Abstract
Background
Work has been associated with cognitive health. We examined whether retirement from work is associated with a decrease in episodic memory and whether this effect differs when considering workers’ occupational class.
Methods
In this prospective study using the English Longitudinal Study of Ageing (ELSA), we examined 1629 persons aged 50–75 years who were in paid work at baseline. A two-slope random effects linear regression centred at retirement was used to study the effect of retirement on episodic memory. The potential effect modification by occupational class was examined.
Results
While memory trajectories show slightly decreasing memory scores before and afterretirement, the decreasing rates for both periods were similar [episodic memory β2b−β2a= −0.03, 95% confidence interval (CI) −0.08, 0.02]. When stratifying by occupational class, there was also no substantial difference in episodic memory trajectories before and after retirement. However, the lower occupational class showed a clear decline in episodic memory with time (pre-retirement β2a = −0.11, 95% CI −0.19, −0.03; post-retirement β2b = −0.13, 95% CI −0.19, −0.07) which was not evident for the higher occupational classes.
Conclusions
Our results show no observable difference in trajectories of change in episodic memory before and after retirement. However, the steeper memory decline in workers belonging to the lower occupational class may limit their prospect of prolonging their working lives. Hence enrichment programmes for the prevention of memory decline for these workers should be considered.
Keywords: Cognitive ageing, longitudinal, cohort studies, episodic memory, retirement, occupational class
Key Messages
In this prospective, population-based study we investigated the association of working status and memory scores in participants who were interviewed and tested every 2 years.
No difference in the rate of memory decline was seen before and after retirement for the whole population, nor in an analysis stratified by occupational class.
Stratification into occupational classes revealed different trajectories for each class: mostly stable memory scores before and after retirement for the professional and intermediate classes, and an indication that the lower occupational class had a steeper rate of memory decline than the higher occupational classes.
Introduction
Cognitive ageing is the decline in cognitive processing that occurs as people get older. Whereas some cognitive aspects such as verbal ability or cumulative knowledge increase with age, a decline occurs for processing-intensive tasks, such as speed of processing, working and long-term memory.1
Three different but correlated theories have steered investigations of work and cognition. The ‘use it or lose it’ hypothesis posits that the individual’s level of cognitive functioning depends on their current mental ability, and that those with higher levels of cognitive functioning throughout their lives maintain their cognition as they age. According to the cognitive reserve theory, stimulating experiences throughout the lifetime, such as educational or occupational attainment, can prevent or decelerate age-related cognitive decline by ‘optimizing performance through differential recruitment of brain networks, which reflect the use of alternate cognitive strategies’.2 Schooler’s theory of environmental influences on cognitive functioning suggests that complex environments have a positive effect, whereas simple environments have a negative effect on cognitive functioning.3
The work environment exposes individuals to social interactions, mental and physical demands and stress. Several studies have found that people with high mental demands at work or high job complexity have less cognitive impairment.4–9 The transition from work into retirement, when people are no longer exposed to the ‘work experience’, may thus affect cognition. A recent review stressed the need for further research on the impact of retirement on cognitive function, citing lack of prospective studies.10 Our study aimed to examine the role of retirement on episodic memory decline. Individual trajectories of memory test scores were analyzed over an 8-year period to examine the effect of retirement on episodic memory and the modifying effect of occupational class.
Methods
Study design
Our study examined data extracted from the English Longitudinal Study of Ageing (ELSA). ELSA is a prospective, population-based cohort study on people aged 50 and over living in private households in England and their partners. The aim of ELSA is to study health, economic status and quality of life among the elderly. The initial sample was drawn from households that responded to the Health Survey for England in 1998, 1999 or 2001. Core sample members were born on or before February 29, 1952, to ensure that they would be 50 or over at the beginning of ELSA wave 1, and were interviewed every 2 years.11 The individual response at wave 1 was 66%, and more information is available elsewhere.12 This study investigated participants from wave 1 to wave 5, spanning a time of 8 years (2002–10).
Standard protocol approvals, registrations and patient consent
Ethical approval for ELSA was obtained by the South Central- Berkshire Research Ethics Committee (reference # 12344) and for this study by the London School of Hygiene and Tropical Medicine Ethics Committee (approval# 09/H0505/124). Written consent was obtained for all study participants, either from the participants or from next of kin, relative, close friend or caregiver per the Mental Capacity Act (2005).
Participants
