Abstract
Background
Open defecation is widespread in rural India, and few households have piped water connections. While government and other efforts have increased toilet coverage in India, and evaluations found limited immediate impacts on health, longer-term effects have not been rigorously assessed.
Methods
We conducted a matched cohort study to assess the longer-term effectiveness of a combined household-level piped water and sanitation intervention implemented by Gram Vikas (an Indian NGO) in rural Odisha, India. Forty-five intervention villages were randomly selected from a list of those where implementation was previously completed at least 5 years before, and matched to 45 control villages. We conducted surveys and collected stool samples between June 2015 and October 2016 in households with a child <5 years of age (n = 2398). Health surveillance included diarrhoea (primary outcome), acute respiratory infection (ARI), soil-transmitted helminth infection, and anthropometry.
Results
Intervention villages had higher improved toilet coverage (85% vs 18%), and increased toilet use by adults (74% vs 13%) and child faeces disposal (35% vs 6%) compared with control villages. There was no intervention association with diarrhoea [adjusted OR (aOR): 0.94, 95% confidence interval (CI): 0.74–1.20] or ARI. Compared with controls, children in intervention villages had lower helminth infection (aOR: 0.44, 95% CI: 0.18, 1.00) and improved height-for-age z scores (HAZ) (+0.17, 95% CI: 0.03–0.31).
Conclusions
This combined intervention, where household water connections were contingent on community-wide household toilet construction, was associated with improved HAZ, and reduced soil-transmitted helminth (STH) infection, though not reduced diarrhoea or ARI. Further research should explore the mechanism through which these heterogenous effects on health may occur.
Keywords: Sanitation, on-premise piped water, diarrhoea, stunting, soil-transmitted helminth infection
Key Messages
An intervention where on-premise piped water coverage was contingent on full community sanitation coverage was associated with improvements in infrastructure coverage and use several years after implementation.
Although there was no evidence the intervention impacted acute conditions such as diarrhoeal disease or respiratory infection, it was associated with a reduction in soil-transmitted helminth infection and improvements in height-for-age that may require longer-term reductions in faecal exposure.
The matched-cohort study design and the time lag between intervention implementation and evaluation allowed for assessment of longer-term effects, including time for children to be born into the potentially less contaminated environment and benefit from birth.
Introduction
Globally, over 2.4 billion people lack access to improved sanitation, and almost one billion people practice open defecation—over half of whom reside in India.1 Efforts to address these massive sanitation shortfalls have primarily focused on construction of pour-flush toilets for selected households within communities. The government of India has implemented a succession of large-scale sanitation campaigns across the country.2 With a focus on reducing open defecation, however, these efforts emphasized toilet construction at the possible expense of sustained coverage and use.3 Health evaluations of these programmes have shown limited impact, possibly due to sub-optimal increases in community-level sanitation coverage and use.2,4,5
The primary purpose of establishing safe water and improved sanitation is to limit exposure to enteric pathogens associated with a range of poor health outcomes, including diarrhoeal diseases and soil-transmitted helminth (STH) infection.6–9 Improved access to water can also increase the quantity available for personal hygiene, which is associated with reduced risk of respiratory infections.10,11 Poor nutritional outcomes are also linked with enteric pathogen exposure, with both underweight and stunting associated with poor household and community-level sanitation.12–14 In India, almost half of children <5 years of age are stunted or severely stunted.15
Coverage of improved community water sources is relatively high in rural India, but may not be sufficient for flushing or post-defecation cleansing.1 While combined water and sanitation interventions have shown limited additive benefits, provision of household piped water, in addition to sanitation, may prove important in increasing use of pour-flush toilets as well as improving water quality for drinking.16,17 However, research on the effects of piped water access on the household premises in combination with sanitation in a rural low-resource context is lacking.
Our objective was to assess the effectiveness of a community-level combined household piped water and sanitation intervention in Odisha, India at least 5 years after intervention completion.
Methods
The intervention
The MANTRA program (Movement and Action Network for the Transformation of Rural Areas) was developed by Gram Vikas an Indian non-governmental organization (NGO).18 It consists of: (i) a household pour-flush toilet with dual soak-away pits, (ii) an attached bathing room, and (iii) household piped water connections in the toilet, bathing room, and kitchen.18 Importantly, for a village to be eligible for participation, every household must commit to constructing their own toilet and bathing room, in addition to other NGO requirements. Gram Vikas assists with the development of a piped water system, which is connected once every household has completed toilet construction. The village is responsible for ongoing costs of operation and maintenance.
Study design and participants
We used a matched cohort design to assess the longer-term impacts of this previously completed intervention.19 We randomly selected 45 villages from a list provided by Gram Vikas of villages with completed interventions in Ganjam and Gajapati districts, Odisha, India, after restriction to those with an intervention start date of 2003–2006. The intervention takes an average of 3 years and the last study village completed implementation in 2010. Forty-five control villages were matched to the 45 intervention villages through a multi-step restriction, matching, and exclusion process to reduce potential bias due to baseline differences.18,20 We used an iterative multivariate matching scheme (R Matching package, version 4.9–2) to match villages on pre-intervention characteristics from the Government of India Census 2001 and Below Poverty Line Survey 2002; balance was achieved on all variables.18
Using Monte Carlo simulation to estimate the log odds of child diarrhoeal disease (the primary outcome) we determined a sample size of 45 villages per study arm and 26 children per village, assuming 8.8% diarrhoea prevalence, 0.20 effect size, 80% power, 0.05 significance level and 10% loss to follow-up, as previously reported.18
Households with a child <5 years of age at any time during surveillance were eligible for enrollment, and no children aged out of the cohort. In each village, we enrolled up to 40 eligible households, and if more were eligible, we systematically randomly selected 40 across the village. The male and/or female household head provided written informed consent for the household.
