cooper CB, Dransfield M. Primary care of the patient with chronic obstructive pulmonary disease-part 4: understanding the clinical manifestations of a progressive disease. Am J Med. 2008;121(7 Suppl):S33–S45. doi: 10.1016/j.amjmed.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Decramer M, Cooper CB. Treatment of COPD: the sooner the better? Thorax. 2010;65(9):837. doi: 10.1136/thx.2009.133355. [DOI] [PubMed] [Google Scholar]
- 10.Xu G, Fan G, Niu W. COPD awareness and treatment in China. Lancet Respir Med. 2018;6(8):e38. doi: 10.1016/S2213-2600(18)30200-5. [DOI] [PubMed] [Google Scholar]
- 11.Shen N, He B. Is the new GOLD classification applicable in China? Lancet Glob Health. 2013;1(5):e247–e2e8. doi: 10.1016/S2214-109X(13)70064-0. [DOI] [PubMed] [Google Scholar]
- 12.Guirguis-Blake Janelle M., Senger Caitlyn A., Webber Elizabeth M., Mularski Richard A., Whitlock Evelyn P. Screening for Chronic Obstructive Pulmonary Disease. JAMA. 2016;315(13):1378. doi: 10.1001/jama.2016.2654. [DOI] [PubMed] [Google Scholar]
- 13.Omori H, Yoshimoto D, Kumar M, Goren A. Prevalence, awareness, characteristics, and health outcomes associated with COPD at-risk status among adults in Japan. Expert Rev Pharmacoecon Outcomes Res. 2016;16(4):501–512. doi: 10.1586/14737167.2016.1104250. [DOI] [PubMed] [Google Scholar]
- 14.China NBoSo. China statistical yearbook. 2016.
- 15.Yawn BP, Mapel DW, Mannino DM, Martinez FJ, Donohue JF, Hanania NA, et al. Development of the lung function questionnaire (LFQ) to identify airflow obstruction. Int J Chron Obstruct Pulmon Dis. 2010;5:1–10. [PMC free article] [PubMed] [Google Scholar]
- 16.Hanania NA, Mannino DM, Yawn BP, Mapel DW, Martinez FJ, Donohue JF, et al. Predicting risk of airflow obstruction in primary care: validation of the lung function questionnaire (LFQ) Respir Med. 2010;104(8):1160–1170. doi: 10.1016/j.rmed.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 17.Zhu S, Ma X, Tang J-L. What is the optimal body mass index for Chinese people? CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2011;183(6):645–646. doi: 10.1503/cmaj.110142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 19.de Marco R, Pesce G, Marcon A, Accordini S, Antonicelli L, Bugiani M, et al. The coexistence of asthma and chronic obstructive pulmonary disease (COPD): prevalence and risk factors in young, middle-aged and elderly people from the general population. PloS One. 2013;8(5):e62985–e6298e. doi: 10.1371/journal.pone.0062985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding B, DiBonaventura M, Karlsson N, Ling X. Asthma-chronic obstructive pulmonary disease overlap syndrome in the urban Chinese population: prevalence and disease burden using the 2010, 2012, and 2013 China National Health and wellness surveys. Int J Chron Obstruct Pulmon Dis. 2016;11:1139–1150. doi: 10.2147/COPD.S103873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ståhl E, Jansson S-A, Jonsson A-C, Svensson K, Lundbäck B, Andersson F. Health-related quality of life, utility, and productivity outcomes instruments: ease of completion by subjects with COPD. Health Qual Life Outcomes. 2003;1(1):18. doi: 10.1186/1477-7525-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ware J, Jr, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Lam CL, Tse EY, Gandek B. Is the standard SF-12 health survey valid and equivalent for a Chinese population? Qual life Res : an international journal of quality of life aspects of treatment, care and rehabilitation. 2005;14(2):539–547. doi: 10.1007/s11136-004-0704-3. [DOI] [PubMed] [Google Scholar]
- 24.EuroQol Group. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. https://www.ncbi.nlm.nih.gov/pubmed/10109801. [DOI] [PubMed]
- 25.Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: EQ-5D and SF-6D. Qual life Res : an international journal of quality of life aspects of treatment, care and rehabilitation. 2005;14(6):1523–1532. doi: 10.1007/s11136-004-7713-0. [DOI] [PubMed] [Google Scholar]