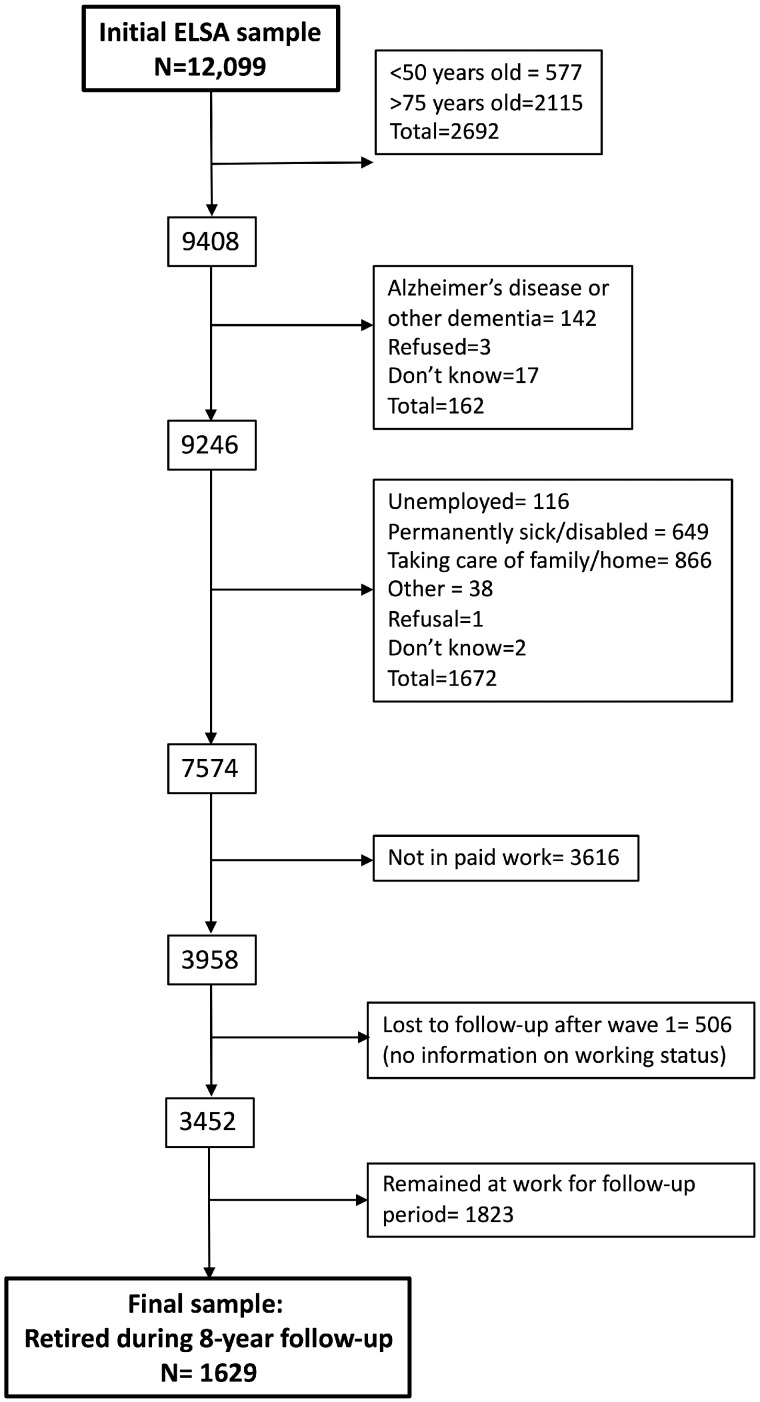
Participants were included in the study if they were between the ages of 50–75 and participated in paid work at the start of the study. Those who reported having dementia or Alzheimer’s disease diagnosis in the study period, and those who were unemployed, permanently sick or at home taking care of family at the start of the study, were excluded. Participants were also excluded if they were already retired at wave 1. At each wave, participants were asked if they had been involved in paid work. We included only participants who had retired at some point during the 8-year follow-up; 506 (13%) of the initially eligible participants were completely lost to follow-up after wave 1. Hence, their work status was not known after wave 1. They were more likely to be men (P = 0.02), to be younger (56.8 vs 58.6, P <0.001) and to be less educated (P = 0.003) than the participants who stayed in the study. Their baseline delayed word recall scores at baseline were also lower (5.86 vs 6.01, P = 0.06), but the other memory scores at baseline were equivalent.
In total 1629 participants who had retired during the 8-year follow-up period remained in the study. They averaged 4.4 interviews [standard deviation (SD) = 1.0, median = 5], of which 3.3 (SD = 1.3, median = 3) were done before retirement, and 1.7 (SD = 0.7, median = 1) occurred after retirement. Figure 1 shows a schematic of the process flow.
Figure 1.
Process flow of study participants.
Retirement age and ‘time until/since retirement’
We defined being retired as being ‘not in paid work’, as has been used in similar studies.13–16 Participants were coded as being ‘in paid work’ or ‘retired’ at each wave. The age of retirement was set as the participant’s age in the last wave in which they were still at work. The mean retirement age was 61.4 years (SD = 5.1, range 50–81).
Outcome measures
Our study focused on episodic memory, assessed through a test of verbal learning and recall. Several studies have found that episodic memory is among the first cognitive functions to decline with ageing.13,14 It has been argued that it is a more sensitive measure than other measures of cognitive function with a limited variability in scores.15 Three tests were used: immediate word recall, delayed word recall and total episodic memory scores, based on the Rey Auditory-Verbal Learning Test.16 In face-to-face interviews, participants were given a list of 10 common words and were asked to recall as many words as possible in any order (immediate word recall, range 0–10). After a period of about 5 min, the participants were asked to recall as many words as they could remember (delayed word recall, range 0–10). The episodic memory score was the sum of both test results. Points were only given to the correct number of words recalled. There were four alternative word lists, so that different lists could be given at different waves. For example for wave 1, the first member of the household to be tested was assigned a list at random and, where there was more than one member of the household in the ELSA sample, the remaining lists were also selected at random. For the next wave, the procedure was similar, but it excluded the list that the respondent had in wave 1.17 A more detailed description of the word recall test and its validity is available elsewhere.18,19
Covariates
All covariate information was taken at each wave through face-to-face interviews and self-completed questionnaires. Baseline age and sex were defined as a priori confounders. Occupational class was categorized into professional, intermediate and lower occupations, according to the National Statistics Socio-Economic Classification. Examples of occupations in the professional category are doctors, scientists/engineers and teachers. Policemen, administrative workers and clerks belong to the intermediate class and construction and maintenance workers, couriers and cooks are examples of those in the lower occupational class.
Self-reported health was obtained based on the questions from the Health and Retirement Study (waves 1, 2, 4 and 5) and from the Health Survey for England (wave 3). A higher score indicates poorer self-reported health. Depression was measured using the Center for Epidemiological Studies-Depression (CES-D) scale.20
Taking test practice effects into account is necessary for longitudinal cognitive studies,21 and we included a re-test effects term in the regression models following the recommendation by Vivot et al.22 We used the square root of the number of previous visits (e.g. 0, 1, 1.4, 1.7…) for re-test effects, considering the methods proposed by Vivot et al. and findings regarding re-test effects for a similar cohort using the same tests as ELSA.23
Statistical analysis
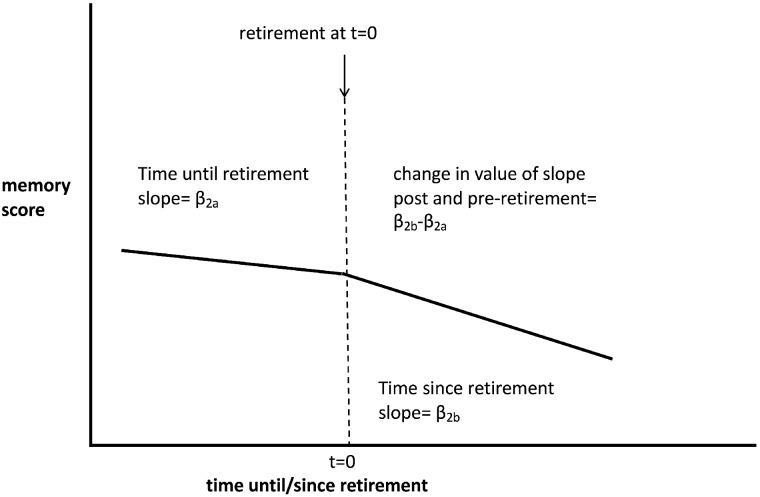
To explore the association between memory scores and covariates, we used mixed effects linear regression. Our regression analysis used all available scores, if they were available in more than one wave. We assessed the possibility of selective attrition bias by correlating the number of waves completed with covariates. We then applied a multivariate mixed effects two-slope linear model with the individual ID as a cluster. The individual’s retirement age was used as the centring point, similar to what has been done in similar studies,24,25 with one linear slope before retirement age and a separate linear slope after retirement. The change in the slope from time before retirement and time after retirement was then estimated. The equation is shown below and a schematic is portrayed in Figure 2.