Procedures and outcome measures
Field workers collected data in four rounds approximately every 4 months from June 2015 to October 2016, with household surveys administered to the primary caregiver in the Odia language. For each of the following, each household member reported his own disease status over the previous 7 days, with the caregiver reporting disease for children.21 Diarrhoeal disease was defined as at least one occasion of three or more loose stools in the previous 24 h.19 Acute respiratory infection (ARI) was defined as cough and/or shortness of breath/difficulty breathing due to chest congestion.24 Both diarrhoeal disease and ARI details were collected every study round. Prevalence of bruising or scrapes (combined) was collected in round 3 (February–June 2016) as a negative control to allow qualitative assessment of differential reporting bias for self-reported outcomes.25
We used direct observation to assess water, santitation and hygiene (WaSH) infrastructure characteristics. We defined improved sanitation, improved water sources, and presence of a handwashing station (a designated location with water and a cleansing agent present), according to Joint Monitoring Programme standard definitions.1 We collected reported interruptions in the preferred drinking water source as: 1) source unavailable for ≥24 h in the previous two weeks, and 2) source unavailable at any time in the previous 24 h. The first measure was collected in all rounds, and the second starting round 2. Interruption in water source was categorized as any interruption, using either measure, across all rounds. Usual defecation location was self-reported for the following categories within each household: elders ≥60 years, men 18–59 years, women 18–59 years, and children 5–17 years. For children <5 years old, the caregiver reported the disposal location for the last defecation event, and improved child faeces disposal was defined as disposal into an improved toilet. We calculated household sanitation use as the proportion of household members each round who reported improved toilet use for defecation (members >5 years old) or for child faeces disposal (members <5 years old), out of the total number of members within each household.
We collected anthropometric measurements for children <5 years old during round 3 (February–June 2016), according to WHO standard methods.26,27 Field workers measured recumbent length for children <2 years old, standing height for children 2–5 years old, and weight for children <5 years old. Height/length were collected in duplicate, and if measurements differed by more than 0.7 cm, a third was collected; the mean of measurements was used to calculate z-scores according to WHO 2006 growth standards (R igrowup macro).28 Back-checks on height/length were conducted on a randomly selected 10% of households.
Field workers collected stool samples in round 2 (October 2015–January 2016) from all household members in a randomly selected subset of 500 households to assess the prevalence of common STHs. We used formol ether concentration to quantify worms and ova for hookworms (Ancylostoma duodenale and Necator americanus), Ascaris lumbricoides, Hymenolepis nana, and Tricuris trichura.29,30 Three slides were examined per sample, with all positives and 10% of negatives examined in duplicate. The mean of measurements was used to estimate eggs per gram of faeces and to quantify worm burden.29
Statistical analysis
We used multilevel logistic regression to estimate intervention association with prevalence of diarrhoeal disease, ARI, bruising/scrapes, and STH infection, and multilevel linear regression to estimate association with height-for-age z score (HAZ), weight-for-age z score (WAZ), and weight-for-height z score (WHZ). Health outcomes measured across all four study rounds, and assessed for all household members during a single round, included random effects for village and household levels to account for repeated measures, and outcomes measured during a single round included a random effect for village level. Profile likelihood confidence intervals (CIs) were estimated to limit potential bias from assumptions of asymptotic normality.
Patterns of missing household-level covariate data were similar across study arms and were handled with multi-level multiple imputation (R pan, version 1.4, and mitml, version 0.3–4, packages).31,32 There was little missing individual-level covariate data; therefore, imputation was restricted to household-level covariates. The imputation model was run for 20 iterations, included all household-level covariates included in regression models, and was adjusted for clustering at the village level. Imputations were used in all subsequent analyses.33
We used principal components analysis (R psych package, version 1.6.12) to construct a household wealth index from 15 variables, including household asset ownership, housing characteristics, agricultural land acreage, and below poverty-line status.34,35 We extracted the component that explained the most variability as the wealth index.36
Adjusted models were fit with an a priori determined set of covariates to adjust for potential confounding, including the individual’s age and sex, household wealth, religion, caste/tribe status, head of household’s education, primary caregiver’s education, and village access-road quality. Outcomes measured across multiple rounds also included the month of data collection. As sensitivity checks, all regressions were repeated including the village matched pair as a random effect, and all regressions were repeated using the original, unimputed data (see Supplementary Material and Supplementary Table S1 for additional details, available as Supplementary data at IJE online). All analyses were completed in R (version 3.3.2).
Deviations from the study protocol
Outcomes and methods were prespecified, with the following exceptions.18 Undernutrition was assessed in children <2 years old in addition to the targeted children <5 years old, to allow comparison with similar studies. Although we intended to assess STH reinfection by collecting a follow-up sample in round 4, this was dropped due to the low stool collection rate in round 2 (75% after two visits) and low STH prevalence.