- 26.Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. PharmacoEconomics. 1993;4(5):353–365. doi: 10.2165/00019053-199304050-00006. [DOI] [PubMed] [Google Scholar]
- 27.Kurmi OP, Li L, Smith M, Augustyn M, Chen J, Collins R, et al. Regional variations in the prevalence and misdiagnosis of air flow obstruction in China: baseline results from a prospective cohort of the China Kadoorie biobank (CKB) BMJ Open Respiratory Res. 2014;1(1):e000025. doi: 10.1136/bmjresp-2014-000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu B, Wang Y, Ming J, Chen W, Zhang L. Disease burden of COPD in China: a systematic review. Int J Chron Obstruct Pulmon Dis. 2018;13:1353–1364. doi: 10.2147/COPD.S161555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yin P, Zhang M, Li Y, Jiang Y, Zhao W. Prevalence of COPD and its association with socioeconomic status in China: findings from China chronic disease risk factor surveillance 2007. BMC Public Health. 2011;11:586. doi: 10.1186/1471-2458-11-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.China National Health Development Research Center. Strengthening evidence-based policy making in support of universal healthcare: Introducing evidence-based clinical pathways for stroke and COPD in rural China [cited 08/06/2019]; Available from: https://www.idsihealth.org/wp-content/uploads/2016/05/Introducing-evidence-based-clinical-pathways-for-stroke-and-COPD-rural-China.pdf. Accessed 08 June 2019.
- 31.Lou P, Zhu Y, Chen P, Zhang P, Yu J, Zhang N, et al. Vulnerability, beliefs, treatments and economic burden of chronic obstructive pulmonary disease in rural areas in China: a cross-sectional study. BMC Public Health. 2012;12:287. doi: 10.1186/1471-2458-12-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y, Xu J, Wang F, Wang B, Liu L, Hou W, et al. Overprescribing in China, driven by financial incentives, results in very high use of antibiotics, injections, And Corticosteroids. Health Affairs. 2012;31(5):1075–1082. doi: 10.1377/hlthaff.2010.0965. [DOI] [PubMed] [Google Scholar]
- 33.Wushouer H, Tian Y, Guan XD, Han S, Shi LW. Trends and patterns of antibiotic consumption in China's tertiary hospitals: based on a 5 year surveillance with sales records, 2011-2015. PLoS One. 2017;12(12):e0190314. doi: 10.1371/journal.pone.0190314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rhee CK, Chau NQ, Yunus F, Matsunaga K, Perng DW. Management of COPD in Asia: A position statement of the Asian Pacific Society of Respirology. Respirology (Carlton, Vic) 2019;24(10):1018–1025. doi: 10.1111/resp.13633. [DOI] [PubMed] [Google Scholar]
- 35.Burnett RT, Pope CA, 3rd, Ezzati M, Olives C, Lim SS, Mehta S, et al. An integrated risk function for estimating the global burden of disease attributable to ambient fine particulate matter exposure. Environ Health Perspect. 2014;122(4):397–403. doi: 10.1289/ehp.1307049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu S, Zhou Y, Liu S, Chen X, Zou W, Zhao D, et al. Association between exposure to ambient particulate matter and chronic obstructive pulmonary disease: results from a cross-sectional study in China. Thorax. 2017;72(9):788–795. doi: 10.1136/thoraxjnl-2016-208910. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Further details on study methodology. Additional information on study methodology including study recruitment, covariates, the Lung Function Questionnaire, and sample weighting.
Additional file 2. Health-related quality of life, healthcare resource use and productivity outcome measures as a function of ‘COPD Diagnosed’ status. Table showing logistic regression for the ‘COPD Diagnosed’ group.
Additional file 3. Supplemental analyses on the impact of provincial air pollution exposure on COPD status. Table and figure showing the results of group comparisons by air pollution exposure status and an odds ratio model where pollution exposure status was added to the list of predictors.
Data Availability Statement
The data that support the findings of this study are available from Kantar Health but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Kantar Health.