Figure 2.
Schematic of statistical model.
Where t = 0 is the time at retirement:
To build the final model we used a forward elimination scheme, in which the model was built in a stepwise fashion with covariates which confounded the association between time to retirement and memory scores, whereas baseline age, sex and re-test effects were always kept in the model. The analysis was based on all available data, but the small proportion of missing data is unlikely to affect results.
Sensitivity analysis
All participants who were at work at baseline, including those who remained at work for the follow-up period, were included in a sensitivity analysis (N = 3445). The process flow is shown in Supplementary Figure 1, available as Supplementary data at IJE online. We used a multivariate mixed effects linear regression model with the individual ID as a cluster to model the association between working status (retired at some point within the follow-up period vs remaining at work) and memory scores. The model was adjusted for age, sex, re-test effects and for factors associated with working status and memory scores. All data analyses were performed with the statistical software STATA v.14.2 (StataCorp., College Station, TX).
Results
Participant characteristics
The participant characteristics at each wave are summarized in Table 1. About 35% of the participants retired at wave 2, 52% by wave 3, 73% by wave 4, and by the end of wave 5, all participants had retired. The proportion of participants suffering from diabetes or hypertension, who had a previous stroke or myocardial infarction, increased with increasing wave. Memory tests scores increased from wave 1 to wave 3, and decreased thereafter.
Table 1.
Distribution of time-varying covariates and memory scores
| Covariate | Wave 1 (0 years) N = 1629 | Wave 2 (2 years) N = 1505 | Wave 3 (4 years) N = 1411 | Wave 4 (6 years) N = 1365 | Wave 5 (8 years) N = 1370 |
|---|---|---|---|---|---|
| Mean age (SD) | 58.6 (5.3) | 60.9 (5.3) | 62.7 (5.3) | 64.7 (5.3) | 66.6 (5.1) |
| Sex | |||||
| Female | 847 (52.0%) | 788 (52.4%) | 746 (52.9%) | 715 (52.4%) | 719 (52.5%) |
| Male | 782 (48.9%) | 717 (47.6%) | 665 (47.1%) | 649 (47.6%) | 651 (47.5%) |
| Total | 1629 (100%) | 1505 (100%) | 1411 (100%) | 1364 (100%) | 1370 (100%) |
| Retired | |||||
| Yes | 0 (0%) | 533 (35.4%) | 729 (51.7%) | 994 (72.8%) | 1370 (100%) |
| No | 1629 (100%) | 972 (64.6%) | 682 (48.3%) | 371 (27.2%) | 0 (0%) |
| Total | 1629 (100%) | 1505 (100%) | 1411 (100%) | 1365 (100%) | 1370 (100%) |
| Diabetes | |||||
| Yes | 67 (4.1%) | 83 (5.5%) | 102 (7.2%) | 110 (8.1%) | 131 (9.6%) |
| No | 1562 (95.9%) | 1422 (94.5%) | 1309 (92.8%) | 1253 (91.9%) | 1239 (9.6%) |
| Total | 1629 (100%) | 1505 (100%) | 1411 (100%) | 1363 (100%) | 1370 (100%) |
| Hypertension | |||||
| Yes | 509 (31.3%) | 555 (36.9%) | 571 (40.5%) | 589 (43.2%) | 619 (45.2%) |
| No | 1120 (68.8%) | 950 (36.9%) | 840 (59.5%) | 775 (56.8%) | 751 (54.8%) |
| Total | 1629 (100%) | 1505 (100%) | 1411 (100%) | 1364 (100%) | 1370 (100%) |
| Stroke | |||||
| Yes | 15 (0.9%) | 24 (1.6%) | 28 (2.0%) | 33 (2.4%) | 40 (2.9%) |
| No | 1614 (99.1%) | 1481 (98.4%) | 1383 (98.0%) | 1330 (97.6%) | 1330 (97.1%) |
| Total | 1629 (100%) | 1505 (100%) | 1411 (100%) | 1363 (100%) | 1370 (100%) |
| Myocardial infarction | |||||
| Yes | 44 (2.7%) | 55 (3.7%) | 68 (4.8%) | 68 (5.0%) | 67 (4.9%) |
| No | 1585 (97.3%) | 1450 (96.4%) | 1343 (95.2%) | 1295 (95.0%) | 1303 (95.1%) |
| Total | 1629 (100%) | 1505 (100%) | 1411 (100%) | 1363 (100%) | 1370 (100%) |
| Depression score | 1.16 (1.67) | 1.28 (1.76) | 1.15 (1.69) | 1.12 (1.73) | 1.23 (1.81) |
| Total | 1592 | 1484 | 1395 | 1327 | 1330 |
| Self-reported health score | 1.64 (0.73) | 1.72 (0.77) | 1.96 (0.73) | 1.76 (0.77) | 1.77 (0.76) |
| Total | 1621 | 1499 | 1404 | 1340 | 1337 |
| Alcohol | |||||
| Abstainer | 538 (33.2%) | 466 (33.6%) | 436 (34.9%) | 410 (33.9%) | 503 (39.3%) |
| Light | 586 (36.1%) | 372 (26.8%) | 342 (27.4%) | 330 (27.3%) | 305 (23.8%) |
| Moderate | 498 (30.7%) | 549 (39.6%) | 472 (37.8%) | 470 (38.8%) | 471 (36.8%) |
| Total | 1622 (100%) | 1387 (100%) | 1250 (100%) | 1210 (100%) | 1279 (100%) |
| Education | |||||
| Low | 671 (41.2%) | 603 (40.1%) | 478 (33.9%) | 502 (36.9%) | 532 (39.0%) |
| Medium | 438 (26.9%) | 415 (27.6%) | 399 (28.3%) | 368 (27.0%) | 351 (25.8%) |
| High | 518 (31.8%) | 486 (32.3%) | 534 (37.9%) | 492 (36.1%) | 480 (35.2%) |
| Total | 1627 (100%) | 1504 (100%) | 1411 (100%) | 1362 (100%) | 1363 (100%) |
| Occupational class | |||||
| Professional/Managerial | 608 (38.6%) | 522 (34.7%) | 479 (34.0%) | 453 (33.2%) | 464 (33.9%) |
| Intermediate | 372 (23.6%) | 396 (26.4%) | 386 (27.4%) | 376 (27.6%) | 369 (27.0%) |
| Lower | 595 (37.8%) | 585 (38.9%) | 544 (38.6%) | 534 (39.1%) | 535 (39.1%) |
| Total | 1575 (100%) | 1503 (100%) | 1409 (100%) | 1363 (100%) | 1368 (100%) |
| Memory tests | |||||
| Immediate recall | 6.01 (1.61) | 6.15 (1.59) | 6.15 (1.65) | 6.13 (1.60) | 6.11 (1.67) |
| Delayed recall | 4.64 (1.92) | 4.86 (1.90) | 4.96 (1.91) | 4.87 (1.90) | 4.85 (2.00) |
| Episodic memory | 10.66 (3.20) | 11.02 (3.18) | 11.10 (3.25) | 11.00 (3.25) | 10.96 (3.41) |
| Total | 1602 | 1498 | 1401 | 1339 | 1333 |
Most participants participated in testing at all waves (68.5%) (Table 2). Participation tended to be lower for less educated participants and for those in the lowest occupational class.