Results
Characteristics of the study population
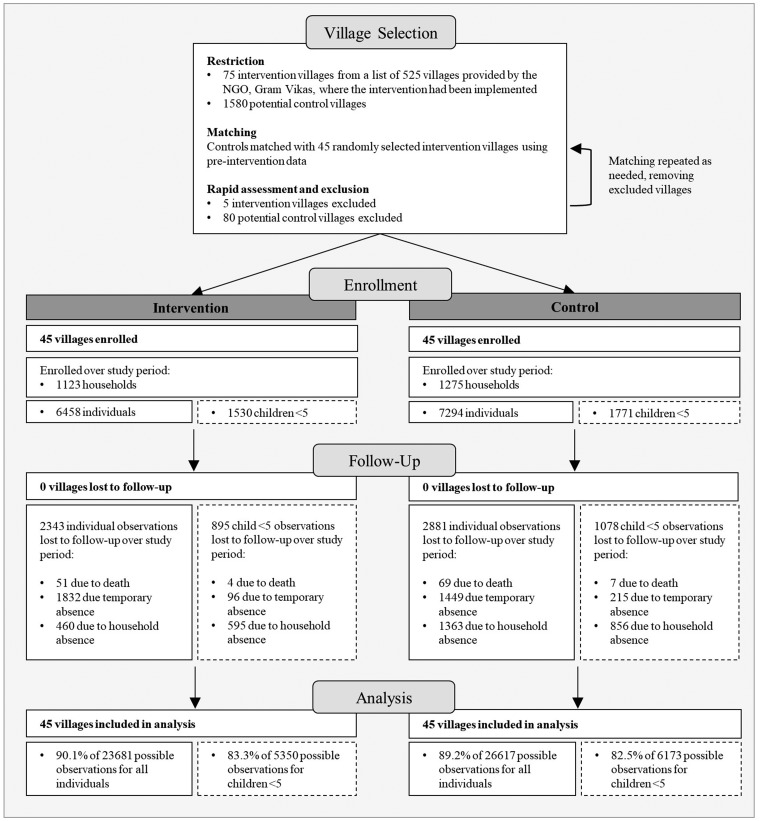
A total of 1123 households in the intervention villages, and 1275 households in the control villages were enrolled over the four study rounds (Figure 1). An average of 26.5 (range: 2–67) child observations per village per round were available and included in analyses. At follow-up, sociodemographic characteristics were generally similar across study arms, though intervention households were less poor (Table 1).
Figure 1.
Village selection and profile of the study population across four rounds of data collection. The total number of individuals included at each stage of enrollment, follow-up and analysis is on the left in the intervention and control columns. The subset of the total population that is <5 years old is on the right in dashed boxes.
Table 1.
Sociodemographic characteristics of the study population, included as covariates in adjusted models
| Control% (n) | Intervention% (n) | P-value | |
|---|---|---|---|
| Village characteristics | n=45 | n=45 | |
| Village size (households), (sd)b | 157.3 (135.0) | 124.0 (92.5) | 0.176 |
| Access road paved | 91.1% (45) | 88.9% (45) | 0.726 |
| Household characteristics | n=1275 | n=1123 | |
| Caregiver education ≥5 years | 48.0% (612) | 57.0% (640) | 0.102 |
| Head of household education ≥5 years | 38.0% (485) | 42.3% (475) | 0.203 |
| Caste/tribe | 0.147 | ||
| Scheduled caste | 23.7% (255) | 13.6% (133) | |
| Scheduled tribe | 15.0% (161) | 12.2% (120) | |
| Other backward caste | 39.7% (426) | 41.5% (407) | |
| Other caste | 21.6% (232) | 32.7% (321) | |
| Religion | 0.632 | ||
| Hindu | 98.8% (1035) | 96.7% (902) | |
| Christian | 1.2% (13) | 2.8% (26) | |
| Other | 0% (0) | 0.5% (5) | |
| Standardized wealth index, (sd) | 0.8 (0.46) | 1.0 (0.46) | 0.026 |
| Wealth quintilea,b | 0.015 | ||
| Poorest | 25.3% (233) | 14.9% (125) | |
| Poor | 20.3% (187) | 19.2% (162) | |
| Middle | 20.6% (190) | 19.4% (163) | |
| Rich | 18.0% (166) | 22.5% (189) | |
| Richest | 15.8% (146) | 24.1% (203) | |
| Individual characteristics | n=7395 all ages n=1797 children <5 years | n=6357 all ages n=1502 children <5 years | |
| Sex, female (all ages) | 52.3% (3802) | 52.0% (3345) | 0.719 |
| Sex, female (children <5 years) | 49.0% (860) | 49.2% (748) | 0.887 |
| Age, years (all ages) (sd) | 24.2 (20.43) | 25.0 (20.60) | 0.082 |
| Age, months (children <5) (sd) | 28.5 (17.72) | 29.4 (17.68) | 0.218 |
Wald P-values are adjusted for clustering at village level for household characteristics, and at village and household levels for individual characteristics. sd = standard deviation.
Wealth quintile captures the proportion of households in each quintile of the standardized wealth index.
Not included as a covariate in adjusted models. Provided here for descriptive purposes.
Coverage, access and use of water, hygiene and sanitation facilities
Access to a household improved toilet was almost five times higher in intervention than control villages (85.0% vs 17.7%; Table 2). Coverage of household piped water for both drinking and other purposes, including cooking, hygiene, and toilet flushing, as well as presence of a functional hand-washing station was substantially higher in the intervention than control arm. The intervention was positively associated with minor improvements in round-trip time to water source, though with higher prevalence of water intermittency, likely due to greater reliance on the piped system in the intervention arm. The proportion of household members using improved sanitation for defecation was also substantially higher in intervention than control villages (59.3% vs 12.9% of members), with almost all remaining members reporting open defecation (Table 2).
Table 2.
Household water, sanitation and hygiene coverage, access and use characteristics across all study rounds, unless otherwise noted
| n | Control% (n) | Intervention% (n) | P-value | |
|---|---|---|---|---|
| Water, sanitation and hygiene coverage | ||||
| Improved toileta | 2105 | 17.7% (198) | 85.0% (837) | <0.001 |
| Toilet with soak-away/septic tank | 2105 | 17.3% (194) | 78.4% (772) | <0.001 |
| Improved drinking water sourcea | 2388 | 72.0% (913) | 92.1% (1031) | <0.001 |
| Household piped watera | 2388 | 8.0% (102) | 72.7% (813) | <0.001 |
| Improved water source for other purposesa | 2110 | 62.9% (707) | 90.1% (888) | <0.001 |
| Household piped watera | 2110 | 8.3% (93) | 73.3% (723) | <0.001 |
| Hand-washing station | 6048 | 61.7% (1934) | 85.3% (2487) | <0.001 |
| Water available | 7529 | 61.5% (2409) | 83.1% (2998) | <0.001 |
| Soap/detergent available | 7528 | 25.1% (982) | 48.9% (1764) | <0.001 |
| Ash/sand available | 7528 | 37.3% (1463) | 27.2% (981) | <0.001 |
| Bathing room | 1902 | 12.1% (121) | 82.1% (739) | <0.001 |
| Water access | ||||
| Interruption in water availability, any | 7807 | 7.1% (291) | 16.5% (609) | <0.001 |
| Anytime in previous 24 hb | 7806 | 4.3% (177) | 9.5% (353) | <0.001 |
| ≥24 h in previous two weeks | 3888 | 6.4% (198) | 15.2% (421) | <0.001 |
| Time to water source (min), (sd) | 5766 | 10.2 (11.5) | 3.5 (6.7) | <0.001 |
| Water storage, any | 7805 | 99.5% (4099) | 97.7% (3601) | <0.001 |
| Water storage, safe | 7786 | 20.6% (849) | 22.6% (831) | <0.001 |
| Narrow-mouthed container (<6 cm) | 7681 | 24.7% (1009) | 26.0% (913) | <0.001 |
| Covered container | 7682 | 83.0% (3398) | 86.2% (3094) | <0.001 |
| Improved sanitation use | ||||
| Proportion of household using, all ages, (sd) | 5890 | 12.9% (28.8%) | 59.3% (36.0%) | <0.001 |
| Toilet use, ≥60 years | 3023 | 17.8% (279) | 76.2% (1107) | <0.001 |
| Toilet use, men 18–59 years | 5395 | 15.0% (428) | 74.5% (1900) | <0.001 |
| Toilet use, women 18–59 years | 5833 | 18.2% (561) | 79.5% (2182) | <0.001 |
| Toilet use, 5–17 years | 3904 | 16.8% (351) | 76.4% (1387) | <0.001 |
| Child faeces disposal, <5 years | 5367 | 8.8% (250) | 39.2% (989) | <0.001 |
P-values are adjusted for clustering at village level. sd = standard deviation.
Reported once for each household.
Data available rounds 2–4.
Health outcomes
Prevalence of 7-day diarrhoea in children <5 years old, the primary study outcome, and 7-day prevalence of ARI were similar across intervention and control villages (5.3 vs 4.9%, and 9.3 vs 10.3%; Table 3). Prevalence of any STH infection among children was almost twice as high in control villages as in intervention (6.8 vs 3.9%; Table 3). No A. lumbricoides and few T. trichiura infections were found in either study arm; the helminth burden was primarily due to infection with hookworms or H. nana. A smaller proportion of children <5 years old were stunted (33.3 vs 40.4%), wasted (10.3 vs 12.3%) or underweight (26.5 vs 34.8%) in intervention villages compared with control (Table 3).
Table 3.
Prevalence of health outcomes in children <2 years old, children <5 years old, and all household members. Prevalence across all study rounds is shown for self-reported health, prevalence at round 2 (Oct 2015–Jan 2016) is shown for STH infection, and prevalence at round 3 (Feb–June 2016) is shown for nutrition and control outcomes
| n | Control % (n) | Intervention% (n) | P-value | |
|---|---|---|---|---|
| Children <5 years old | ||||
| Self-reported health | ||||
| Diarrhoea | 8875 | 5.3% (251) | 4.9% (199) | 0.557 |
| Acute respiratory infection | 8964 | 9.3% (127) | 10.3% (122) | 0.959 |
| Soil-transmitted helminth infection | ||||
| Any STH prevalence | 775 | 6.8% (28) | 3.9% (14) | 0.044 |
| Ascaris lumbricoides prevalence | 775 | 0.0% (0) | 0.0% (0) | 1.000 |
| Trichuris trichiura prevalence | 775 | 0.0% (0) | 0.0% (0) | 1.000 |
| Hymenolepis nana prevalence | 775 | 1.5% (6) | 1.1% (4) | 0.659 |
| Hymenolepis nana intensity (epg), (sd) | 775 | 2.4 (13.72) | 1.1 (9.32) | 0.270 |
| Hookworm prevalence | 775 | 5.3% (22) | 2.8% (10) | 0.095 |
| Hookworm intensity (epg), (sd) | 775 | 1.8 (24.04) | 0.4 (3.62) | 0.115 |
| Nutrition outcomes | ||||
| HAZ, (sd) | 1826 | −1.77 (1.12) | −1.48 (1.17) | <0.001 |
| Stunted (HAZ<-2) | 1826 | 40.4% (402) | 33.3% (277) | 0.063 |
| Severely stunted (HAZ<-3) | 1826 | 14.0% (139) | 7.9% (66) | 0.356 |
| WAZ, (sd) | 1893 | −1.61(1.08) | −1.36 (1.11) | 0.019 |
| Underweight (WAZ<-2) | 1893 | 34.8% (362) | 26.5% (226) | 0.030 |
| Severely underweight (WAZ<-3) | 1893 | 9.8% (102) | 6.2% (53) | 0.602 |
| WHZ, (sd) | 1829 | −0.85 (1.03) | −0.75 (1.06) | 0.146 |