Table 2.
Characteristics of participants at baseline and their participation in interviewsa
| Covariate at baseline | Number of total interviews completed |
|||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | P-valueb | |
| Number of participants | 20 (1.2%) | 115 (7.1%) | 172 (10.6%) | 205 (12.6%) | 1116 (68.5%) | |
| (Total = 1629) | ||||||
| Mean age (SD) | 58.7 (4.0) | 60.2 (5.3) | 59.1 (5.5) | 59.0 (5.5) | 58.3 (5.2) | 0.40c |
| Female | 9 (45.0%) | 55 (47.8%) | 81 (47.1%) | 103 (50.2%) | 599 (53.7%) | 0.36 |
| Diabetes | ||||||
| Yes | 0 (0%) | 6 (5.2%) | 7 (4.1%) | 9 (4.4%) | 45 (4.0%) | 0.87 |
| Hypertension | ||||||
| Yes | 7 (35%) | 41 (35.7%) | 58 (33.7%) | 70 (34.2%) | 332 (29.8%) | 0.46 |
| Stroke | ||||||
| Yes | 0 (0%) | 2 (1.7%) | 2 (1.2%) | 4 (2.0%) | 7 (0.6%) | 0.33 |
| Myocardial infarction | ||||||
| Yes | 3 (5.7%) | 12 (5.0%) | 24 (4.4%) | 41 (4.8%) | 222 (4.0%) | 0.72 |
| Depression score | 0.87 (1.25) | 1.32 (1.83) | 1.51 (2.01) | 1.24 (1.75) | 1.09 (1.57) | <0.001c |
| Self-reported health score | 2.05 (0.78) | 1.62 (0.73) | 1.70 (0.77) | 1.70 (0.74) | 1.61 (0.72) | <0.001c |
| Alcohol | ||||||
| Abstainer | 8 (42.1%) | 38 (33.0%) | 63 (36.8%) | 67 (33.3%) | 362 (32.4%) | 0.48 |
| Light drinker | 9 (43.4%) | 38 (33.0%) | 56 (32.8%) | 66 (32.8%) | 417 (37.4%) | |
| Moderate | 2 (10.5%) | 39 (33.9%) | 52 (30.4%) | 68 (33.8%) | 337 (30.3%) | |
| Drinker | ||||||
| Education | ||||||
| Low | 15 (79.0%) | 65 (56.5%) | 88 (51.2%) | 97 (47.6%) | 405 (36.3%) | <0.001 |
| Medium | 1 (5.3%) | 23 (20.0%) | 40 (23.3%) | 50 (24.5%) | 324 (29.0%) | |
| High | 3 (15.8%) | 27 (23.5%) | 44 (25.6%) | 57 (28.0%) | 387 (34.7%) | |
| Occupational class | ||||||
| Professional/ | 5 (31.3%) | 34 (31.2%) | 54 (34.2%) | 63 (32.1%) | 452 (41.2%) | 0.003 |
| Managerial | ||||||
| Intermediate | 4 (25.0%) | 24 (22.0%) | 30 (19.0%) | 42 (21.4%) | 272 (24.8%) | |
| Lower | 7 (43.8%) | 51 (46.8%) | 74 (46.8%) | 91 (46.4%) | 372 (33.9%) | |
Five interviews completed means full participation, including testing, at waves 1–5; 1 interview completed means tests completed for only one wave.
Chi square.
One-way analysis of variance (ANOVA).
Univariate analysis
Results from univariate linear regressions for the memory test scores are presented in Table 3. Delayed memory recall scores slightly increased before retirement and remained stable after retirement, but no effect was observed for immediate memory recall scores. All covariates were associated either to immediate or to delayed recall scores, and therefore they were all included when building the model.
Table 3.
Crude regression coefficients (ß) for association of participant characteristics with memory test outcomes
| Characteristic | Immediate word recall |
Delayed word recall |
Episodic memory |
|||
|---|---|---|---|---|---|---|
| ß (95% CI) | P-valuea | ß (95% CI) | P-valuea | ß (95% CI) | P-valuea | |
| Baseline age | −0.06 | <0.001 | −0.09 | <0.001 | −0.15 | <0.001 |
| (−0.07, −0.05) | (−0.10, −0.07) | (−0.17, −0.13) | ||||
| Sex | 0.49 | <0.001 | 0.67 | <0.001 | 1.16 | <0.001 |
| (ref. = male) | (0.37, 0.61) | (0.52, 0.82) | (0.90, 1.40) | |||
| Education (ref. =.less than O-level or equivalent) | ||||||
| O-level or equivalent | 0.47 | <0.001 | 0.52 | <0.001 | 0.93 | <0.001 |
| (0.35, 0.60) | (0.37, 0.67) | (0.68, 1.19) | ||||
| Higher than A-level | 0.75 | <0.001 | 0.86 | <0.001 | 1.53 | <0.001 |
| (0.63, 0.87) | (0.72, 1.01) | (1.29, 1.78) | ||||
| Alcohol (ref. = abstainer) | ||||||
| Light drinker | 0.13 | 0.02 | 0.11 | 0.06 | 0.22 | 0.03 |
| (0.02, 0.23) | (−0.01, 0.23) | (0.02, 0.42) | ||||
| Moderate drinker | 0.31 | <0.001 | 0.37 | <0.001 | 0.65 | <0.001 |
| (0.20, 0.42) | (0.24, 0.51) | (0.42, 0.87) | ||||
| Diabetes (ref. =.no) | −0.47 | <0.001 | −0.50 | <0.001 | −0.92 | <0.001 |
| (−0.66, −0.29) | (−0.72, −0.29) | (−1.28, −0.56) | ||||
| Stroke (ref. = no) | −0.71 | <0.001 | −0.56 | 0.003 | −1.26 | <0.001 |
| (−1.03, −0.39) | (−0.93, −0.19) | (−1.77, −0.64) | ||||
| Hypertension (ref. = no) | −0.12 | 0.01 | −0.08 | 0.09 | −0.20 | 0.04 |
| (−0.22, −0.03) | (−0.21, 0.02) | (−0.40, −0.01) | ||||
| Cardiac infarct (ref. =.no) | −0.05 | <0.001 | −0.18 | 0.21 | −0.39 | 0.12 |
| (−0.07, −0.03) | (−0.47, 0.10) | (−0.87, 0.10) | ||||
| Self-reported health | −0.15 | <0.001 | −0.16 | <0.001 | −0.27 | <0.001 |
| (−0.20, −0.10) | (−0.21, −0.10) | (−0.37, −0.17) | ||||
| CES-D Depression | −0.05 | <0.001 | −0.05 | <0.001 | −0.10 | <0.001 |
| (−0.08, −0.03) | (−0.07, −0.02) | (−0.14, −0.05) | ||||
| Occupational class (ref. = professional) | ||||||
| Intermediate | −0.31 | <0.001 | −0.32 | <0.001 | −0.61 | <0.001 |
| (−0.44, −0.18) | (−0.49, −0.19) | (−0.87, −0.36) | ||||
| Lower | −0.61 | <0.001 | −0.68 | <0.001 | −1.19 | <0.001 |
| (−0.72, −0.49) | (−0.82, −0.54) | (−1.44, −0.95) | ||||
| Time until retirement (years) | 0.01 | 0.58 | 0.04 | 0.02 | 0.05 | 0.08 |
| (−0.02, 0.04) | (0.01, 0.08) | (−0.01, 0.12) | ||||
| Time since retirement (years) | −0.01 | 0.21 | −0.01 | 0.09 | −0.02 | 0.12 |
| (−0.02, 0.01) | (−0.03, 0.00) | (−0.05, 0.01) | ||||
| Square root of previous tests taken | 0.05 | 0.04 | 0.11 | <0.001 | 0.15 | <0.001 |
| (0.00, 0.09) | (0.07, 0.16) | (0.07, 0.23) | ||||
P-value partial F test.
Multivariate analysis
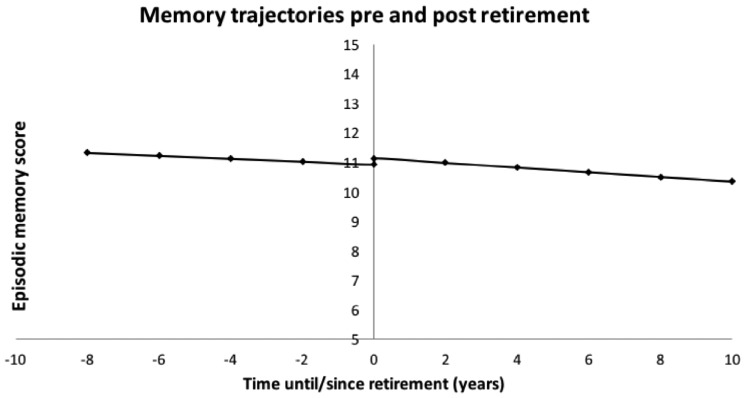
After adjusting for baseline age, sex, depression, alcohol, education and occupational class, there was a slight indication that memory scores decreased slightly with time preceding retirement (episodic memory β2a = −0.05, 95% CI −0.10, 0.01) (Table 4). After retirement, memory scores continued to decrease with time (episodic memory β2b = −0.08, 95% CI −0.12, −0.04). There was an indication of a slightly steeper negative slope for the time before retirement compared with the time after retirement for the delayed word recall scores (delayed word recall β2b −β2a = −0.03, 95% CI −0.06, 0.00), but overall no difference observed for the episodic memory scores (episodic memory β2b −β2a = −0.03, 95% CI −0.08, 0.02). Figure 3 shows the trajectory for the episodic memory scores before and after retirement. Results were alike when stratifying by gender (not shown).
Table 4.
Regression coefficients (ß) for a two-slope multivariate linear regression with spline at retirement
| ßa (95% CI) | P-value | ßb (95% CI) | P-value | ßc (95% CI) | P-value | |
|---|---|---|---|---|---|---|
| Immediate word recall | ||||||
| Time until retirement | −0.06 | <0.001 | −0.04 | 0.006 | −0.02 | 0.14 |
| (β2a) | (−0.09, −0.04) | (−0.07, −0.01) | (−0.05, 0.01) | |||
| Time since retirement | −0.06 | <0.001 | −0.04 | <0.001 | −0.02 | 0.03 |
| (β2b) | (−0.08, −0.04) | (−0.06, −0.02) | (−0.04, 0.00) | |||
| Difference in slope | 0.00 | 0.73 | 0.00 | 0.91 | 0.00 | 0.89 |
| (β2b-β2a) | (−0.02, 0.03) | (−0.03, 0.03) | (−0.03, 0.03) | |||
| Delayed word recall | ||||||
| Time until retirement | −0.08 | <0.001 | −0.05 | 0.002 | −0.03 | 0.08 |
| (β2a) | (−0.11, −0.05) | (−0.08, −0.02) | (−0.06, 0.00) | |||
| Time since retirement | −0.10 | <0.001 | −0.07 | <0.001 | −0.06 | <0.001 |
| (β2b) | (−0.12, −0.07) | (−0.10, −0.05) | (−0.08, −0.03) | |||
| Difference in slope | −0.02 | 0.25 | −0.03 | 0.09 | −0.03 | 0.05 |
| (β2b-β2a) | (−0.05, 0.01) | (−0.05, 0.00) | (−0.06, 0.00) | |||
| Episodic memory | ||||||
| Time until retirement | −0.14 | <0.001 | −0.08 | 0.002 | −0.05 | 0.06 |
| (β2a) | (−0.19, −0.08) | (−0.14, −0.03) | (−0.10, 0.01) | |||
| Time since retirement | −0.15 | <0.001 | −0.11 | <0.001 | −0.08 | <0.001 |
| (β2b) | (−0.15, −0.07) | (−0.15, −0.07) | (−0.12, −0.04) | |||
| Difference in slope | −0.01 | 0.57 | −0.02 | 0.27 | −0.03 | 0.21 |
| (β2b-β2a) | (−0.06, 0.04) | (−0.08, 0.02) | (−0.08, 0.02) | |||
Adjusted for re-test effects.
Adjusted by baseline age, sex and re-test effects.
Adjusted by baseline age, sex, re-test effects, depression, alcohol, education and occupational class.
Figure 3.