| Wasted (WHZ<-2) | 1829 | 12.3% (123) | 10.3% (86) | 0.808 |
| Severely wasted (WHZ<-3) | 1829 | 1.5% (15) | 1.0% (8) | 0.303 |
| Control | ||||
| Bruising/scrapes | 2172 | 3.8% (45) | 3.5% (35) | 0.738 |
| Children <2 years old | ||||
| Nutrition outcomes | ||||
| HAZ, (sd) | 655 | −1.67 (1.20) | −1.35 (1.33) | 0.013 |
| Stunted (HAZ<-2) | 655 | 38.0% (136) | 30.0% (89) | 0.070 |
| Severely stunted (HAZ<-3) | 655 | 15.1% (54) | 9.1% (27) | 0.311 |
| WAZ, (sd) | 685 | −1.49 (1.11) | −1.21(1.22) | 0.038 |
| Underweight (WAZ<-2) | 685 | 30.3% (115) | 21.6% (66) | 0.054 |
| Severely underweight (WAZ<-3) | 685 | 10.3% (39) | 5.9% (18) | 0.384 |
| WHZ, (sd) | 659 | −0.76 (1.09) | −0.67 (1.05) | 0.244 |
| Wasted (WHZ<-2) | 659 | 12.2% (44) | 8.4% (25) | 0.413 |
| Severely wasted (WHZ<-3) | 659 | 1.7% (6) | 0.7% (2) | 0.130 |
| All household members | ||||
| Self-reported health | ||||
| Diarrhoea | 40436 | 2.8% (593) | 2.4% (485) | 0.092 |
| Acute respiratory infection | 40999 | 4.3% (254) | 6.6% (241) | 0.678 |
| Soil-transmitted helminth infection | ||||
| Any STH prevalence | 1452 | 11.5% (86) | 8.6% (61) | 0.273 |
| Ascaris lumbricoides prevalence | 1452 | 0.0% (0) | 0.0% (0) | 1.000 |
| Trichuris trichiura prevalence | 1452 | 0.0% (0) | 0.0% (1) | 0.997 |
| Trichuris trichiura intensity (epg), (sd) | 1452 | 0.0 (0) | 0.0 (0.1) | 0.318 |
| Hymenolepis nana prevalence | 1452 | 1.9% (14) | 1.6% (11) | 0.714 |
| Hymenolepis nana intensity (epg), (sd) | 1452 | 3.8 (66.2) | 0.78 (9.7) | 0.238 |
| Hookworm prevalence | 1452 | 9.7% (72) | 7.2% (51) | 0.366 |
| Hookworm intensity (epg), (sd) | 1452 | 5.8 (24.2) | 3.7 (18.4) | 0.333 |
| Control | ||||
| Bruising/scrapes | 10091 | 1.7% (93) | 1.5% (70) | 0.276 |
P-values adjusted for clustering at village and household levels. sd = standard deviation.
There was no intervention association with 7-day diarrhoea prevalence for children <5 years old (adjusted OR (aOR): 0.98, 95% CI: 0.77, 1.25) or with 7-day ARI prevalence (aOR: 1.03, 95% CI: 0.84–1.25; Table 4). There was also no intervention effect on prevalence of bruising/scrapes, collected as a negative control for self-reported health outcomes. However, there was evidence that the intervention had a protective effect on infection with any STH in children (aOR: 0.44, 95% CI: 0.18, 1.00); though not in all household members (Table 4). The intervention was positively associated with increased HAZ in children <5 years old (+0.17 HAZ, 95% CI: 0.03, 0.31) (Table 4). The association between the intervention and HAZ in children <2 years old was similar in magnitude to that in children <5 years old, but was not as strong. This may be due to not being sufficiently powered to detect an effect in the child <2 years age group. There was no intervention association with either WAZ or WHZ (Table 4).
Table 4.
Effect of the intervention on health in children <2 years old, children <5 years old, and all household members
| Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|
| n | OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Children under 5 years | |||||
| Self-reported health | |||||
| Diarrhoea | 8875 | 0.93 (0.73, 1.18) | 0.557 | 0.98 (0.77, 1.25) | 0.855 |
| Acute respiratory infection | 8964 | 1.00 (0.84, 1.18) | 0.959 | 1.03 (0.84, 1.25) | 0.363 |
| Soil-transmitted helminth infection | |||||
| STH infection, anyb | 777 | 0.49 (0.20, 1.08) | 0.077 | 0.44 (0.18, 1.00) | 0.049 |
| Nutrition outcomes | |||||
| Height-for-age z scorea | 1826 | 0.26 (0.06, 0.46) | 0.011 | 0.17 (0.03, 0.31) | 0.015 |
| Weight-for-age z scorea | 1893 | 0.22 (0.01, 0.42) | 0.038 | 0.13 (-0.01, 0.27) | 0.068 |
| Weight-for-height z scorea | 1829 | 0.08 (-0.07, 0.24) | 0.288 | 0.04 (-0.09, 0.16) | 0.587 |
| Control | |||||
| Bruising/scrapes | 2172 | 0.93 (0.59, 1.45) | 0.737 | 0.88 (0.55, 1.41) | 0.601 |
| Children <2 years | |||||
| Nutrition outcomes | |||||
| Height-for-age z scorea | 655 | 0.31 (0.04, 0.57) | 0.026 | 0.17 (-0.04, 0.38) | 0.110 |
| Weight-for-age z scorea | 685 | 0.23 (-0.03, 0.49) | 0.077 | 0.08 (-0.11, 0.28) | 0.390 |
| Weight-for-height z scorea | 659 | 0.07 (-0.13, 0.27) | 0.481 | 0.00 (-0.17, 0.18) | 0.958 |
| All household members | |||||
| Self-reported health | |||||
| Diarrhoea | 40409 | 0.85 (0.72, 1.01) | 0.063 | 0.86 (0.74, 1.03) | 0.122 |
| Acute respiratory infection | 40999 | 1.03 (0.90, 1.18) | 0.688 | 1.08 (0.94, 1.24) | 0.288 |
| Soil-transmitted helminth infection | |||||
| STH infection | 1452 | 0.69 (0.40, 1.16) | 0.161 | 0.72 (0.42, 1.19) | 0.192 |
| Control | |||||
| Bruising/scrapes | 10091 | 0.89 (0.42, 1.88) | 0.764 | 0.86 (0.41, 1.39) | 0.660 |
Marginal effect, not odds ratio.
Household religion excluded from adjusted model due to lack of variability.
Discussion
To our knowledge, this study is the first evaluating the effectiveness of a combined on-premise water and sanitation intervention in rural India, and the first to assess the longer-term impacts of such an intervention. In contrast to interventions that involve only community water supplies and/or partial community sanitation coverage, the Gram Vikas MANTRA intervention was designed to provide piped water at each home and ensure every household had an improved toilet and bathing room. However, there was no evidence the intervention was protective against diarrhoeal disease, the primary study outcome, or ARI, despite increases in water and hand-washing station coverage. In contrast, our findings suggest the intervention was protective against child STH infection as well as effective in improving HAZ in children <5 years old.
The lack of a protective effect on diarrhoea is consistent with previous evaluations of sanitation interventions in India.4,5,37 Despite sanitation and hygiene deficiencies, diarrhoea prevalence is comparatively low, providing limited opportunity for improvements. The lack of an association with ARI may be due to continued insults from indoor and ambient air pollution not impacted by this intervention. The protective effect on STH infection is in contrast with previous studies in India, where community sanitation coverage and use was not as high, but consistent with overall evidence on sanitation impacts.38,39
The protective effect of the intervention on HAZ is noteworthy given the high levels of stunting in India and the hypothesis that this may be attributable to environmental enteric dysfunction.40 The observed effect was similar in magnitude to that in a previous study with similarly large reductions in reported open defecation within a community-level approach.13 However, unlike in this previous study, there was a similar magnitude effect in both children <2 years old and all children <5 years old.13 Since our study began years after intervention completion, there was the opportunity for children to be born into potentially less fecally contaminated environments, benefit from the intervention from birth, and thus have sustained nutritional benefits past the key developmental window of 6–24 months. Other recent trials have reported no effect on linear growth from combined WaSH interventions, though these were not community-wide interventions and were implemented in settings where open defecation was uncommon.41,42
Notwithstanding these heterogenous effects on health, there were substantial gains in WaSH coverage, access, and use. In these respects, the intervention was effective in accomplishing the target outputs of many WaSH initiatives. However, intermittent availability of preferred water sources and subsequent high levels of drinking water storage provided a possible source of continued exposure to enteric pathogens. The increase in household piped water coverage may have indirectly impacted child health through increasing toilet use, instead of expected direct impacts if the piped system provided microbiologically high-quality water.
Our study design and methods presented certain limitations. First, as we were interested in assessing longer-term effects, we employed a study design in which the intervention status was not randomly assigned. Although study arms were well balanced at the village-level after matching on available pre-intervention characteristics, we cannot rule out imbalance on unobserved variables and the potential for residual confounding. The intervention involves the commitment and active participation of the entire village, attributes that are difficult to measure, especially retrospectively, and thus balance. In addition, pre-intervention disease prevalence was not available for matching. To limit bias, a set of a priori determined potential confounders were included in all models. The time lapse between intervention completion and study initiation necessitated matching villages on pre-intervention characteristics measured several years prior to the evaluation process, and prevented assessment of immediate impacts. On the other hand, the retrospective design allowed us to assess longer-term impacts, a challenge for experimental designs with limited funding and follow-up. In addition to its policy relevance, this longer-term assessment also provided a biologically plausible length of time for die-off of even the most persistent pathogens in the environment, and time for the target population, children <5 years old, to be born into this environment. Another limitation is that diarrhoeal disease and ARI were collected using self- and caregiver reports—a method that may be subject to measurement bias.43,44 However, we found no effect on our negative control outcome, indicating any potential measurement bias for self-reported health was not differential by study arm. Moreover, we found no protective intervention effects on these reported outcomes, diarrheal disease and ARI. In contrast, we found protective effects on STH infection and anthropometrics, outcomes that were objectively assessed and therefore not susceptible to reporting bias.
Finally, there are limitations to generalizability. Although intervention study villages were randomly selected from those where the implementation was complete, and so results should be representative of those on the list, we understand from Gram Vikas that there are villages that received a motivation visit but declined participation. While we excluded these from the list of potential controls, non-participating villages may be different from participating villages in their awareness of health risks, collective efficacy, or other characteristics. Thus, it should not be assumed that the MANTRA intervention can be successfully implemented across all villages in this setting or elsewhere. Future planned analysis of collective efficacy may shed light on its contribution to programme implementation and effectiveness.
In conclusion, this study provides evidence that a combined intervention, where provision of household piped water connections is contingent on community sanitation coverage, can substantially decrease open defecation. Although we found no evidence these reduced child diarrhoea or ARI, our results suggest a protective effect against STH infection and HAZ. Future planned analyses, including assessment of fecal environmental contamination, environmental enteric dysfunction, and collective efficacy, may provide a fuller understanding of both the biological and behavioural mechanisms for these heterogeneous effects on child health. Given previous evidence that increasing sanitation use, even with high coverage, is especially difficult in rural India, this study provides evidence to support a combined community-level implementation of household piped water and sanitation.4,5,45
Funding
This work was supported by Bill & Melinda Gates Foundation [grant numbers OPP1008048 and OOP1125067].