Trajectories of episodic memory estimated by the two-slope mixed effects multivariate model.
Results were similar when restricting only those who retired at or before 65 years of age in the analysis (Table 5). Here, pre- and post-retirement slopes were comparable for all memory measures.
Table 5.
Regression coefficients (ß) for a two-slope multivariate linear regression with spline at retirement (retirement age ≤65 years)
| ßa (95% CI) | P-value | |
|---|---|---|
| Immediate word recall | ||
| Time until retirement | −0.03 | 0.05 |
| (β2a) | (-0.06, 0.00) | |
| Time since retirement | −0.02 | 0.04 |
| (β2b) | (-0.04, 0.00) | |
| Difference in slope | 0.01 | 0.74 |
| (β2b-β2a) | (-0.02, 0.03) | |
| Delayed word recall | ||
| Time until retirement | −0.04 | 0.03 |
| (β2a) | (-0.07, 0.00) | |
| Time since retirement | −0.06 | <0.001 |
| (β2b) | (-0.08, -0.04) | |
| Difference in slope | −0.03 | 0.12 |
| (β2b-β2a) | (-0.06, 0.01) | |
| Episodic memory | ||
| Time until retirement | −0.06 | 0.02 |
| (β2a) | (-0.12, 0.01) | |
| Time since retirement | −0.08 | <0.001 |
| (β2b) | (-0.12, -0.04) | |
| Difference in slope | −0.02 | 0.47 |
| (β2b-β2a) | (-0.07, 0.03) | |
Adjusted by baseline age, sex, re-test effects, depression, alcohol, education and occupational class.
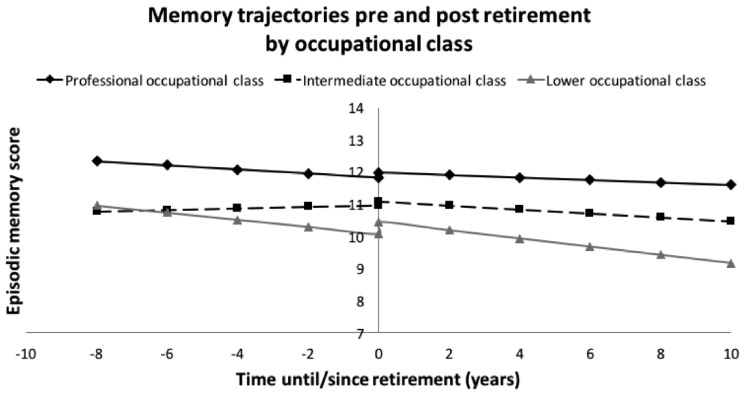
When restricting the analysis to those in the professional occupational class (Table 6, Figure 4), immediate word recall and episodic memory scores were stable before retirement. The delayed word recall scores might have been already decreasing before retirement (β2a = −0.04, 95% CI −0.09, 0.01), but after retirement there is evidence that they were decreasing β2b = −0.05, 95% CI −0.09, −0.01).No association was seen for immediate word recall scores after retirement, which were reflected in the results for episodic memory (β2b = −0.04, 95% CI −0.10, 0.03). There were no observed differences in the slopes before and after retirement for any of the memory scores.
Table 6.
Regression coefficients (ß) for a two-slope multivariate linear regression with spline at retirement, by occupational class
| Immediate word recall |
Delayed word recall |
Episodic memory |
||||
|---|---|---|---|---|---|---|
| ßa (95% CI) | P-value | ßa (95% CI) | P-value | ßa (95% CI) | P-value | |
| Professional/managerial occupational classb | ||||||
| Time until retirement | −0.02 | 0.30 | −0.04 | 0.11 | −0.06 | 0.14 |
| (β2a) | (−0.06, 0.02) | (−0.09, 0.01) | (−0.14, 0.02) | |||
| Time since retirement | 0.02 | 0.37 | −0.05 | 0.007 | −0.04 | 0.23 |
| (β2b) | (−0.02, 0.05) | (−0.09, −0.01) | (−0.10, 0.03) | |||
| Difference in slope | 0.04 | 0.14 | −0.02 | 0.53 | 0.02 | 0.64 |
| (β2b-β2a) | (−0.01, 0.08) | (−0.07, 0.03) | (−0.06, 0.11) | |||
| Intermediate occupational class | ||||||
| Time until retirement | 0.02 | 0.43 | 0.01 | 0.68 | 0.03 | 0.64 |
| (β2a) | (−0.03, 0.08) | (−0.05, 0.08) | (−0.08, 0.13) | |||
| Time since retirement | −0.01 | 0.59 | −0.04 | 0.07 | −0.06 | 0.11 |
| (β2b) | (−0.05, 0.03) | (−0.09, 0.00) | (−0.14, 0.01) | |||
| Difference in slope | −0.03 | 0.27 | −0.06 | 0.07 | −0.09 | 0.10 |
| (β2b−β2a) | (−0.09, 0.03) | (−0.12, 0.01) | (−0.19, 0.02) | |||
| Lower occupational classb | ||||||
| Time until retirement | −0.05 | 0.02 | −0.06 | 0.02 | −0.11 | 0.01 |
| (β2a) | (−0.09, −0.01) | (−0.11, −0.01) | (−0.19, −0.03) | |||
| Time since retirement | −0.05 | <0.001 | −0.07 | <0.001 | −0.13 | <0.001 |
| (β2b) | (−0.08, −0.02) | (−0.11, −0.04) | (−0.19, −0.07) | |||
| Difference in slope | 0.00 | 0.84 | −0.01 | 0.57 | −0.02 | 0.67 |
| (β2b−β2a) | (−0.05, 0.04) | (−0.07, 0.04) | (−0.10, 0.07) | |||
Adjusted by baseline age, sex, re-test effects, depression, alcohol, education and occupational class.
Model did not include jump in intercept (β02).
Figure 4.
Trajectories of episodic memory preceding and following retirement, by occupational class.
Those in the intermediate occupational class had stable memory scores before retirement (episodic memory β2a = 0.03, 95% CI −0.08, 0.13). After retirement, delayed word recall scores may have been slightly decreasing, but overall, the decreasing trend in episodic memory scores after retirement was not significant (β2b = −0.06, 95% CI −0.14, 0.01). There was only a weak indication, if at all, that the post-retirement slope was negative compared with the pre-retirement slope (episodic memory β2b −β2a = −0.09, 95% CI −0.19, 0.02).
Those in the lower occupational class had clearly decreasing pre-retirement and post-retirement scores in all memory tests (episodic memory β2a = −0.11, 95% CI −0.19, −0.03; β2b = −0.13, 95% CI −0.19, −0.07). However, there was no difference in the slopes before and after retirement (episodic memory β2b −β2a = −0.02, 95% CI −0.10, 0.07).