Supplementary Material
Acknowledgements
We greatly appreciate the cooperation of Gram Vikas and the study communities, and would also like to thank our field team. The study protocol was approved by the London School of Hygiene and Tropical Medicine, London, U.K (No. 9071) and the Kalinga Institute of Medical Sciences of KIIT University, Bhubaneswar, India (KIMS/KIIT/IEC/053/2015) ethics committees. Anonymized data were provided to Emory University, Atlanta, U.S. under a data transfer agreement and analysis was approved by the Emory University IRB (IRB00079717). This study was registered at ClinicalTrials.gov (NCT02441699).
Author Contributions
T.C., H.H.C. and H.R. designed the study, with contributions from S.S.S. H.R. and S.S.S. developed data collection tools. H.R., S.S.S., B.T. and P.R. were involved in training. H.R. and P.R. oversaw field work. H.R. analysed the data and drafted the manuscript. All authors contributed to editing and revising the manuscript.
Conflict of interest: none declared.
References
- 1.UNICEF and WHO. Progress and Sanitation and Drinking Water: 2015 Update and MDG Assessment Geneva, 2015. https://www.unicef.org/publications/index_82419.html.
- 2.Water and Sanitation Program. A Decade of the Total Sanitation Campaign: Rapid Assessment of Processes and Outcomes Vol. 1, 2010. https://openknowledge.worldbank.org/handle/10986/17287.
- 3. Garn JV, Sclar GD, Freeman MC. et al. The impact of sanitation interventions on latrine coverage and latrine use: a systematic review and meta-analysis. Int J Hyg Environ Health 2017;220:329–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Patil SR, Arnold BF, Salvatore AL. et al. The effect of India’s total sanitation campaign on defecation behaviors and child health in rural Madhya Pradesh: a cluster randomized controlled trial. PLoS Med 2014;11:e1001709.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clasen T, Boisson S, Routray P. et al. Effectiveness of a rural sanitation programme on diarrhoea, soil-transmitted helminth infection, and child malnutrition in Odisha, India: a cluster-randomised trial. Lancet Glob Heal 2014;2:e645–653. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Soil-Transmitted Helminthiasis. Number of Children (Pre-SAC and SAC) Requiring Preventative Chemotherapy for Soil-Transmitted Helminthiases, 2015. http://apps.who.int/neglected_diseases/ntddata/sth/sth.html (20 April 2017, date last accessed).
- 7. Ziegelbauer K, Speich B, Mäusezahl D, Bos R, Keiser J, Utzinger J.. Effect of sanitation on soil-transmitted helminth infection: systematic review and meta-analysis. PLoS Med 2012;9:e1001162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Strunz EC, Addiss DG, Stocks ME, Ogden S, Utzinger J, Freeman MC.. Water, sanitation, hygiene, and soil-transmitted helminth infection: a systematic review and meta-analysis. PLoS Med 2014;11:e1001620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.United Nations Childrens Fund (UNICEF). One is Too Many: Ending Child Deaths from Pneumonia and Diarrhoea New York: UNICEF, 2016.
- 10. Rabie T, Curtis V.. Handwashing and risk of respiratory infections: a quantitative systematic review. Trop Med Int Health 2006;11:258–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stelmach RD, Clasen T, Household water quantity and health: a systematic review. Int J Environ Res Public Health 2015; 12: 5954–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spears D, Ghosh A, Cumming O.. Open defecation and childhood stunting in India: an ecological analysis of new data from 112 districts. PLoS One 2013;8:e73784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pickering AJ, Djebbari H, Lopez C, Coulibaly M, Alzua ML.. Effect of a community-led sanitation intervention on child diarrhoea and child growth in rural Mali: a cluster-randomised controlled trial. Lancet Glob Heal 2015;3:e701–711. [DOI] [PubMed] [Google Scholar]
- 14. Fuller JA, Villamor E, Cevallos W, Trostle J, Eisenberg J.. I get height with a little help from my friends: herd protection from sanitation on child growth in rural Ecuador. Int J Epidemiol 2016;45:460–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.NFHS-4. National Family Health Survey 4 2015–2016: India Fact Sheet Mumbai, India, 2016. http://rchiips.org/nfhs/factsheet_nfhs-4.shtml (1 May 2019, date last accessed).
- 16. Schmidt W-P, Cairncross S, Barreto ML, Clasen T, Genser B.. Recent diarrhoeal illness and risk of lower respiratory infections in children under the age of 5 years. Int J Epidemiol 2009;38:766–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Routray P, Schmidt W-P, Boisson S, Clasen T, Jenkins MW.. Socio-cultural and behavioural factors constraining latrine adoption in rural coastal Odisha: an exploratory qualitative study. BMC Public Health 2015;15:880.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reese H, Routray P, Torondel B. et al. Design and rationale of a matched cohort study to assess the effectiveness of a combined household-level piped water and sanitation intervention in rural Odisha, India. BMJ Open 2017;7:e012719.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arnold B, Arana B, Mäusezahl D, Hubbard A, Colford JM.. Evaluation of a pre-existing, 3-year household water treatment and handwashing intervention in rural Guatemala. Int J Epidemiol 2009;38:1651–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Diamond A, Sekhon J.. Genetic matching for estimating causal effects. Rev Econ Stat 2012;95:932–45. [Google Scholar]
- 21. Schmidt W-P, Luby SP, Genser B, Barreto ML, Clasen T.. Estimating the longitudinal prevalence of diarrhea and other episodic diseases: continuous versus intermittent surveillance. Epidemiology 2007;18:537–43. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization. The Treatment of Diarrhoea: A Manual for Physicians and Other Senior Health Workers, 4th Rev. Geneva, Switzerland, 2005. http://www.who.int/iris/handle/10665/43209 (5 May 2019, date last accessed).
- 23.World Health Organization/The United Nations Children’s Fund (UNICEF). Ending Preventable Child Deaths from Pneumonia and Diarrhoea by 2025: The integrated Global Action Plan for Pneumonia and Diarrhoea (GAPPD); 2013. https://www.who.int/maternal_child_adolescent/documents/global_action_plan_pneumonia_diarrhoea/en/ (5 May 2019, date last accessed).
- 24.MEASURE DHS. DHS Model Questionnaires http://dhsprogram.com/What-We-Do/Survey-Types/DHS-Questionnaires.cfm (14 May 2017, date last accessed).
- 25. Lipsitch M, Tchetgen ET, Cohen T.. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology 2010;21:383–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. de Onis M, Onyango AW, Van den Broeck J, Chumlea WC, Martorell R.. Measurement and standardization protocols for anthropometry used in the construction of a new international growth reference. Food Nutr Bull 2004;25(1 Suppl): S27–36. [DOI] [PubMed] [Google Scholar]
- 27.Cogill B. Anthropometric Indicators Measurement Guide. Revised Edition Washington, DC: Academy for Educational Development [AED], Food and Nutrition Technical Assistance, 2003: p. 92. http://www.developmentgateway.org/download/202582/anthro_2003.pdf
- 28.WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/Height-For-Age, Weight-For-Age, Weight-For-Length, Weight-For-Height and Body Mass Index-For-Age: Methods and development Geneva, 2006. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed8&NEWS=N&AN=2008031311.
- 29. Cheesbrough M, District Laboratory Practice in Tropical Countries. 2nd edn Cambridge: Cambridge University Press, 2009. [Google Scholar]
- 30. Funk AL, Boisson S, Clasen T, Ensink J.. Comparison of Kato-Katz, ethyl-acetate sedimentation, and Midi Parasep® in the diagnosis of hookworm, Ascaris and Trichuris infections in the context of an evaluation of rural sanitation in India. Acta Trop 2013;126:265–68. [DOI] [PubMed] [Google Scholar]
- 31. Díaz-Ordaz K, Kenward MG, Cohen A, Coleman CL, Eldridge S.. Are missing data adequately handled in cluster randomised trials? A systematic review and guidelines. Clin Trials 2014;11:590–600. [DOI] [PubMed] [Google Scholar]
- 32. Lüdtke O, Robitzsch A, Grund S, Lüdtke O, Robitzsch A, Grund S.. Multiple imputation of missing data in multilevel designs: a comparison of different strategies. Psychol Methods 2017;22:141–65. [DOI] [PubMed] [Google Scholar]
- 33. Enders CK, Mistler SA, Keller BT.. Multilevel multiple imputation: a review and evaluation of joint modeling and chained equations imputation. Psychol Methods 2016;21:222–40. [DOI] [PubMed] [Google Scholar]
- 34. Filmer D, Pritchett LH.. Estimating wealth effects without expenditure data—or tears: an application to education enrollments in states of India. Demography 2001;38:115–32. [DOI] [PubMed] [Google Scholar]
- 35. Bassani DG, Corsi DJ, Gaffey MF, Barros A.. Local distributions of wealth to describe health inequalities in india: a new approach for analyzing nationally representative household survey data, 1992–2008. PLoS One 2014;9:e110694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kolenikov S, Angeles G.. The Use of Discrete Data in PCA: Theory, Simulations, and Applications to Socioeconomic Indices. Chapel Hill: Chapel Hill Carolina Population Center, University of North Carolina, 2004, pp. 1–59. [Google Scholar]
- 37. Arnold BF, Khush RS, Ramaswamy P. et al. Causal inference methods to study nonrandomized, preexisting development interventions. Proc Natl Acad Sci U S A 2010;107:22605–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Overbo A, Williams AR, Evans B, Hunter PR, Bartram J.. On-plot drinking water supplies and health: a systematic review. Int J Hyg Environ Health 2016;219:317–30. [DOI] [PubMed] [Google Scholar]
- 39. Freeman MC, Garn JV, Sclar GD. et al. The impact of sanitation on infectious disease and nutritional status: a systematic review and meta-analysis. Int J Hyg Environ Health 2017;220:329–40. [DOI] [PubMed] [Google Scholar]
- 40. Humphrey JH. Child undernutrition, tropical enteropathy, toilets, and handwashing. Lancet 2009;374:1032–035. [DOI] [PubMed] [Google Scholar]
- 41. Luby SP, Rahman M, Arnold BF. et al. Effect of water quality, sanitation, handwashing and nutritional interventions on diarrhoea and child linear growth in rural Bangladesh: A cluster randomized trial. Lancet Glob Heal 2018;6:e302–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Null C, Stewart CP, Pickering AJ. et al. Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhoea and child growth in rural Kenya: a cluster randomised controlled trial. Lancet Glob Heal 2018;30490–0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sinha A, Nagel CL, Thomas E. et al. Assessing latrine use in rural India: A cross-sectional study comparing reported use and passive latrine use monitors. Am J Trop Med Hyg 2016;95:720–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Arnold BF, Galiani S, Ram PK. et al. Optimal Recall Period for Caregiver-reported Illness in Risk Factor and Intervention Studies: A Multicountry Study. Am J Epidemiol 2013;177:361–70. [DOI] [PubMed] [Google Scholar]
- 45. Coffey D, Gupta A, Hathi P, Spears D, Srivastav N, Vyas S, Culture and the Health Transition: Understanding Sanitation Behavior in Rural North India Working Paper. 2015. Available at: https://www.theigc.org/wp-content/uploads/2015/04/Coffey-et-al-2015-Working-Paper-1.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.