Sensitivity analysis
In the sensitivity analysis, there was no association between the effect of retirement or of remaining in work on episodic memory (ß= −0.01, 95% CI −0.14, 0.12). Results can be seen in Supplementary material (Supplementary Tables 1–3, available as Supplementary data at IJE online).
Discussion
Our study showed equally declining memory scores preceding and following retirement. These memory score trajectories depended on occupational class: those in the professional class had relatively stable episodic memory scores before and after retirement. Those in the intermediate class had stable pre-retirement scores and if at all, a slight decrease of scores afterretirement. Those in the lower occupational class had equally decreasing memory scores before retirement and after retirement, but the rate of their memory decline was faster than for the higher classes. Our sensitivity analysis investigating the association of retirement with episodic memory scores in the larger cohort of worker,s in which some retired and some remained at work, also corroborated the lack of an association between retiring and memory decline.
Olaya et al. 2017 mapped trajectories of episodic memory by age in the ELSA population, and found that among other factors, wealth and education were associated with more favourable trajectories.26 Similarly, our data indicate that higher occupational status is associated with better memory scores. The findings of this study are similar to those of Coe and Zamarro,27 de Grip et al.28 and Roberts et al.,29 who did not find an association between retirement and episodic memory. However, several studies (Adam et al.,30 Wickrama et al.,31 Bonsang et al.15 and Mazzona and Perachi32) found that retirees had lower episodic memory scores than those in work. Coe et al.33 studied the effect of occupation and found an increase in memory scores after retirement for blue-collar workers but not for white-collar workers. Differences in cohorts and in the methodology used might explain the differences in our results.
It has been reported that individuals with higher education and socioeconomic status are more likely to develop engaged lifestyles that help maintain intellectual functioning later in life.34 Hultsch et al. 1999 found that intellectually engaging activities buffer against decline in cognitive function, and postulated that high-ability individuals lead intellectually active lives until cognitive decline in old age limits their activities.35 Furthermore, workers in the higher occupational classes experience greater mental demands and control than those in the lower ones.36,37 Then et al. (2014) also found an association between high mental demands at work and better cognitive functioning in retirees of older age.38 Our findings suggest rather constant episodic memory trajectories independent of retirement for the professional and intermediate occupational classes, and seem to support the cognitive reserve theory.
Our findings should be considered in light of the study’s limitations. Although cognition is a multifaceted construct, this study only focused on episodic memory. Nonetheless, episodic memory has been found to be a central factor in cognitive ageing.39 Our model did not include measures of social engagement or leisure activities, yet these may affect episodic memory before or after retirement. Individuals who were lost to follow-up had lower memory scores at the start of the study. To consider the effect of attrition, a subgroup of participants who did not participate in all waves were analysed, yielding similar results (Supplementary Table 4, available as Supplementary data at IJE online). Depression and alcohol consumption were considered confounders and were included in the models, although it is plausible that they could also be the reason for or the consequence of retirement. We also estimated models without these covariates, with similar results (data not shown). There are other alternatives for adjusting for practice effects, such as using the previous number of tests or considering a boost in performance only after initial testing.22 However, we believe that our method using the square root of the previous test was appropriate, as this would allow for the possibility for the participants to learn more at each test but with reduced results over time. For a similar cohort using the same tests as ELSA, there was evidence that despite well-matched alternate tests, there were still practice effects seen for subsequent waves, especially for memory measures.23 Because individuals who were unemployed, permanently ill or were taking care of family at baseline were excluded, this study is not generalizable to this population. Since only 4% of our population was 70 years or older at baseline, our study may also not be generalizable to this older segment of the population. However, this study is generalizable to healthy working populations between 50 and 70 years of age. In addition, our results reflect the role of retirement in an English setting, and thus may be generalizable only to countries with a similar population and social structure.
The current trend is to delay retirement age, but these results show that there is no cognitive benefit by remaining at work as measured by episodic memory. However, people working in occupations characterized by manual labour appeared to experience a more rapid memory decline with time than those in the higher occupational classes. As a consequence, these workers may face cognitive and, more specifically, memory limitations, as their working life progresses. Governments, policy makers and employers should hence consider providing cognitive enrichment programmes to potentially affected workers, to help prevent or slow their episodic memory decline.
Funding
This work was supported by the English Longitudinal Study of Ageing (ELSA) and has been developed by a team of researchers based at University College London, NatCen Social Research and the Institute for Fiscal Studies. ELSA is supported by the National Institute on Aging and a consortium of UK government departments coordinated by the Office for National Statistics. The developers and funders of ELSA and the UK Data Archive do not bear any responsibility for the analyses or interpretations presented here. No funding (industrial, governmental or institutional) for this study was received.
Supplementary Material
Acknowledgements
The data were made available through the UK Data Archive. ELSA was developed by a team of researchers based at the NatCen Social Research, University College London and the Institute for Fiscal Studies.
Author Contributions
K.R.S., data analysis and interpretation, research conduct; A.S., adviser for data analysis and interpretation; J.H. adviser for study design and data analysis; A.K., adviser for data analysis; K.P., primary adviser for data analysis and interpretation.
Conflict of interest: None declared.
References
- 1. Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK.. Models of visuospatial and verbal memory across the adult life span. Psychol Aging 2002;17:299. [PubMed] [Google Scholar]
- 2. Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc 2002;8:448–60. [PubMed] [Google Scholar]
- 3. Schooler C, Mulatu MS, Oates G.. The continuing effects of substantively complex work on the intellectual functioning of older workers. Psychol Aging 1999;14:483–506. [DOI] [PubMed] [Google Scholar]
- 4. Bosma H, van Boxtel MP, Ponds RW, Houx PJ, Burdorf A, Jolles J.. Mental work demands protect against cognitive impairment: MAAS prospective cohort study. Exp Aging Res 2003;29:33–45. [DOI] [PubMed] [Google Scholar]
- 5. Potter GG, Helms MJ, Plassman BL.. Associations of job demands and intelligence with cognitive performance among men in late life. Neurology 2008;70:1803–08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Andel R, Kåreholt I, Parker MG, Thorslund M, Gatz M.. Complexity of primary lifetime occupation and cognition in advanced old age. J Aging Health 2007;19:397–415. [DOI] [PubMed] [Google Scholar]
- 7. Fisher GG, Chaffee DS, Tetrick LE, Davalos DB, Potter GG.. Cognitive functioning, aging, and work: a review and recommendations for research and practice. J Occup Health Psychol 2017;22:314.. [DOI] [PubMed] [Google Scholar]
- 8. Seidler A, Nienhaus A, Bernhardt T, Kauppinen T, Elo A, Frölich L.. Psychosocial work factors and dementia. Occup Environ Med 2004;61:962–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Then FS, Luck T, Luppa M.. Systematic review of the effect of the psychosocial working environment on cognition and dementia. Occup Environ Med 2014;71:358–65. [DOI] [PubMed] [Google Scholar]
- 10. Meng A, Nexø MA, Borg V.. The impact of retirement on age related cognitive decline–a systematic review. BMC Geriatr 2017;17:160.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marmot M, Oldfield Z, Clemens S. et al. English Longitudinal Study of Ageing: Waves 0–7, 1998–2015. 27th edn. Colchester, UK: UK Data Service, 2015.
- 12. Steptoe A, Breeze E, Banks J, Nazroo J.. Cohort Profile: The English Longitudinal Study of Ageing. Int J Epidemiol 2013;42:1640–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Petersen RC, Smith G, Kokmen E, Ivnik RJ, Tangalos EG.. Memory function in normal aging. Neurology 1992;42:396.. [DOI] [PubMed] [Google Scholar]
- 14. Nilsson LG. Memory function in normal aging. Acta Neurol Scand 2003;179:7–13. [DOI] [PubMed] [Google Scholar]
- 15. Bonsang E, Adam S, Perelman S.. Does retirement affect cognitive functioning? J Health Econ 2012;31:490–501. [DOI] [PubMed] [Google Scholar]
- 16. Rey A. L’examen clinique en psychologie [The Clinical Psychological Examination]. Paris: Presses Universitaires de France, 1964. [Google Scholar]
- 17. Huppert FA, Gardener E, McWilliams B.. Retirement, Health, and Relationships of the Older Population in England: The 2004 English Longitudinal Study of Ageing. London: The Institute for Fiscal Studies, 2006. [Google Scholar]
- 18. Ofstedal MBF, Gwenith G, Herzog AR.. Documentation of cogntive functioning measures in the Health and Retirement Study. Survey Research Center, University of Michingan, 2005DR-006. [Google Scholar]
- 19. Herzog AR, Wallace RB.. Measures of cognitive functioning in the AHEAD Study. J Gerontol B Psychol Sci Soc Sci 1997;52:37–48. [DOI] [PubMed] [Google Scholar]
- 20. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401. [Google Scholar]
- 21. Salthouse TA, Schroeder DH, Ferrer E.. Estimating retest effects in longitudinal assessments of cognitive functioning in adults between 18 and 60 years of age. Dev Psychol 2004;40:813.. [DOI] [PubMed] [Google Scholar]
- 22. Vivot A, Power MC, Glymour MM. et al. Jump, hop, or skip: modeling practice effects in studies of determinants of cognitive change in older adults. Am J Epidemiol 2016;183:302–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rodgers WL, Ofstedal MB, Herzog AR.. Trends in scores on tests of cognitive ability in the elderly US population, 1993–2000. J Gerontol B Psychol Sci Soc Sci 2003;58:S338–46. [DOI] [PubMed] [Google Scholar]
- 24. Finkel D, Andel R, Gatz M, Pedersen NL.. The role of occupational complexity in trajectories of cognitive aging before and after retirement. Psychol Aging 2009;24:563–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Andel R, Infurna FJ, Hahn Rickenbach EA, Crowe M, Marchiondo L, Fisher GG.. Job strain and trajectories of change in episodic memory before and after retirement: results from the Health and Retirement Study. J Epidemiol Community Health 2015;69:442–46. [DOI] [PubMed] [Google Scholar]
- 26. Olaya B, Bobak M, Haro JM, Demakakos P.. Trajectories of verbal episodic memory in middle‐aged and older adults: evidence from the English Longitudinal Study of Ageing. J Am Geriatr Soc 2017;65:1274–81. [DOI] [PubMed] [Google Scholar]
- 27. Coe NB, Zamarro G.. Retirement effects on health in Europe. J Health Econ 2011;30:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. de Grip A, Dupuy A, Jolles J, van Boxtel M.. Retirement and cognitive development in the Netherlands: are the retired really inactive? Econ Hum Biol 2015;19:157–69. [DOI] [PubMed] [Google Scholar]
- 29. Roberts BA, Fuhrer R, Marmot M, Richards M.. Does retirement influence cognitive performance? The Whitehall II Study. J Epidemiol Community Health 2011;65:958–63. [DOI] [PubMed] [Google Scholar]
- 30. Adam S, Bonsang E, Germain S, Perelman S.. Retirement and Cognitive Reserve: A Stochastic Frontier Approach Applied to Survey Data CREPP, HEC Management School, University of Liege, 2007.
- 31. Wickrama KKAS, O'Neal CW.. The influence of working later in life on memory functioning. Adv Life Course Res 2013;18:288–95. [DOI] [PubMed] [Google Scholar]
- 32. Mazzonna F, Peracchi F.. Ageing, cognitive abilities and retirement. Eur Econ Rev 2012;56:691–710. [Google Scholar]
- 33. Coe NB, von Gaudecker HM, Lindeboom M, Maurer J.. The effect of retirement on cognitive functioning. Health Econ 2012;21:913–27. [DOI] [PubMed] [Google Scholar]
- 34. Gold DP, Andres D, Etezadi J, Arbuckle T, Schwartzman A, Chaikelson J.. Structural equation model of intellectual change and continuity and predictors of intelligence in older men. Psychol Aging 1995;10:294–303. [DOI] [PubMed] [Google Scholar]
- 35. Hultsch DF, Hertzog C, Small BJ, Dixon RA.. Use it or lose it: engaged lifestyle as a buffer of cognitive decline in aging? Psychol Aging 1999;14:245–63. [DOI] [PubMed] [Google Scholar]
- 36. Raittila S, Rahkonen O, Lahelma E, Alho J, Kouvonen A.. Occupational class differences in trajectories of working conditions in women. Int J Environ Res Public Health 2017;14:790.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schrijvers CTM, van de MH, Stronks K, Mackenbach JP.. Socioeconomic inequalities in health in the working population: the contribution of working conditions. Int J Epidemiol 1998;27:1011–18. [DOI] [PubMed] [Google Scholar]
- 38. Then FS, Luck T, Luppa M. et al. Association between mental demands at work and cognitive functioning in the general population—results of the health study of the Leipzig Research Center For Civilization Diseases (LIFE). J Occup Med Toxicol 2014;9:23.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bäckman L, Jones S, Berger A-K, Laukka EJ, Small BJ.. Cognitive impairment in preclinical Alzheimer's disease: a meta-analysis. Neuropsychology 2005;19:520–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.